Abstract
Although polyethylene glycol (PEG)-based surfactants are widely used in various industries due to their wide range of hydrophile–lipophile balance (HLB) values, their possible by-product, 1,4-dioxane, has been listed as a reasonably anticipated human carcinogen, which may limit their applications in fields closely related to the human body. Polyglycerol fatty acid esters (PGFEs), a class of surfactants based on polyglycerol (another polyether), also have a wide range of HLB values that can be adjust by varying the degree of polymerization of the polyglycerol, the length of the fatty acid carbon chain, or the degree of esterification, but do not have the risks caused by 1,4-dioxane. In addition, all the raw materials (glycerol and fatty acids) required for the preparation of PGFEs can be obtained via hydrolysis of renewable vegetable oils. Therefore, PGFEs, as new eco-friendly and biodegradable non-ionic surfactants, have been proposed as potential green alternatives to PEG-based non-ionic surfactants. This review summarizes the properties of PGFEs specifically including their HLB, surface properties, phase behaviors, stabilizing effect on foams and emulsions, and stability, and highlights their potential applications in food, cosmetics, and pharmaceuticals observed in the last decade.
1. Introduction
Surfactants are amphiphilic organic molecules, usually composed of a hydrophobic “tail” and a hydrophilic “head” linked by various covalent bonds. The hydrophobic “tail” can be aromatic group or alkyl chains containing 8–22 carbons, and the hydrophilic “head” usually includes polar moieties (e.g., a hydroxyl group and ether bond) or ionic moieties (e.g., carboxylate and sulfonate). Surfactants are usually divided into non-ionic, anionic, cationic, and amphoteric types based on the composition of their hydrophilic heads. Among them, non-ionic surfactants, including polyethylene glycol (PEG)-based surfactants [1,2], glycerol-based surfactants [3,4], and sugar-based surfactants [5,6], have attracted widespread attention in the fields of cosmetics, food, and pharmaceuticals due to their lower toxicity and reduced irritant effects compared to ionic surfactants [7].
PEG-based surfactants, one of the most important non-ionic surfactants, include alkyl PEG ethers, PEG fatty acid esters, block copolymers of PEG and polypropylene glycol (PPG) (e.g., poloxamers 184), and alkyl PEG/PPG ethers. However, during the manufacturing process of PEG-based surfactants, 1,4-dioxane may be generated as a by-product, which is reasonably anticipated to be a human carcinogen (the 15th Report on Carcinogens) and unsafe for the human body [8]. Moreover, PEG is a synthetic polyether compound derived from petroleum (a non-renewable resource), and its wide-scale use in the preparation of surfactants does not meet the needs of sustainable development. Polyglycerol (PG) is a polyether that can be polymerized from glycerol obtained through the hydrolysis of renewable vegetable oils. Polyglycerol also can be used as the hydrophilic part of surfactants, replacing PEG in the preparation of non-ionic surfactants, with no potential risk caused by 1,4-dioxane. Therefore, with the increasing awareness of the importance of health and sustainable development, polyglycerol-based non-ionic surfactants have attracted extensive attention.
Polyglycerol fatty acid esters (PGFEs) are the most extensively investigated polyglycerol-based non-ionic surfactants because the raw materials (e.g., glycerol and fatty acids) required to prepare PGFEs can be derived from renewable vegetable oils. Glycerol-based non-ionic surfactants are also renewable surfactants, but they are usually used as water-in-oil (w/o) emulsifiers due to their low hydrophile–lipophile balance (HLB) [3]. Sugar-based non-ionic surfactants, including sorbitan esters, alkyl polyglucosides, and sucrose esters, are another type of renewable surfactants that have been extensively studied [9]. However, the typical HLB value of sorbitan esters is usually in the range of 1–8, which is mainly suitable for oil-based systems [9], while the HLB value of alkyl polyglycosides is usually above 9, making it mainly suitable for water-based systems [10]. Compared with glycerol-based surfactants, sorbitan esters, and alkyl polyglucosides, PGFEs can be used not only in oil-based systems but also in water-based systems, because high lipophilicity (a low HLB value) can be obtained by decreasing the degree of polymerization of PG or increasing the degree of esterification, while high hydrophilicity (high HLB value) can be obtained by increasing the degree of polymerization of PG or decreasing the degree of esterification.
Although sucrose esters are also suitable for both oil-based and water-based systems due to their wide HLB value range of 1–16 [5,11], the esterification of sucrose requires perfect control due to sucrose’s sensitivity to temperature. In addition, the use of harmful DMSO and DMF as solvents in the production process of sucrose esters is of great concern [9]. Compared with the production of sucrose esters, the production of PGFEs does not require the use of toxic organic solvents, and PG is not sensitive to temperature, which makes it very attractive.
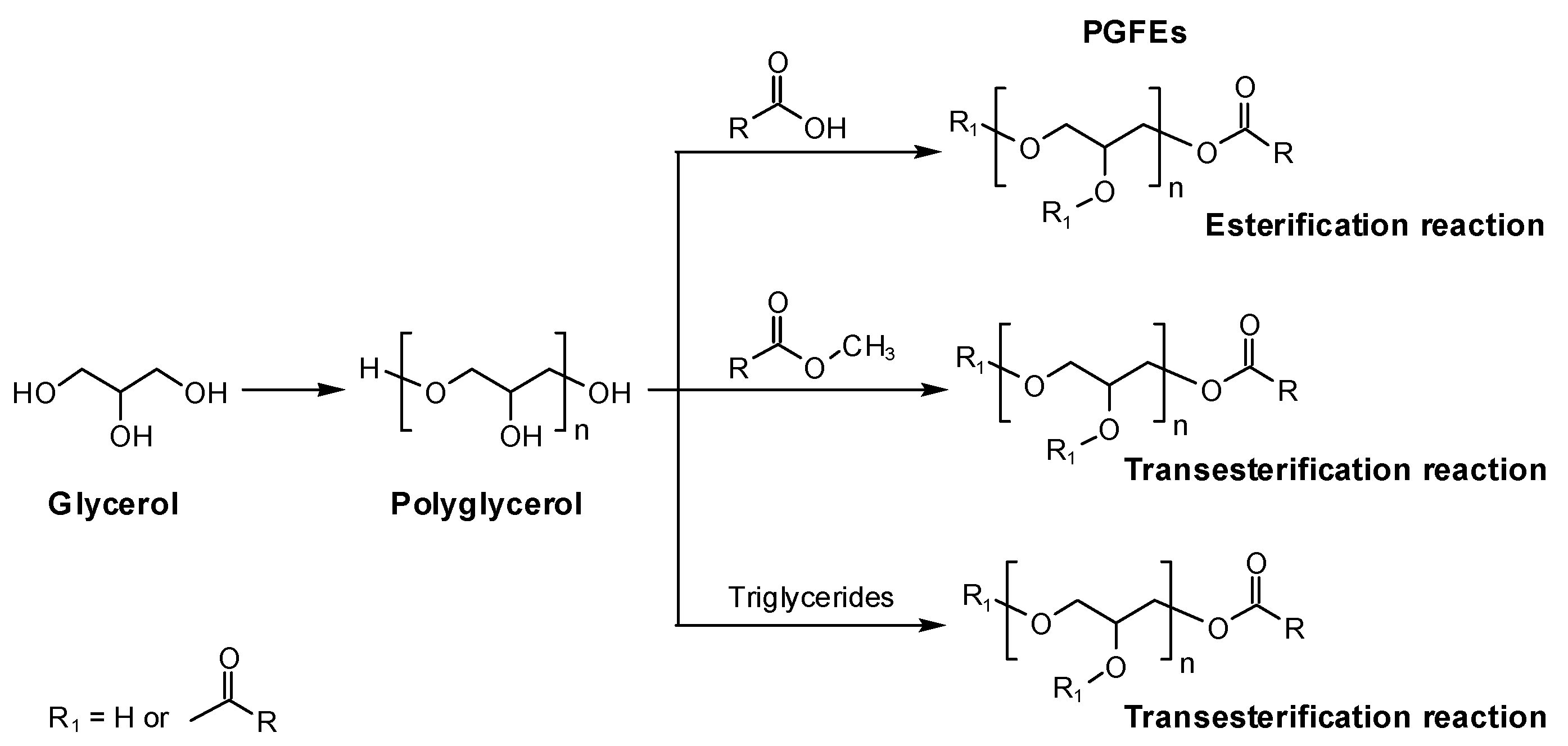
The typical preparation of PGFEs is as follows: (1) glycerol is dehydrated and polymerized using alkali (e.g., NaOH and K2CO3) or acid (e.g., H2SO4) as a catalyst to prepare polyglycerol, and the average degree of the polymerization of PG can be regulated via controlling the dehydration; (2) then, PGFEs can be prepared using (a) the esterification reaction between PG and fatty acids or (b) the transesterification reaction of PG with fatty acid esters or triglycerides in the absence of solvents (Scheme 1).
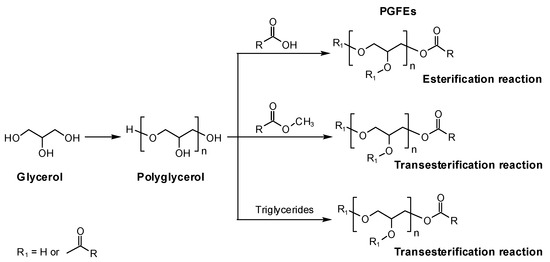
Scheme 1.
The typical synthesis routes of polyglycerol fatty acid esters.
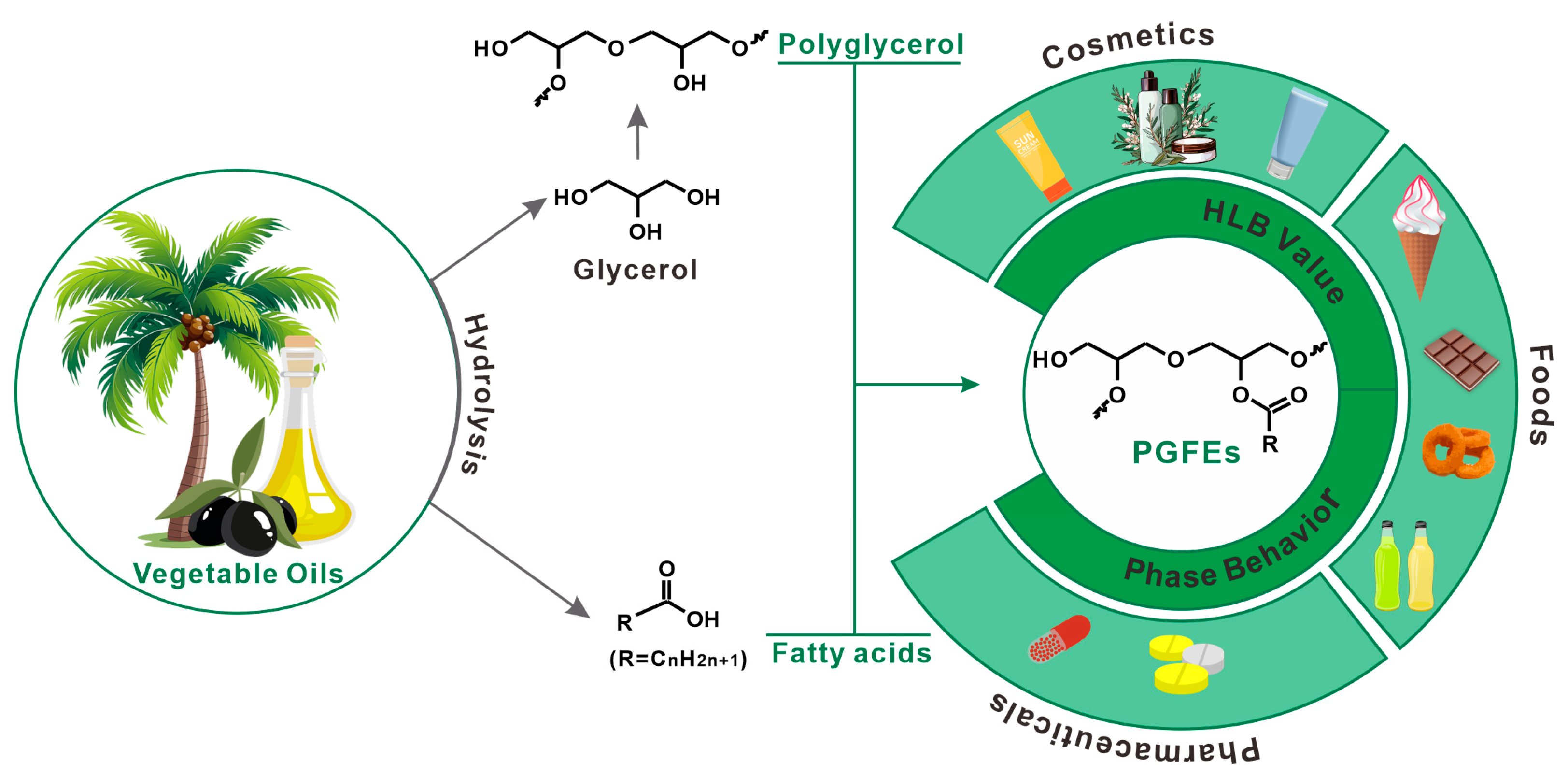
PGFEs are not only eco-friendly and free of 1,4-dioxane during preparation, but they also have outstanding application performances in various fields (Figure 1). In the food field, PGFEs have been approved as food additives (E475) [12] in many countries, and are mainly used as emulsifiers and crystallization-adjusters. In the cosmetic field, PGFEs have been used as emulsifiers for the preparation of moisturizing creams or as detergents for the preparation of cleaning products. In the pharmaceutical field, PGFEs have been investigated for their use as lipid-based excipients. Moreover, the cost of PGFEs is comparable to that of glycerol-based surfactants and sugar-based surfactants [13], and this cost can be further reduced when they are prepared via transesterification with vegetable oils [14].
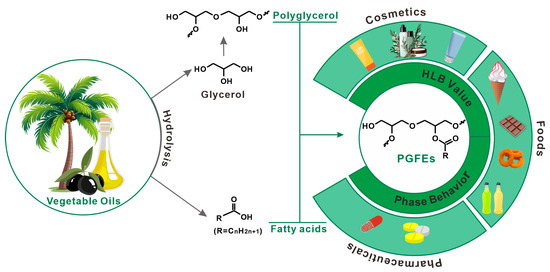
Figure 1.
The typical preparation route and applications of polyglycerol fatty acid esters.
Although the synthesis and methods of analysis of PGFEs have been summarized [15], their applications and related important properties (e.g., HLB value and phase behavior) have not been reviewed in detail in recent years. In this review, the properties of PGFEs, including their HLB values and phase behaviors in various mixture systems, are summarized, with an emphasis on their potential applications in food, cosmetics, and pharmaceuticals observed in the last decade.
2. Properties of PGFEs
The hydrophile–lipophile balance (HLB) is one of the most important parameters when evaluating the suitability of a surfactant for a specific application, as it reflects the partitioning tendency of a surfactant between oil and water. In addition to the HLB value, the phase behavior also plays a significant role in the practical application of surfactant mixtures. This not only reflects how surfactant molecules aggregate in a specific surfactant system, but is also closely related to physical properties such as viscosity and rheological properties.
2.1. Hydrophile–Lipophile Balance
The HLB value was first proposed by Griffin and generally ranges from 0 to 20 [16]. For non-ionic surfactants, the HLB value is defined as the hydrophilicity to hydrophobicity ratio of a surfactant, and can be calculated using Equation (1):
where Mh is the molecular weight of the hydrophilic moiety of the surfactant, Mt is the molecular weight of the entire surfactant, S is the saponification value of the surfactant, and A is the acid value of the surfactant. Davies’ group numbers contribution method is another commonly used method to estimate the HLB value of surfactants, as shown in Equation (2).
In addition to the methods of Griffin and Davies, the HLB values of PGFEs also can be estimated from organic conceptual diagrams [17] via converting the inorganic/organic balance (IOB) value to the HLB value with Equation (3) [18].
Based on the methods used to estimate HLB value, the HLB values of PGFEs increase with an increase in the degree of polymerization of the polyglycerol (hydrophilic moiety), and decrease with an increase in the length of the fatty acid (FA) carbon chain (hydrophobic moiety) or the degree of esterification of PGFEs. The chemical structures, HLB values and uses of investigated PGFEs are summarized in Table 1. As shown in Table 1, similarly to PEG-based surfactants, the HLB values of PGFEs can be adjusted in a wide range from 1.4 to 19.0.

Table 1.
The chemical structures, HLB values, and uses of PGFEs.
For practical purposes, the HLB value is extensively used in the design of formulations of water/oil systems in surfactants. Generally, surfactants with a range of HLB values, e.g., 3–6, can be used to prepare water-in-oil (w/o) emulsions, while surfactants with an HLB range of 8–18 can be used to prepare oil-in-water (o/w) emulsions [16]. As shown in Table 1, PGFEs are most commonly studied for their use as emulsifiers. Regarding emulsification, a series of PG-5 fatty acid esters with HLB values ranging from 1.4 to 16.6 were designed and synthesized to investigate the relationship between emulsifying properties and HLB. The results showed that when the oil–water ratio was 1:1, PG-5 fatty acid esters with HLB values of greater than nine formed o/w emulsions, while those with HLB values less than nine formed w/o emulsions [21]. Of course, the type of PGFEs used as emulsifiers depends not only on the HLB value, but also on the oil-–water ratio of the formulation system. In addition to emulsification, PGFEs with low HLB values in the range of 1.6–6.0 can be used as crystallization-adjusters [23,24,25] and lipid-based excipients [26] due to their good lipophilicity; some PGFEs with high HLB values of around 14 have been reported to be applied as detergents [37]. The detailed applications of PGFEs will be further discussed in Section 3.
2.2. Surface Properties of PGFEs
Changes in the hydrophilic “head” (PG) and hydrophobic “tail” (fatty acid) not only alter the HLB values of the surfactants, but also alter their surface properties, including their critical micelle concentration (CMC), surface tension, and interfacial tension (Table 2).
As shown in Table 2, the CMC of PGFEs usually increases with an increase in the average degree of polymerization of PG moieties and decreases with an increase in the aliphatic chain length or the degree of esterification. PGFEs can effectively reduce the surface tension of water (about 72 mN/m). The surface tension value of most PGFEs at CMC reported in the literature was around 30 mN/m, and a few PGFEs (PG-2 monolaurate [38], PG-2 monooleate [39], and PG-10 mono-caprylate/caprate [22,40]) can reduce the surface tension of water to 27 mN/m.

Table 2.
Surface properties of PGFEs.
Table 2.
Surface properties of PGFEs.
| PGFEs | CMC (mg/L) | γCMC (mN/m) | Interfacial Tension (mN/m) | Ref. |
|---|---|---|---|---|
| PG-2 monolaurate | 36.54 | 27.7 | 5.5 a | [38] |
| PG-2 monooleate | 0.12 mmol/L | 27 | 5.5 | [39] |
| PG-2 stearate (Enzymatic or chemical method) | - | - | ~2 b (0.4 g/L) | [41] |
| PG-2 monoester | 39.09 | 31.96 | 4.81 c | [14] |
| PG-2 diester | 28.12 | 33.54 | 7.22 c | [14] |
| PG-2 triester | 15.80 | 35.99 | 7.45 c | [14] |
| PG-3 monoester | 69.70 | 31.55 | 4.99 c | [14] |
| PG-3 diester | 53.22 | 33.89 | 5.80 c | [14] |
| PG-3 triester | 31.04 | 35.76 | 6.35 c | [14] |
| PG-3 monolaurate | 78.91 | 31.4 | 1.7 a | [38] |
| PG-4 monolaurate | 118.05 | 35.2 | 3.4 a | [38] |
| PG-5 monolaurate | 168.15 | 39.6 | 5.3 a | [38] |
| PG-10 mono-caprylate/caprate | 10–30 | 27.13 | 14 b | [22,40] |
| PG-10 monolaurate | 30 | ~29 | 20 b | [22] |
| PG-10 monostearate | - | - | 25 b | [22] |
a corn oil/aqueous solution of PGFEs (corn oil/water: 23.9 mN/m), 30 °C; b sunflower oil/aqueous solution of PGFEs (sunflower oil/water: 31 mN/m); c n-heptane/aqueous solution of PGFEs (n-heptane/water: 48.5 mN/m). “-” = Not provided.
In addition, a notable reduction in the interfacial tensions (IFT) in various oil/water systems was also observed. It is worth noting that the purified PG-3 monolaurate significantly reduced the interfacial tension of corn oil/water from 23.9 mN/m to 1.7 mN/m at a concentration of 0.1% [38]. Another study revealed that as the concentration of PG-2 stearate increased, the interfacial tension of the sunflower oil/water system decreased from approximately 20 mN/m (0.04 g/L) to approximately 2 mN/m (0.4 g/L). Interestingly, although both chemically and enzymatically synthesized PG-2 stearate showed a similar minimum interfacial tension (around 2 mN/m), chemically synthesized PG-2 stearate showed a constant IFT decrease from 0.25 g/L, while enzymatically synthesized PG-2 stearates showed an abrupt decrease at 0.37 g/L [41]. This may be due to the fact that the composition of enzymatically synthesized PG-2 stearate (PG-2: 6%; PG-2 stearate: 90%; other: 4%) is relatively simple compared to that of chemically synthesized PG-2 stearate (PG-2: 5%; PG-2 stearate: 70%; PG-3 stearate + PG-4 stearate + other: 25%), which was ascribed to the higher selectivity and fewer side reactions of enzymatic synthesis. The PG-3 stearate and PG-4 stearate found in chemically synthesized PG-2 stearate may be attributed to the further polymerization of PG-2.
2.3. Phase Behaviors of Various Systems Containing PGFEs
Although the HLB value of a surfactant is a crucial and effective parameter for its practical applications, its phase behavior also plays an important role in practice because this can reflect the arrangement of surfactant molecules in a system or at an interface, which generally has a great influence on the viscosity [42], rheological properties [43], and stability of emulsions or foams [44]. For example, in the same mixture system, the viscosity of the micellar phase is usually significantly lower than that of the liquid crystal phase, and the formation of various phases depends on their different compositions. Therefore, the phase behaviors of various mixture systems containing PGFEs are worthy of attention and are summarized in Table 3.
Hashizaki et al. investigated the effects of PGFEs on the lecithin/n-decane system, and found that PGFEs can induce the formation of a long cylindrical reverse worm-like micellar phase (PGFE/lecithin/n-decane) from the spherical or ellipsoidal reverse micellar phase (lecithin/n-decane). Due to the addition of PGFEs, the lecithin/n-decane solution was transformed into a highly viscoelastic solution composed of reverse wormlike micelles and exhibited the rheological property of “shear-thickening”. In addition, with the increase in PGFEs concentration, the number and length of the reverse worm-like micelles increased, resulting in a significant increase in the zero-shear-rate viscosity (η0) of PGFE/lecithin/n-decane solution [18]. Additionally, lamellar gels formed by PG-10 monostearate/cetyl isooctanoate/water ternary system also can be used to prepare o/w emulsions with a high dispersion stability just by diluting them with water (low-energy emulsification method) [45].

Table 3.
The phases of mixed systems containing PGFEs.
Table 3.
The phases of mixed systems containing PGFEs.
| PGFEs | Systems | Phases 1 | Ref. |
|---|---|---|---|
| PGL6FA10 | PGFE/lecithin/n-decane | Om | [18] |
| PGL10FA6 | Om | ||
| PGL10FA10 | Om | ||
| PGL10FA14 | Om | ||
| PGL10FA18 | - | ||
| PGL20FA10 | Om | ||
| PGL40FA10 | Om | ||
| PG-10 monolaurate | PGFE/ethanol/water | lamellar gel | [31] |
| PG-10 monomyristate | |||
| PG-10 monopalmitate | |||
| PG-10 monostearate | |||
| PG-2 monostearate | |||
| PG-10 monostearate | PGFE/cetyl isooctanoate/water | Lα | [45] |
| PG-x didodecanoates (x = 4.5, 7.3, 10.7, 21.3) | PGFE/water | Lα, Lα + W, H1, Wm | [46] |
| PG-5 monostearate | PGFE/water | Lα + W, Lα (>46 °C), | [47] |
| α-gel + W, α-gel (<46 °C) | |||
| PG-5 monooleate | PGFE/water | Lα + W, Lα, Om | |
| PGE 55 | PGFE/water | MLV + W, Lα (>58 °C) MLV + α-gel, α-gel (<58 °C) | [48,49] |
| SY-Glyster ML-750 (PG-10 monolaurate) | (PGFE/polyglycerol polyricinoleate)/ | L3, Lc + L3, L3 + Wm + O, Wm + O | [50] |
| SY-Glyster ML-550 (PG-6 monolaurate) | water/vegetable oil | Lα + L3, Lc + L3, L3 + Wm + O, Wm + O | |
| SY-Glyster MM-750 (PG-10 monomyristate) | PGFE/polyglycerol polyricinoleate = 1:1 | L3, Lc + L3, L3 + Wm + O, Wm + O | |
| SY-Glyster ML-550 (PG-6 monolaurate) | (PGFE/polyglycerol polyricinoleate)/ glycerol/vegetable oil PGFE/polyglycerol polyricinoleate = 1:1 | Lc + L3 + G, Lc + L3, Lc + L3 + O, Lc + L3 + G + O | [51] |
1 Om = reverse micellar phase; Lα = lamellar phase; W = isotropic aqueous phase; H1 = normal hexagonal phase; Wm = normal micellar phase; MLV = multilamellar vesicles; L3 = sponge phase; Lc = liquid crystalline phase; O = oil phase; G = glycerol phase. “-” = Not observed.
2.4. Stabilizing Effect on Foams and Emulsions
Foam is an important sensory indicator of certain products. Therefore, foaming ability and foam stability are critical for surfactant applications. The initial foam heights of 0.1% (1%) PG-10 mono-caprylate/caprate, PG-10 monolaurate, PG-10 monostearate and PG-10 monooleate were 22 mm (56 mm), 17 mm (44 mm), 5 mm (36 mm), and negligible (2 mm), respectively (stirring method), indicating that when PG-10 is used as the hydrophilic head, the shorter the fatty chain, the better the foaming ability [22]. Although the initial foam heights of the PGFEs aqueous solution were relatively low, the foam heights did not change much over time [22,38], indicating that the foam formed by PGFE has good stability. In addition, PGE 55, a commercial PGFE prepared from oligoglycerol (the average degree of polymerization is about 3) is an efficient surfactant for foam generation that can generate a large amount of foam with very small bubble size (about 7 mg surfactant for 1 m2 surface area) [28]. Another report showed that pH has a significant impact on foam volume. At a pH of 3, nearly 3 L foam formed in just 60 s, while at a pH of 9, only 100 mL foam was produced after 5 min of whipping [52].
To maintain the stability of foams and emulsions, interfacial film is one of the key factors. Taking the o/w emulsion as an example, the more rigid the interfacial film is the greater the amount of energy required to destroy it. Thus, the more difficult it is for the emulsion to coalesce, the more stable the emulsion. Adding surfactants that can form a lamellar phase is an effective way to improve the stability of emulsions because the ordered lamellar structure can enhance the rigidity of the interfacial film.
For instance, Sakai et al. prepared ethanol-containing o/w emulsions (ethanol content: 40 wt.%) with long-term stability (more than 4 months) using a mixture of PG-10 monostearate and PG-2 monostearate as an emulsifier. The good stability was attributed to the adsorption of the lamellar phase at the o/w interface, forming a rigid interfacial film with excellent viscoelastic properties [31].
2.5. Stability of PGFEs
The thermal stability of PGFEs under neutral conditions is relatively good and can be considered stable below 100 °C [53]. When the storage temperature exceeds 100 °C, the instability will be enhanced. In terms of hydrolysis, PGFEs are comparable to monoglycerides [54]. Their hydrolysis will be accelerated in the presence of bases or acids due to the ester linkage between PG and fatty acids. In addition, similarly to other glycerides, PGFEs can also be degraded by lipases [55].
3. Applications of PGFEs
Since the raw materials (e.g., glycerol and fatty acids) for the preparation of PGFEs are derived from natural sources, they are considered to possess high bio-safety and are approved as food additives [53] and cosmetic ingredients [55]. Their safety as pharmaceutical excipients has been evaluated in recent years [56].
3.1. Food Field
In the food field, fats and oils are important ingredients. The crystallization behaviors of fats and oils used in food products has a strong influence on the overall structures of fat-/oil-containing products and ultimately affects the physical properties of the final products.
3.1.1. Oils and Fats
Palm oil, an edible oil derived from palm fruit, exhibits a semi-solid state at room temperature. It can be fractionated into a high-melting solid fraction (palm stearin) and a low-melting liquid fraction (palm olein) [57]. The quality of the two fractions is related to the crystallization and separation steps. For the crystallization step, PGFEs can be used as a crystallization-adjuster to retard crystal growth. For example, PGEmix-8 (a polyglycerol mixed-fatty acid ester with melting point of 42.8 °C) can promote nucleation and retard crystal growth, thereby significantly affecting the crystallization process of palm oil and reducing the fractionation time [24,25]. Interestingly, the effects of PGFEs on the crystallization behavior of palm olein are not consistent. Palsgaard® PGE 1105 and PGE 1117 decreased the crystallization induction time and increased the crystallization rate of palm olein, while Palsgaard® PGE1155 significantly decreased the crystallization rate [58].
Oils rich in diacylglycerols (DAG) are a promising alternative to conventional edible oils in healthy daily diets because DAG have health benefits such as reducing serum triacylglycerol levels and suppressing body weight and the amount of visceral fat. However, compared with triglyceride-based oils, DAG-rich oils are more susceptible to precipitation at low temperatures, limiting their applications. According to the literature, different PGFEs had different effects on DAG crystallization. Commercial PG-10 oleate (Q-1710) did not retard the crystallization of DAG-rich oils, whereas polyglycerol fatty acid ester, which was fully esterified with a mixture of palmitic acid/oleic acid (50/50) and PG-10, significantly retarded the crystallization of DAG-rich oils [59,60]. In addition to PG-10 fatty acid esters, PG-2 linoleates also can retard the crystallization of DAG-rich oil and the retardation effect increased as the esterification degree of PG-2 linoleates decreased [61].
PGFEs also can adjust the crystallization of fats. For example, the polyglycerol fatty acid ester, fully esterified with PG-3 and stearic acid, can effectively promote the nucleation of fat blend to form small and uniform crystals, thereby improving the quality of whipped cream [62]. From the results of the effects of various PGFEs on the crystallization of oils and fats, the chemical structure of PGFEs, such as the type and composition of fatty acids and the degree of esterification of hydrophilic polyglycerol, may be an important factor regulating the crystallization.
In addition to adjusting the crystallization, PGFEs (e.g., polyglycerol stearate and polyglycerol behenate) have also been reported to act as oleogelators [63,64,65] in the structure of liquid oils (e.g., soybean oil [66] and sunflower oil [67]) or low-saturated fat blends [68], or to act as antioxidants to inhibit the lipid oxidation and improve the shelf life of soybean oil [69].
3.1.2. Bioactive Ingredients
Bioactive ingredients such as polyphenols provide some potential health benefits, but adding bioactive ingredients to foods to enhance their functionality leads to many challenges. How to improve the stability and bioaccessibility of bioactive ingredients in food has always been an urgent problem to be solved.
Curcumin (CUR) is a polyphenol that has attracted much attention in the development of functional foods due to its antioxidant, anti-inflammatory, and other physiological activities. However, CUR is highly hydrophobic with a very low oral bioavailability of less than 0.5%. In order to improve the water-solubility of CUR, PGFEs can be used to prepare CUR-loaded emulsions [27,70] or enhance the stability of CUR-loaded emulsions [71,72]. For example, Zhang et al. prepared CUR-loaded nano-emulsions stabilized by PGFEs and investigated the effect of the aliphatic chain length in PGFEs on the digestive profiles using an in vitro gastrointestinal tract (GIT) model [73]. Moreover, Nagano et al. reported an amorphous CUR formulation (CUR/polyvinylpyrrolidone (PVP)/PGFE = 11:64:25, w/w) with high and stable water-solubility using PGFE as an emulsifier, and revealed that PGFE can reduce the interaction between CUR, thereby improving the dispersibility and solubility of CUR in water [74].
In addition to CUR, lycopene also can be prepared in the nanodispersion using PGFE (M-7D) as an emulsifier. Interestingly, PGFEs can inhibit the conversion of the Z-isomer of lycopene to the all-E-isomer during storage [75]. Moreover, PGFEs have been reported to be effective emulsifiers for other lipophilic bioactive ingredients or nutrients, including co-enzyme Q10 [76], β-carotene [77], and peppermint oil [78].
3.1.3. Other
In addition to the above applications in food, PGFEs also can be used to improve the quality of recombined dairy cream [29], increase the solubility of a flavor (e.g., lemon essential oil) to produce transparent flavor emulsions for beverages [79], or in combination with mono- and di-glycerides as a crystal-promoter to improve the quality of heat-resistant compound chocolates [80,81]. In addition, PG-3 monostearate was reported to be used as an additive to prepare stabilized 3D-printable capsanthin hydrogels for innovative foods [82], and polyglycerol monolaurates (PG-2 monolaurate, PG-3 monolaurate, and PG-6 monolaurate) were reported to exhibit antibacterial effects against Gram-positive bacteria, S. aureus, and B. subtilis, and may be utilized as a safe preservative in foods [83].
3.2. Cosmetic Field
PGFEs have become increasingly popular in cosmetic formulations as a potential alternative to PEG-based surfactants to avoid the risks caused by 1,4-dioxane, and have been used as emulsifying agents, emulsion stabilizers, cleansing agents, solubilizing agents, skin-conditioning agents, and hair-conditioning agents [55].
3.2.1. Emulsifying Agent
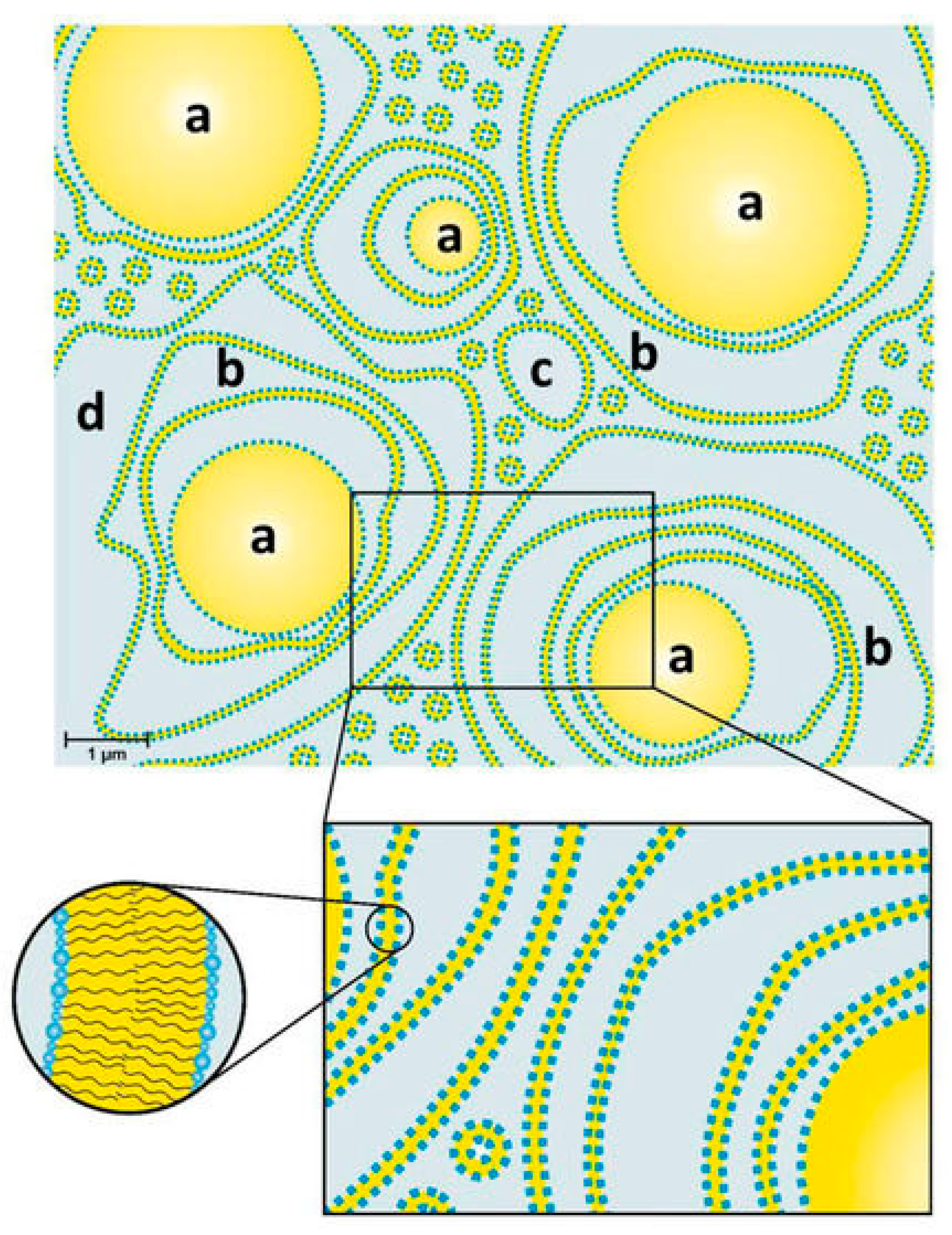
Most cosmetic products come as emulsions. Although their uses are very different, their bases are very similar; they are primarily a mixture of water and oils (fats and lipids), along with some other ingredients. For example, Rajput et al. synthesized a series of PGFEs and used them as an emulsifying agent to successfully prepare moisturizing creams [14]. Dahl et al. investigated the structure of a typical cosmetic oil-in-water emulsion (o/w-emulsion) based on the emulsifier TEGO® Care PSC 3 (an esterification product of PG-3 with stearic acid and a substoichiometric amount of citric acid) and the consistency enhancer (glyceryl stearate/stearyl alcohol). The results showed that pure TEGO® Care PSC 3 forms lamellar stacked bilayers in water with a spacing of 7 nm, coexisting with polydisperse unilamellar vesicles. When the consistency enhancer was added, multilamellar vesicles could be obtained. In the final cosmetic o/w-emulsion with a cream-like consistency containing 25 wt.% oils, the oil droplets were surrounded by multiple irregularly spaced bilayers and vesicles, and about 30% of water was restricted in this system in the form of encapsulated water, as shown in Figure 2. This steric interaction between oil droplets and crystalline bilayers in water phase may be an important factor in enhancing the consistency of cosmetic emulsions [32].
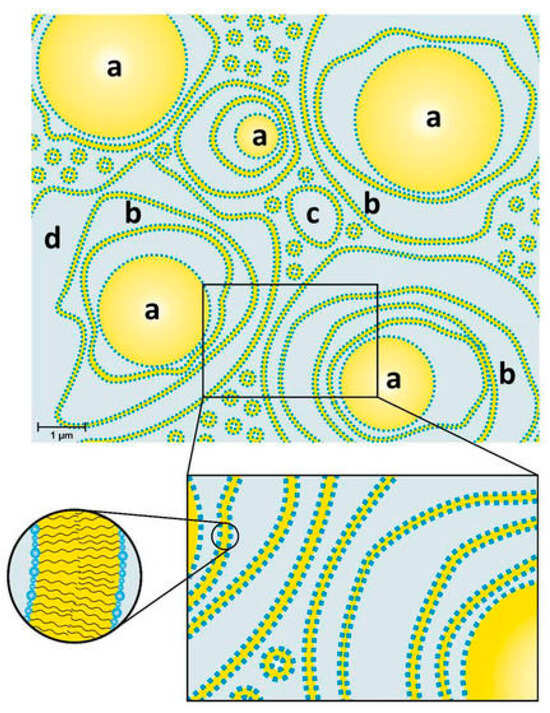
Figure 2.
Model of an o/w-emulsion containing TEGO® Care PSC 3 (an esterification product of PG-3 with stearic acid and a substoichiometric amount of citric acid) as emulsifier and glyceryl stearate/stearyl alcohol as consistency enhancer: (a) Oil droplets surrounded by (b) several layers of bilayers and (c) polydisperse vesicles in (d) a water phase [32].
In addition, PG-3 rice bran fatty acid esters have also been reported as being used as an emulsifier in sunscreen formulations containing UVA (butyl methoxydibenzoylmethane) and UVB (ethylhexyl methoxycinnamate) filters, and the protective effects of the sunscreen emulsions has been evaluated. Regarding the emulsions containing the UVA filter, the SPF value of the emulsion prepared with the PG-3 rice bran fatty acid esters was comparable to that of the emulsion prepared with the glucoside derivative Montanov 68 (cetearyl alcohol and cetearyl glucoside), but the decrease in the SPF value of the emulsion prepared with PG-3 rice bran fatty acid esters (9.62%) was clearly lower than that of Montanov 68 (12.28%), indicating that PG-3 rice bran fatty acid esters can improve UVA filters’ photostability [84].
3.2.2. Cleansing Agent
Another use of PGFEs is as a cleaning agent. For instance, Iwanaga et al. successfully prepared an oil-based makeup remover that can be used even in a bath by utilizing the polyglycerol ester of middle-chain fatty acids [85]. For water-based makeup-removers, Zhang et al. investigated the aggregation behavior of PG-10 monocaprylate in water and the cleaning ability of its aqueous solution. The results showed that PG-10 monocaprylate could self-assemble into nanoparticles in the water with a transparent appearance (the concentration of PG-10 monocaprylate: 2.5–10 wt.%). Compared with the PEG-based non-ionic surfactants (e.g., PEG-6 caprylic/capric glycerides) used in transparent makeup removers, PG-10 monocaprylate is less irritating and exhibits a good removal effect against makeup, especially pen eyeliner [37]. It has been used as a sustainable alternative to PEG-6 caprylic/capric glycerides for the preparation of makeup-removers.
3.2.3. Other
In addition to acting as an emulsifying agent and cleansing agent, PGFEs can also serve as a solubilizing agent to increase the solubility of red raspberry seed oil and/or hydro-glycolic acid fruit extract for the protection and hydration of skin [30]. The active ingredients (Bakuchiol and N-prolyl palmitoyl tripeptide-56 acetate) have also been reported to be incorporated into oil-in-water dermato-cosmetic emulsions using PG-6 stearate and PG-6 behenate, and the results showed that the obtained emulsions may be suitable for the preparation of cosmetics with potential anti-aging effects [86].
3.3. Pharmaceutical Field
In recent years, the application of PGFEs in the pharmaceutical industry has also received much attention. PGFEs can be used as lipid-based excipients, drug carriers, or for modifying nanomedicines.
3.3.1. Lipid-Based Excipients
PGFEs were considered as an approach for overcoming the stability challenges faced by conventional lipid-based excipients, allowing for them to have advanced pharmaceutical applications due to their stable solid state, microstructure, and physicochemical properties [26,87,88]. For instance, ibuprofen-loaded inhalable lipid-microparticles were successfully prepared using PG behenates as lipid-based excipients via spray-drying [89]. Witepsol® PMF 166 (PG-6 palmitate) can be used as a high-performing lipid material that is processed into blank filaments or felodipine-loaded filaments, and the obtained filaments can be further used to fabricate placebo dosage forms and personalized oral solid dosage forms via 3D-printing without using additional materials [90,91].
3.3.2. Drug Carriers
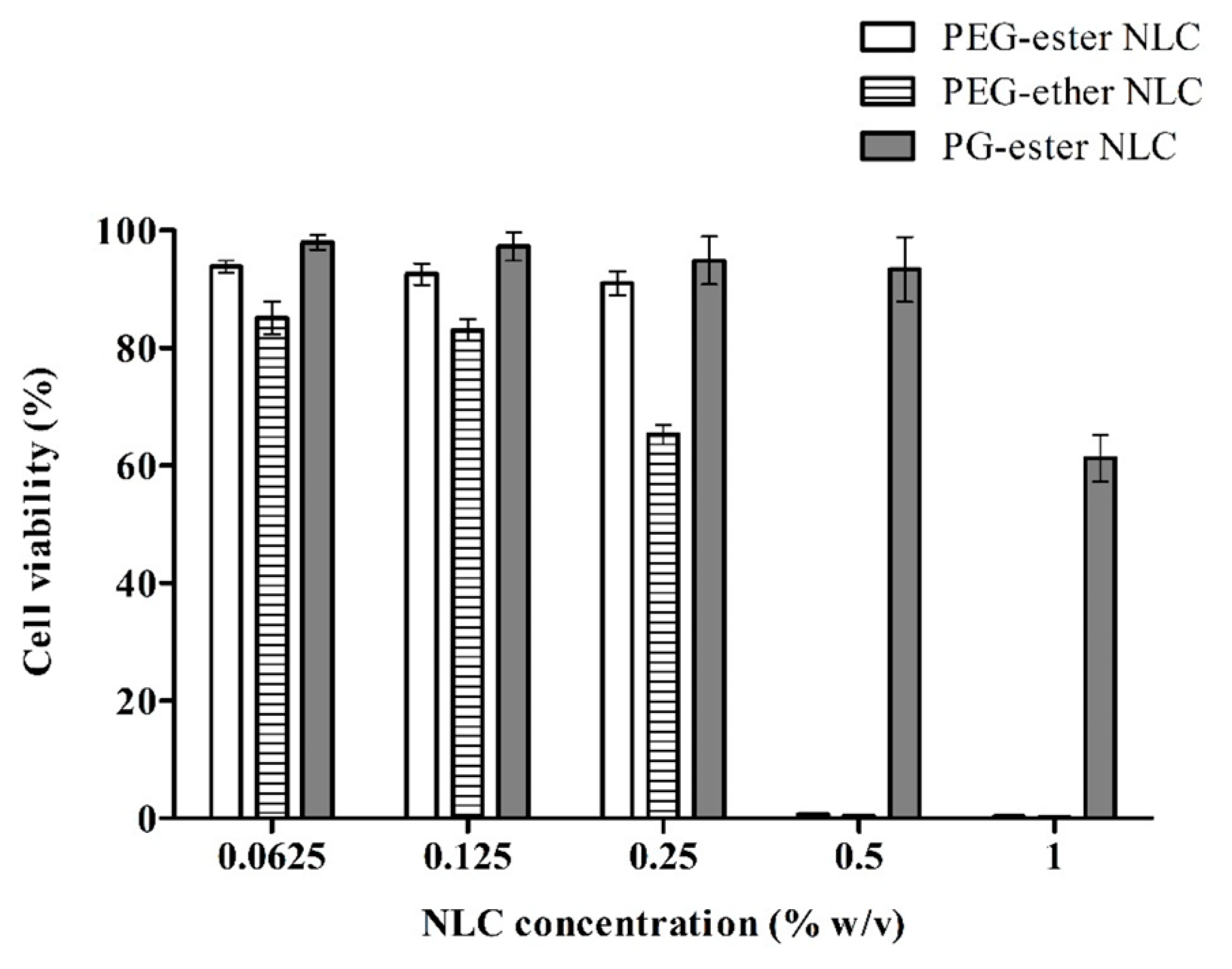
In addition to lipid-based excipients, PGFEs can also be used as carriers for the delivery of proteins [92], peptides (e.g., insulin [33]), and hydrophobic drugs (e.g., cannabidiol [93]). For oral formulations, Bernkop-Schnürch’s group prepared three insulin/sodium dodecyl sulfate complex-loaded NLC (nanostructured lipid carrier) formulations containing PEG-ester, PEG-ether, and PGFE (TEGOSOFT® PC 41 and TEGO® Solve 90 MB) surfactants, respectively, and evaluated their cytotoxicity by resazurin assay against Caco-2 cells. The results showed that the non-cytotoxic concentration of PGFE-based NLC (0.5%) was significantly higher than that of PEG-based NLCs (PEG-ester NLC: 0.25%; PEG-ether NLC: 0.125%), as shown in Figure 3 [33].
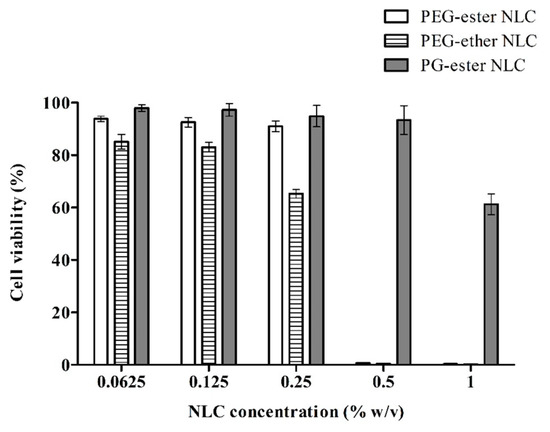
Figure 3.
The viability of Caco-2 cells after 4 h incubation with insulin/sodium dodecyl sulfate complex-loaded NLCs, determined via resazurin assay [33].
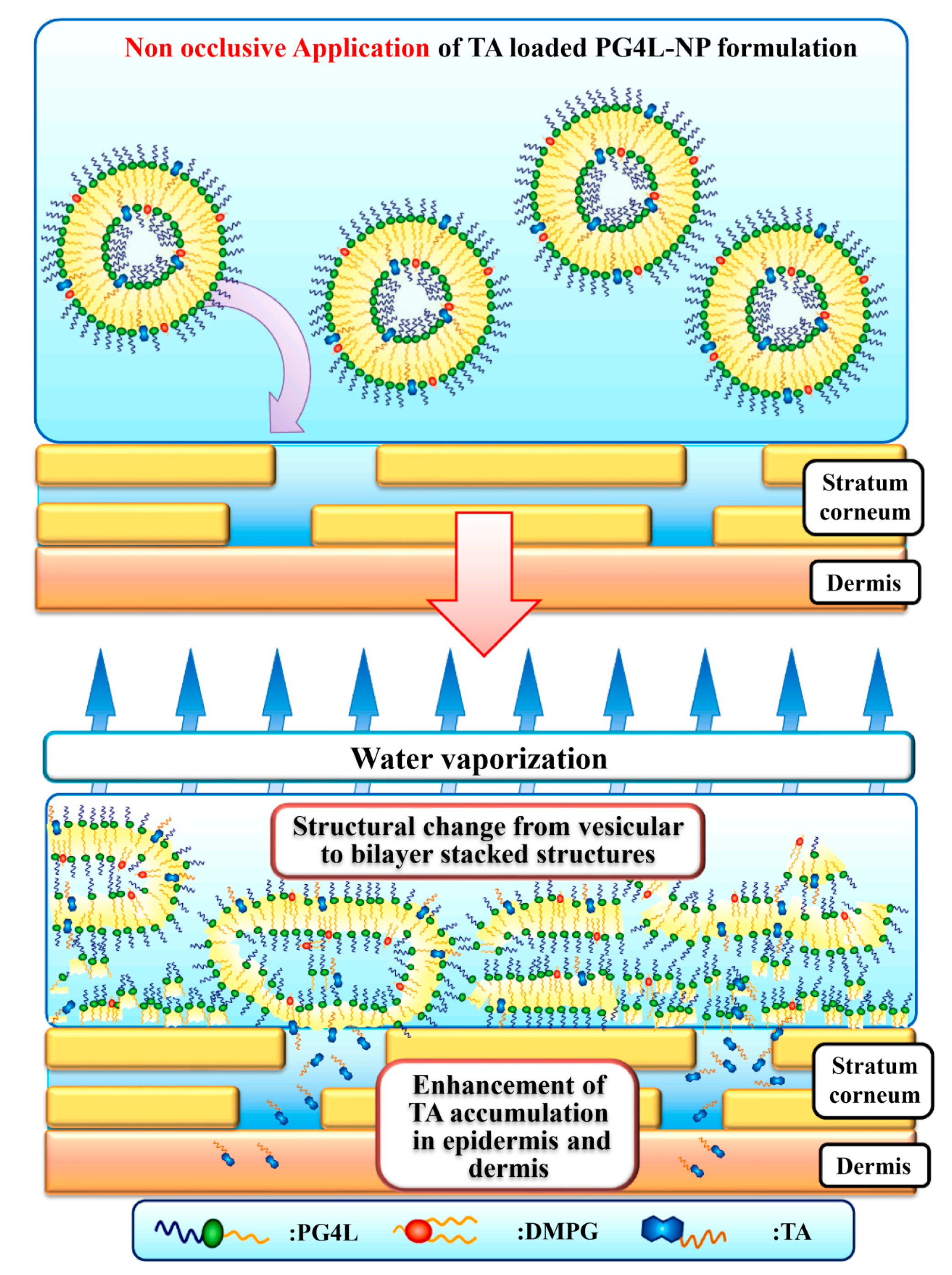
For topical applications, Uchino’s group developed PG-4 laurate-based tocopherol acetate-loaded nanoparticles (PG4L-NP) and evaluated the accumulation of tocopherol acetate (TA) in the epidermis and dermis. Interestingly, compared with other emulsifiers (e.g., soya phosphatidylcholine), only PG4L-NP induced TA accumulation in the dermis under non-occlusive applications. This may be due to the transformation of PG4L-NP from vesicles to bilayer stacked structures following water evaporation (Figure 4) [35].
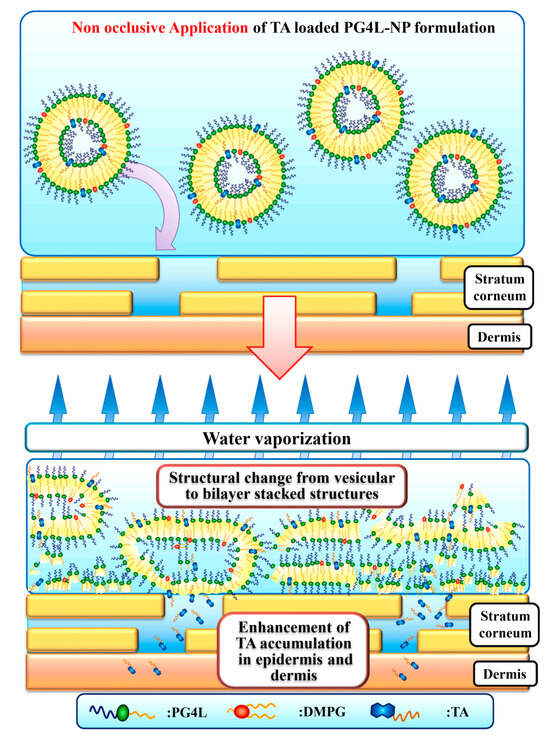
Figure 4.
Speculated mechanism of TA accumulation from PG4L-NP under non-occlusive application. Reprinted from Ref. [35], Copyright (2021), with permission from Elsevier.
3.3.3. Other
PEGylation is a common strategy to enhance the stability, reduce the immunogenicity, and prolong the circulation time of protein drugs and nanomedicines (e.g., liposomes) [94,95]. However, the induced anti-PEG IgM production is thought to be associated with loss of therapeutic efficacy and increased adverse effects [96,97,98].
Chen et al. modified liposomes with PG-10-monostearate, and investigated their pharmacokinetics, in vivo distribution, and capacity to induce anti-PEG IgM. The results showed that, compared with unmodified liposomes, PG-10-monostearate significantly prolonged the blood circulation time of liposomes. Importantly, the biodistribution pattern of PG-10-monostearate-modified liposomes was similar to that of PEGylated liposomes, but they were less likely to induce the production of anti-PEG IgM [99]. Therefore, PGFEs may be a potential good choice to replace PEG for modifying liposomes.
4. Conclusions
Polyglycerol fatty acid esters (PGFEs) are a class of nonionic surfactants with a wide HLB value range. In the food field, PGFEs can not only be used to improve the quality of oils/fats (crystallization-adjusters), cream, and chocolate, but can also be used as emulsifiers to prepare emulsions or nanoparticles loaded with bioactive ingredients or flavors to improve their water-solubility, stability, and bioaccessibility. In addition, PGFEs also have antibacterial effects on specific bacteria and are expected to be potential food preservatives. In the field of cosmetics, PGFEs are commonly used as emulsifiers, emulsion stabilizers, cleansing agents, solubilizers, etc. The safety of 274 PGFEs was assessed by the Expert Panel for Cosmetic Ingredient Safety [55], and PGFEs are considered safe for use in cosmetics in their present uses and concentrations, as described in their safety assessment. In the pharmaceutical field, PGFEs can be used for the delivery of drugs and peptides, and can also be used as lipid-based excipients to improve the processing of formulations.
Although PGFEs have a broad range of applications and prospective applications, many problems remain. For example, considering the complexity of polyglycerol, what effects do the polydispersity and branching degree of polyglycerol have on the properties of PGFEs, and how do these further affect the practical applications of PGFEs? The phase behaviors of PGFEs in more mixed systems also require further in-depth research to expand their applications. In addition, the safety of PGFEs in food and cosmetics has been systematically evaluated, but safety assessments regarding their use in pharmaceuticals require more extensive research.
Author Contributions
Conceptualization, G.Z.; writing—original draft preparation, M.Z. and G.Z.; writing—review and editing, M.Z. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, J.; Ding, C.; Xu, B.; Wang, S.; Wu, Z.; Zhao, W. The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant. Prog. Nat. Sci. Mater. Int. 2023, 33, 295–300. [Google Scholar] [CrossRef]
- Kandeel, E.M. Synthesis and Performance of Glycerol Ester-Based Nonionic Surfactants. J. Dispers. Sci. Technol. 2012, 33, 949–954. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability—Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Cholakova, D.; Tcholakova, S. Sucrose ester surfactants: Current understanding and emerging perspectives. Curr. Opin. Colloid Interface Sci. 2024, 73, 101832. [Google Scholar] [CrossRef]
- Das, R.; Mallik, N.; Adhikari, A.; Bhattarai, A. A Comprehensive Review on the Creation, Description, and Utilization of Surfactants Containing Multiple Hydroxyl Groups. Int. J. Polym. Sci. 2024, 2024, 6120535. [Google Scholar] [CrossRef]
- Lémery, E.; Briançon, S.; Chevalier, Y.; Bordes, C.; Oddos, T.; Gohier, A.; Bolzinger, M.-A. Skin toxicity of surfactants: Structure/toxicity relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 166–179. [Google Scholar] [CrossRef]
- Lafranconi, M.; Anderson, J.; Budinsky, R.; Corey, L.; Forsberg, N.; Klapacz, J.; LeBaron, M.J. An integrated assessment of the 1,4-dioxane cancer mode of action and threshold response in rodents. Regul. Toxicol. Pharmacol. 2023, 142, 105428. [Google Scholar] [CrossRef]
- Pal, A.; Mondal, M.H.; Adhikari, A.; Bhattarai, A.; Saha, B. Scientific information about sugar-based emulsifiers: A comprehensive review. RSC Adv. 2021, 11, 33004–33016. [Google Scholar] [CrossRef]
- Ji, S.; Shen, W.; Chen, L.; Zhang, Y.; Wu, X.; Fan, Y.; Fu, F.; Chen, G. Synthesis and properties of sugar-based surfactants alkoxyethyl β-D-glucopyranoside. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 59–68. [Google Scholar] [CrossRef]
- Szűts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Gundert-Remy, U.; Gurtler, R.; Husøy, T.; et al. Follow-up of the re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2022, 20, e07308. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; Aserin, A.; Zaidman, B. Polyglycerol esters: Optimization and techno-economic evaluation. J. Am. Oil Chem. Soc. 1981, 58, 878–883. [Google Scholar] [CrossRef]
- Rajput, Y.N.; Kulkarni, R.D. Unlocking the potential: Cost-efficient and sustainable synthesis of polyglycerol ester derivatives from industrial by-products, surface properties evaluation and development of moisturizing creams. Ind. Crops Prod. 2023, 204, 117291. [Google Scholar] [CrossRef]
- Damle, S.; Madankar, C. An overview on eco-friendly polyglycerol esters of fatty acid, synthesis and applications. Tenside Surfactants Deterg. 2023, 60, 611–621. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Fujita, A. Prediction of Organic Compounds by a Conceptional Diagram. Pharm. Bull. 1954, 2, 163–173. [Google Scholar] [CrossRef]
- Hashizaki, K.; Imai, M.; Yako, S.; Tsusaka, H.; Sakanishi, Y.; Saito, Y.; Fujii, M. Highly Viscoelastic Reverse Wormlike Micellar Systems from a Mixture of Lecithin, Polyglycerol Fatty Acid Monoesters, and an Oil. J. Oleo Sci. 2017, 66, 997–1007. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Sun, Y.; Zaaboul, F.; Tan, C.-P.; Liu, Y. Different hydrophilic polyglycerol fatty acid esters interact with fat crystals and proteins at the interface to co-stabilize highly unsaturated whipped emulsions. Food Hydrocoll. 2024, 146, 109232. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Zaaboul, F.; Sun, Y.; Tan, C.P.; Liu, Y. Synergistic effect of hydrophilic polyglycerol fatty acid esters and protein on the stability of interfacial membrane in low-fat aerated emulsions with different homogenization conditions. Food Chem. 2024, 435, 137584. [Google Scholar] [CrossRef]
- Shikhaliev, K.S.; Stolpovskaya, N.V.; Krysin, M.Y.; Zorina, A.V.; Lyapun, D.V.; Zubkov, F.I.; Yankina, K.Y. Production and Emulsifying Effect of Polyglycerol and Fatty Acid Esters with Varying Degrees of Esterification. J. Am. Oil Chem. Soc. 2016, 93, 1429–1440. [Google Scholar] [CrossRef]
- Stanimirova, R.D.; Danov, K.D.; Georgiev, M.T.; Petkov, J.T. Colloid, interface, and foam properties of water-soluble polyglycerol esters solutions. J. Colloid Interface Sci. 2025, 677, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Kato, H.; Ito, N.; Takase, Y.; Nanbu, H.; Mishima, S.; Sakaki, H.; Sato, K. Effects of polyglycerol esters of fatty acids and ethylene-vinyl acetate co-polymer on crystallization behavior of biodiesel. Eur. J. Lipid Sci. Technol. 2010, 112, 1323–1330. [Google Scholar] [CrossRef]
- Kuriyama, J.; Miyaji, Y.; Tamura, K.; Zaliha, O.; Chong, C.L. Improved sustainable fractionation of palm oil using polyglycerol fatty acid esters. J. Oil Palm Res. 2011, 23, 1141–1145. [Google Scholar]
- Saw, M.H.; Hishamuddin, E.; Chong, C.L.; Yeoh, C.B.; Lim, W.H. Effect of polyglycerol esters additive on palm oil crystallization using focused beam reflectance measurement and differential scanning calorimetry. Food Chem. 2017, 214, 277–284. [Google Scholar] [CrossRef]
- Corzo, C.; Lopes, D.G.; Lochmann, D.; Reyer, S.; Stehr, M.; Salar-Behzadi, S. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 1: Screening of solid-state and physical properties of polyglycerol esters of fatty acids as advanced pharmaceutical excipients. Eur. J. Pharm. Biopharm. 2020, 148, 134–147. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Y.; Zhang, S.; Xu, X.; Yang, J. Synthesis of polyglycerol fatty acid esters catalyzed by lipase and evaluation of its emulsification properties. LWT 2023, 182, 114919. [Google Scholar] [CrossRef]
- Mustan, F.; Politova-Brinkova, N.; Vinarov, Z.; Rossetti, D.; Rayment, P.; Tcholakova, S. Interplay between bulk aggregates, surface properties and foam stability of nonionic surfactants. Adv. Colloid Interface Sci. 2022, 302, 102618. [Google Scholar] [CrossRef]
- Yan, G.; Wang, S.; Li, Y.; Zhang, J.; Ding, H.; Li, Y.; Zhang, L. Effect of Different Polymerization Degrees and Fatty Acids of Polyglycerol Esters on the Physical Properties and Whippability of Recombined Dairy Cream. Foods 2023, 12, 22. [Google Scholar] [CrossRef]
- Gupta, M.; Hooghten, R.V.; Fischer, P.; Gunes, D.Z.; Vermant, J. Limiting coalescence by interfacial rheology: Over-compressed polyglycerol ester layers. Rheol. Acta 2016, 55, 537–546. [Google Scholar] [CrossRef]
- Motoyama, T.; Katsuumi, Y.; Sasakura, H.; Nakamura, T.; Suzuki, H.; Tsuchiya, K.; Akamatsu, M.; Sakai, K.; Sakai, H. Preparation of Highly Stable Oil-in-Water Emulsions with High Ethanol Content Using Polyglycerol Monofatty Acid Esters as Emulsifiers. J. Oleo Sci. 2022, 71, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.; Friedrich, A.; Meyer, J.; Venzmer, J.; Belkoura, L.; Strey, R.; Mayer, C.; Michel, R.; Gradzielski, M. Structural Analysis of a Modern o/w-Emulsion Stabilized by a Polyglycerol Ester Emulsifier and Consistency Enhancers. Colloids Interfaces 2018, 2, 3. [Google Scholar] [CrossRef]
- Shahzadi, I.; Furst, A.; Knoll, P.; Bernkop-Schnurch, A. Nanostructured Lipid Carriers (NLCs) for Oral Peptide Drug Delivery: About the Impact of Surface Decoration. Pharmaceutics 2021, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gledovic, A.; Janosevic Lezaic, A.; Nikolic, I.; Tasic-Kostov, M.; Antic-Stankovic, J.; Krstonosic, V.; Randjelovic, D.; Bozic, D.; Ilic, D.; Tamburic, S.; et al. Polyglycerol Ester-Based Low Energy Nanoemulsions with Red Raspberry Seed Oil and Fruit Extracts: Formulation Development toward Effective In Vitro/In Vivo Bioperformance. Nanomaterials 2021, 11, 217. [Google Scholar] [CrossRef]
- Uchino, T.; Fujimori, S.; Hatta, I.; Miyazaki, Y.; Kamiya, D.; Fujino, H.; Suzuki, R.; Kirishita, Y.; Eda, T.; Murashima, K.; et al. Development of novel polyglycerol fatty acid ester-based nanoparticles for the dermal delivery of tocopherol acetate. Int. J. Pharm. 2021, 592, 120004. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kimura, Z.; Misono, T.; Tsuchiya, K.; Sakai, K.; Abe, M.; Sakai, H. Preparation and Properties of Nonionic Vesicles Prepared with Polyglycerol Fatty Acid Esters Using the Supercritical Carbon Dioxide Reverse Phase Evaporation Method. J. Oleo Sci. 2016, 65, 201–206. [Google Scholar] [CrossRef]
- Zhang, G.; Bao, C.; Fu, K.; Lin, Y.; Li, T.; Yang, H. Synthesis, Characterization, Self-Assembly, and Irritation Studies of Polyglyceryl-10 Caprylates. Polymers 2020, 12, 294. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, T.; Yamashita, M.; Kawaguchi, M.; Kato, T.; Itoh, T. Surfactant properties of purified polyglycerol monolaurates. J. Surfactants Deterg. 2003, 6, 331–337. [Google Scholar] [CrossRef]
- Martínez, M.; Oliveros, R.; Aracil, J. Synthesis of Biosurfactants: Enzymatic Esterification of Diglycerol and Oleic Acid. 1. Kinetic Modeling. Ind. Eng. Chem. Res. 2011, 50, 6609–6614. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and stability of nanoemulsions: How relevant is the type of surfactant? J. Drug Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Amari, J.; Sangiorgio, S.; Pargoletti, E.; Rabuffetti, M.; Zaccheria, F.; Usuelli, F.; Quaranta, V.; Speranza, G.; Cappelletti, G. Chemically vs. Enzymatically Synthesized Polyglycerol-Based Esters: A Comparison between Their Surfactancy. ACS Omega 2023, 8, 26405–26413. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, G.; Wang, P. Phase Behavior and Rheological Properties of AES/CAPB/H2O Ternary System. Appl. Sci. 2024, 14, 3605. [Google Scholar] [CrossRef]
- Godoy, C.A.; Valiente, M.; Muñoz, W.; Bonilla, P.; Pons, R.; Montalvo, G. Microstructural and Viscoelastic Properties of Liquid Crystal Phases of Soybean Lecithin with Olive and Castor Oils at Low Water Contents. J. Chem. Eng. Data 2023, 68, 1534–1542. [Google Scholar] [CrossRef]
- Denkov, N.; Tcholakova, S.; Cholakova, D. Surface phase transitions in foams and emulsions. Curr. Opin. Colloid Interface Sci. 2019, 44, 32–47. [Google Scholar] [CrossRef]
- Kuwabara, H.; Ogura, T.; Tsuchiya, K.; Akamatsu, M.; Arakawa, K.; Sakai, K.; Sakai, H. Structural Analysis of Interfacial Films of Oil/Water Emulsions Prepared by Emulsification via Lamellar Gels. J. Oleo Sci. 2024, 73, 1467–1477. [Google Scholar] [CrossRef]
- Kunieda, H.; Akahane, A.; Jin, F.; Ishitobi, M. Phase Behavior of Polyglycerol Didodecanoates in Water. J. Colloid Interface Sci. 2002, 245, 365–370. [Google Scholar] [CrossRef]
- Izquierdo, P.; Acharya, D.P.; Hirayama, K.i.; Asaoka, H.; Ihara, K.; Tsunehiro, T.; Shimada, Y.; Asano, Y.; Kokubo, S.; Kunieda, H. Phase Behavior of Pentaglycerol Monostearic and Monooleic Acid Esters in Water. J. Dispers. Sci. Technol. 2006, 27, 99–103. [Google Scholar] [CrossRef]
- Duerr-Auster, N.; Kohlbrecher, J.; Zuercher, T.; Gunde, R.; Fischer, P.; Windhab, E. Microstructure and Stability of a Lamellar Liquid Crystalline and Gel Phase Formed by a Polyglycerol Ester Mixture in Dilute Aqueous Solution. Langmuir 2007, 23, 12827–12834. [Google Scholar] [CrossRef]
- Curschellas, C.; Kohlbrecher, J.; Geue, T.; Fischer, P.; Schmitt, B.; Rouvet, M.; Windhab, E.J.; Limbach, H.J. Foams Stabilized by Multilamellar Polyglycerol Ester Self-Assemblies. Langmuir 2013, 29, 38–49. [Google Scholar] [CrossRef]
- Wakisaka, S.; Nakanishi, M.; Gohtani, S. Phase behavior and formation of o/w nano-emulsion in vegetable oil/ mixture of polyglycerol polyricinoleate and polyglycerin fatty acid ester/water systems. J. Oleo Sci. 2014, 63, 229–237. [Google Scholar] [CrossRef]
- Wakisaka, S.; Nishimura, T.; Gohtani, S. O/W nano-emulsion formation using an isothermal low-energy emulsification method in a mixture of polyglycerol polyricinoleate and hexaglycerol monolaurate with glycerol system. J. Oleo Sci. 2015, 64, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Duerr-Auster, N.; Eisele, T.; Wepf, R.; Gunde, R.; Windhab, E.J. Influence of pH on colloidal properties and surface activity of polyglycerol fatty acid ester vesicles. J. Colloid Interface Sci. 2008, 327, 446–450. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2017, 15, e05089. [Google Scholar] [CrossRef]
- Norn, V. Polyglycerol Esters. In Emulsifiers in Food Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 181–208. [Google Scholar]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Polyglyceryl Fatty Acid Esters as Used in Cosmetics. Int. J. Toxicol. 2023, 42, 5S–101S. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Öhlinger, K.; Meindl, C.; Corzo, C.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. In vitro toxicity screening of polyglycerol esters of fatty acids as excipients for pulmonary formulations. Toxicol. Appl. Pharmacol. 2020, 386, 114833. [Google Scholar] [CrossRef]
- Tong, S.C.; Tang, T.K.; Lee, Y.Y. A Review on the Fundamentals of Palm Oil Fractionation: Processing Conditions and Seeding Agents. Eur. J. Lipid Sci. Technol. 2021, 123, 2100132. [Google Scholar] [CrossRef]
- Tangsanthatkun, J.; Sonprasert, T.; Sonwai, S. The Effect of Polyglycerol Esters of Fatty Acids on the Crystallization of Palm Olein. J. Oleo Sci. 2021, 70, 309–319. [Google Scholar] [CrossRef]
- Saitou, K.; Taguchi, K.; Homma, R.; Shimizu, M.; Yasunaga, K.; Katsuragi, Y.; Ueno, S.; Sato, K. Retardation Mechanism of Crystallization of Diacylglycerols Resulting from the Addition of Polyglycerol Fatty Acid Esters. Cryst. Growth Des. 2017, 17, 4749–4756. [Google Scholar] [CrossRef]
- Saitou, K.; Homma, R.; Kudo, N.; Katsuragi, Y.; Sato, K. Retardation of Crystallization of Diacylglycerol Oils Using Polyglycerol Fatty Acid Esters. J. Am. Oil Chem. Soc. 2014, 91, 711–719. [Google Scholar] [CrossRef]
- Wan, F.; Teng, Y.; Zou, L.; Yang, X.; Chen, Q.; Li, A.; Wang, Y. Effect of Addition of Purified Diglycerol Linoleic Acid Esters on the Crystallization Behavior of Diacylglycerol Oils. J. Am. Oil Chem. Soc. 2016, 93, 1605–1614. [Google Scholar] [CrossRef]
- Li, Y.; Liao, T.; Liu, T.; Yan, R.; Sun, Z.; Zhao, M.; Deng, X.; Zhao, Q. The quality of whipped cream: Effect of polyglycerol ester on the crystallization of fat blend and the properties of interface. Food Hydrocoll. 2023, 145, 109145. [Google Scholar] [CrossRef]
- Mei Huey, S.A.W. Effect of Storage Temperature and Duration on Rheological and Thermal Characteristics of Superolein Oleogels. J. Oil Palm Res. 2022, 35, 256–267. [Google Scholar] [CrossRef]
- Saw, M.H.; Lim, W.H.; Yeoh, C.B.; Badlishah, S.B.; Tan, C.P. Screening of Organogelators for Structuring Palm Superolein. J. Oil Palm Res. 2020, 32, 152–158. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, Y.; Wang, Y.; Liu, Y.F. Organogels based on the polyglyceryl fatty acid ester and sunflower oil: Macroscopic property, microstructure, interaction force, and application. Lwt-Food Sci. Technol. 2019, 116, 108590. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Serna-Saldívar, S.O.; Pérez-Carrillo, E.; Jordânia-Silva, T.; Barrera-Arrellano, D.; Buitimea-Cantúa, G.V. Textural and rheological properties of soybean oil organogels structured with polyglycerol and propylene glycol esters during storage. Grasas Aceites 2022, 73, e443. [Google Scholar] [CrossRef]
- Aydeniz-Guneser, B.; Yilmaz, E. Sunflower Oil-Polyglycerol Stearate Oleogels: Alternative Deep-fat Frying Media for Onion Rings. J. Oleo Sci. 2022, 71, 651–662. [Google Scholar] [CrossRef]
- Mahjoob, R.; Hakimzadeh, V.; Salehi, E.A.; Farmani, J. The interaction of polyglycerol esters with sorbitan tristearate, and sorbitan monostearate in structuring a low-saturated fat. J. Food Meas. Charact. 2022, 16, 4174–4184. [Google Scholar] [CrossRef]
- Li, B.; Sun, S.; Chen, X.; Zhang, H. Enzymatic conversion of soapstock fatty acids from oil refining waste to biosurfactant using a low-cost liquid lipase and a new application as an antioxidant. Biomass Convers. Biorefin. 2022, 14, 3839–3851. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, F.; Zhang, J.; Yu, L.; Zhang, G.; Liu, C.; Wang, N.; Xu, B. Development of polyglycerol fatty acid ester-based low-energy nanoemulsion for the improvement of curcumin stability. J. Dispers. Sci. Technol. 2020, 43, 605–611. [Google Scholar] [CrossRef]
- Shi, L.; Cheng, Y.; Jia, C.; Lin, H.; Zhang, W.; He, J. Stable complex of sodium caseinate and hexaglycerol monooleate with improved oil-in-water emulsion stability and curcumin encapsulation. Food Biophys. 2024, 19, 321–333. [Google Scholar] [CrossRef]
- Peng, B.; Xiong, C.Y.; Huang, Y.; Hu, J.N.; Zhu, X.M.; Deng, Z.Y. Enzymatic Synthesis of Polyglycerol Fatty Acid Esters and Their Application as Emulsion Stabilizers. J. Agric. Food Chem. 2018, 66, 8104–8113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, X.; Yang, J.; Ren, J. Impact of Emulsifier Structure and Concentration on Lipolysis Dynamics and Curcumin Bioaccessibility in the Nanoemulsions Stabilized by Polyglycerol Fatty Acid Esters. Food Biophys. 2022, 17, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Nagano, K.; Nakao, T.; Takeda, M.; Hirai, H.; Maekita, H.; Nakamura, M.; Imakawa, N.; Egawa, A.; Fujiwara, T.; Gao, J.Q.; et al. Polyglycerol fatty acid ester contributes to the improvement and maintenance of water solubility of amorphous curcumin by suppressing the intermolecular interaction and the diffusion rate of curcumin. Food Chem. 2024, 437, 137866. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kageyama, H.; Hibino, T.; Zhang, Y.; Goto, M.; Honda, M. Preparation of Highly Stable Z-Isomer-Rich Lycopene Nanodispersions via a Continuous-Flow System with Selected Emulsifiers. Eur. J. Lipid Sci. Technol. 2022, 124, 2200034. [Google Scholar] [CrossRef]
- Iwase, H.; Kobayashi, J.; Kasama, Y.; Fujii, W.; Nanbu, H. Structural analysis of polyglycerol fatty acid ester-coenzyme Q10 aggregates in solution. Food Res. Int. 2024, 175, 113741. [Google Scholar] [CrossRef]
- Das, N.M.A.; Kobayashi, I.; Nakajima, M. Nanotechnology for bioactives delivery systems. J. Food Drug Anal. 2020, 20, 52. [Google Scholar] [CrossRef]
- Xie, H.; Ni, F.; Liu, C.; Shi, J.; Ren, G.; Wu, Z.; Song, Z. Characterization and stability of peppermint oil emulsions using polyglycerol esters of fatty acids and milk proteins as emulsifiers. J. Food Sci. 2021, 86, 5148–5158. [Google Scholar] [CrossRef]
- Sakai, T.; Seki, H.; Yoshida, S.; Hori, H.; Suzuki, H.; Nakamura, T.; Kawamura, I. Interaction of Clear Flavor Emulsions Containing Lemon Essential Oils with Lipid Bilayers via a Quartz Crystal Microbalance. Food Sci. Technol. Res. 2019, 25, 879–884. [Google Scholar] [CrossRef]
- Rosales, C.K.; Suwonsichon, S.; Klinkesorn, U. Influence of crystal promoters on sensory characteristics of heat-resistant compound chocolate. Int. J. Food Sci. Technol. 2018, 53, 1459–1467. [Google Scholar] [CrossRef]
- Rosales, C.K.; Suwonsichon, S.; Klinkesorn, U. Ability of crystal promoters to delay fat bloom development in heat-resistant compound chocolate with or without the presence of crystal inhibitor. Int. J. Food Sci. Technol. 2017, 52, 2343–2351. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.; Zhu, J.; Cheng, Y.; Yang, Y.; Qiao, S.; Jiao, B.; Ma, L.; Fu, Y.; Chen, H.; et al. Stabilization of capsanthin in physically-connected hydrogels: Rheology property, self-recovering performance and syringe/screw-3D printing. Carbohydr. Polym. 2023, 319, 121209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, W.; Zhang, G.; Zhang, J.; Liu, C.; Xu, B. In vitro Antibacterial Effect of Polyglycerol Monolaurates against Gram-Bacteria and Understanding the Underlying Mechanism. J. Oleo Sci. 2021, 70, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green Cosmetic Surfactant from Rice: Characterization and Application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Iwanaga, T.; Uchida, K.; Takeuchi, N.; Abe, Y. Development of Oil-Type Make-up Remover Prepared with Polyglycerol Fatty Acid Esters. Int. J. Cosmet. Sci. 2006, 28, 73. [Google Scholar] [CrossRef]
- Barna, A.S.; Maxim, C.; Trifan, A.; Blaga, A.C.; Cimpoesu, R.; Turcov, D.; Suteu, D. Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 7004. [Google Scholar] [CrossRef]
- Salar-Behzadi, S.; Corzo, C.; Gomes Lopes, D.; Meindl, C.; Lochmann, D.; Reyer, S. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 2: Application of polyglycerol esters of fatty acids as hot melt coating excipients. Eur. J. Pharm. Biopharm. 2020, 148, 107–117. [Google Scholar] [CrossRef]
- Corzo, C.; Meindl, C.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 3: Application of polyglycerol esters of fatty acids for the next generation of solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2020, 152, 44–55. [Google Scholar] [CrossRef]
- Corzo, C.; Fuchsbichler, A.; Savencu, I.; Afonso Urich, J.; Zimmer, A.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. Lipid-microparticles for pulmonary delivery of active pharmaceutical ingredients: Impact of lipid crystallization on spray-drying processability. Int. J. Pharm. 2021, 610, 121259. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Corzo, C.; Afonso Urich, J.A.; Slama, E.; Fröhlich, E.; Lochmann, D.; Reyer, S.; Freichel, T.; Spoerk, M.; Salar-Behzadi, S. Personalization of lipid-based oral dosage forms via filament-based 3D-printing. Appl. Mater. Today 2024, 40, 102399. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Koutsamanis, I.; Corzo, C.; Maisriemler, M.; Ocampo, A.B.; Slama, E.; Alva, C.; Lochmann, D.; Reyer, S.; Freichel, T.; et al. Filament-based 3D-printing of placebo dosage forms using brittle lipid-based excipients. Int. J. Pharm. 2022, 624, 122013. [Google Scholar] [CrossRef]
- Yamagata, Y.; Iga, K.; Ogawa, Y. Novel sustained-release dosage forms of proteins using polyglycerol esters of fatty acids. J. Controll. Release 2000, 63, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Sandmeier, M.; Hoeng, J.; Skov Jensen, S.; Nykjaer Nikolajsen, G.; Ziegler Bruun, H.; To, D.; Ricci, F.; Schifferle, M.; Bernkop-Schnurch, A. Oral formulations for highly lipophilic drugs: Impact of surface decoration on the efficacy of self-emulsifying drug delivery systems. J. Colloid Interface Sci. 2025, 677, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Mehrizi, T.Z.; Mirzaei, M.; Ardestani, M.S. Pegylation, a Successful Strategy to Address the Storage and Instability Problems of Blood Products: Review 2011-2021. Curr. Pharm. Biotechnol. 2024, 25, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Joshi, M.; Zhao, Z.; Mitragotri, S. PEGylated therapeutics in the clinic. Bioeng. Transl. Med. 2024, 9, e10600. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Controll. Release 2016, 244, 184–193. [Google Scholar] [CrossRef]
- Takata, H.; Shimizu, T.; Yamade, R.; Elsadek, N.E.; Emam, S.E.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production induced by PEGylated liposomes as a function of administration route. J. Controll. Release 2023, 360, 285–292. [Google Scholar] [CrossRef]
- Emam, S.E.; Elsadek, N.E.; Abu Lila, A.S.; Takata, H.; Kawaguchi, Y.; Shimizu, T.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production and accelerated blood clearance phenomenon after the administration of PEGylated exosomes in mice. J. Controll. Release 2021, 334, 327–334. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q. Polyglycerol Fatty Acid Esters as Alternatives to PEGylated Lipids for Liposome Coating. Nanomedicine 2022, 17, 1027–1035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).