Synthesis, Characterization and Sensor Application of Novel PCL-Based Triblock Copolymers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Measurement
2.3. Construction Sensor and Electrochemical Measurement
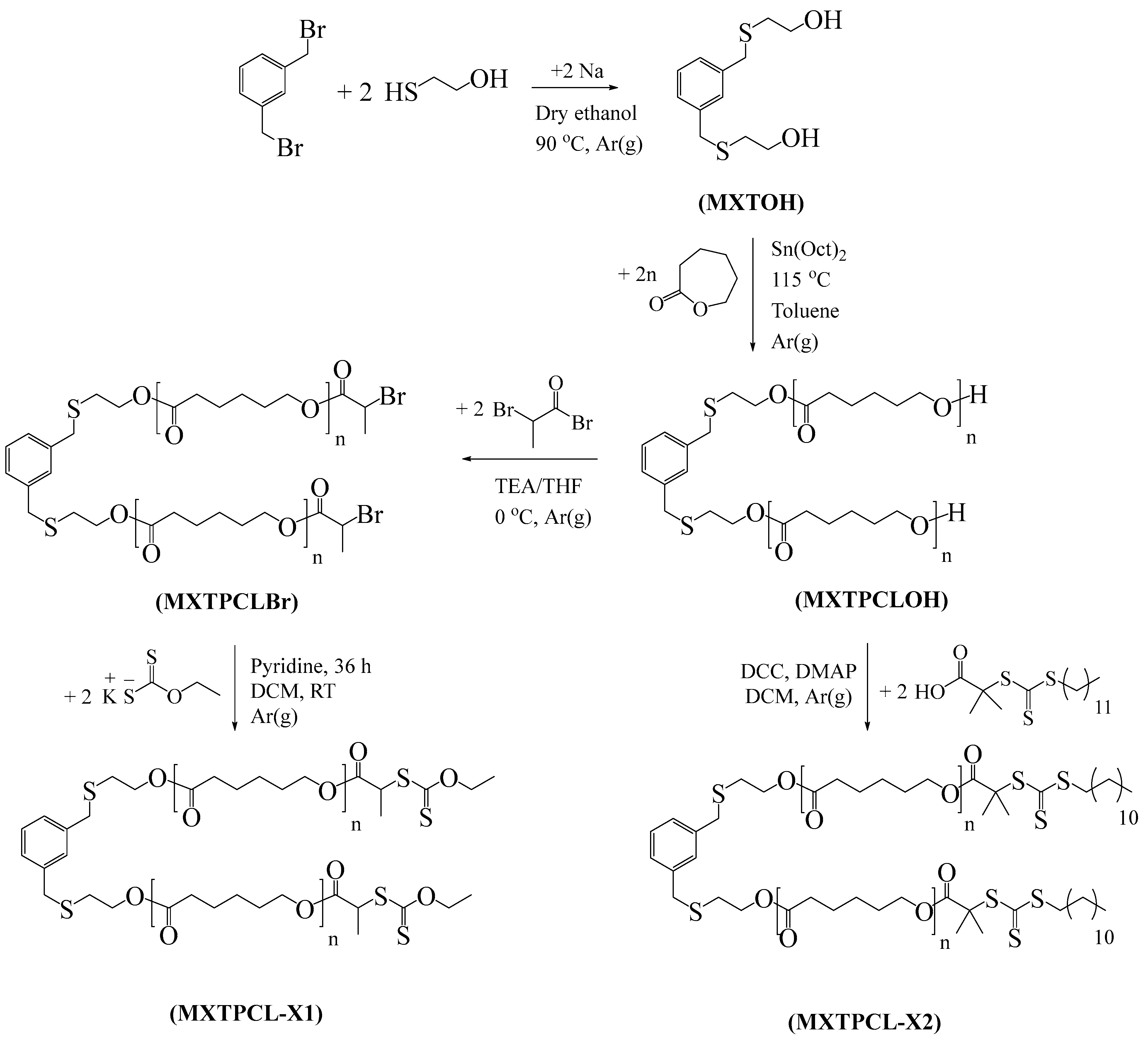
2.4. Synthesis of ROP Initiator (MXTOH)
2.5. Synthesis of MXPCLOH
2.6. Synthesis of MXPCLBr
2.7. Synthesis of Dixanthate Terminated PCL-Based Macro-RAFT CTA (MXTPCL-X1)
2.8. Synthesis of Trithiocarbonate-Capped PCL-Based Macro-RAFT CTA (MXTPCL-X2)
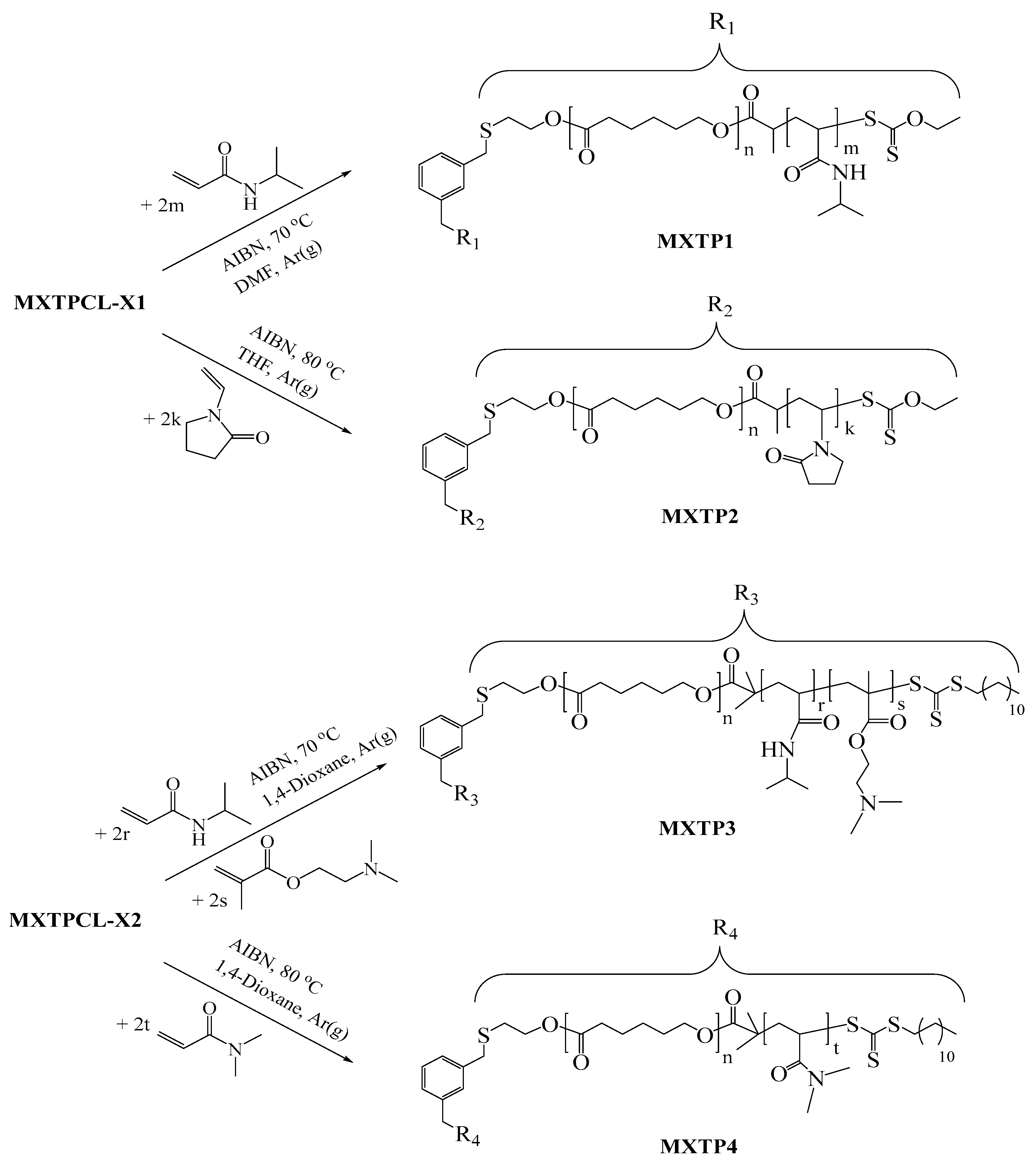
2.9. Synthesis of Block Copolymers Using MXTPCL-X1 as macroCTA (MXTP1 and MXTP2)
2.10. Synthesis of Block Copolymers Using MXTPCL-X2 as macroCTA (MXTP3 and MXTP4)
3. Results and Discussion
3.1. Characterization of MXTPCLOH and MXTPCLBr
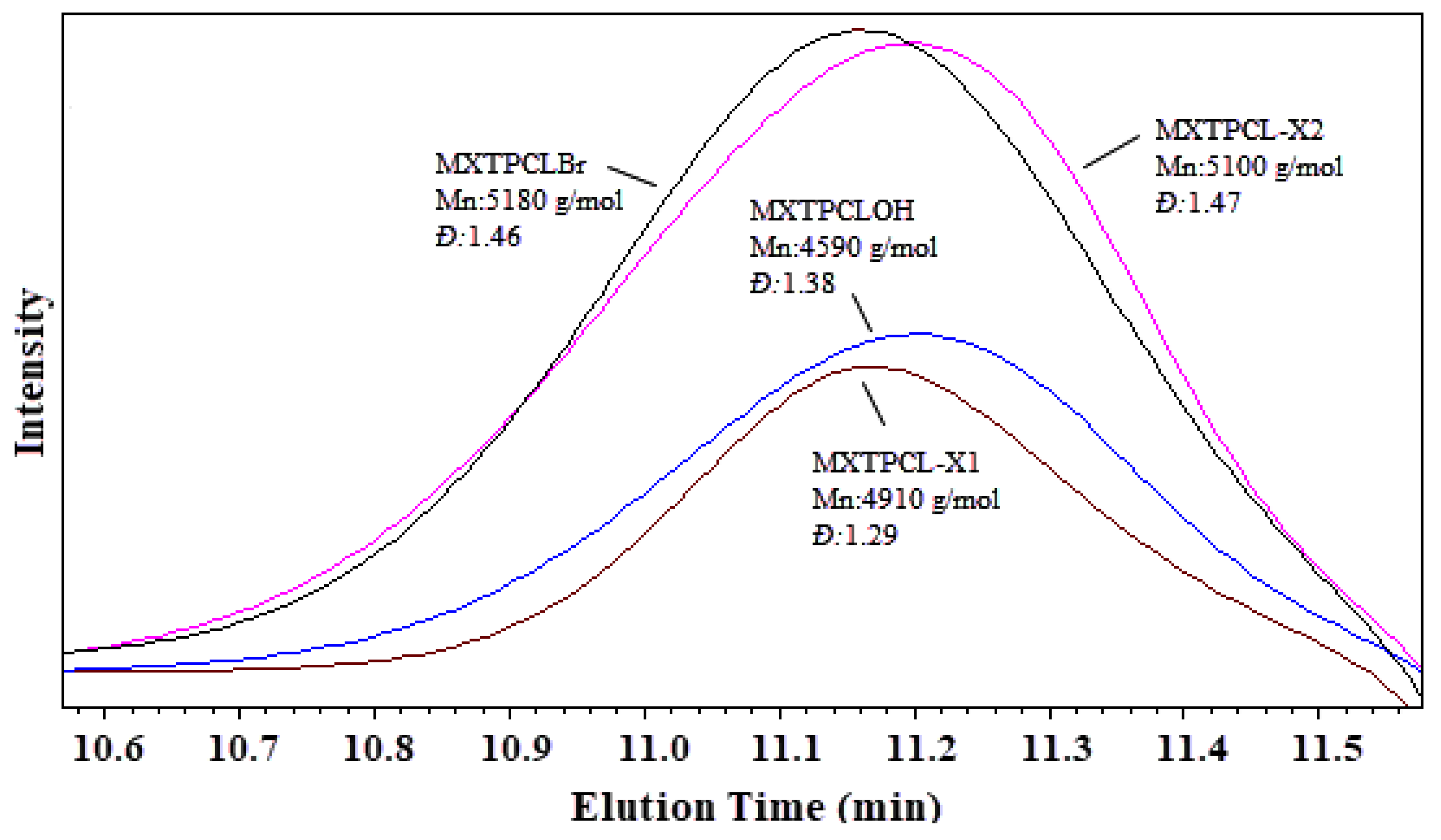
3.2. Characterization of MXTPCL-X1 and MXTPCL-X2
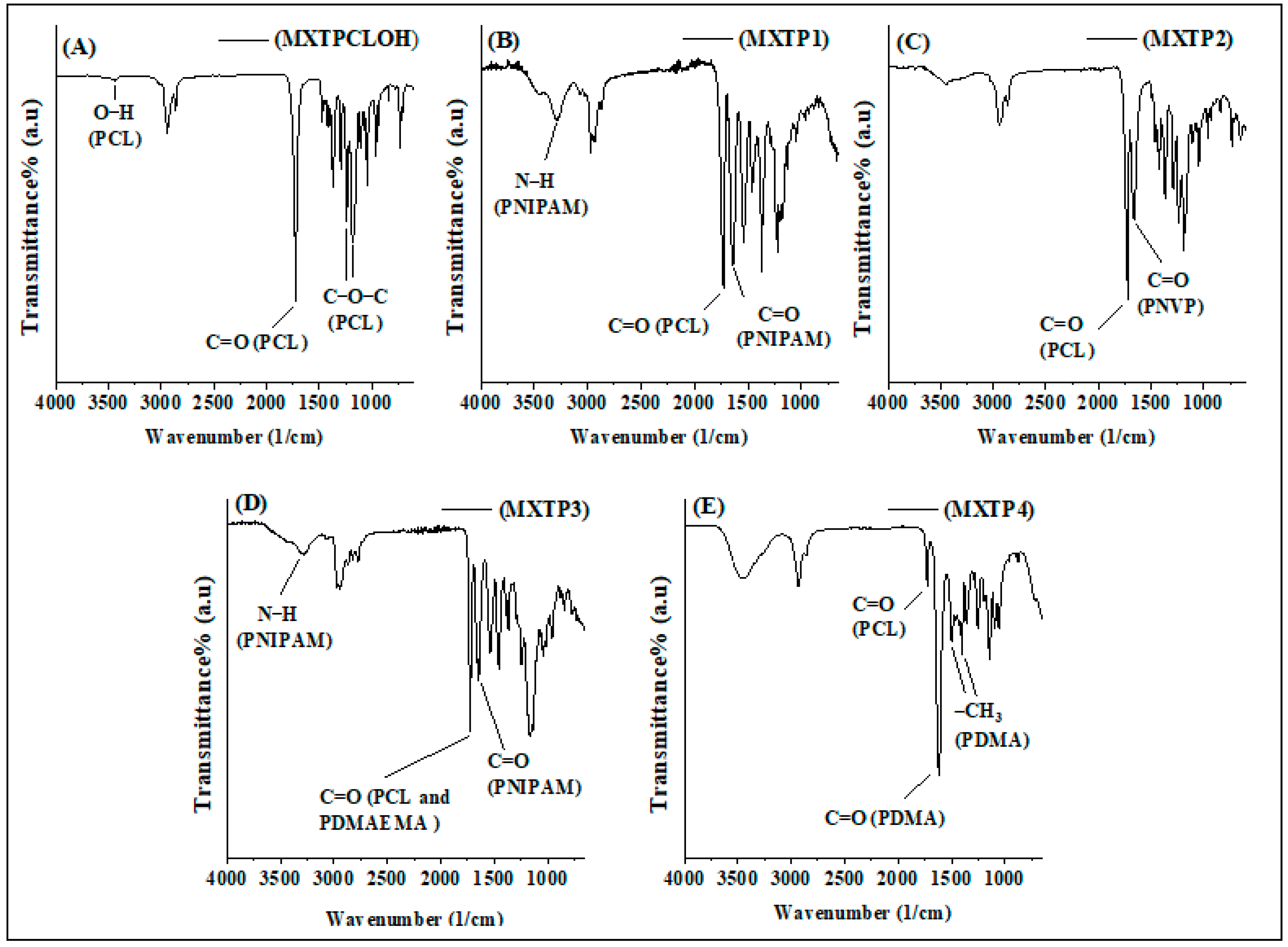
3.3. Characterization of Block Copolymers
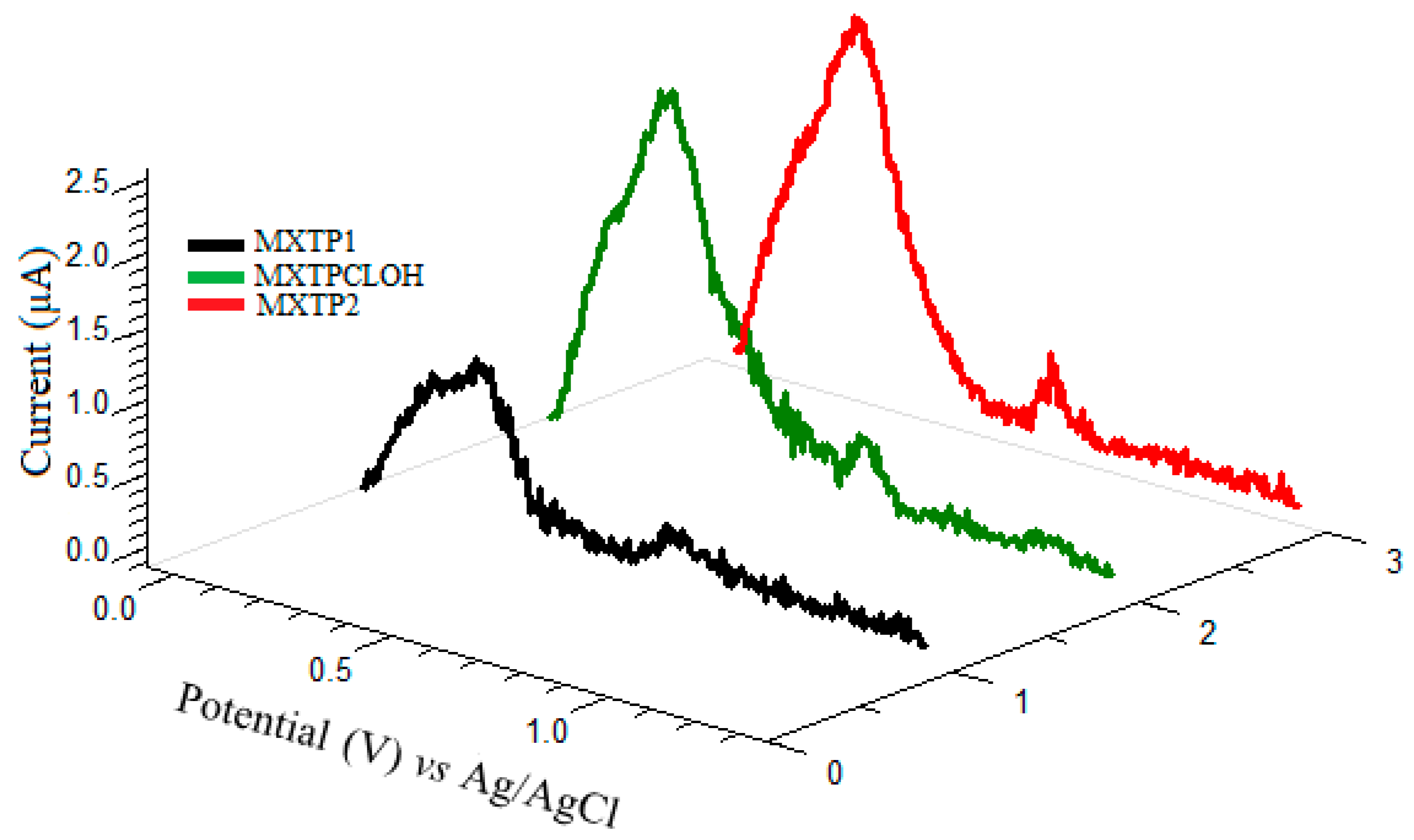
3.4. Sensor Ability
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. Part A 2015, 103, 2482–2498. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Dzib-Cauich, D.A.; de Jesús Ramírez-Rivera, E.; Cabal-Prieto, A.; Can-Herrera, L.A. Applications of polycaprolactone in the food industry: A review. Czech J. Food Sci. 2024, 42, 77–84. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Z.; Li, X.; Ding, X.; Guo, M.; Zhao, H.; Yao, J.; Wang, L.; Cai, Q.; Fan, Y. The effect of mechanical loads on the degradation of aliphatic biodegradable polyesters. Regen. Biomater. 2017, 4, 179–190. [Google Scholar] [CrossRef]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Wang, K.; Ni, M.; Dundas, A.A.; Dimitrakis, G.; Irvine, D.J. Ring opening polymerisation of ɛ-caprolactone with novel microwave magnetic heating and cyto-compatible catalyst. Front. Bioeng. Biotechnol. 2023, 11, 1123477. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Weidner, S.M. ROP of L-lactide and ε-caprolactone catalyzed by tin (ii) and tin (iv) acetates–switching from COOH terminated linear chains to cycles. J. Polym. Sci. 2021, 59, 439–450. [Google Scholar] [CrossRef]
- Politakos, N.; Avgeropoulos, A. Advances and Applications of Block Copolymers. Polymers 2023, 15, 2930. [Google Scholar] [CrossRef]
- Mısır, M.; Savaskan Yılmaz, S.; Bilgin, A. Synthesis and Characterization of ABA-Type Triblock Copolymers Using Novel Bifunctional PS, PMMA, and PCL Macroinitiators Bearing p-xylene-bis (2-mercaptoethyloxy) Core. Polymers 2023, 15, 3813. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Abetz, V. Polymerizations by RAFT: Developments of the Technique and Its Application in the Synthesis of Tailored (Co) polymers. Macromol. Chem. Phys. 2021, 222, 2000311. [Google Scholar] [CrossRef]
- Sofla, S.F.I.; Abbasian, M.; Mirzaei, M. Synthesis and micellar characterization of novel pH-sensitive thiol-ended triblock copolymer via combination of RAFT and ROP processes. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 297–307. [Google Scholar] [CrossRef]
- Lee, Y.; Boyer, C.; Kwon, M.S. Photocontrolled RAFT polymerization: Past, present, and future. Chem. Soc. Rev. 2023, 52, 3035–3097. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem.—Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef]
- Lee, R.-S.; Lin, C.-H.; Aljuffali, I.A.; Hu, K.-Y.; Fang, J.-Y. Passive targeting of thermosensitive diblock copolymer micelles to the lungs: Synthesis and characterization of poly (N-isopropylacrylamide)-block-poly (ε-caprolactone). J. Nanobiotechnol. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly (N-vinylpyrrolidone)-modified surfaces for biomedical applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly (N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Ramesh, S.; Ramesh, K. Flexible and self-healable poly (N, N-dimethylacrylamide) hydrogels for supercapacitor prototype. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126377. [Google Scholar] [CrossRef]
- Algi, M.P.; Okay, O. Highly stretchable self-healing poly (N, N-dimethylacrylamide) hydrogels. Eur. Polym. J. 2014, 59, 113–121. [Google Scholar] [CrossRef]
- Roka, N.; Kokkorogianni, O.; Kontoes-Georgoudakis, P.; Choinopoulos, I.; Pitsikalis, M. Recent advances in the synthesis of complex macromolecular architectures based on poly (n-vinyl pyrrolidone) and the RAFT polymerization technique. Polymers 2022, 14, 701. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Kang, H.U.; Youk, J.H. Synthesis and micellar characterization of thermosensitive amphiphilic poly (ε-caprolactone)-b-poly (N-vinylcaprolactam) block copolymers. Colloid Polym. Sci. 2012, 290, 1107–1113. [Google Scholar] [CrossRef]
- Mishra, A.K.; Patel, V.K.; Vishwakarma, N.K.; Biswas, C.S.; Raula, M.; Misra, A.; Mandal, T.K.; Ray, B. Synthesis of well-defined amphiphilic poly (ε-caprolactone)-b-poly (N-vinylpyrrolidone) block copolymers via the combination of ROP and xanthate-mediated raft polymerization. Macromolecules 2011, 44, 2465–2473. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramesh, K.; Paira, T.K.; Srivastava, D.N.; Mandal, T.K.; Misra, N.; Ray, B. Synthesis and self-assembly properties of well-defined four-arm star poly (ε-caprolactone)-b-poly (N-vinylpyrrolidone) amphiphilic block copolymers. Polym. Bull. 2013, 70, 3201–3220. [Google Scholar] [CrossRef]
- de Moraes, R.M.; de Carvalho, L.T.; Teixeira, A.J.R.; Medeiros, S.F.; dos Santos, A.M. Well-defined amphiphilic poly (ε-caprolactone)-b-poly (N-isopropylacrylamide) and thermosensitive micelles formulation. Iran. Polym. J. 2023, 32, 1627–1641. [Google Scholar] [CrossRef]
- Mishra, A.K.; Vishwakarma, N.K.; Patel, V.K.; Biswas, C.S.; Paira, T.K.; Mandal, T.K.; Maiti, P.; Ray, B. Synthesis, characterization, and solution behavior of well-defined double hydrophilic linear amphiphilic poly (N-isopropylacrylamide)-b-poly (ε-caprolactone)-b-poly (N-isopropylacrylamide) triblock copolymers. Colloid Polym. Sci. 2014, 292, 1405–1418. [Google Scholar] [CrossRef]
- Peña, J.A.; Gutiérrez, S.J.; Villamil, J.C.; Agudelo, N.A.; Pérez, L.D. Policaprolactone/polyvinylpyrrolidone/siloxane hybrid materials: Synthesis and in vitro delivery of diclofenac and biocompatibility with periodontal ligament fibroblasts. Mater. Sci. Eng. C 2016, 58, 60–69. [Google Scholar] [CrossRef]
- Bian, Q.; Xiao, Y.; Lang, M. R-RAFT approach for the polymerization of N-isopropylacrylamide with a star poly (ε-caprolactone) core. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 571–580. [Google Scholar] [CrossRef]
- Sheng, L.; Zhu, X.; Sun, M.; Lan, Z.; Yang, Y.; Xin, Y.; Li, Y. Tumor Microenvironment-Responsive Magnetic Nanofluid for Enhanced Tumor MRI and Tumor multi-treatments. Pharmaceuticals 2023, 16, 166. [Google Scholar] [CrossRef]
- Kim, T.; Mays, J.; Chung, I. Porous poly (ε-caprolactone) microspheres via UV photodegradation of block copolymers prepared by RAFT polymerization. Polymer 2018, 158, 198–203. [Google Scholar] [CrossRef]
- Yuan, W.; Shen, J.; Zou, H. Amphiphilic block copolymer terminated with pyrene group: From switchable CO2-temperature dual responses to tunable fluorescence. RSC Adv. 2015, 5, 13145–13152. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- de Groot, B.; Loeb, S.J.; Shimizu, G.K. Large-ring S6-thiacyclophanes as ditopic macrocycles. Synthesis and structures of 2, 5, 8, 17, 20, 23-hexathia [9.9]-o-cyclophane, HT [9.9] OC, 2, 5, 8, 17, 20, 23-hexathia [9.9]-m-cyclophane, HT [9.9] MC, and [Ag2 (CH3CN) 2 (HT [9.9] OC)][BF4] 2. Inorg. Chem. 1994, 33, 2663–2667. [Google Scholar] [CrossRef]
- Mısır, M.; Azarkan, S.Y. Synthesis, characterization, and cytotoxicity analyses of ABA-type block copolymer bearing p-xylene-bis (2-mercaptoethyloxy) core. J. New Results Sci. 2024, 13, 271–283. [Google Scholar] [CrossRef]
- Mısır, M. Synthesis, characterization and cytotoxicity analyzes of novel AB-Type Amphiphilic Block Copolymers. Health Sci. Q. 2025, 5, 51–63. [Google Scholar] [CrossRef]
- Yu, W.; Foster, J.C.; Dove, A.P.; O’Reilly, R.K. Length control of biodegradable fiber-like micelles via tuning solubility: A self-seeding crystallization-driven self-assembly of poly (ε-caprolactone)-containing triblock copolymers. Macromolecules 2020, 53, 1514–1521. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ye, D.; Zhang, J.; Guo, Y.; You, R.; Yan, S.; Li, M.; Qu, J. Fabrication and characterization of electrospun PCL/Antheraea pernyi silk fibroin nanofibrous scaffolds. Polym. Eng. Sci. 2017, 57, 206–213. [Google Scholar] [CrossRef]
- Banerjee, S.L.; Hoskins, R.; Swift, T.; Rimmer, S.; Singha, N.K. A self-healable fluorescence active hydrogel based on ionic block copolymers prepared via ring opening polymerization and xanthate mediated RAFT polymerization. Polym. Chem. 2018, 9, 1190–1205. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Chen, G.-J.; Feng, T.-H.; Li, M.-H.; Wang, L.-F. Synthesis and characterization of S-PCL-PDMAEMA for co-delivery of pDNA and DOX. RSC Adv. 2014, 4, 11089–11098. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Shen, J.; Xu, Y.; An, Y.; Shi, L. Controlled release of ionic drugs from complex micelles with charged channels. Biomacromolecules 2012, 13, 1307–1314. [Google Scholar] [CrossRef]
- Dhumale, V.A.; Gangwar, R.K.; Datar, S.S.; Sharma, R.B. Reversible aggregation control of polyvinylpyrrolidone capped gold nanoparticles as a function of pH. Mater. Express 2012, 2, 311–318. [Google Scholar] [CrossRef]
- Debuigne, A.; Schoumacher, M.; Willet, N.; Riva, R.; Zhu, X.; Rütten, S.; Jérôme, C.; Detrembleur, C. New functional poly (N-vinylpyrrolidone) based (co) polymers via photoinitiated cobalt-mediated radical polymerization. Chem. Commun. 2011, 47, 12703–12705. [Google Scholar] [CrossRef]
- Motamedi, S.; Massoumi, B.; Jaymand, M.; Hamishehkar, H. A dual stimuli-responsive star-shaped nanocarrier as de novo drug delivery system for chemotherapy of solid tumors. J. Polym. Res. 2020, 27, 272. [Google Scholar] [CrossRef]
- Egghe, T.; Cools, P.; Van Guyse, J.F.; Asadian, M.; Khalenkow, D.; Nikiforov, A.; Declercq, H.; Skirtach, A.G.; Morent, R.; Hoogenboom, R. Water-stable plasma-polymerized N, N-dimethylacrylamide coatings to control cellular adhesion. ACS Appl. Mater. Interfaces 2019, 12, 2116–2128. [Google Scholar] [CrossRef]
- Demir, E.; Silah, H.; Aydogdu, N. Electrochemical applications for the antioxidant sensing in food samples such as citrus and its derivatives, soft drinks, Supplementary Food and Nutrients. In Citrus-Research, Development and Biotechnology; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Demır, E. Sensitive and selective pathway of total antioxidant capacity in commercially lemon, watermelon and mango-pineapple cold teas by square wave adsorptive stripping voltammetry. Gazi Univ. J. Sci. 2019, 32, 1123–1136. [Google Scholar] [CrossRef]
- Yildirim, S.; Gok, I.; Demir, E.; Tokusoglu, O. Use of electrochemical techniques for determining the effect of brewing techniques (espresso, Turkish and filter coffee) and roasting levels on total antioxidant capacity of coffee beverage. J. Food Process. Preserv. 2022, 46, e16626. [Google Scholar] [CrossRef]
- Öztürk, M.; Demir, E.; Ozdal, T. Voltammetric and spectrophotometric pathways for the determination of total antioxidant capacity in commercial turnip juice. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 163–172. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Nag, S.; Das, D.; Roy, R.B. Detection of syringic acid in food extracts using molecular imprinted polyacrylonitrile infused graphite electrode. J. Food Compos. Anal. 2024, 132, 106280. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mısır, M. Synthesis, Characterization and Sensor Application of Novel PCL-Based Triblock Copolymers. Polymers 2025, 17, 873. https://doi.org/10.3390/polym17070873
Mısır M. Synthesis, Characterization and Sensor Application of Novel PCL-Based Triblock Copolymers. Polymers. 2025; 17(7):873. https://doi.org/10.3390/polym17070873
Chicago/Turabian StyleMısır, Murat. 2025. "Synthesis, Characterization and Sensor Application of Novel PCL-Based Triblock Copolymers" Polymers 17, no. 7: 873. https://doi.org/10.3390/polym17070873
APA StyleMısır, M. (2025). Synthesis, Characterization and Sensor Application of Novel PCL-Based Triblock Copolymers. Polymers, 17(7), 873. https://doi.org/10.3390/polym17070873





