Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood
Abstract
:1. Introduction
2. Materials and Methods
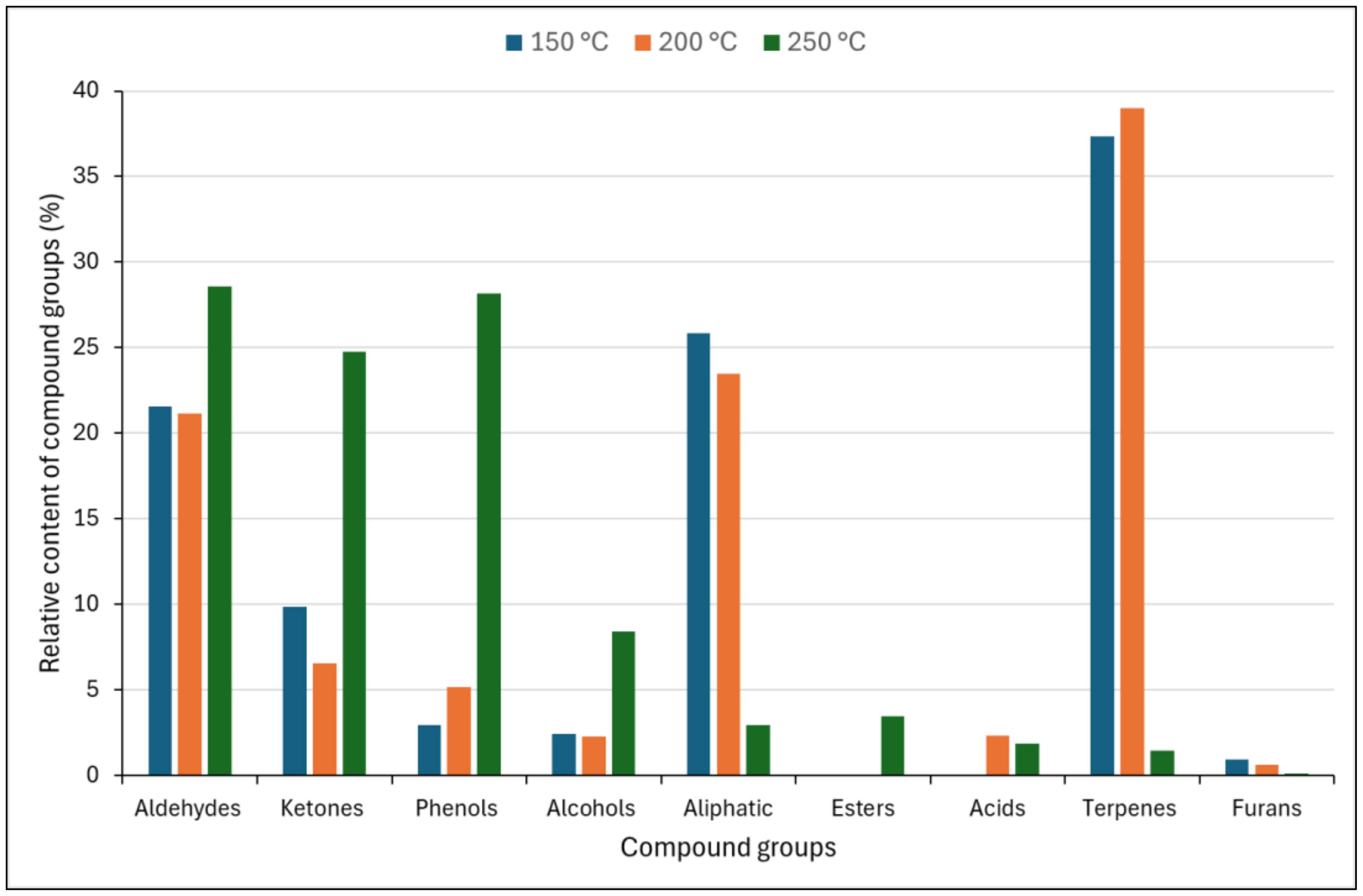
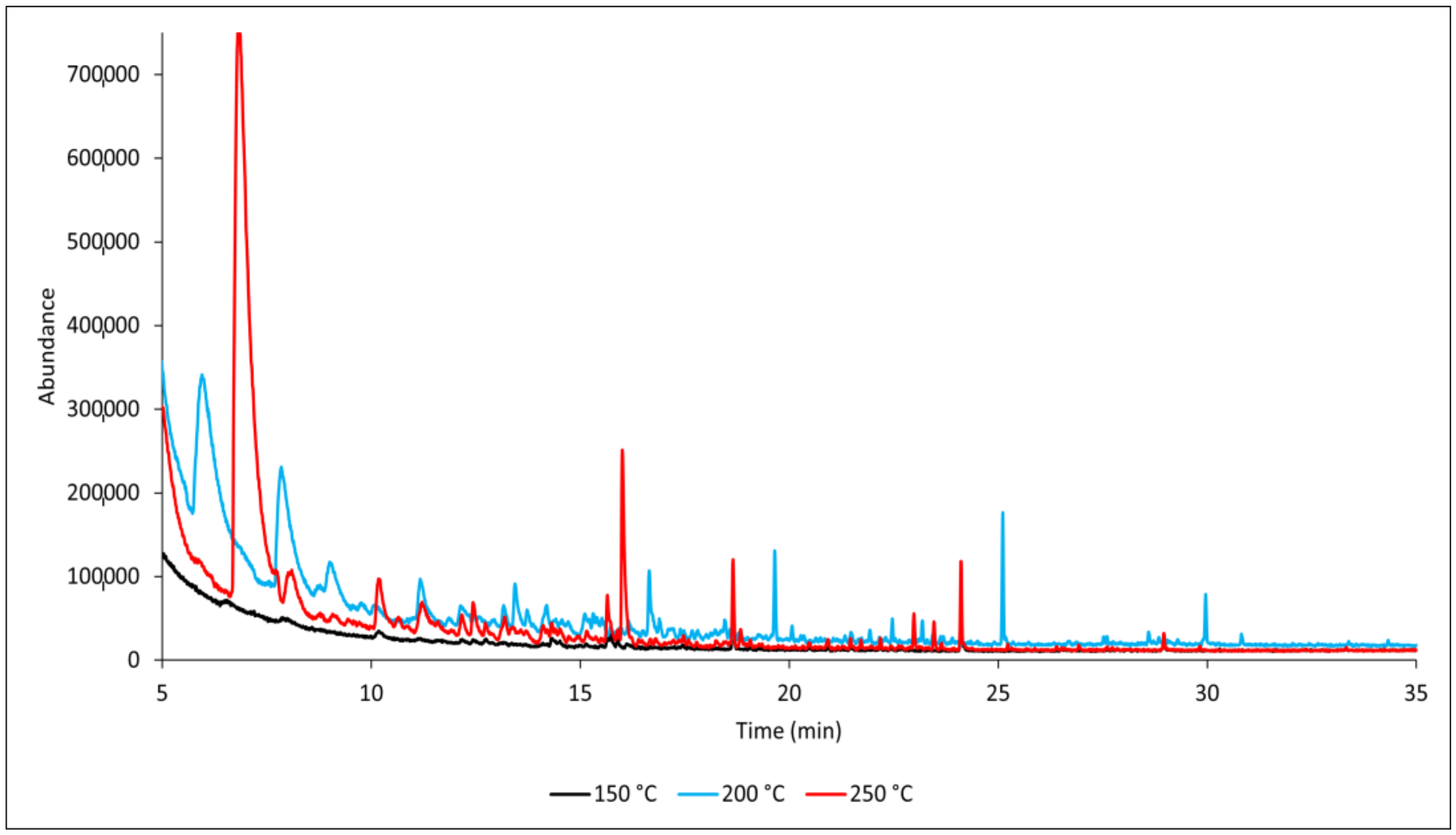
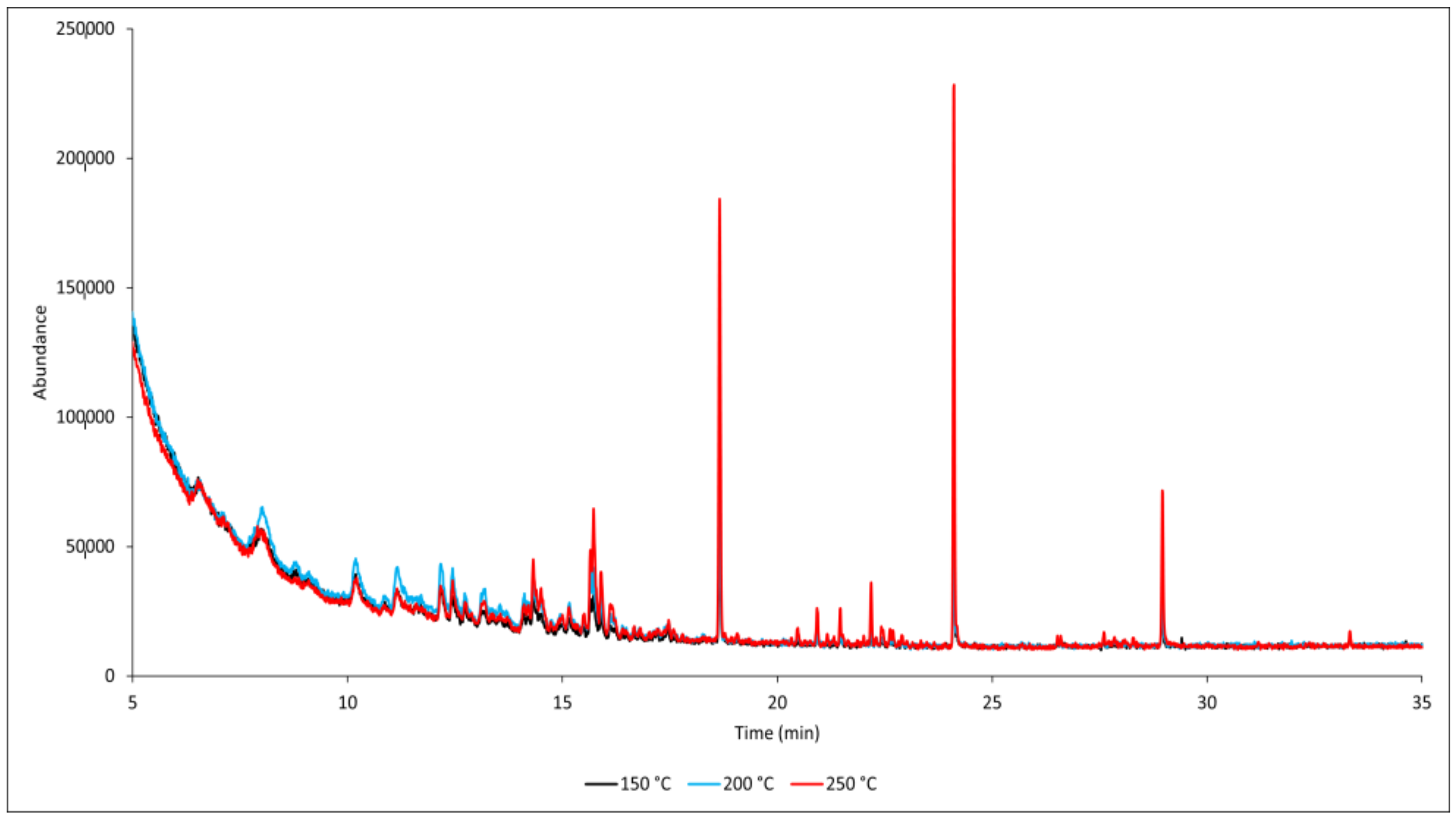
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lowden, L.A.; Hull, T.R. Flammability Behaviour of Wood and a Review of the Methods for Its Reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Mai, C.; Zhang, K. Wood Chemistry. In Springer Handbook of Wood Science and Technology; Niemz, P., Teischinger, A., Sandberg, D., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 179–279. ISBN 978-3-030-81315-4. [Google Scholar]
- Mensah, R.A.; Jiang, L.; Renner, J.S.; Xu, Q. Characterisation of the Fire Behaviour of Wood: From Pyrolysis to Fire Retardant Mechanisms. J. Therm. Anal. Calorim. 2023, 148, 1407–1422. [Google Scholar] [CrossRef]
- Lee, Y.X.; Wang, W.; Lei, Y.; Xu, L.; Agarwal, V.; Wang, C.; Yeoh, G.H. Flame-Retardant Coatings for Wooden Structures. Prog. Org. Coat. 2025, 198, 108903. [Google Scholar] [CrossRef]
- Reinprecht, L. Wood Deterioration, Protection and Maintenance; Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-119-10650-0. [Google Scholar]
- Júda, M.; Sydor, M.; Rogoziński, T.; Kučerka, M.; Pędzik, M.; Kminiak, R. Effect of Low-Thermal Treatment on the Particle Size Distribution in Wood Dust after Milling. Polymers 2023, 15, 1059. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Gašparík, M.; Todaro, L.; Jones, D.; Corleto, R.; Makovická Osvaldová, L.; Čekovská, H. The Effect of Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Thermally Modified Teak (Tectona Grandis L. f.) Wood. Constr. Build. Mater. 2019, 200, 551–558. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Lu, W.; Zhang, J.; Yue, Z.; Jie, Y.; Zhang, J.; Cheng, Z.; Ji, W.; Wu, J. Effects of Different Accelerated Aging Modes on the Mechanical Properties, Color and Microstructure of Wood. J. Build. Eng. 2024, 98, 111026. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-351-02822-6. [Google Scholar]
- Candelier, K.; Chaouch, M.; Dumarçay, S.; Pétrissans, A.; Pétrissans, M.; Gérardin, P. Utilization of Thermodesorption Coupled to GC–MS to Study Stability of Different Wood Species to Thermodegradation. J. Anal. Appl. Pyrol. 2011, 92, 376–383. [Google Scholar] [CrossRef]
- Candelier, K.; Dumarçay, S.; Pétrissans, A.; Pétrissans, M.; Kamdem, P.; Gérardin, P. Thermodesorption Coupled to GC–MS to Characterize Volatiles Formation Kinetic during Wood Thermodegradation. J. Anal. Appl. Pyrol. 2013, 101, 96–102. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Shen, Y.; Li, C.; Wang, Y.; Ma, Z.; Sun, W. New Perspective on Wood Thermal Modification: Relevance between the Evolution of Chemical Structure and Physical-Mechanical Properties, and Online Analysis of Release of VOCs. Polymers 2019, 11, 1145. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Thermal Decomposition of Wood: Kinetics and Degradation Mechanisms. Bioresour. Technol. 2012, 126, 7–12. [Google Scholar] [CrossRef]
- Vargun, E.; Baysal, E.; Turkoglu, T.; Yuksel, M.; Toker, H. Thermal Degradation of Oriental Beech Wood Impregnated with Different Inorganic Salts. Maderas Cienc. Tecnol. 2019, 21, 163–170. [Google Scholar] [CrossRef]
- Dietenberger, M.; Hasburgh, L. Wood Products Thermal Degradation and Fire. Ref. Modul. Mater. Sci. Mater. Eng. 2016, 1, 9712–9716. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Sandberg, D.; Babiak, M.; Turčani, M.; Niemz, P.; Hanzlík, P. The Effect of Chemical Changes during Thermal Modification of European Oak and Norway Spruce on Elasticity Properties. Compos. Struct. 2019, 220, 529–538. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Ornaghi, F.G.; Motta Neves, R.; Magalhães De Oliveira, D.; Poletto, M. Thermal Decomposition of Wood Fibres: Thermal Simulation Using the F-Test Statistical Tool. Cellul. Chem. Technol. 2021, 55, 231–241. [Google Scholar] [CrossRef]
- Albert, C.M.; Liew, K.C. Recent Development and Challenges in Enhancing Fire Performance on Wood and Wood-Based Composites: A 10-Year Review from 2012 to 2021. J. Bioresour. Bioprod. 2024, 9, 27–42. [Google Scholar] [CrossRef]
- De Angelis, M.; Humar, M.; Kržišnik, D.; Tamantini, S.; Romagnoli, M. Influence of Thermal Modification and Impregnation with Biocides on Physical Properties of Italian Stone Pine Wood (Pinus Pinea L.). Appl. Sci. 2022, 12, 3801. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Pfriem, A. Treatments and Modification to Improve the Reaction to Fire of Wood and Wood Based Products—An Overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-Retardant Surface Treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, X.; Davies, M.; Fisk, C.; Holliday, M.; King, D.; Zhang, Y.; Willmott, J. Characterisation of Wood Combustion and Emission under Varying Moisture Contents Using Multiple Imaging Techniques. Fuel 2024, 373, 132397. [Google Scholar] [CrossRef]
- Pohleven, J.; Burnard, M.D.; Kutnar, A. Volatile Organic Compounds Emitted from Untreated and Thermally Modified Wood—A Review. Wood Fiber Sci. 2019, 51, 231–254. [Google Scholar] [CrossRef]
- Sokamte Tegang, A.; Mbougueng, P.D.; Sachindra, N.M.; Douanla Nodem, N.F.; Tatsadjieu Ngoune, L. Characterization of Volatile Compounds of Liquid Smoke Flavourings from Some Tropical Hardwoods. Sci. Afr. 2020, 8, e00443. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Pánek, M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers 2020, 12, 2289. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, V.; Fleitmann, L.; Kangas, J.; Leonhard, K.; Tanskanen, J. Conceptual Design of Furfural Extraction, Oxidative Upgrading and Product Recovery: COSMO-RS-Based Process-Level Solvent Screening. Comput. Chem. Eng. 2024, 191, 108835. [Google Scholar] [CrossRef]
- Palai, Y.N.; Fukuoka, A.; Shrotri, A. Unlocking the Potential of 5-Hydroxy-2(5H)-Furanone as a Platform for Bio-Based Four Carbon Chemicals. ACS Catal. 2024, 14, 2545–2551. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Yuan, D.; Li, C.; Ma, Y.; Wang, S.; Wang, S. Fire Hazards of PMMA-Based Composites Combined with Expandable Graphite and Multi-Walled Carbon Nanotubes: A Comprehensive Study. Fire Saf. J. 2023, 135, 103727. [Google Scholar] [CrossRef]
- Mizera, K.; Borucka, M.; Przybysz, J.; Gajek, A. Identification of Substances Emitted During Combustion and Thermal Decomposition of Wood-Based Materials. Chem. Eng. Trans. 2024, 111, 283–288. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC Emissions between Air-Dried and Heat-Treated Scots Pine Wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Kačík, F.; Veľková, V.; Šmíra, P.; Nasswettrová, A.; Kačíková, D.; Reinprecht, L. Release of Terpenes from Fir Wood during Its Long-Term Use and in Thermal Treatment. Molecules 2012, 17, 9990–9999. [Google Scholar] [CrossRef]
- Atiku, F.A.; Lea-Langton, A.R.; Bartle, K.D.; Jones, J.M.; Williams, A.; Burns, I.; Humphries, G. Some Aspects of the Mechanism of Formation of Smoke from the Combustion of Wood. Energy Fuels 2017, 31, 1935–1944. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Prajer, M. VOC Emissions from Spruce Strands and Hemp Shive: In Search for a Low Emission Raw Material for Bio-Based Construction Materials. Materials 2019, 12, 2026. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the Pyrolytic Behaviour of Xylan-Based Hemicellulose Using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrol. 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Peters, J.; Fischer, K.; Fischer, S. Characterization of Emissions from Thermally Modified Wood and Their Reduction by Chemical Treatment. BioResources 2008, 3, 491–502. [Google Scholar] [CrossRef]
- Hyttinen, M.; Masalin-Weijo, M.; Kalliokoski, P.; Pasanen, P. Comparison of VOC Emissions between Air-Dried and Heat-Treated Norway Spruce (Picea Abies), Scots Pine (Pinus Sylvesteris) and European Aspen (Populus Tremula) Wood. Atmos. Environ. 2010, 44, 5028–5033. [Google Scholar] [CrossRef]
- Liang, Y.; Jian, H.; Deng, C.; Xu, J.; Liu, Y.; Park, H.; Wen, M.; Sun, Y. Research and Application of Biomass-Based Wood Flame Retardants: A Review. Polymers 2023, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.D.; Zielinska, B.; Fujita, E.M.; Sagebiel, J.C.; Chow, J.C.; Watson, J.G. Fine Particle and Gaseous Emission Rates from Residential Wood Combustion. Environ. Sci. Technol. 2000, 34, 2080–2091. [Google Scholar] [CrossRef]
- Sivrikaya, H.; Tesařová, D.; Jeřábková, E.; Can, A. Color Change and Emission of Volatile Organic Compounds from Scots Pine Exposed to Heat and Vacuum-Heat Treatment. J. Build. Eng. 2019, 26, 100918. [Google Scholar] [CrossRef]


| Group | Compound | RT (min) | Temperature (°C) | ||
|---|---|---|---|---|---|
| 150 | 200 | 250 | |||
| Aldehydes | Vanillin | 24.097 | 10.1 | 13.2 | 52.7 |
| Furfural | 6.841 | 0.0 | 0.0 | 306.7 | |
| 2-Furancarboxaldehyde, 5-methyl- | 11.230 | 0.0 | 0.0 | 53.1 | |
| 5-Hydroxymethylfurfural | 19.406 | 0.3 | 0.3 | 20.7 | |
| 5-Acetoxymethyl- 2-furaldehyde | 21.724 | 0.0 | 0.0 | 44.3 | |
| 4-Hydroxy- 2-methoxycinnamaldehyde | 31.938 | 0.3 | 0.0 | 18.7 | |
| 5-Norbornane- 2-carboxaldehyde | 12.783 | 0.0 | 0.0 | 11.4 | |
| 1,2-Dimethoxy- 4-n-propylbenzene | 26.847 | 0.0 | 0.0 | 10.5 | |
| Ketones | 2(5H)-Furanone | 9.472 | 0.0 | 0.0 | 79.9 |
| 4-Methyl-5H-furan-2-one | 11.662 | 0.0 | 0.0 | 31.4 | |
| 2-Furanone, 2,5-dihydro-3,5-dimethyl | 12.460 | 1.5 | 1.1 | 5.1 | |
| 4-Methyl-5H-furan-2-one | 13.797 | 0.0 | 0.0 | 18.6 | |
| 2(3H)-Furanone, 5-acetyldihydro- | 16.483 | 0.6 | 0.4 | 2.7 | |
| 4-Methoxycarbonyl- 4-butanolide | 19.675 | 0.0 | 0.0 | 6.1 | |
| 1-(acetyloxy)-2-Propanone (Acetoxyacetone) | 8.081 | 0.0 | 0.0 | 150.5 | |
| 2-Butanone, 4-(acetyloxy)- | 12.083 | 0.0 | 0.0 | 12.2 | |
| 1,2-Cyclopentanedione, 3-methyl- | 13.236 | 1.4 | 1.4 | 28.9 | |
| Levoglucosenone | 15.997 | 1.0 | 0.9 | 37.7 | |
| 2-Propanone, 1-(4-hydroxy- 3-methoxyphenyl)- | 27.408 | 0.0 | 0.0 | 73.8 | |
| Phenols | Phenol, 2-methyl- | 14.196 | 0.0 | 5.3 | |
| Phenol, 2-methoxy- | 15.286 | 0.0 | 1.3 | 74.8 | |
| Creosol | 18.446 | 0.0 | 0.0 | 108.5 | |
| Phenol, 4-ethyl-2-methoxy- | 20.905 | 0.7 | 0.6 | 60.3 | |
| 2-Methoxy-4-vinylphenol | 21.854 | 0.0 | 0.0 | 42.3 | |
| Eugenol | 23.040 | 0.4 | 0.4 | 73.5 | |
| Phenol, 2-methoxy-4-propyl- | 23.288 | 0.0 | 0.0 | 21.2 | |
| trans-Isoeugenol | 24.345 | 0.0 | 0.3 | 25.2 | |
| Phenol, 2-methoxy-4-propyl- | 25.629 | 0.0 | 0.0 | 21.2 | |
| (Z)-4-(But-1-en-1-yl)guaiacol | 26.082 | 0.0 | 0.0 | 4.2 | |
| Apocynin | 26.286 | 0.0 | 0.0 | 38.3 | |
| (E)-4-(But-1-en-1-yl)guaiacol | 27.764 | 0.3 | 0.7 | 4.3 | |
| 4-(1-Hydroxyallyl)- 2-methoxyphenol | 28.422 | 0.0 | 0.0 | 31.7 | |
| Phenol, 2-methoxy- 4-(1-propenyl)-, Acetate | 29.360 | 0.0 | 0.0 | 5.4 | |
| Alcohols | 3-Pyridinol | 14.488 | 1.2 | 0.8 | 7.1 |
| Orcinol | 14.887 | 0.0 | 0.3 | 11.2 | |
| Benzenepropanol, 4-hydroxy-3-methoxy- | 30.072 | 0.0 | 0.3 | 1.6 | |
| 2-furanmethanol (Furfuryl alcohol) | 7.617 | 0.0 | 0.0 | 132.9 | |
| Aliphatic | Undecane | 15.652 | 5.2 | 3.6 | 4.9 |
| Heptane, 4-ethyl- | 18.607 | 0.0 | 0.3 | 19.4 | |
| Tridecane | 21.465 | 1.4 | 1.4 | 0.0 | |
| Hexadecane | 28.961 | 5.0 | 7.8 | 15.6 | |
| Heptadecane | 33.329 | 0.8 | 1.5 | 7.4 | |
| Oktadecane | 37.126 | 0.4 | 0.4 | 6.4 | |
| Esters | Benzoic acid, 4-hydroxy- 3-methoxy-, methyl ester | 27.052 | 0.0 | 0.0 | 15.4 |
| Benzeneacetic acid, 4-hydroxy-3-methoxy-, methyl ester | 28.594 | 0.0 | 0.0 | 5.5 | |
| Ethyl-.beta.-(4-hydroxy-3-methoxy-phenyl)propionate | 32.790 | 0.0 | 0.0 | 18.0 | |
| 7-Oxodehydroabietic acid, methyl ester | 42.475 | 0.0 | 0.0 | 15.7 | |
| 1-Phenanthrenecarboxylic acid,1,2,3,4,4a,10a-hexahydro-1,4a-dimethyl-7-(1-methyl ethyl)-,methyl ester, [1R-(1.alpha.,4a.beta.,10a.alpha.)]- | 40.534 | 0.0 | 0.0 | 8.1 | |
| Terpenes | α-Pinene | 10.173 | 13.0 | 18.8 | 26.6 |
| β-Pinene | 11.597 | 2.5 | 6.0 | 0.0 | |
| Δ3-carene | 12.729 | 3.1 | 0.0 | 0.0 | |
| Acids | Phenylacetylformic acid, 4-hydroxy-3-methoxy- | 31.140 | 0.0 | 0.0 | 10.6 |
| Methyl dehydroabietate | 40.577 | 0.0 | 1.5 | 23.0 | |
| Furans | Furane, 2,5-dihydro-2,5-dimethoxy- | 20.312 | 0.5 | 0.4 | 2.7 |
| Group | Compound | RT (min) | Temperature (°C) | ||
|---|---|---|---|---|---|
| 150 | 200 | 250 | |||
| Aldehydes | Furfural | 6.827 | 0.00 | 31.04 | 391.10 |
| Ketones | Levoglucosenone | 16.016 | 0.37 | 0.99 | 5.86 |
| 4-Hexen-3-one | 23.504 | 0.00 | 0.26 | 6.86 | |
| Terpenes | α-pinene | 10.184 | 0.00 | 5.27 | 9.68 |
| Aliphatic | Undecane | 15.649 | 0.43 | 2.41 | 3.81 |
| Dodecane | 18.658 | 1.52 | 3.67 | 3.99 | |
| 4-Methyl-2-hexene, c&t | 22.986 | 0.00 | 0.42 | 7.05 | |
| Tetradecane | 24.105 | 1.96 | 13.95 | 6.35 | |
| Hexadecane | 28.958 | 0.89 | 12.13 | 2.07 | |
| Group | Compound | RT (min) | Temperature (°C) | ||
|---|---|---|---|---|---|
| 150 | 200 | 250 | |||
| Aliphatic | Dodecane | 18.658 | 1.6 | 1.7 | 3.8 |
| Tetradecane | 24.105 | 1.8 | 1.6 | 5.3 | |
| Hexadecane | 28.958 | 0.6 | 0.6 | 1.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trojanová, K.; Veľková, V.; Kačík, F. Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood. Polymers 2025, 17, 875. https://doi.org/10.3390/polym17070875
Trojanová K, Veľková V, Kačík F. Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood. Polymers. 2025; 17(7):875. https://doi.org/10.3390/polym17070875
Chicago/Turabian StyleTrojanová, Katarína, Veronika Veľková, and František Kačík. 2025. "Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood" Polymers 17, no. 7: 875. https://doi.org/10.3390/polym17070875
APA StyleTrojanová, K., Veľková, V., & Kačík, F. (2025). Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood. Polymers, 17(7), 875. https://doi.org/10.3390/polym17070875







