Application of 3D Printing Technology in Dentistry: A Review
Abstract
1. Overview of 3D Printing Technology
2. Three-Dimensional Printing Technologies of Different Principles
2.1. Selective Laser Melting (SLM)
2.2. Selective Laser Sintering (SLS)
2.3. Fused Deposition Modeling (FDM)
2.4. Stereolithography (SLA)
2.5. Digital Light Processing (DLP)
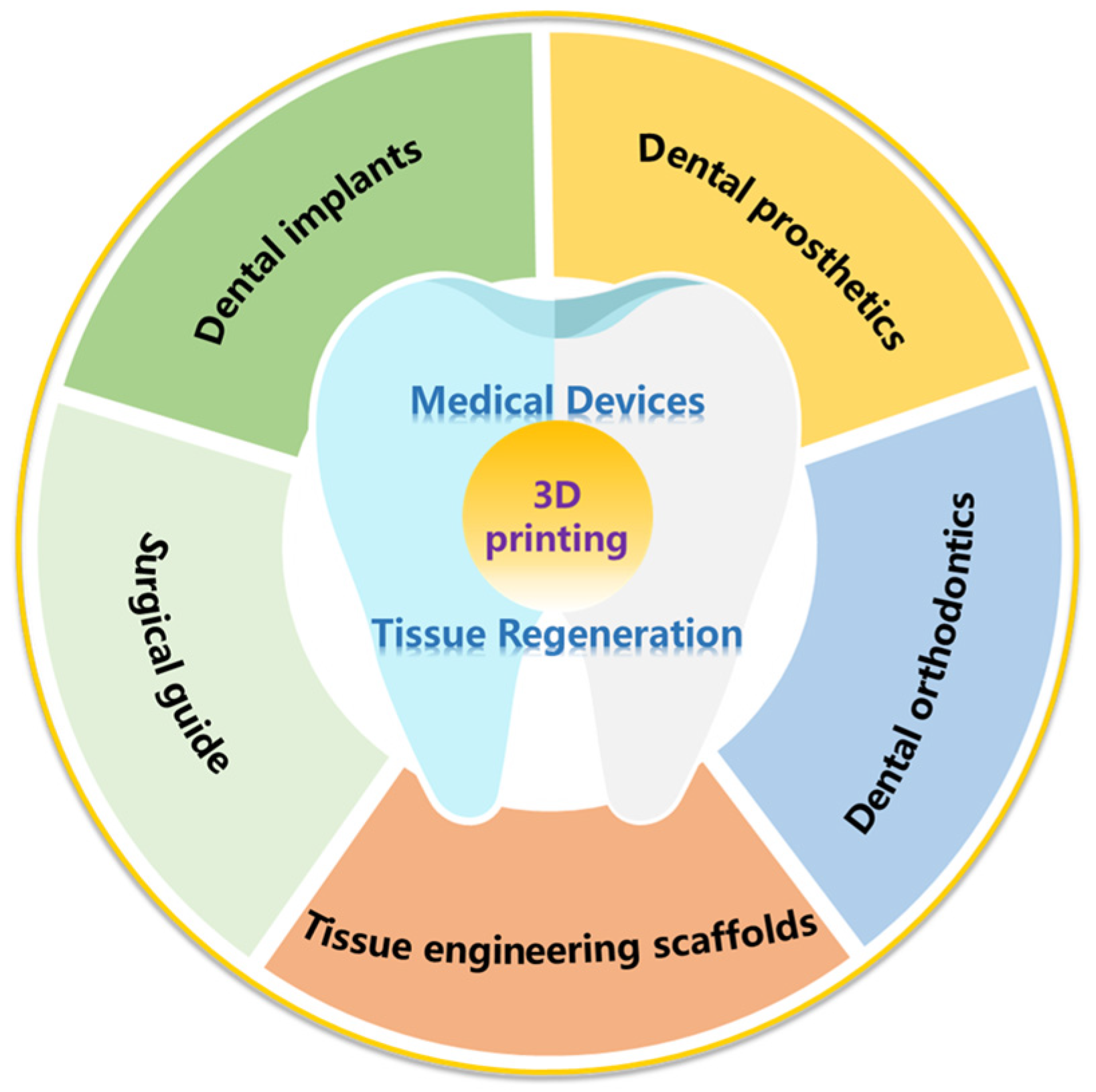
3. Application of 3D Printing Technology in Dentistry
3.1. The Application of 3D Printing Technology in Medical Devices
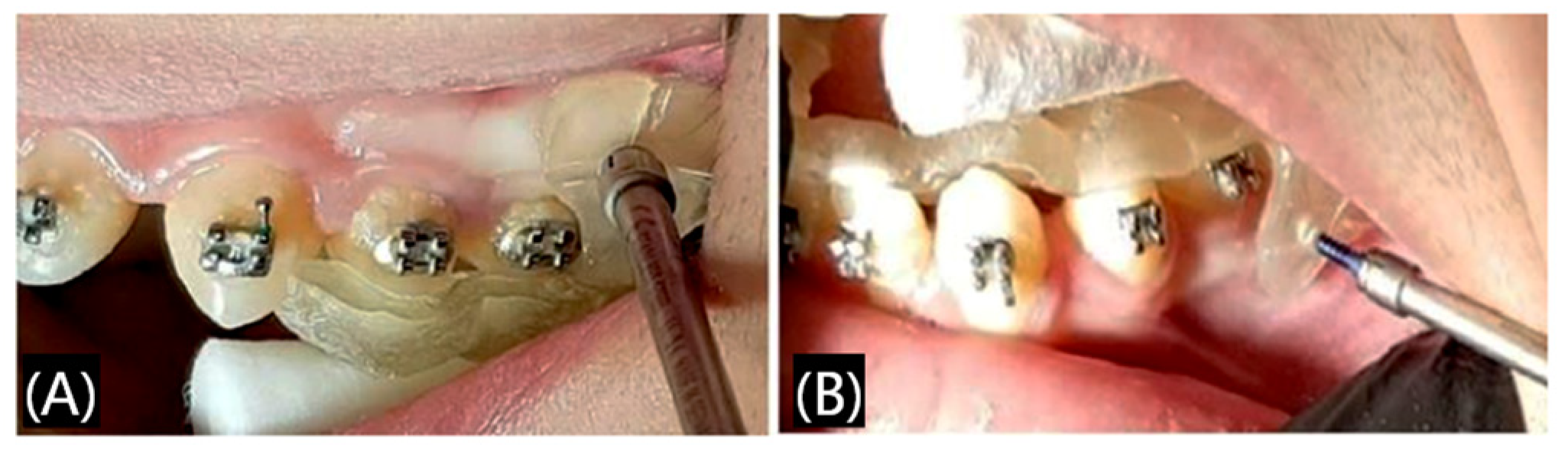
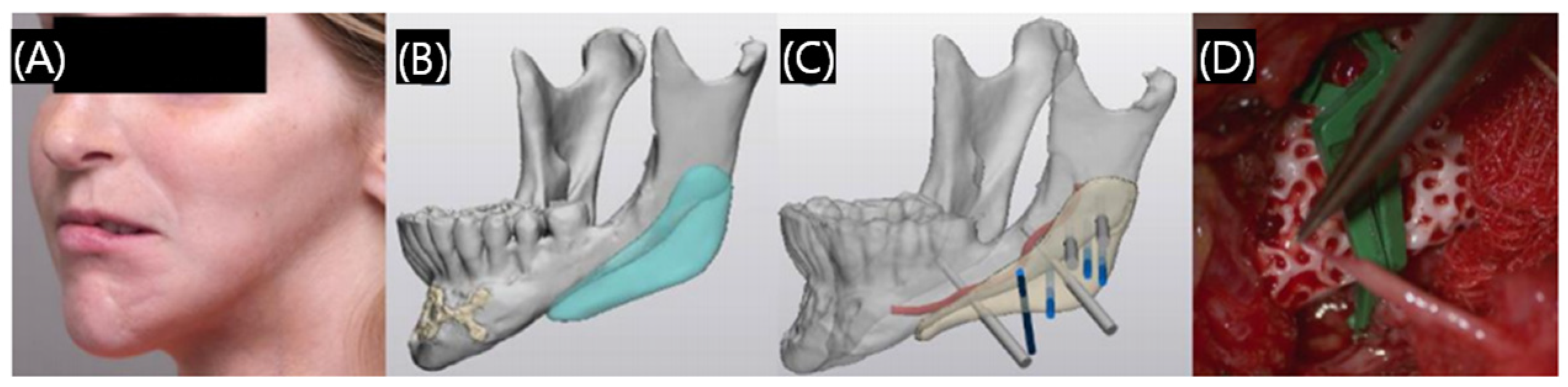
3.1.1. Surgical Guide
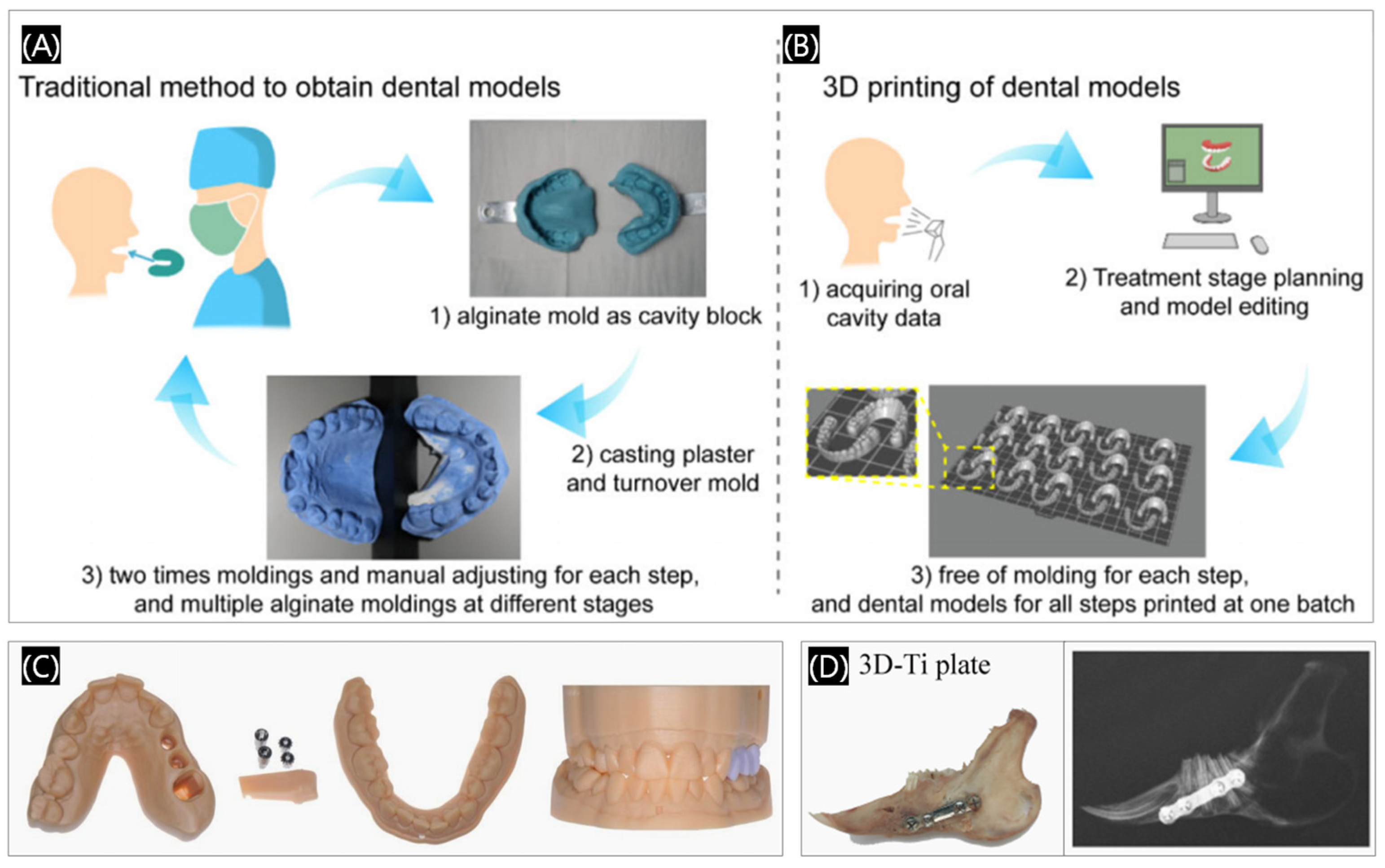
3.1.2. Dental Implants
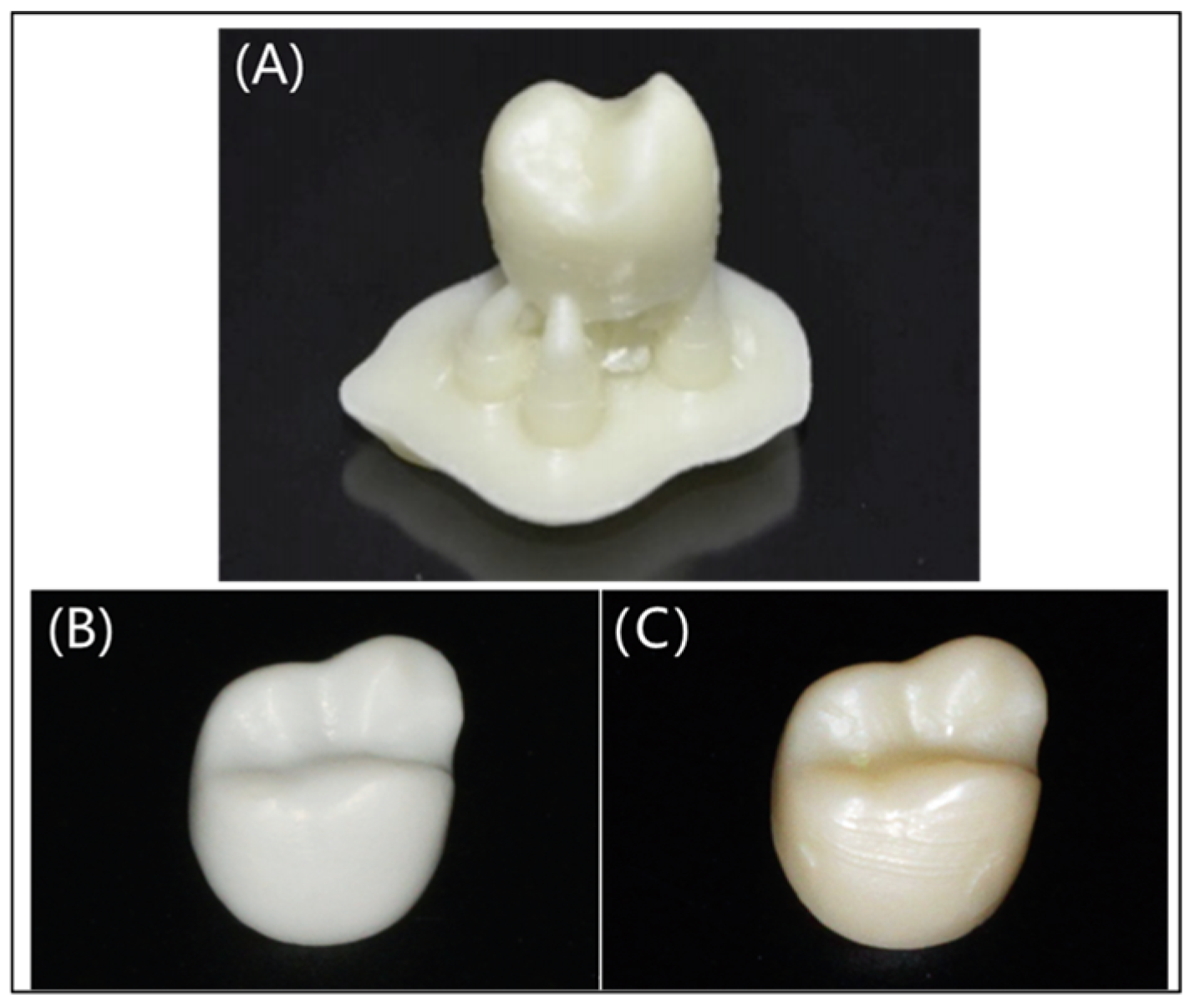
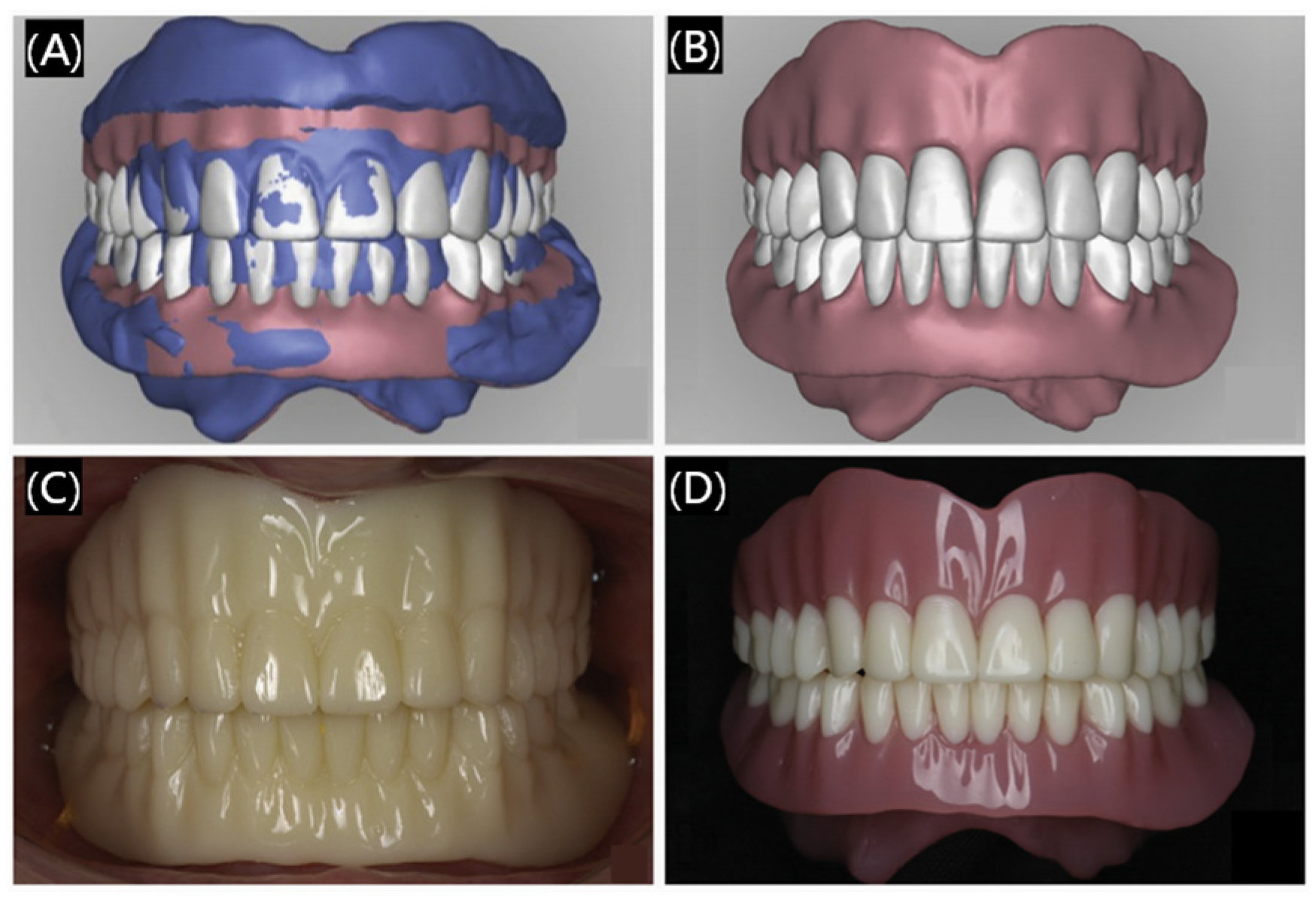
3.1.3. Dental Prosthetics
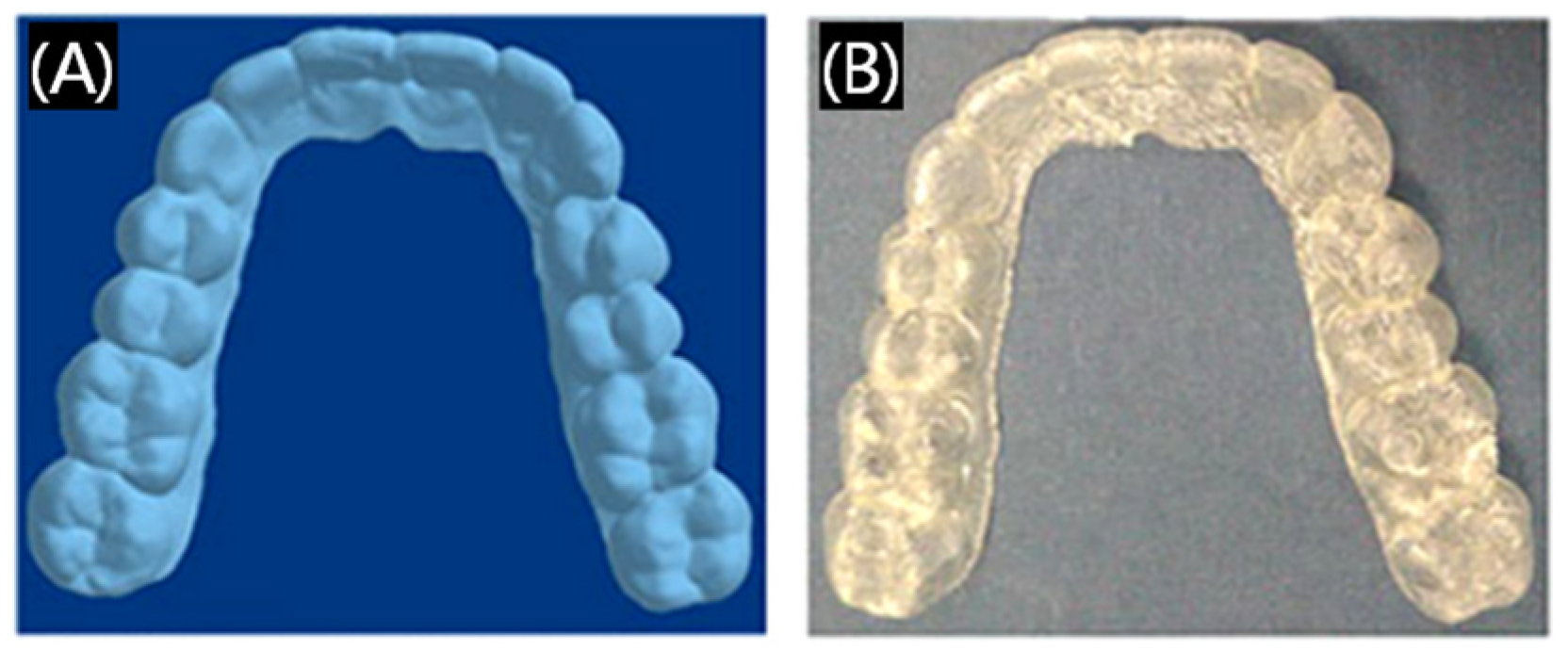
3.1.4. Dental Orthodontics
3.1.5. Others
3.2. The Application of 3D Printing Technology in Oral Tissue Regeneration
3.2.1. Three-Dimensional Printed Metal-Based Scaffolds
3.2.2. Three-Dimensional Printed Ceramic-Based Scaffolds
3.2.3. Three-Dimensional Printed Polymer-Based Scaffolds
3.2.4. Three-Dimensional Printed Composite Bioactive Scaffolds
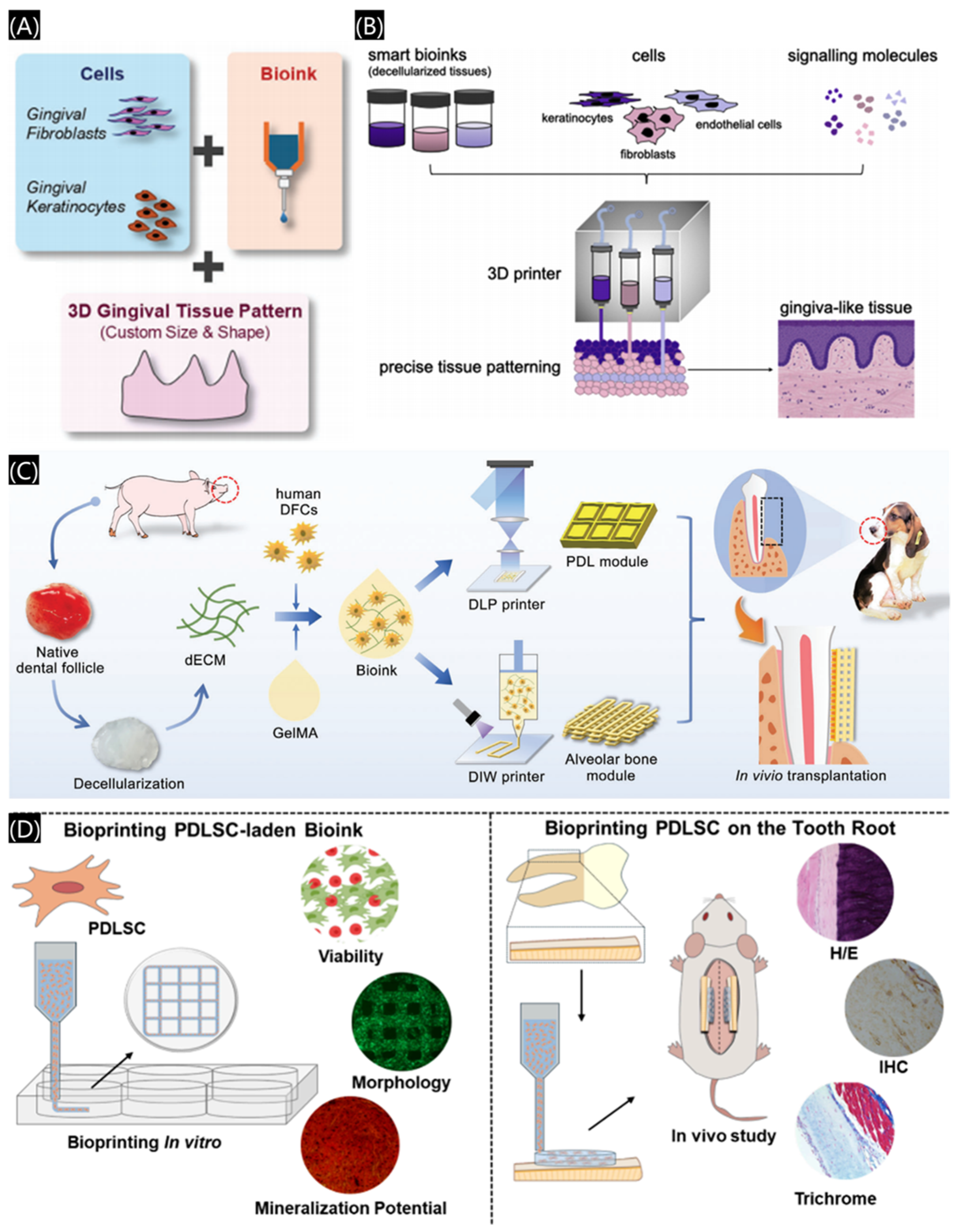
3.2.5. Three-Dimensional Bioprinted Scaffolds
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Laleh, M.; Sadeghi, E.; Revilla, R.I.; Chao, Q.; Haghdadi, N.; Hughes, A.E.; Xu, W.; Graeve, I.D.; Qian, M.; Gibson, I.; et al. Heat treatment for metal additive manufacturing. Prog. Mater. Sci. 2023, 133, 101051. [Google Scholar]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X.Q. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.; Mihut, D. Enhancing durability of 3D printed polymer structures by metallization. J. Mater. Sci. Technol. 2020, 53, 185–191. [Google Scholar]
- Liu, G.; He, Y.H.; Liu, P.C.; Chen, Z.; Chen, X.L.; Wan, L.; Li, Y.; Lu, J. Development of bioimplants with 2D, 3D, and 4D additive manufacturing materials. Engineering 2020, 6, 1232–1243. [Google Scholar] [CrossRef]
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar]
- Yu, J.; Bian, H.L.; Zhao, Y.N.; Guo, J.M.; Yao, C.M.; Liu, H.; Shen, Y.; Yang, H.Y.; Huang, C. Epigallocatechin-3-gallate/mineralization precursors co-delivery hollow mesoporous nanosystem for synergistic manipulation of dentin exposure. Bioact. Mater. 2023, 23, 394–408. [Google Scholar]
- Tigmeanu, C.V.; Ardelean, L.C.; Rusu, L.C.; Negrutiu, M.L. Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers 2022, 14, 3658. [Google Scholar] [CrossRef]
- Meng, L.; Bai, J.X.; Tao, H.Q.; Hao, L.; Yin, W.L.; Ren, X.X.; Gao, A.; Li, N.; Wang, M.; Fang, S.Y.; et al. Rational integration of defense and repair synergy on PEEK osteoimplants via biomimetic peptide clicking strategy. Bioact. Mater. 2022, 8, 309–324. [Google Scholar]
- Lin, L.W.; Fang, Y.F.; Liao, Y.X.; Chen, G.; Gao, C.X.; Zhu, P.Z. 3D printing and digital processing techniques in dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar]
- Liu, X.M.; Zhao, D.; Wang, J. Challenges and opportunities in preserving key structural features of 3D-printed metal/covalent organic framework. Nano-Micro Lett. 2024, 16, 157. [Google Scholar]
- Wang, C.Z.; Hu, Y.X.; Zhong, C.; Lan, C.X.; Li, W.; Wang, X.J. Microstructural evolution and mechanical properties of pure Zn fabricated by selective laser melting. Mat. Sci. Eng. A 2022, 846, 143276. [Google Scholar]
- Kouhi, M.; Araújo, I.J.S.; Asa’ad, F.; Zeenat, L.; Bojedla, S.S.R.; Pati, F. Recent advances in additive manufacturing of patient-specific devices for dental and maxillofacial rehabilitation. Dental Mats. 2024, 40, 700–715. [Google Scholar]
- Takahashi, A.; Inoue, K.; Imagawa-Fujimura, N.; Matsumoto, K.; Yamada, K.; Sawai, Y.; Nakajima, Y.; Mano, T.; Kato-Kogoe, N.; Ueno, T. Clinical study of 14 cases of bone augmentation with selective laser melting titanium mesh plates. Materials 2023, 16, 6842. [Google Scholar] [CrossRef]
- Park, J.H.; Odkhuu, M.; Cho, S.; Li, J.W.; Park, B.-Y.; Kim, J.-W. 3D-printed titanium implant with premounted dental implants for mandible reconstruction: A case report. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 28. [Google Scholar] [PubMed]
- Zhang, F.; Zhou, S.X.; You, H.Y.; Zhang, G.; Yang, J.Q.; Shi, Y.S. 3D printing of ceramic matrix composites: Strengthening and toughening strategies. Compos. Part B 2025, 297, 112335. [Google Scholar]
- Ahn, S.J.; Lee, H.; Cho, K.J. 3D printing with a 3D printed digital material filament for programming functional gradients. Nat. Commun. 2024, 15, 3605. [Google Scholar] [PubMed]
- Bagheri, A.; Jin, J.Y. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar]
- Zhou, X.; Yu, X.; You, T.; Zhao, B.; Dong, L.; Huang, C.; Zhou, X.; Qian, W.; Luo, G. 3D Printing-Based Hydrogel Dressings for Wound Healing. Adv. Sci. 2024, 11, 2404580. [Google Scholar]
- González, G.; Baruffaldi, D.; Martinengo, C.; Angelini, A.; Chiappone, A.; Roppolo, I.; Pirri, C.F.; Frascella, F. Materials testing for the development of biocompatible devices through vat-polymerization 3D printing. Nanomaterials 2020, 10, 1788. [Google Scholar] [CrossRef]
- Dhand, A.P.; Davidson, M.D.; Burdick, J.A. Lithography-based 3D printing of hydrogels. Nat. Rev. Bioeng. 2025, 3, 108–125. [Google Scholar]
- Wan, X.; Xiao, Z.M.; Tian, Y.J.; Chen, M.; Liu, F.; Wang, D. Recent advances in 4D printing of advanced materials and structures for functional applications. Adv. Mater. 2024, 36, 2312263. [Google Scholar]
- Song, C.H.; Liu, R.; Kong, B.; Gu, Z.X.; Chen, G.P. Functional hydrogels for treatment of dental caries. Biomed. Technol. 2024, 5, 73–81. [Google Scholar] [CrossRef]
- Tarce, M.; Joe Merheb, J.; Meeus, M.; Vasconcelos, K.D.F.; Marc Quirynen, M. Surgical guides for guided bone augmentation: An in vitro study. Clin. Oral. Impl. Res. 2022, 33, 558–567. [Google Scholar]
- Tian, Y.; Chen, C.X.; Xu, X.T.; Wang, J.Y.; Hou, X.Y.; Li, K.L.; Lu, X.Y.; Shi, H.Y.; Lee, E.S.; Jian, H.B.; et al. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar]
- Xu, L.W.; You, J.; Zhang, J.X.; Liu, Y.F.; Peng, W. Impact of surgical template on the accuracy of implant placement. J. Prosthodont. 2016, 25, 641–646. [Google Scholar] [CrossRef]
- Kholy, K.E.; Lazarin, R.; Janner, S.F.M.; Faerber, K.; Buser, R.; Buser, D. Influence of surgical guide support and implant site location on accuracy of static Computer-Assisted Implant Surgery. Clin. Oral Implants Res. 2019, 30, 1067–1075. [Google Scholar]
- Vasoglou, G.; Patatou, A.; Vasoglou, M. Bimaxillary dentoalveolar protrusion case treated with anchorage by buccally implemented mini-implants using a 3D-printed surgical guide. Children 2023, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Sonaye, S.Y.; Bokam, V.K.; Saini, A.; Nayak, V.V.; Witek LCoelho, P.G.; Bhaduri, S.B.; Bottino, M.C.; Sikder, P. Patient-specific 3D printed Poly-ether-ether-ketone (PEEK) dental implant system. J. Mech. Behav. Biomed. Mater. 2022, 136, 105510. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Wilkins, G.N. 3D printing in dentistry—Exploring the new horizons. J. Dent. Sci. 2021, 16, 1037–1038. [Google Scholar]
- Brink, T.; Damanik, F.; Rotmans, J.; Moroni, L. Unraveling and harnessing the immune response at the cell–biomaterial interface for tissue engineering purposes. Adv. Healthcare Mater. 2024, 13, 2301939. [Google Scholar]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar]
- Tahayeri, A.; Morgana, M.C.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [PubMed]
- Wang, W.N.; Yu, H.; Liu, Y.F.; Jiang, X.L.; Ga, B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J. Prosthet. Dent. 2019, 121, 285–291. [Google Scholar]
- Takeda, Y.; Lau, J.; Nouh, H.; Hirayama, H. A 3D printing replication technique for fabricating digital dentures. J. Prosthet. Dent. 2020, 124, 251–256. [Google Scholar]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D printing-encompassing the facets of dentistry. Front. Bioeng. Biotech. 2018, 6, 172. [Google Scholar]
- Schweiger, J.; Edelhoff, D.; Güth, J.F. 3D printing in digital prosthetic dentistry: An overview of recent developments in additive manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Kuang, Y.; Hu, B.; Feng, G.; Huang, L.; Song, J. The Application of a 3-dimensional printing technique in refining the orthodontic trans-palatal. Arch. Appl. Sci. 2022, 12, 7497. [Google Scholar]
- Makvandi, P.; Esposito Corcione, C.E.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Poly. Advan. Technol. 2018, 29, 364–371. [Google Scholar]
- Yu, X.Y.; Li, G.H.; Zheng, Y.K.; Pan, X.G.; Ding, J.D. ‘Invisible’ orthodontics by polymeric ‘clear’ aligners molded on 3D-printed personalized dental models. Regen. Biomater. 2022, 9, rbac007. [Google Scholar]
- Wang QTelha, W.; Wu, Y.; Abotaleb, B.; Jiang, N.; Zhu, S. Evaluation of the properties of 3D-printed Ti alloy plates: In Vivo and In Vitro Comparative Experimental Study. J. Clin. Med. 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, W.; Qian, C.Y.; Xiao, W.S.; Zhu, H.J.; Guo, J.; Meng, Z.B.; Zhu, J.Y.; Ge, Z.L.; Cui, W.G. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater. Sci. Eng. C 2019, 103, 109858. [Google Scholar]
- Guo, J.X.; Yao, H.; Li, X.; Chang, L.; Zhu, W.Y.; Su, Y.X.; Qin, L.; Xu, J.K. Advanced hydrogel systems for mandibular reconstruction. Bioact. Mater. 2023, 21, 175–193. [Google Scholar] [PubMed]
- Zhao, F.J.; Yang, Z.; Xiong, H.C.; Yan, Y.; Chen, X.F.; Shao, L.Q. A bioactive glass functional hydrogel enhances bone augmentation via synergistic angiogenesis, self-swelling and osteogenesis. Bioact. Mater. 2023, 22, 201–210. [Google Scholar]
- Cidonioa, G.; Glinkaa, M.; Dawsona, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [PubMed]
- Masri, S.; Zawani, M.; Zulkiflee, I.; Salleh, A.; Fadilah, N.I.M.; Maarof, M.; Wen, A.P.Y.; Duman, F.; Tabata, Y.; Aziz, I.A.; et al. Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 476. [Google Scholar] [CrossRef]
- Gao, J.M.; Yu, X.Y.; Wang, X.L.; He, Y.N.; Ding, J.D. Biomaterial-related cell microenvironment in tissue engineering and regenerative medicine. Engineering 2022, 13, 31–45. [Google Scholar]
- Yan, N.; Hu, B.; Xu, J.C.; Cai, R.; Liu, Z.H.; Fu, D.P.; Huo, B.B.; Liu, Z.H.; Zhao, Y.L.; Chen, C.Y.; et al. Stem cell Janus patch for periodontal regeneration. Nano Today 2022, 42, 101336. [Google Scholar]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.B.; He, Z.; Chen, Z.L.; He, X.; Tian, S.; Liao, J.M.; Lu, B.H.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.T.; Xu, S.; Feng, Q.; Dai, Q.Y.; Yao, L.T.; Zhang, Y.C.; Gao, H.C.; Dong, H.; Chen, D.F.; Cao, X.D. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Wang, C.J.; Fang, Y.C.; Ko, J.; Chen, L.; Zhang, T.; Xiong, Z.; Zhang, L.; Sun, W. 3D printed conductive multiscale nerve guidance conduit with hierarchical fibers for peripheral nerve regeneration. Adv. Sci. 2023, 10, 2205744. [Google Scholar]
- Gong, H.; Fei, H.S.; Xu, Q.F.; Gou, M.L.; Chen, H.H. 3D-engineered GelMA conduit filled with ECM promotes regeneration of peripheral nerve. J. Biomed. Mater. Res. A 2020, 108, 805–813. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Daikuara, L.Y.; Chen, X.F.; Yue, Z.L.; Skropeta, D.; Wood, F.M.; Fear, M.W.; Wallace, G.G. 3D bioprinting constructs to facilitate skin regeneration. Adv. Funct. Mater. 2022, 32, 2105080. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Zhao, D.L.; Han, C.J.; Peng, B.; Cheng, T.; Fan, J.X.; Yang, L.; Chen, L.L.; Wei, Q.S. Corrosion fatigue behavior and anti-fatigue mechanisms of an additively manufactured biodegradable zinc-magnesium gyroid scaffold. Acta Biomater. 2022, 153, 614–629. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Höland, W. Editorial: Inorganic Biomaterials. Front. Bioeng. Biotechnol. 2016, 4, 2. [Google Scholar] [CrossRef]
- Richter, R.F.; Ahlfeld, T.; Gelinsky, M.; Lode, A. Composites consisting of calcium phosphate cements and mesoporous bioactive glasses as a 3D plottable drug delivery system. Acta Biomater. 2023, 156, 146–157. [Google Scholar] [CrossRef]
- Tian, P.F.; Zhao, L.M.; Kim, J.; Li, X.; Liu, C.Y.; Cui, X.; Liang, T.; Du, Y.B.; Chen, X.H.; Pan, H.B. Dual stimulus responsive borosilicate glass (BSG) scaffolds promote diabetic alveolar bone defectsrepair by modulating macrophage phenotype. Bioact. Mater. 2023, 26, 231–248. [Google Scholar] [PubMed]
- Verbist, M.; Vandevelde, A.-L.; Geusens, J.; Sun, Y.; Shaheen, E.; Willaert, R. Reconstruction of craniomaxillofacial bone defects with 3D-printed bioceramic implants: Scoping review and clinical case series. J. Clin. Med. 2024, 13, 2805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Wang, J.R.; Gao, R.; Liu, X.; Feng, Z.J.; Zhang, C.N.; Huang, P.S.; Dong, A.J.; Kong, D.L.; Wang, W.W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials 2022, 285, 121538. [Google Scholar]
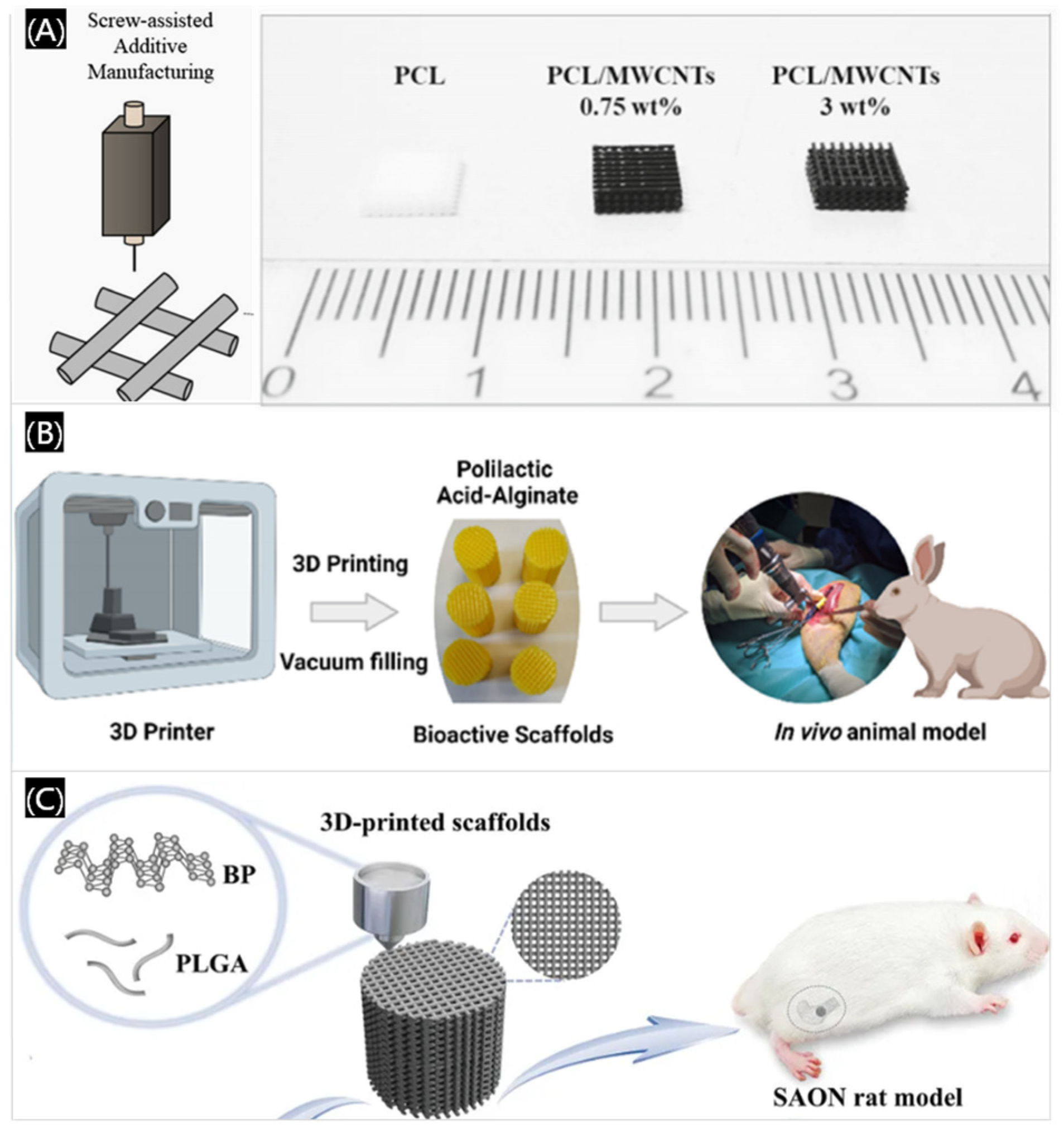
- Silva, E.P.; Huang, B.Y.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria-Jr, M.; et al. In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Bio-Des. Manuf. 2021, 4, 190–202. [Google Scholar]
- Serra-Aguado, C.I.; Llorens-Gamez, M.; Vercet-Llopis, M.; Martinez-Chicote, V.; Deb, S.; Serrano-Aroca, A. Engineering Three-dimensional-printed bioactive polylactic acid alginate composite scaffolds with antibacterial and in vivo osteoinductive capacity. ACS Appl. Mater. Inter. 2022, 14, 53593–53602. [Google Scholar]
- Ma, L.M.; Wang, X.L.; Zhao, N.R.; Zhu, Y.; Qiu, Z.Y.; Li, Q.T.; Zhou, Y.; Lin, Z.F.; Li, X.; Zeng, X.L.; et al. Integrating 3D printing and biomimetic mineralization for personalized enhanced osteogenesis, angiogenesis, and osteointegration. ACS Appl. Mater. Inter. 2018, 10, 42146–42154. [Google Scholar]
- Wang, Y.; Liu, Y.; Chen, S.S.; Siu, M.F.F.; Liu, C.; Bai, J.M.; Wang, M. Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes. Bioact. Mater. 2025, 46, 21–36. [Google Scholar]
- Zhong, L.N.; Chen, J.Y.; Ma, Z.Y.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.Y.; Pei, X.B.; Wang, J.; Wan, Q.B.; et al. 3D printing of metal–organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar]
- Long, J.; Yao, Z.Y.; Zhang, W.; Liu, B.; Chen, K.M.; Li, L.; Teng, B.; Du, X.F.; Li, C.R.; Yu, X.F.; et al. Regulation of osteoimmune microenvironment and osteogenesis by 3D-printed PLAG/black phosphorus scaffolds for bone regeneration. Adv. Sci. 2023, 10, 2302539. [Google Scholar]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; SLee, S.; Shin, H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater. 2019, 8, 1801106. [Google Scholar]
- Niu, Y.M.; Wang, Z.Z.; Shi, Y.C.; Dong, L.; Wang, C.M. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Bioact. Mater. 2021, 6, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhang, H.; Hu, Y.; Jing, Y.Y.; Geng, Z.; Su, J.C. Bone repair biomaterials: A perspective from immunomodulation. Adv. Funct. Mater. 2022, 32, 2208639. [Google Scholar] [CrossRef]
- Wang, J.W.; Xia, Y.H.; Hao, Z.W.; Shi, G.; Zhang, Q.; Wang, C.L.; Zhu, M.Y.; Huang, Y.L.; Guo, L.H.; Luan, T.; et al. A triple-integrated 3D-printed composite scaffold of high-activity peptide-metal ion-bone cement facilitates osteo-vascular regenerative repair of diabetic bone defects. Adv. Funct. Mater. 2025, 2422950. [Google Scholar] [CrossRef]
- Wu, J.P.; Liu, Y.T.; Cao, Q.D.; Yu, T.; Zhang, J.; Liu, Q.Y.; Yang, X.Y. Growth factors enhanced angiogenesis and osteogenesis on polydopamine coated titanium surface for bone regeneration. Mater. Design 2020, 196, 109162. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Zhang, L.L.; Zhang, B.Q.; Zhang, M.; Luo, Z.Y.; Tan, Y.; Tang, R.; Sun, J.X.; Zhou, X.D.; et al. Coaxial 3D printing scaffolds with sequential antibacterial and osteogenic functions to effectively repair infected mandibular defects. Adv. Funct. Mater. 2024, 34, 2407483. [Google Scholar] [CrossRef]
- Su, N.; Villicana, C.; Yang, F. Immunomodulatory strategies for bone regeneration: A review from the perspective of disease types. Biomaterials 2022, 286, 121604. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hong, S.B.; Jiang, W.D.; Li, X.J.; Zhou, X.Y.; He, X.Y.; Liu, J.Q.; Lin, K.L.; Mao, L.X. Engineering mesoporous bioactive glasses for emerging stimuli-responsive drug delivery and theranostic applications. Bioact. Mater. 2024, 34, 436–462. [Google Scholar] [CrossRef]
- He, X.T.; Li, X.; Zhang, M.; Tian, B.M.; Sun, L.J.; Bi, C.S.; Deng, D.K.; Zhou, H.; Qu, H.L.; Wu, C.T.; et al. Role of molybdenum in material immunomodulation and periodontal wound healing: Targeting immunometabolism and mitochondrial function for macrophage modulation. Biomaterials 2022, 283, 121439. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wang, P.; Mishra, A.; You, K.; Zong, Y.H.; Lu, W.F.; Chow, E.K.H.; Preshaw, P.M.; Huang, D.J.; Chew, J.R.J.; et al. 3D bioprinting and artificial intelligence-assisted biofabrication of personalized oral soft tissue constructs. Adv. Healthc. Mater. 2024, 2402727. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhou, X.H.; Fang, Y.C.; Xiong, Z.; Zhang, T. AI-driven 3D bioprinting for regenerative medicine: From bench to bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.T.; Cannata, S.; Gargioli, C.; et al. 3D bioprinting in skeletal muscle tissue engineering. Small 2019, 15, 1805530. [Google Scholar]
- Martyniak, K.; Lokshina, A.; Cruz, M.A.; Karimzadeh, M.; Kemp, R.; JKean, T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022, 152, 221–234. [Google Scholar]
- Rutz, A.L.; Gargus, E.S.; Hyland, K.E.; Lewis, P.L.; Setty, A.; Burghardt, W.R.; Shah, R.N. Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties. Acta Biomat. 2019, 99, 121–132. [Google Scholar] [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-dimensional bioprinting of mesenchymal stem cells using an osteoinductive bioink containing alginate and BMP-2-loaded PLGA nanoparticles for bone tissue engineering. Biomater. Adv. 2022, 136, 212789. [Google Scholar] [CrossRef] [PubMed]
- Nesic, D.; Durual, S.; Marger, L.; Mekki, M.; Sailer, I.; Scherrer, S.S. Could 3D printing be the future for oral soft tissue regeneration? Bioprinting 2020, 20, e00100. [Google Scholar]
- Yang, X.T.; Ma, Y.; Wang, X.T.; Yuan, S.M.; Huo, F.J.; Yi, G.Z.; Zhang, J.Y.; Yang, B.; Tian, W.D. A 3D-bioprinted functional module based on decellularized extracellular matrix bioink for periodontal regeneration. Adv. Sci. 2023, 10, 2205041. [Google Scholar]
- de Souza Araujo, I.J.; Perkins, R.S.; Ibrahim, M.M.; Huang, G.T.-J.; Zhang, W. Bioprinting PDLSC-laden collagen scaffolds for periodontal ligament regeneration. ACS Appl. Mater. Interfaces 2024, 16, 59979–59990. [Google Scholar] [PubMed]
- Liu, J.; Ruan, J.P.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.F.; Xu, H.H.K. Periodontal bone-liga-ment-cementum regeneration via scaffolds and stem cells. Cells 2019, 8, 537. [Google Scholar]
- Hu, Y.; Zhu, T.; Cui, H.T.; Cui, H.J. Integrating 3D bioprinting and organoids to better recapitulate the complexity of cellular microenvironments for tissue engineering. Adv. Healthc. Mater. 2025, 14, 2403762. [Google Scholar]
- Cadamuro, F.; Nicotra, F.; Russo, L. 3D printed tissue models: From hydrogels to biomedical applications. J. Control. Release 2023, 354, 726–745. [Google Scholar]
- Reyes, M.G.; Torras, A.B.; Carrillo, J.A.C.; García, J.M.V.; Aguilar, J.J.C. A study of tensile and bending properties of 3D-printed biocompatible materials used in dental appliances. J. Mater. Sci. 2022, 57, 2953–2968. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Woo, B.H.; Kim, N.; Jun, Y.C. Multicolor 4D printing of shapememory polymers for light-induced selective heating and remote actuation. Sci. Rep. 2020, 10, 6258. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; He, Y.; Liu, Y.J.; Leng, J.S. 4D printing of multiple shape memory polymer and nanocomposites with biocompatible, programmable and selectively actuated properties. Addit. Manuf. 2022, 53, 102689. [Google Scholar]






| Techniques | Raw Materials | Advantages | Challenges | Costs | Refs. |
|---|---|---|---|---|---|
| Selective laser melting | Metal powders: stainless steel powder, iron powder, etc. | High precision, efficiency and complex structure manufacturing | Limited materials and time-consuming | The manufacturing cost is higher | [12,13,14,15] |
| Selective laser sintering | Polymers, metals, ceramics, gypsum, and their mixed powders | Wide range of material adaptability, suitable for mass production | Slow printing speeds and complex post-processing | The cost of equipment is higher | [16] |
| Fused deposition modeling | Filamentous thermoplastic materials: PLA, nylon, etc. | High efficiency and high material utilization | Slow printing speed and limited accuracy | Economical and practical | [17] |
| Stereolithography | Liquid photopolymer resin | High precision, smooth printing surface | Time-consuming and complex post-processing | The cost of materials, equipment and its maintenance are higher | [18,19] |
| Digital light processing | Photopolymer liquid and ceramic mixture slurry | High precision, high efficiency and fast printing speed | Limited print size and operating temperature | Cost-effective | [20,21,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wei, J. Application of 3D Printing Technology in Dentistry: A Review. Polymers 2025, 17, 886. https://doi.org/10.3390/polym17070886
Chen Y, Wei J. Application of 3D Printing Technology in Dentistry: A Review. Polymers. 2025; 17(7):886. https://doi.org/10.3390/polym17070886
Chicago/Turabian StyleChen, Yangqing, and Junchao Wei. 2025. "Application of 3D Printing Technology in Dentistry: A Review" Polymers 17, no. 7: 886. https://doi.org/10.3390/polym17070886
APA StyleChen, Y., & Wei, J. (2025). Application of 3D Printing Technology in Dentistry: A Review. Polymers, 17(7), 886. https://doi.org/10.3390/polym17070886







