Improved Performances of Zn//MnO2 Batteries with an Electrolyte Containing Co-Additives of Polyethylene Glycol and Lignin Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Quaternized Kraft Lignin (QKL)
2.3. Fabrication of Batteries
2.4. Electrochemical Tests
2.5. Material Characterization
3. Results and Discussion
3.1. FTIR, 1H NMR, Zeta Potential, and Elemental Analysis for KL and QKL
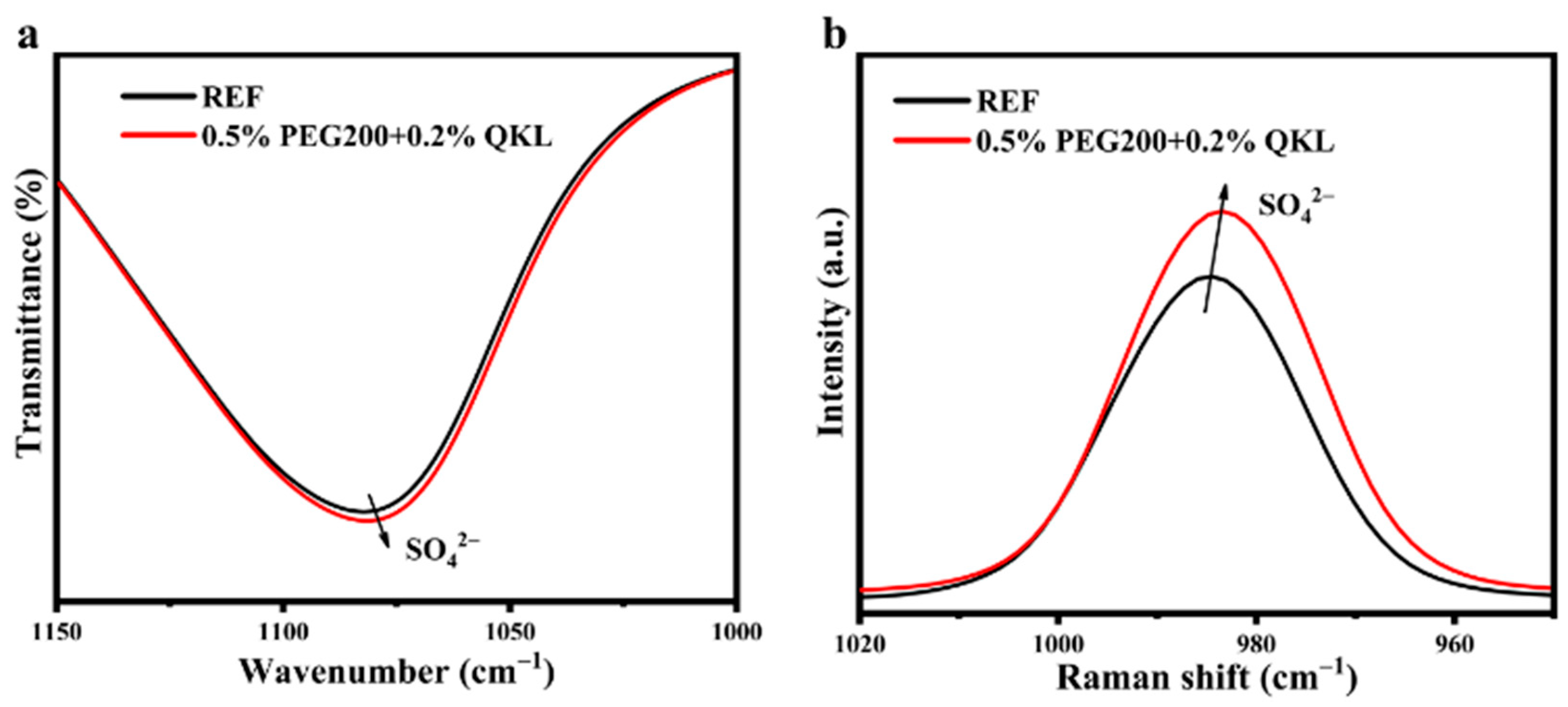
3.2. Changes in Zn2+ Solvation Structure
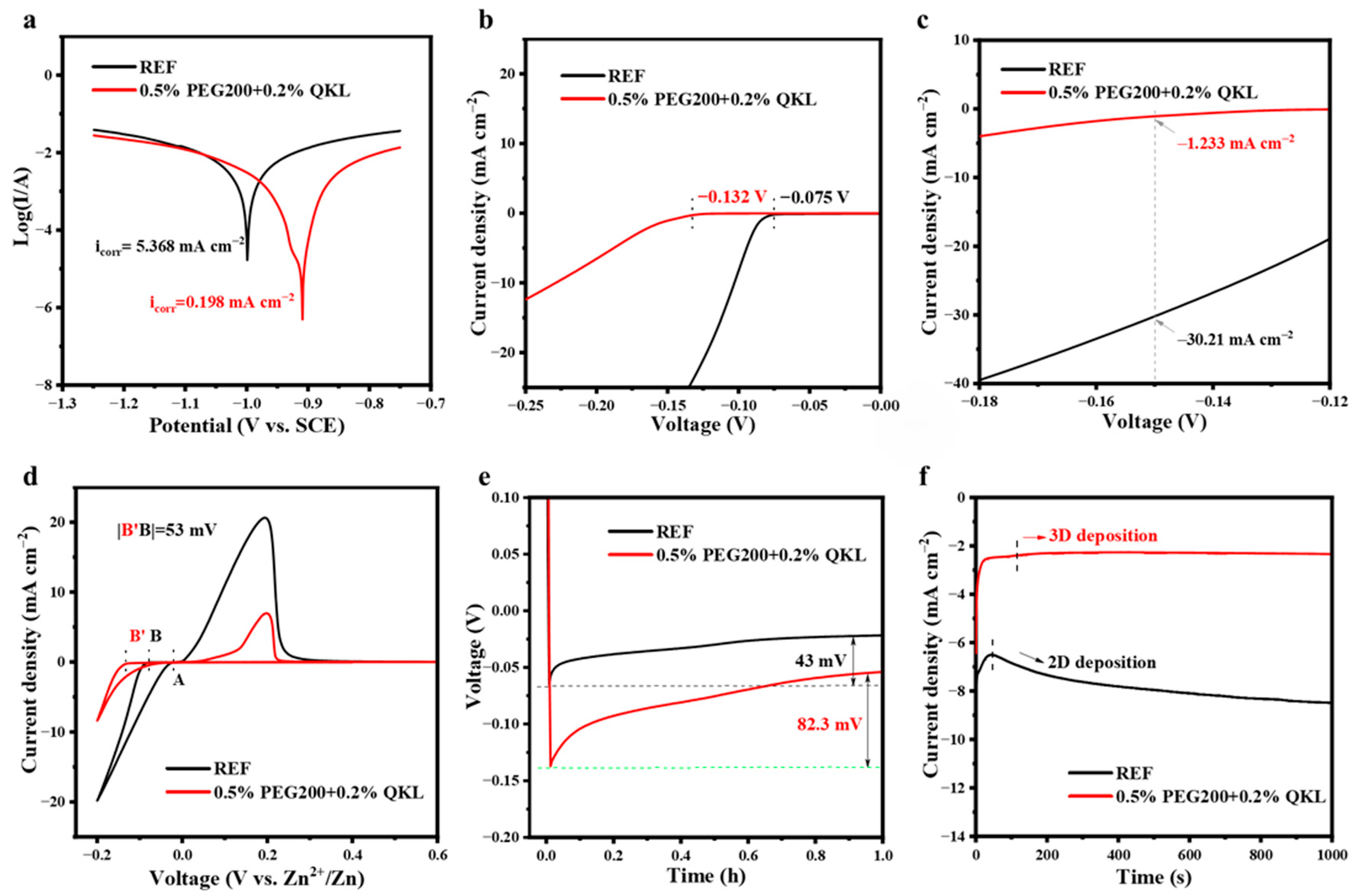
3.3. Zn Deposition Behavior in REF and 0.5% PEG200 + 0.2% QKL
3.4. Stability and Reversibility of the Zn Anode
3.5. Zn//MnO2 Battery Performance
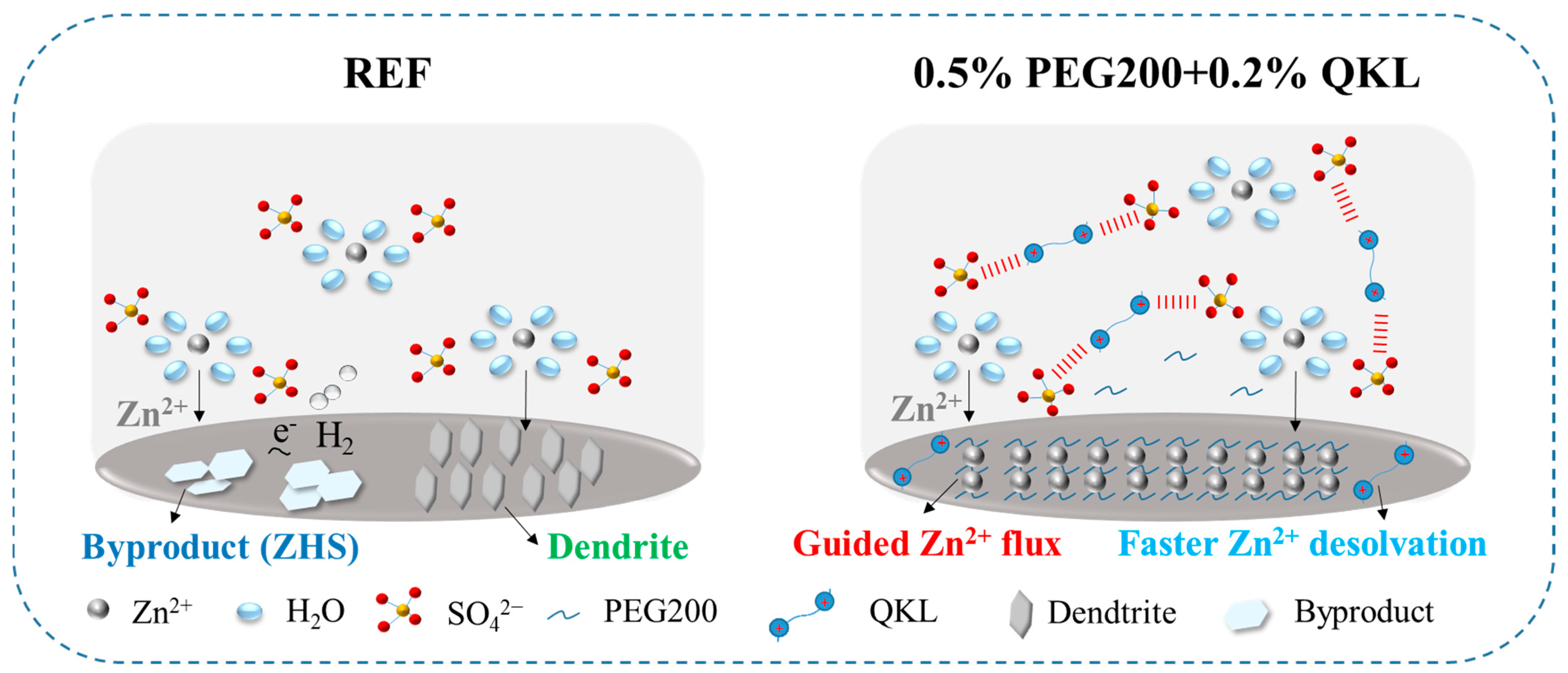
3.6. Mechanism of Protection on the Zn Anode by Electrolyte Co-Additive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, C.; Li, H.; Chen, Y.; Hao, J.; Liu, J.; Shan, J.; Qiao, S.-Z. The role of electrocatalytic materials for developing post-lithium metal||sulfur batteries. Nat. Commun. 2024, 15, 4797. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Xu, S.; Li, H.; Shan, J.; Qiao, S.-Z. Developing Cathode Films for Practical All-Solid-State Lithium-Sulfur Batteries. Adv. Mater. 2024, 2407738. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, R.; Wang, C.; Mao, J.; Chao, D.; Zhang, C.; Zhang, S.; Guo, Z. Zinc ion Batteries: Bridging the Gap from Academia to Industry for Grid-Scale Energy Storage. Angew. Chem. Int. Ed. 2024, 63, e202400045. [Google Scholar] [CrossRef]
- Innocenti, A.; Bresser, D.; Garche, J.; Passerini, S. A critical discussion of the current availability of lithium and zinc for use in batteries. Nat. Commun. 2024, 15, 4068. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Ding, J.; Han, X.; Deng, Y.; Wu, T.; Amine, K.; Hu, W.; Zhong, C.; Lu, J. Understanding the Gap between Academic Research and Industrial Requirements in Rechargeable Zinc-Ion Batteries. Batter. Supercaps 2020, 4, 60–71. [Google Scholar] [CrossRef]
- Liu, M.; Wang, P.; Zhang, W.; He, H.; He, G.; Xu, S.; Yao, L.; Miller, T.S. Strategies for pH regulation in aqueous zinc ion batteries. Energy Storage Mater. 2024, 67, 103248. [Google Scholar] [CrossRef]
- Lee, B.; Seo, H.R.; Lee, H.R.; Yoon, C.S.; Kim, J.H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Critical Role of pH Evolution of Electrolyte in the Reaction Mechanism for Rechargeable Zinc Batteries. ChemSusChem 2016, 9, 2948–2956. [Google Scholar] [CrossRef]
- Lim, W.-G.; Li, X.; Reed, D. Understanding the Role of Zinc Hydroxide Sulfate and its Analogues in Mildly Acidic Aqueous Zinc Batteries: A Review. Small Methods 2024, 8, 2300965. [Google Scholar] [CrossRef]
- Nie, C.; Wang, G.; Wang, D.; Wang, M.; Gao, X.; Bai, Z.; Wang, N.; Yang, J.; Xing, Z.; Dou, S. Recent Progress on Zn Anodes for Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300606. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J.-P.; Fu, N.; Zhou, W.-B.; Liu, B.; Deng, Q.; Wu, X.-W. Comprehensive review on zinc-ion battery anode: Challenges and strategies. InfoMat 2022, 4, e12306. [Google Scholar] [CrossRef]
- He, H.; Qin, H.; Wu, J.; Chen, X.; Huang, R.; Shen, F.; Wu, Z.; Chen, G.; Yin, S.; Liu, J. Engineering interfacial layers to enable Zn metal anodes for aqueous zinc-ion batteries. Energy Storage Mater. 2021, 43, 317–336. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Tian, Z.; Zhao, J.; Ma, Y.; Alshareef, H.N. Recent developments in three-dimensional Zn metal anodes for battery applications. InfoMat 2024, 6, e12485. [Google Scholar] [CrossRef]
- Tian, H.; Feng, G.; Wang, Q.; Li, Z.; Zhang, W.; Lucero, M.; Feng, Z.; Wang, Z.-L.; Zhang, Y.; Zhen, C.; et al. Three-dimensional Zn-based alloys for dendrite-free aqueous Zn battery in dual-cation electrolytes. Nat. Commun. 2022, 13, 7922. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Yang, Z.; Hou, Q.; Huang, T.; Liu, H. Artificial protective layers of zinc metal anodes for reversible aqueous zinc ion batteries. Curr. Opin. Electrochem. 2024, 48, 101594. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, F.; Guan, W.; Yang, X.; Zhao, Q.; Gao, L.; Ren, X.; Wu, G.; Liu, A. Additives for Aqueous Zinc-Ion Batteries: Recent Progress, Mechanism Analysis, and Future Perspectives. Small 2024, 20, 2400221. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Pan, Y.; Yu, Y.; Zhu, D.; Prabowo, J.; Wei, L.; Chen, Y. Electrolyte additives toward practical aqueous zinc-ion batteries: Recent advances and future challenges. Next Energy 2023, 1, 100073. [Google Scholar] [CrossRef]
- Deng, W.; Li, G.; Wang, X. Zinc-Ion Battery Chemistries Enabled by Regulating Electrolyte Solvation Structure. Adv. Funct. Mater. 2024, 34, 2405012. [Google Scholar] [CrossRef]
- Han, D.; Wang, Z.; Lu, H.; Li, H.; Cui, C.; Zhang, Z.; Sun, R.; Geng, C.; Liang, Q.; Guo, X.; et al. A Self-Regulated Interface toward Highly Reversible Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2102982. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Yue, S.; Jia, S.; Li, H.; Liu, W.; Li, L. Organic Electrolyte Additives for Aqueous Zinc Ion Batteries: Progress and Outlook. Chem. Rec. 2024, 24, e202400142. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, K.; Liu, S.; Zhang, S.; Hao, J.; Liu, J.; Pang, W.K.; Liu, J.; Rao, P.; Wang, Q.; et al. Bio-inspired design of anin situmultifunctional polymeric solid–electrolyte interphase for Zn metal anode cycling at 30 mA cm−2 and 30 mA h cm−2. Energy Environ. Sci. 2021, 14, 5947–5957. [Google Scholar] [CrossRef]
- Mitha, A.; Yazdi, A.Z.; Ahmed, M.; Chen, P. Surface Adsorption of Polyethylene Glycol to Suppress Dendrite Formation on Zinc Anodes in Rechargeable Aqueous Batteries. ChemElectroChem 2018, 5, 2409–2418. [Google Scholar] [CrossRef]
- Trejo, G.; Ruiz, H.; Borges, R.O.; Meas, Y. Influence of polyethoxylated additives on zinc electrodeposition from acidic solutions. J. Appl. Electrochem. 2001, 31, 685–692. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, D.; Ouyang, K.; Yang, M.; Shen, S.; Wang, Y.; Mi, H.; Sun, L.; He, C.; Zhang, P. A Multifunctional Anti-Proton Electrolyte for High-Rate and Super-Stable Aqueous Zn-Vanadium Oxide Battery. Nano-Micro Lett. 2022, 14, 154. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Z.; Shen, D.; Chen, L.; Song, T.; Kang, T.; Tong, Z.; Tang, Y.; Wang, H.; Lee, C.S. Electrolyte engineering enables stable Zn-Ion deposition for long-cycling life aqueous Zn-ion batteries. Energy Storage Mater. 2022, 45, 1084–1091. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Lin, C.; Hou, Q.; Nie, S. Advances in application of sustainable lignocellulosic materials for high-performance aqueous zinc-ion batteries. Nano Energy 2024, 123, 109416. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Asraful Alam, M.; Hoang, T.K.A.; Zhang, Y.; Li, H.; Lv, Y.; Zhao, A.; Xiong, W. Employing cationic kraft lignin as electrolyte additive to enhance the electrochemical performance of rechargeable aqueous zinc-ion battery. Fuel 2023, 333, 126450. [Google Scholar] [CrossRef]
- Chen, H.-B.; Meng, H.; Zhang, T.-R.; Ran, Q.; Liu, J.; Shi, H.; Han, G.-F.; Wang, T.-H.; Wen, Z.; Lang, X.-Y.; et al. Dynamic Molecular Interphases Regulated by Trace Dual Electrolyte Additives for Ultralong-Lifespan and Dendrite-Free Zinc Metal Anode. Angew. Chem. Int. Ed. 2024, 136, e202402327. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wang, T.; He, Z.; Lu, B.; Liang, S.; Zhou, J. Interfacial Engineering Strategy for High-Performance Zn Metal Anodes. Nano-Micro Lett. 2021, 14, 6. [Google Scholar] [CrossRef]
- Zhai, C.; Zhao, D.; He, Y.; Huang, H.; Chen, B.; Wang, X.; Guo, Z. Electrolyte Additive Strategies for Suppression of Zinc Dendrites in Aqueous Zinc-Ion Batteries. Batteries 2022, 8, 153. [Google Scholar] [CrossRef]
- Rauschkolb, J.C.; Ribeiro, B.C.; Feiden, T.; Fischer, B.; Weschenfelder, T.A.; Cansian, R.L.; Junges, A. Parameter Estimation of Mark-Houwink Equation of Polyethylene Glycol (PEG) Using Molecular Mass and Intrinsic Viscosity in Water. Biointerface Res. Appl. Chem. 2021, 12, 1778–1790. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Khamsawat, J.; Danwong, P.; Buasri, A.; Pattananuwat, P. Application of Coffee Silverskin Cellulose/Polyacrylamide Gel Polymer Electrolytes for Rechargeable Zinc-Ion Batteries. Sci 2024, 6, 50. [Google Scholar] [CrossRef]
- Shen, P.; Hu, Y.; Ji, S.; Luo, H.; Zhai, C.; Yang, K. A self-healing nanocomposite hydrogel electrolyte for rechargeable aqueous Zn-MnO2 battery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129195. [Google Scholar] [CrossRef]
- Xie, Q.; Li, X.; Wang, L.; Dong, R.; Xie, W.; Alam, M.A.; Xu, J.; Xiong, W. An efficient electrolyte additive of quaternized hardwood kraft lignin enabling dendrite-free aqueous zinc-ion batteries. Int. J. Biol. Macromol. 2025, 307, 142020. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Huang, P.; Zhong, L.; Gan, S.; Wang, W.; Niu, L. Nitrogen-doped lignin-derived porous carbons for supercapacitors: Effect of nanoporous structure. Chem. Eng. J. 2023, 471, 144817. [Google Scholar] [CrossRef]
- Lin, X.; Chen, D.; Qiu, X.; Liu, B.; Liu, J.; Wang, X.; Sun, S.; Qin, Y. Lignin-Metal Supramolecular Framework Strategy of Self-Healing Carbon-Coated CoRu Alloy Nanocatalyst for Efficient Overall Water Splitting. Adv. Energy Mater. 2024, 14, 2303442. [Google Scholar] [CrossRef]
- Feng, P.; Wang, H.; Gan, S.; Liao, B.; Niu, L. Novel Lignin-Functionalized Waterborne Epoxy Composite Coatings with Excellent Anti-Aging, UV Resistance, and Interfacial Anti-Corrosion Performance. Small 2024, 20, 2312085. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hulteberg, C.P. Physicochemical Characterisation of Technical Lignins for Their Potential Valorisation. Waste Biomass Valorization 2017, 8, 859–869. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Gao, W.; Fatehi, P. Novel Process for Generating Cationic Lignin Based Flocculant. Ind. Eng. Chem. Res. 2018, 57, 6595–6608. [Google Scholar] [CrossRef]
- Dai, Z.; Chanajaree, R.; Yang, C.; Zhang, X.; Okhawilai, M.; Pattananuwat, P.; Zhang, X.; He, G.; Qin, J. Dual-Functional Additives Boost Zinc-Ion Battery Electrolyte over Wide Temperature Range. Energy Mater. Adv. 2025, 6, 0139. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Zhang, S.; Sun, L.; Li, M.; Yuwono, J.A.; Mao, J.; Hao, J.; Vongsvivut, J.; Xing, L.; et al. A biocompatible electrolyte enables highly reversible Zn anode for zinc ion battery. Nat. Commun. 2023, 14, 6526. [Google Scholar] [CrossRef]
- Wu, X.; Xia, Y.; Chen, S.; Luo, Z.; Zhang, X.; Shahzad, M.W.; Xu, B.B.; Pan, H.; Yan, M.; Jiang, Y. Boosting the performance of aqueous zinc-ion battery by regulating the electrolyte solvation structure. EcoMat 2024, 6, e12438. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: A mini review. Carbon Energy 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, F.; Zhou, A.; Hu, X.; Yan, Q.; Liu, Y.; Arshad, F.; Li, Z.; Chen, R.; Wu, F.; et al. Highly Reversible Zn Metal Anodes Enabled by Increased Nucleation Overpotential. Nano-Micro Lett. 2023, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Z.; Qin, H.; Zeng, X.; Meng, J. Advanced electrochemical performance of ZnMn2O4/N-doped graphene hybrid as cathode material for zinc ion battery. J. Power Sources 2019, 425, 162–169. [Google Scholar] [CrossRef]
- Yang, W.; Dong, L.; Yang, W.; Xu, C.; Shao, G.; Wang, G. 3D Oxygen-Defective Potassium Vanadate/Carbon Nanoribbon Networks as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Small Methods 2020, 4, 1900670. [Google Scholar] [CrossRef]
- Cao, J.; Wu, J.; Wu, H.; Jin, Y.; Luo, D.; Yang, X.; Zhang, L.; Zhang, D.; Qin, J.; Lu, J. Dendrite-Free Zinc Anode via Oriented Plating with Alkaline Earth Metal Ion Additives. Adv. Funct. Mater. 2024, 34, 2401537. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, B.; Cai, Y.; Chen, X.; Li, L.; Zhao, Q.; Chou, S.-L.; Li, F. Interfacial Engineering for Oriented Crystal Growth toward Dendrite-Free Zn Anode for Aqueous Zinc Metal Battery. Angew. Chem. Int. Ed. 2024, 63, e202402342. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Li, Z.; Gao, L.; Xu, B.; Wang, C. Surface Modification Induces Oriented Zn(002) Deposition for Highly Stable Zinc Anode. Batteries 2024, 10, 178. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, K.; Jiang, W.; Yang, H.; Ma, M.; Zhao, L.; Yang, W. Highly 002-Oriented Dendrite-Free Anode Achieved by Enhanced Interfacial Electrostatic Adsorption for Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 21184–21197. [Google Scholar] [CrossRef]
- Song, Z.; Yang, C.; Kiatwisarnkij, N.; Lu, A.; Tunghathaithip, N.; Lolupiman, K.; Bovornratanaraks, T.; Zhang, X.; He, G.; Qin, J. Polyethylene Glycol-Protected Zinc Microwall Arrays for Stable Zinc Anodes. ACS Appl. Mater. Interfaces 2024, 16, 64834–64845. [Google Scholar] [CrossRef]
- Nian, Q.; Luo, X.; Ruan, D.; Li, Y.; Xiong, B.-Q.; Cui, Z.; Wang, Z.; Dong, Q.; Fan, J.; Jiang, J.; et al. Highly reversible zinc metal anode enabled by strong Brønsted acid and hydrophobic interfacial chemistry. Nat. Commun. 2024, 15, 4303. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhang, X.; Wei, H.; Jiang, J.; Chen, G.; Li, H.; Liu, Z. Suppressing Zinc Metal Corrosion by an Effective and Durable Corrosion Inhibitor for Stable Aqueous Zinc Batteries. Adv. Funct. Mater. 2024, 2418594. [Google Scholar] [CrossRef]
- Sun, S.; Billings, A.; Wang, B.; Huang, K. Combined Effect of Dissolved Oxygen and pH in Aqueous Electrolytes on Zn-Anode Corrosion Behavior in Aqueous Zn-Ion Batteries. ACS Electrochem. 2025, 1, 195–204. [Google Scholar] [CrossRef]
- Shi, X.; Xie, J.; Wang, J.; Xie, S.; Yang, Z.; Lu, X. A weakly solvating electrolyte towards practical rechargeable aqueous zinc-ion batteries. Nat. Commun. 2024, 15, 302. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Hong, H.; Wang, S.; Zhu, J.; Zhi, C. Interface regulation and electrolyte design strategies for zinc anodes in high-performance zinc metal batteries. iScience 2025, 28, 111751. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.; Chen, Y.; Meng, X.; Azizi, A.; He, Q.; Li, H.; Chen, L.; Han, C.; Pan, A. Self-Smoothing Deposition Behavior Enabled by Beneficial Potential Compensating for Highly Reversible Zn-Metal Anodes. Adv. Funct. Mater. 2023, 33, 2307201. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, P.; Wang, W.; Liu, J. Tuning the Electrode/Electrolyte Interface Enabled by a Trifunctional Inorganic Oligomer Electrolyte Additive for Highly Stable and High-Rate Zn Anodes. Small Methods 2023, 7, 2300546. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Chanajaree, R.; Yue, Y.; Zeng, Z.; Zhang, X.; Qin, J. Stabilizing zinc anode via a chelation and desolvation electrolyte additive. Adv. Powder Mater. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Li, C.; Gou, Q.; Tang, R.; Deng, J.; Wang, K.; Luo, H.; Cui, J.; Geng, Y.; Xiao, J.; Zheng, Y.; et al. Electrolyte Modulation of Biological Chelation Additives toward a Dendrite-Free Zn Metal Anode. J. Phys. Chem. Lett. 2023, 14, 9150–9158. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The Three-Dimensional Dendrite-Free Zinc Anode on a Copper Mesh with a Zinc-Oriented Polyacrylamide Electrolyte Additive. Angew. Chem. Int. Ed. 2019, 58, 15841–15847. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, M.H.; Alam, M.A.; Xie, Q.; Abbasi, A.R.; Wang, L.; Xu, J.; Xiong, W. Improved Performances of Zn//MnO2 Batteries with an Electrolyte Containing Co-Additives of Polyethylene Glycol and Lignin Derivatives. Polymers 2025, 17, 888. https://doi.org/10.3390/polym17070888
Memon MH, Alam MA, Xie Q, Abbasi AR, Wang L, Xu J, Xiong W. Improved Performances of Zn//MnO2 Batteries with an Electrolyte Containing Co-Additives of Polyethylene Glycol and Lignin Derivatives. Polymers. 2025; 17(7):888. https://doi.org/10.3390/polym17070888
Chicago/Turabian StyleMemon, Muzammil Hussain, Md. Asraful Alam, Qiyuan Xie, Abdul Rahman Abbasi, Lele Wang, Jingliang Xu, and Wenlong Xiong. 2025. "Improved Performances of Zn//MnO2 Batteries with an Electrolyte Containing Co-Additives of Polyethylene Glycol and Lignin Derivatives" Polymers 17, no. 7: 888. https://doi.org/10.3390/polym17070888
APA StyleMemon, M. H., Alam, M. A., Xie, Q., Abbasi, A. R., Wang, L., Xu, J., & Xiong, W. (2025). Improved Performances of Zn//MnO2 Batteries with an Electrolyte Containing Co-Additives of Polyethylene Glycol and Lignin Derivatives. Polymers, 17(7), 888. https://doi.org/10.3390/polym17070888






