Smart Biomimetic 3D Scaffolds Based on Shape Memory Polyurethane for Soft Tissue Repair
Abstract
1. Introduction
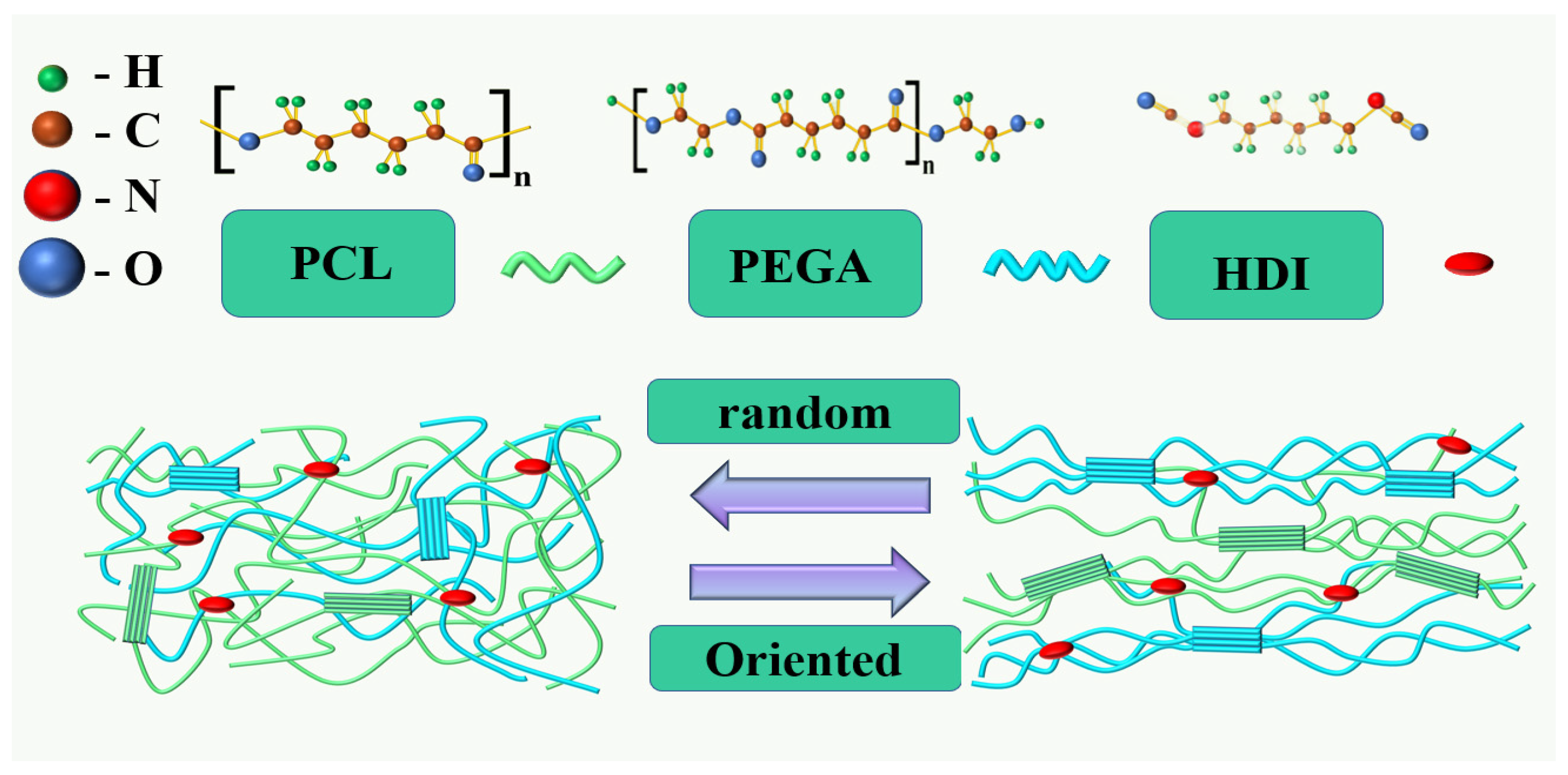
Preparation of PCL/PEGA Porous Scaffolds
2. Materials and Methods
2.1. Materials
2.2. XRD
2.3. SEM
2.4. FTIR
2.5. Thermal Analysis Testing
2.6. Mechanical Performance Testing
2.7. Hydrophilicity and Gel Swelling Testing
2.8. Shape Memory Properties Testing
2.9. Statistical Analysis
3. Results
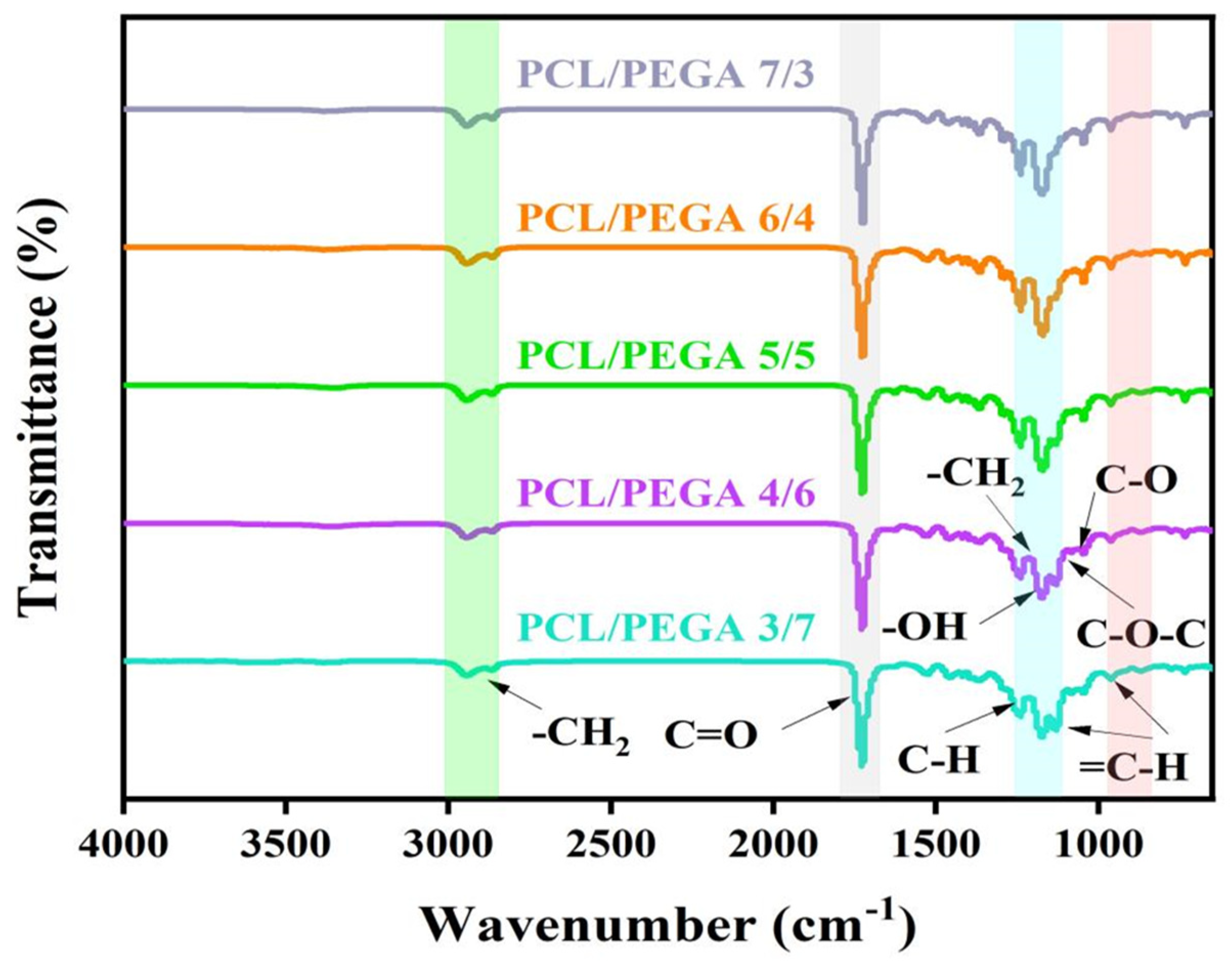
3.1. Fourier Transform Infrared Spectra (FTIR) Analysis of PCL/PEGA Porous Tissue Engineering Scaffolds
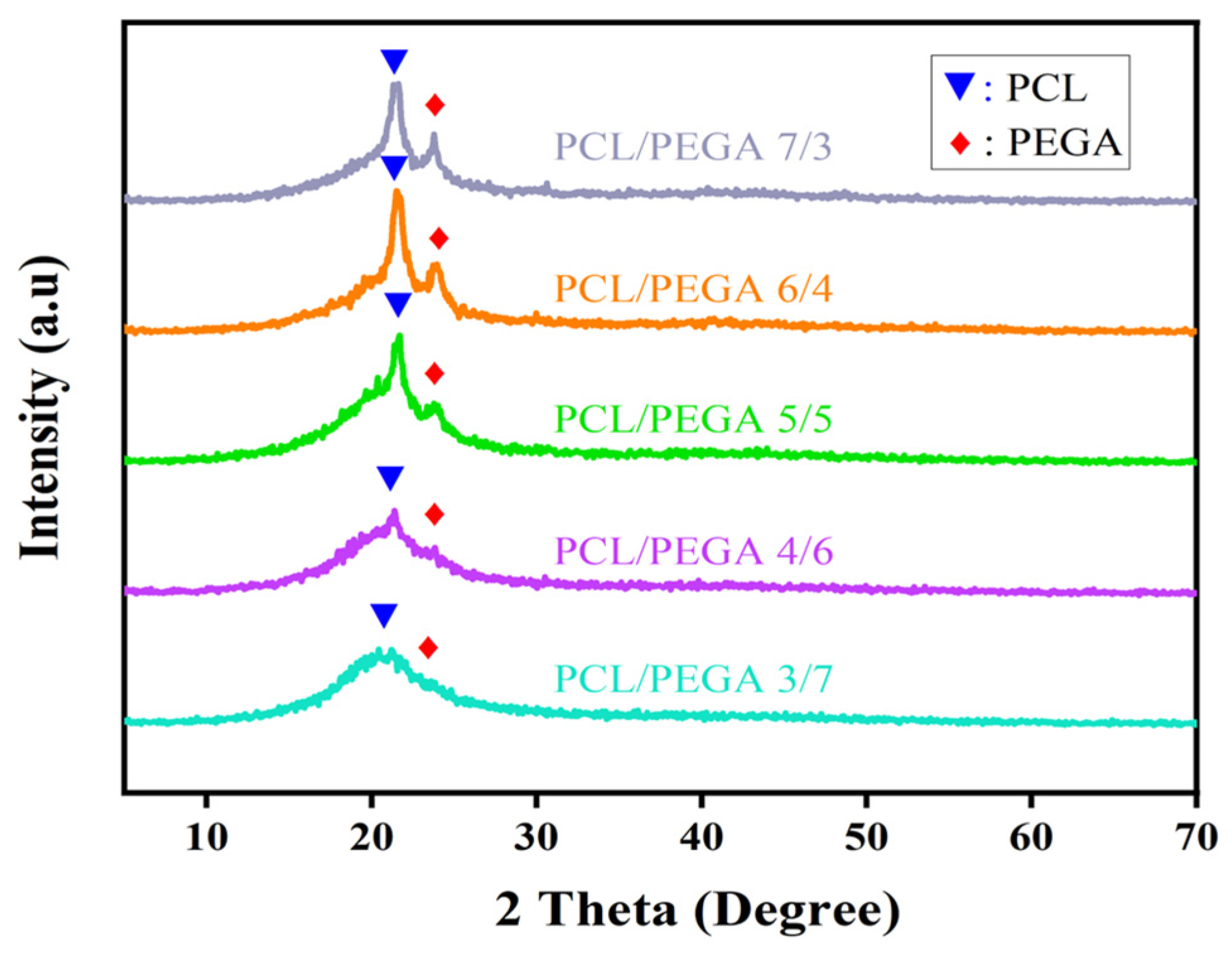
3.2. X-Ray Diffraction Analysis of PCL/PEGA Porous Tissue Engineering Scaffolds
3.3. Swelling Degree and Gel Content Analysis
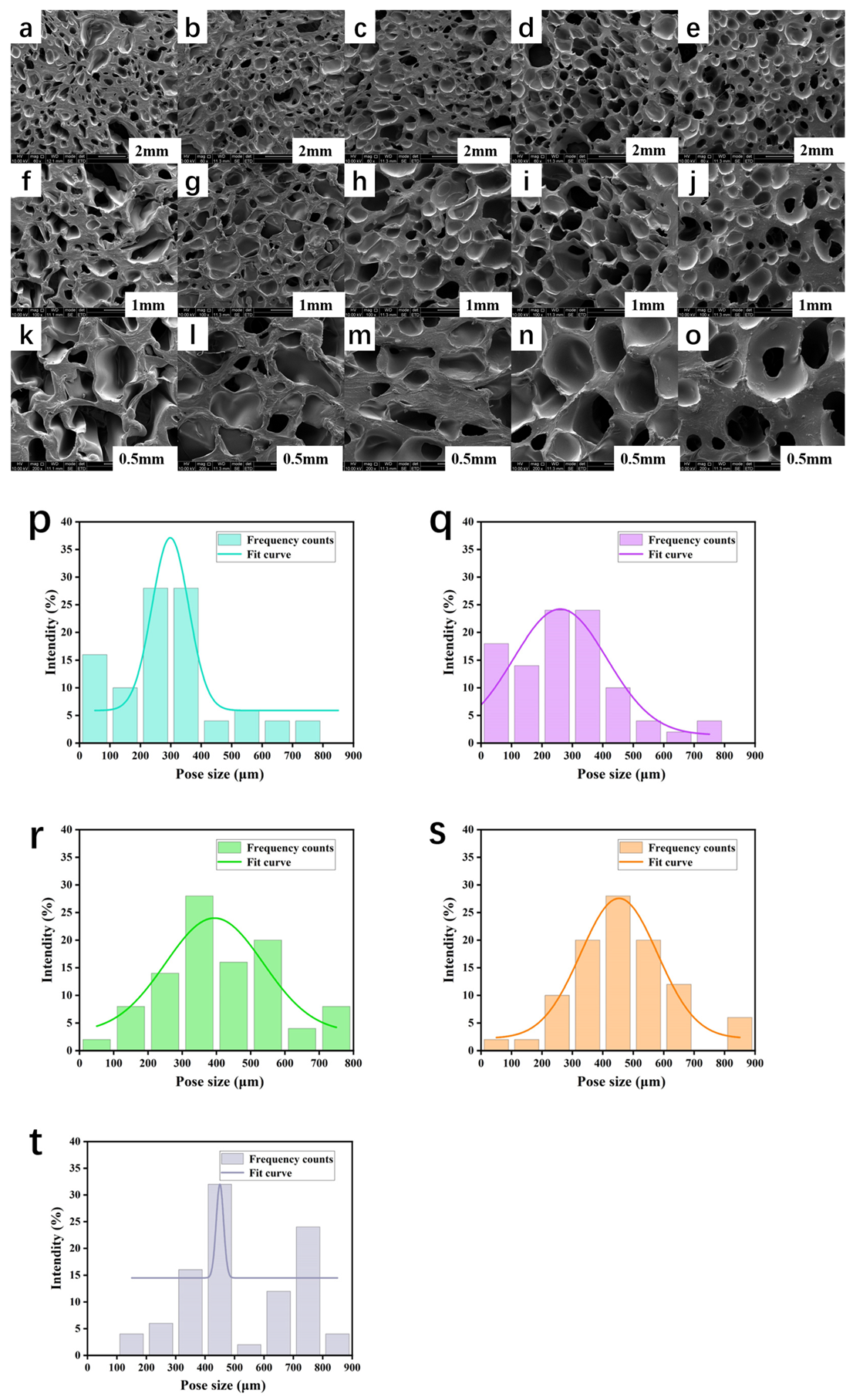
3.4. Morphological Analysis of PCL/PEGA Scaffolds
3.5. Thermal Analysis of PCL/PEGA Scaffolds
3.6. Porosity and Hydrophilicity of PCL/PEGA Scaffolds
3.7. Mechanical Properties of PCL/PEGA Scaffolds
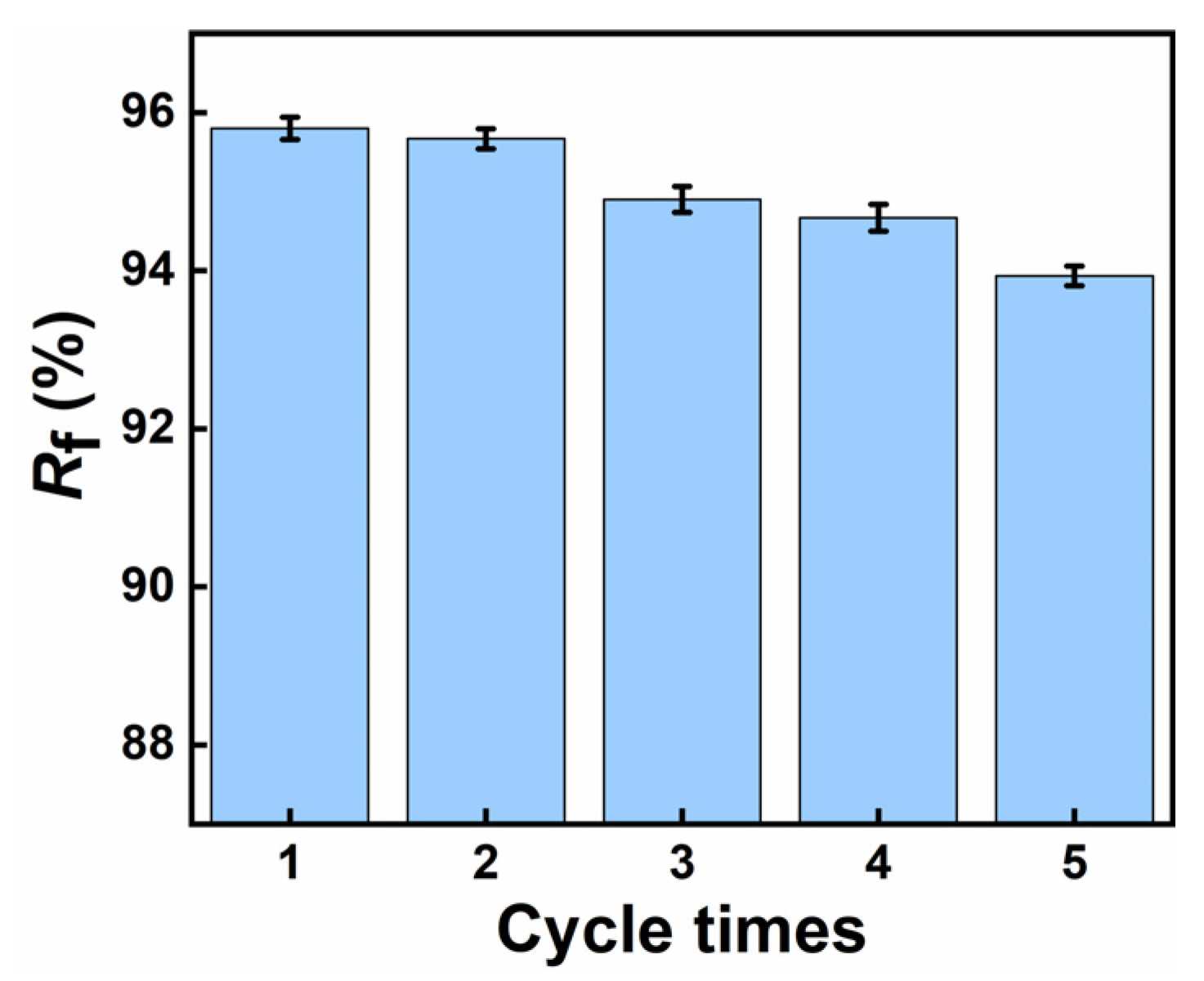
3.8. Shape Memory Effect Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds. J. Eur. Ceram. Soc. 2023, 44, 23–42. [Google Scholar] [CrossRef]
- Gorgun, B.; Gamlı, A.; Duran, M.E.; Bayram, B.; Ulku, T.K.; Kocaoglu, B. Collagen Scaffold Application in Arthroscopic Recon-struction of Osteochondral Lesions of the Talus with Autologous Cancellous Bone Grafts. Orthop. J. Sports Med. 2023, 11, 23259671221145733. [Google Scholar] [CrossRef]
- Christensen, B.B.; Olesen, M.L.; Hede, K.T.C.; Bergholt, N.L.; Foldager, C.B.; Lind, M. Particulated cartilage for chondral and oste-ochondral repair: A review. Cartilage 2021, 13 (Suppl. S1), 1047S–1057S. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Krishna, N.G.; Fox, M.G.; Blankenbaker, D.G.; Frick, M.A.; Jawetz, S.T.; Li, G.; Reitman, C.; Said, N.; Stensby, J.D.; et al. ACR Appropriateness Criteria® Osteoporosis and Bone Mineral Density: 2022 Update. J. Am. Coll. Radiol. 2022, 19, S417–S432. [Google Scholar] [CrossRef]
- Choi, K.; Ryu, Y.-H.; Song, M.; Han, S.-K.; Lim, H.; Kim, H.-J.; Choi, S.-W. Biodegradable Polyurethane Scaffolds with Hard and Soft Compartments for Potential Bone-to-tendon Regeneration. Polym. Korea 2022, 46, 171–178. [Google Scholar] [CrossRef]
- Luo, K.; Gao, P.; Yang, W.; Lei, X.; Wong, T.-W.; Ismail, A.F.; Wang, L. Antibacterial Poly-urethane Composite Scaffolds for Minimally Invasive Alveolar Bone Repair. Appl. Mater. Today 2023, 31, 202752. [Google Scholar]
- Rosales-Ibáñez, R.; Viera-Ruiz, A.E.; Cauich-Rodríguez, J.V.; Carrillo-Escalante, H.J.; González-González, A.; Rodríguez-Martínez, J.J.; Hernández-Sánchez, F. Electrospun/3D-printed PCL bioactive scaffold for bone regeneration. Polym. Bull. 2022, 80, 2533–2552. [Google Scholar] [CrossRef]
- Jin, S.; Yang, R.; Hu, C.; Xiao, S.; Zuo, Y.; Man, Y.; Li, Y.; Li, J. Plant-derived polyphenol and ll-37 peptide-modified nanofibrous scaffolds for promotion of antibacteri-al activity, anti-inflammation, and type h vascularized bone regeneration. ACS Appl. Mater. Interfaces 2023, 15, 7804–7820. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Zeng, X.; Luo, K.; Chen, T.; Zhang, T.; Yan, G. A Multiple Remote-ly Controlled Platform from Recyclable Polyurethane Compsite Nework with Shape-Memory Effect and Self-Healing Ability. Small 2022, 18, 2205286. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumari, K.; Kundu, P.P.; Dhiman, V.; Bhadada, S. Recycled Polyurethane (rPU)-Block-Hydroxybutyl-Terminated Poly (dime-thylsiloxane)(hbPDMS)(rPU-b-hbPDMS) Copolymer Nanocomposites for Osteoblast Cell Regeneration. ACS Appl. Polym. Mater. 2024, 6, 2659–2674. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegradation 2023, 180, 105599. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Wang, Y.; Han, W.; Wei, Y.; Hu, Y.; Liang, Z.; Lian, X.; Huang, D. Hydroxyapatite/polyurethane scaffolds for bone tissue engi-neering. Tissue Eng. Part B Rev. 2024, 30, 60–73. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Rational design of multifunctional CuS nanoparticle-PEG composite soft hydrogel-coated 3D hard polycaprolactone scaffolds for efficient bone re-generation. Adv. Funct. Mater. 2022, 32, 2202470. [Google Scholar] [CrossRef]
- Kalbali, N.; Hashemi-Najafabadi, S.; Bagheri, F. Improving pore size of electrospun gelatin scaffolds containing graphene oxide using PEG as a sacrificial agent for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 1068–1077. [Google Scholar] [CrossRef]
- Dang, X.; Du, Y.; Wang, X.; Liu, X.; Yu, Z. New indoleacetic acid-functionalized soluble oxidized starch-based nonionic biopolymers as natural antibacterial materials. Int. J. Biol. Macromol. 2023, 242, 125071. [Google Scholar] [CrossRef]
- Kulkarni, D.; Gadade, D.; Kapare, H.; Dhas, N.L.; Ban, M. Characterization Techniques for Stimuli-Responsive Delivery Nanoplatforms in Cancer Treatment. In Site-Specific Cancer Nanotheranostics; CRC Press: Boca Raton, FL, USA, 2024; pp. 322–338. [Google Scholar]
- Abate, K.M.; Nazir, A.; Yeh, Y.-P.; Chen, J.-E.; Jeng, J.-Y. Design, optimization, and validation of mechanical properties of different cellular structures for biomedical application. Int. J. Adv. Manuf. Technol. 2019, 106, 1253–1265. [Google Scholar] [CrossRef]
- Thenard, T.; Catapano, A.; Allena, R.; El May, M.; Saintier, N.; Mesnard, M. Topography and wettability characterization of sur-faces manufactured by SLM and treated by chemical etching. Mech. Adv. Mater. Struct. 2022, 29, 1674–1691. [Google Scholar] [CrossRef]
- Basak, S. Redesigning the modern applied medical sciences and engineering with shape memory polymers. Adv. Compos. Hybrid Mater. 2021, 4, 223–234. [Google Scholar] [CrossRef]
- Furlan, P.Y.; Furlan, A.Y.; Bryant, P.L.; Thorn, N.R.; Reckline, C.I. Collagen-Mediated Calcium Carbonate Polymorph Modulation—A Nature-Inspired General Chemistry Experiment Utilizing Modern Characterization Tools, Including SEM, EDS, and FTIR-ATR Spectroscopy. J. Chem. Educ. 2023, 100, 4828–4837. [Google Scholar] [CrossRef]
- Yang, C.; Wang, G.; Zhang, A.; Zhao, J.; Xu, Z.; Li, S.; Zhao, G. High-elastic and strong hexamethylene diisocyanate (HDI)-based thermoplastic polyu-rethane foams derived by microcellular foaming with co-blowing agents. J. CO2 Util. 2023, 74, 102543. [Google Scholar] [CrossRef]
- Samperisi, L.; Zou, X.; Huang, Z. Three-dimensional electron diffraction: A powerful structural characterization technique for crystal engineering. CrystEngComm 2022, 24, 2719–2728. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Ramos, E.V.; Martínez, I.; Ambrosi, M.; Raudino, M. Assessment of the structures contribution (crystalline and mesophases) and mechanical properties of polycaprolactone/pluronic blends. J. Mech. Behav. Biomed. Mater. 2023, 139, 105668. [Google Scholar] [CrossRef] [PubMed]
- Wulin, S.; Shiu, B.-C.; Yuan, Q.-Y.; Zhangjian, H.-q.; Lin, J.-H.; Lou, C.-W. Evaluation of Mechanical Properties of Porous Chitosan/Gelatin/Polycaprolactone Bone Scaffold Prepared by Microwave Foaming Method. Polymers 2022, 14, 4668. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Raj, N.S.; Saiprasad, C.; Sudhanva, A.R. Investigation on the Morphological and Thermal Properties of Polylactic Ac-id/Polycaprolactone Loaded with Halloysite Nanotubes and Basalt Fiber. Surf. Rev. Lett. 2022, 29, 2250037. [Google Scholar]
- Aguiar, V.O.; Tavares, M.I.B. Polycaprolactone, clay and vanadium oxide nanocomposites: Development and performance evaluation. Polym. Bull. 2024, 81, 14801–14821. [Google Scholar] [CrossRef]
- Doke, S.D.; Patel, C.M.; Lad, V.N. Improving physical properties of silica aerogel using compatible additives. Chem. Pap. 2021, 75, 215–225. [Google Scholar] [CrossRef]
- Zusmanovitch, I.; Asbi, T.; Regev, O.; Giladi, H.Z.; Bianco-Peled, H. An integrative approach for the design of bioactive polycaprolactone-based scaffold for bone tissue engineering. Polym. Eng. Sci. 2023, 63, 2905–2919. [Google Scholar] [CrossRef]
- Song, Y.; Joo, K.; Seo, J.H. Evaluation of mechanical and thermal properties of hydroxyapatite-levan composite bone graft. Biotechnol. Bioprocess Eng. 2021, 26, 201–207. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Sun, Y.; Sun, B.; Gu, B.; Leng, J.; Zhang, W. Electrothermal shape memory behavior and recovery force of four-dimensional printed continuous carbon fiber/polylactic acid composite. Smart Mater. Struct. 2021, 30, 025040. [Google Scholar] [CrossRef]
- Guo, X.; Song, P.; Li, F.; Yan, Q.; Bai, Y.; He, J.; Che, Q.; Cao, H.; Guo, J.; Su, Z. Research Progress of Design Drugs and Composite Biomaterials in Bone Tissue Engineering. Int. J. Nanomed. 2023, ume 18, 3595–3622. [Google Scholar] [CrossRef]
- Bistac, S.; Brogly, M.; Bindel, D. Crystallinity of Amphiphilic PE-b-PEG Copolymers. Polymers 2022, 14, 3639. [Google Scholar] [CrossRef]
- Worch, J.C.; Weems, C.; Yu, J.; Arno, M.C.; Wilks, T.R.; Huckstepp, R.T.; O’Reilly, R.K.; Becker, M.L.; Dove, A.P. Elastomeric polyamide biomaterials with stereochemically tuneable mechanical properties and shape memory. Nat. Commun. 2020, 11, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, L.; Yuan, Y.; Shi, R. The Current Status, Prospects, and Challenges of Shape Memory Polymers Application in Bone Tissue Engineering. Polymers 2023, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, X.; Zeng, X.; Luo, K.; Zhou, S.; Zhang, P.; Li, J. Degradable smart composite foams for bone regeneration. Compos. Sci. Technol. 2021, 217, 109124. [Google Scholar] [CrossRef]





| Samples | Porosity (%) | Water Absorption (%) | Density (g/cm3) |
|---|---|---|---|
| PCL/PEGA 7/3 | 78.6 ± 4.2 | 234.6 ± 22.4 | 0.5 ± 0.01 |
| PCL/PEGA 6/4 | 76.3 ± 4.9 | 214.8 ± 15.4 | 0.4 ± 0.03 |
| PCL/PEGA 5/5 | 70.8 ± 3.2 | 199.6 ± 32.4 | 0.3 ± 0.01 |
| PCL/PEGA 4/6 | 68.5 ± 3.5 | 180.3 ± 26.4 | 0.3 ± 0.02 |
| PCL/PEGA 3/7 | 60.2 ± 1.8 | 176.5 ± 10.8 | 0.2 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Sun, W.; Wu, Y.; Gu, H.; Chen, T.; Zhang, T.; Liu, X.; Zhang, J.; Wang, L. Smart Biomimetic 3D Scaffolds Based on Shape Memory Polyurethane for Soft Tissue Repair. Polymers 2025, 17, 872. https://doi.org/10.3390/polym17070872
Zuo X, Sun W, Wu Y, Gu H, Chen T, Zhang T, Liu X, Zhang J, Wang L. Smart Biomimetic 3D Scaffolds Based on Shape Memory Polyurethane for Soft Tissue Repair. Polymers. 2025; 17(7):872. https://doi.org/10.3390/polym17070872
Chicago/Turabian StyleZuo, Xiaoling, Weijing Sun, Yutong Wu, Hanliu Gu, Tao Chen, Ting Zhang, Xiaoying Liu, Jianwei Zhang, and Li Wang. 2025. "Smart Biomimetic 3D Scaffolds Based on Shape Memory Polyurethane for Soft Tissue Repair" Polymers 17, no. 7: 872. https://doi.org/10.3390/polym17070872
APA StyleZuo, X., Sun, W., Wu, Y., Gu, H., Chen, T., Zhang, T., Liu, X., Zhang, J., & Wang, L. (2025). Smart Biomimetic 3D Scaffolds Based on Shape Memory Polyurethane for Soft Tissue Repair. Polymers, 17(7), 872. https://doi.org/10.3390/polym17070872





