Efficient Recovery of Waste Cotton Fabrics Using Ionic Liquid Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Determination of Polymerization Degree of Cotton Fabrics
2.2.2. Determination of Accessibility of Cotton Fabrics (Iodine Equilibrium Adsorption Value)
2.2.3. Instrument Testing
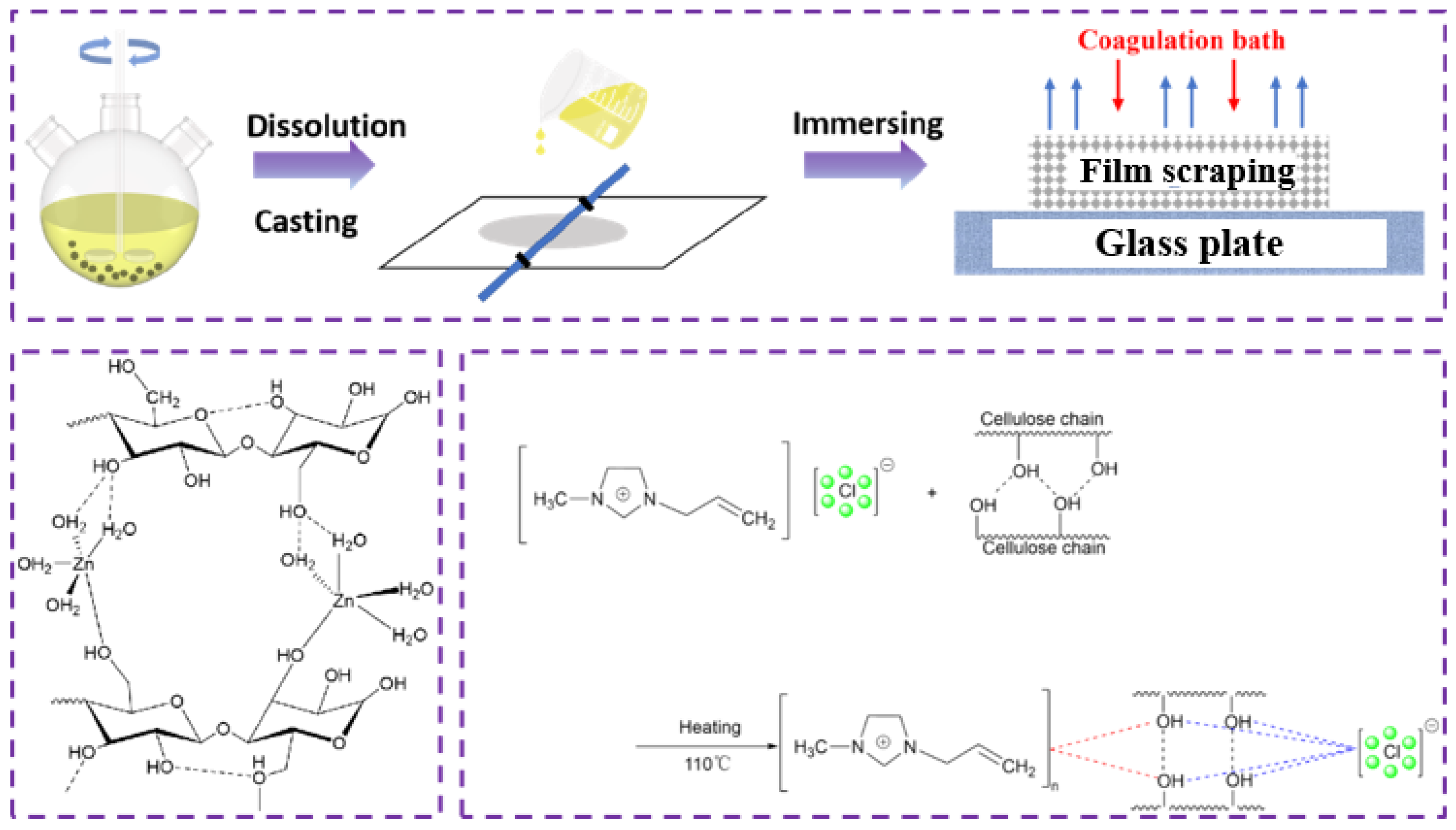
2.3. Dissolution of Waste Cotton Fabric
2.3.1. Zinc Chloride System
2.3.2. Ionic Liquid System
2.4. Preparation of the Regenerated Cellulose Film
3. Results
3.1. Comparison of Properties Between Old and New Cotton
3.1.1. Analysis of Breaking Strength and Elongation at Break of Old and New Cotton Fabrics
3.1.2. Analysis of Polymerization Degree of Old and New Cotton Fabrics
3.1.3. Analysis of Accessibility of Old and New Cotton Fabrics
3.1.4. Analysis of Crystallinity of Old and New Cotton Fabrics
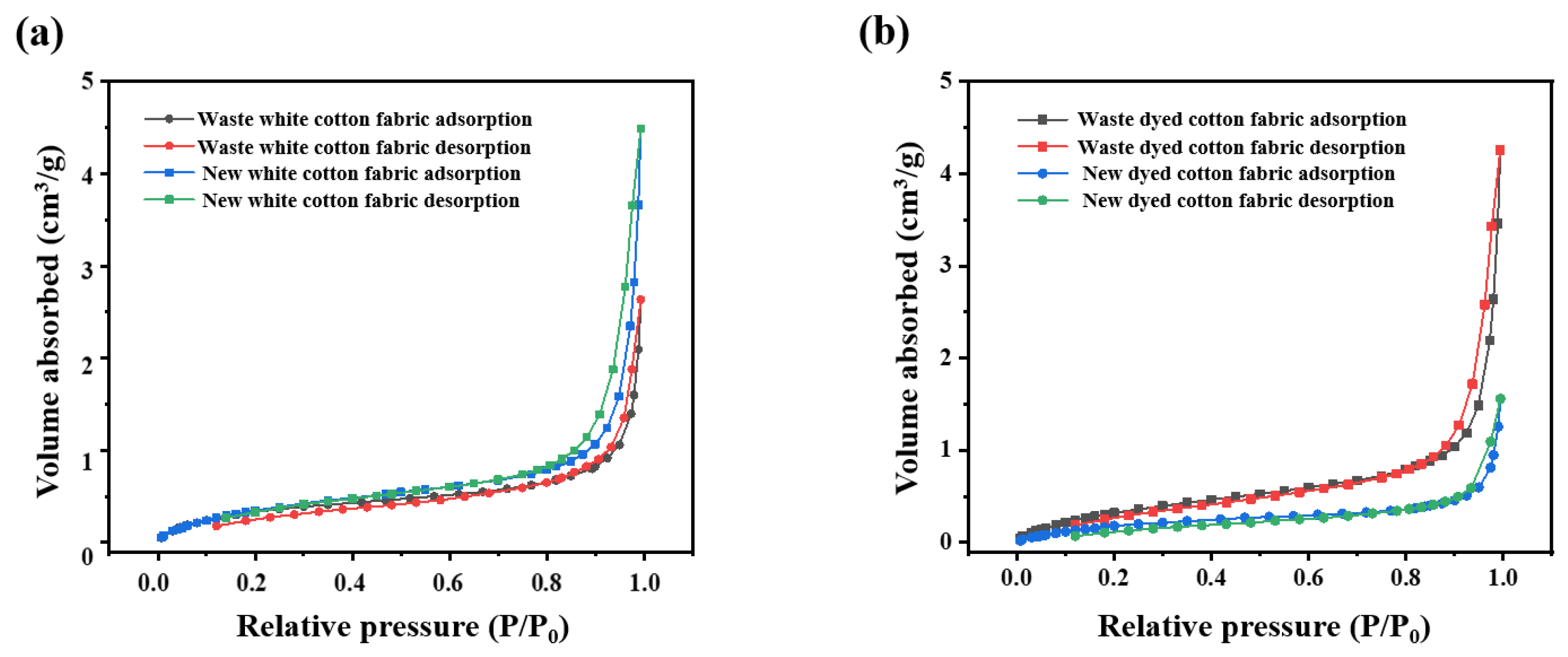
3.1.5. Analysis of Specific Surface Area of Old and New Cotton Fabrics
3.2. Process Investigation
3.2.1. Dissolving Process
3.2.2. Regeneration Process
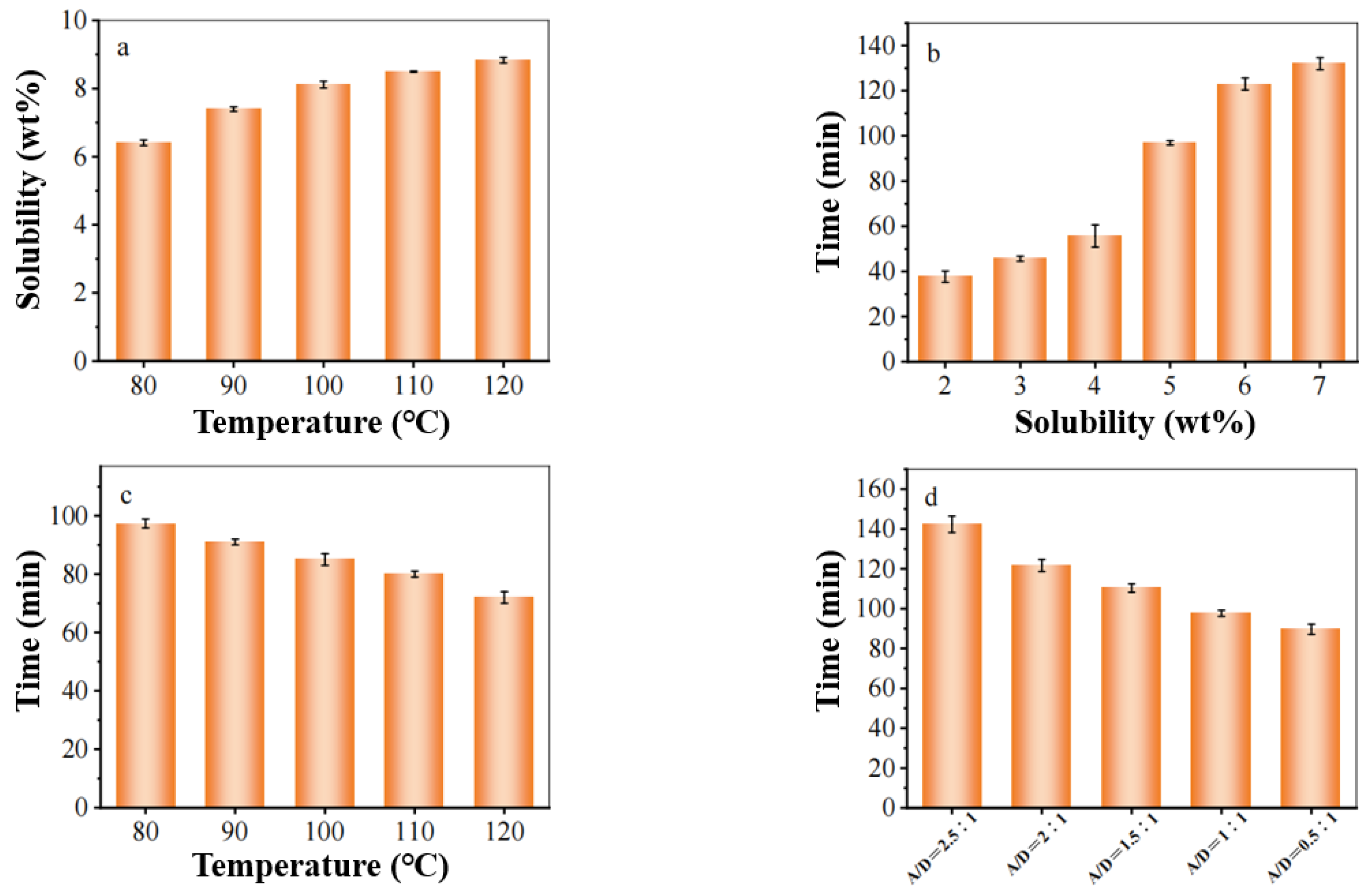
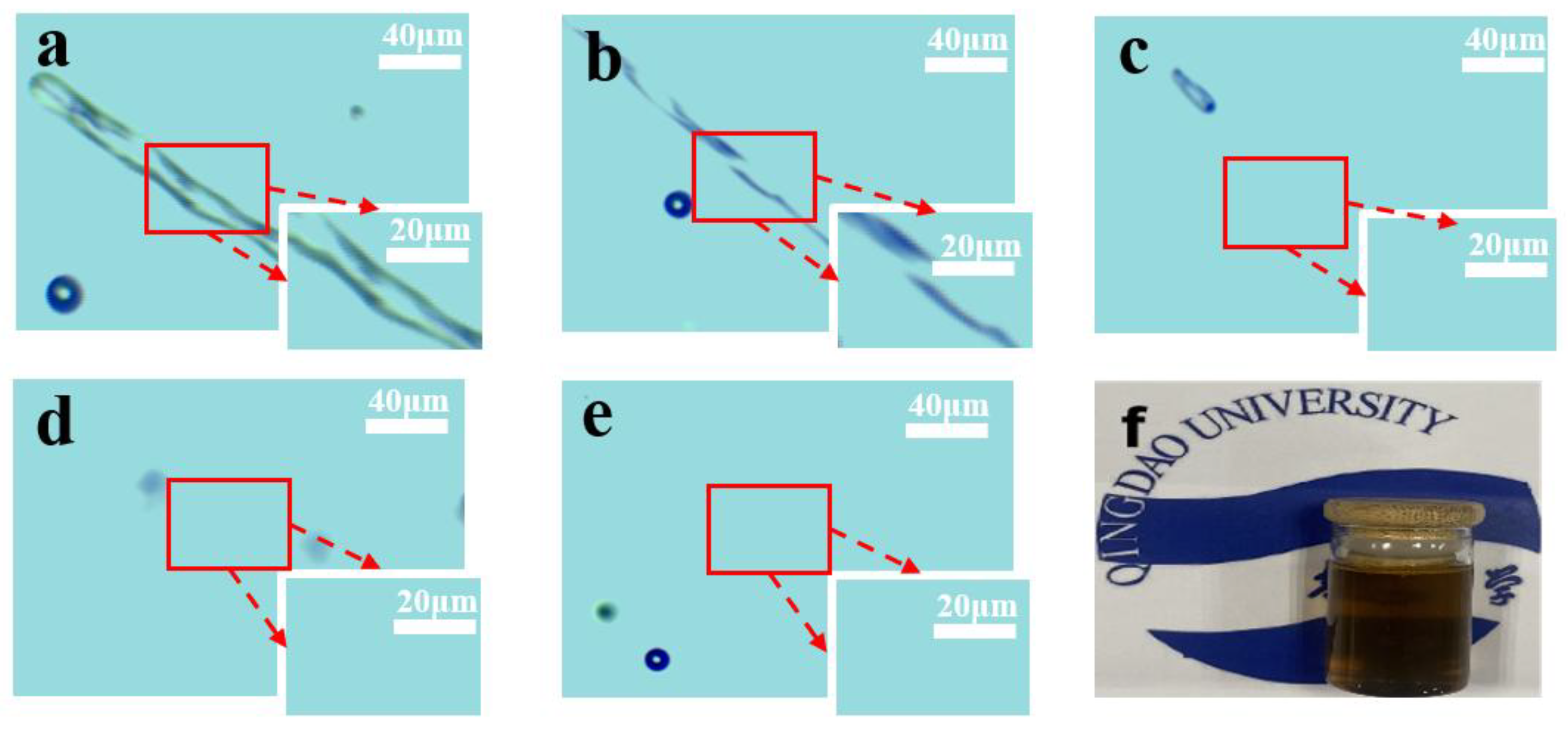
3.2.3. Solubility of Waste Cotton Fabric
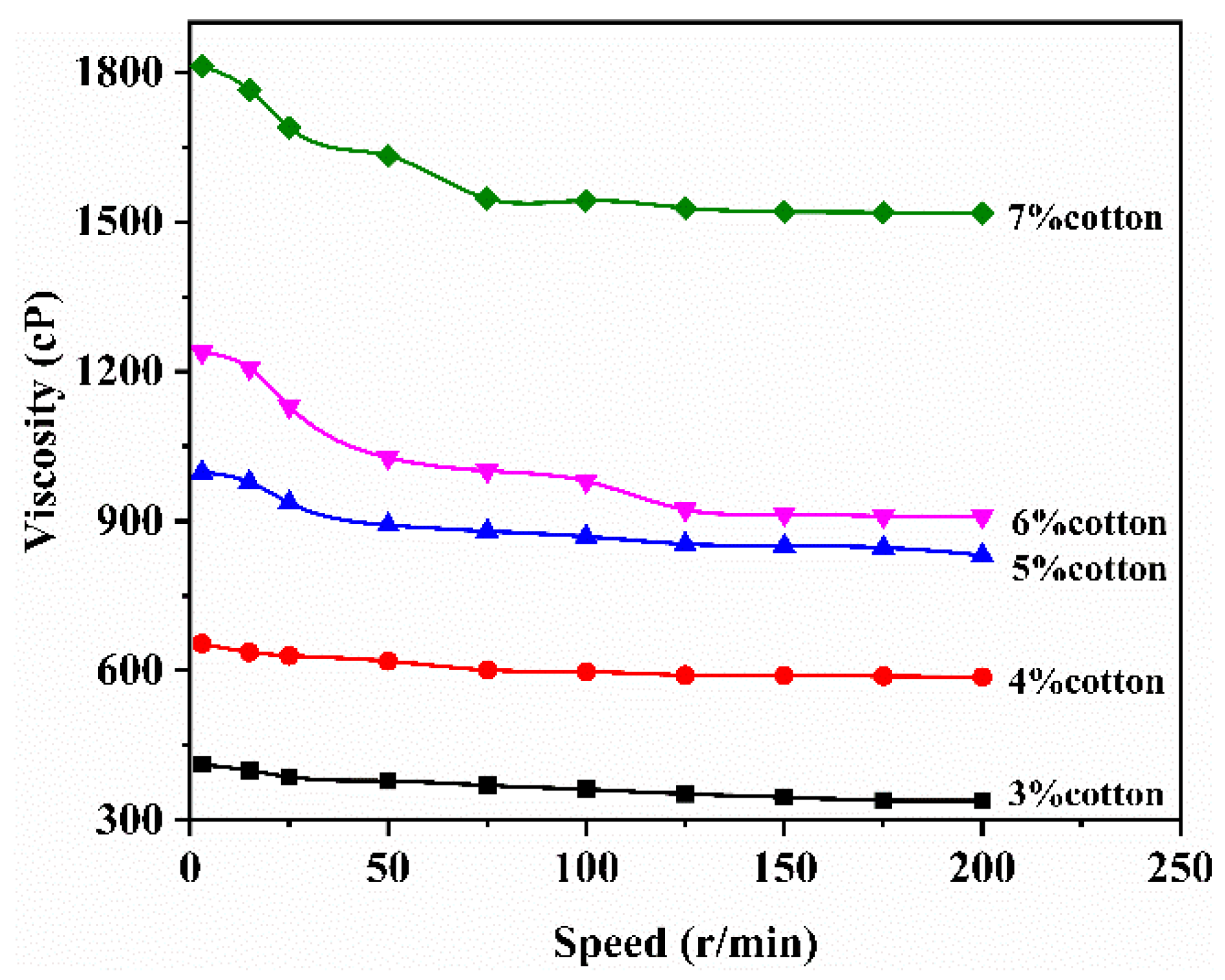
3.2.4. Rheological Properties of Waste Cotton Fabric
3.3. Representation
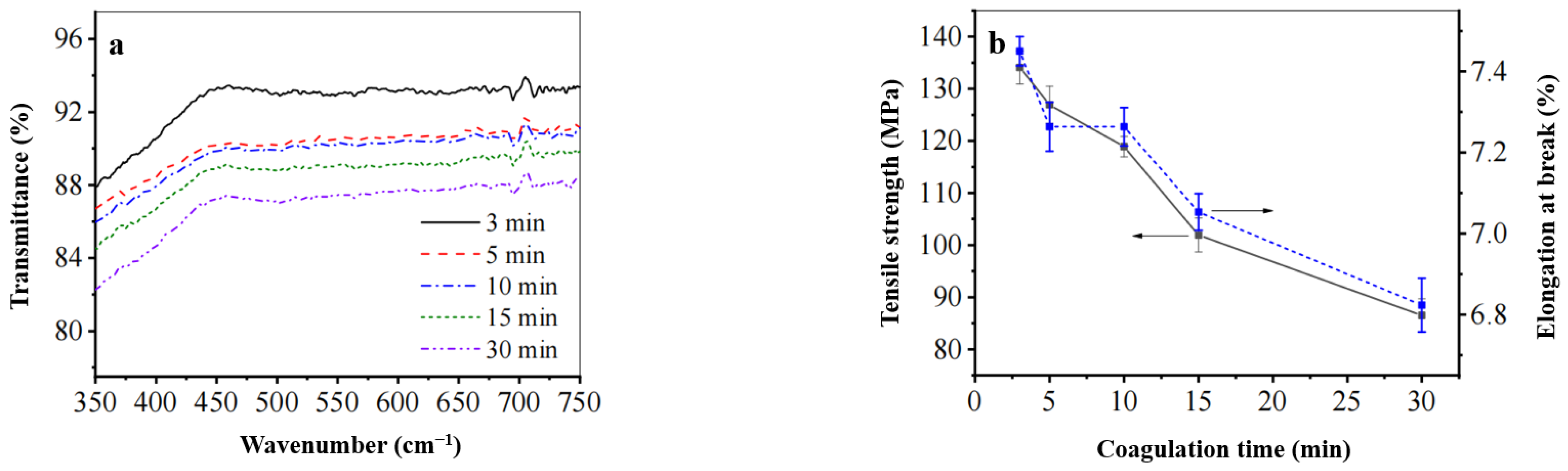
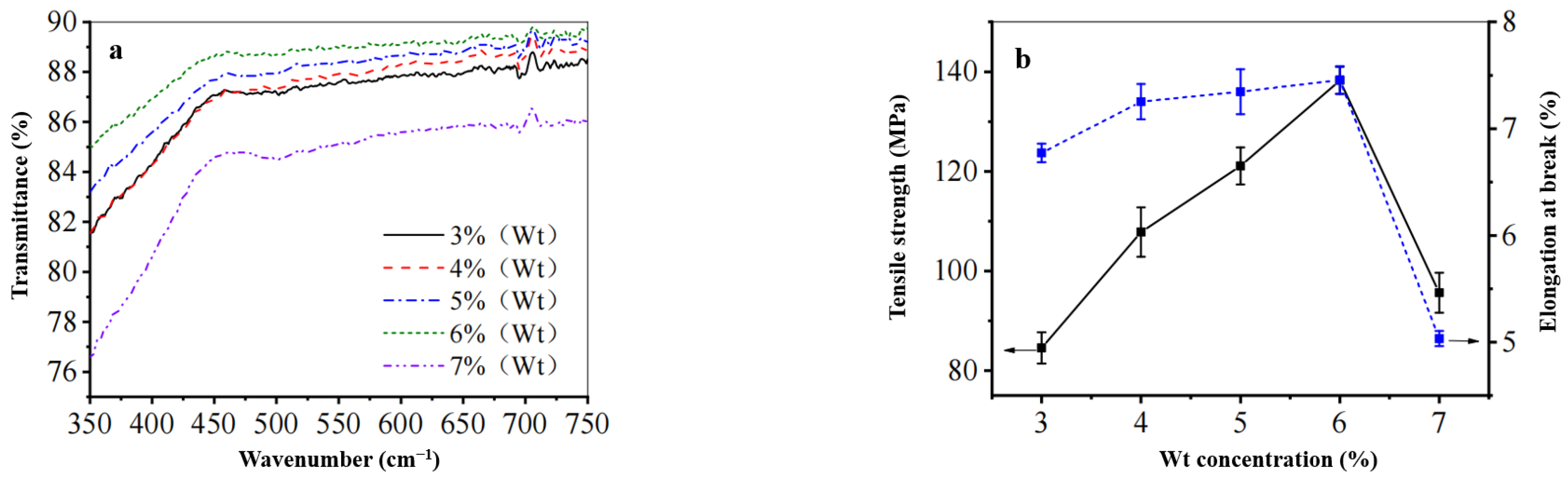
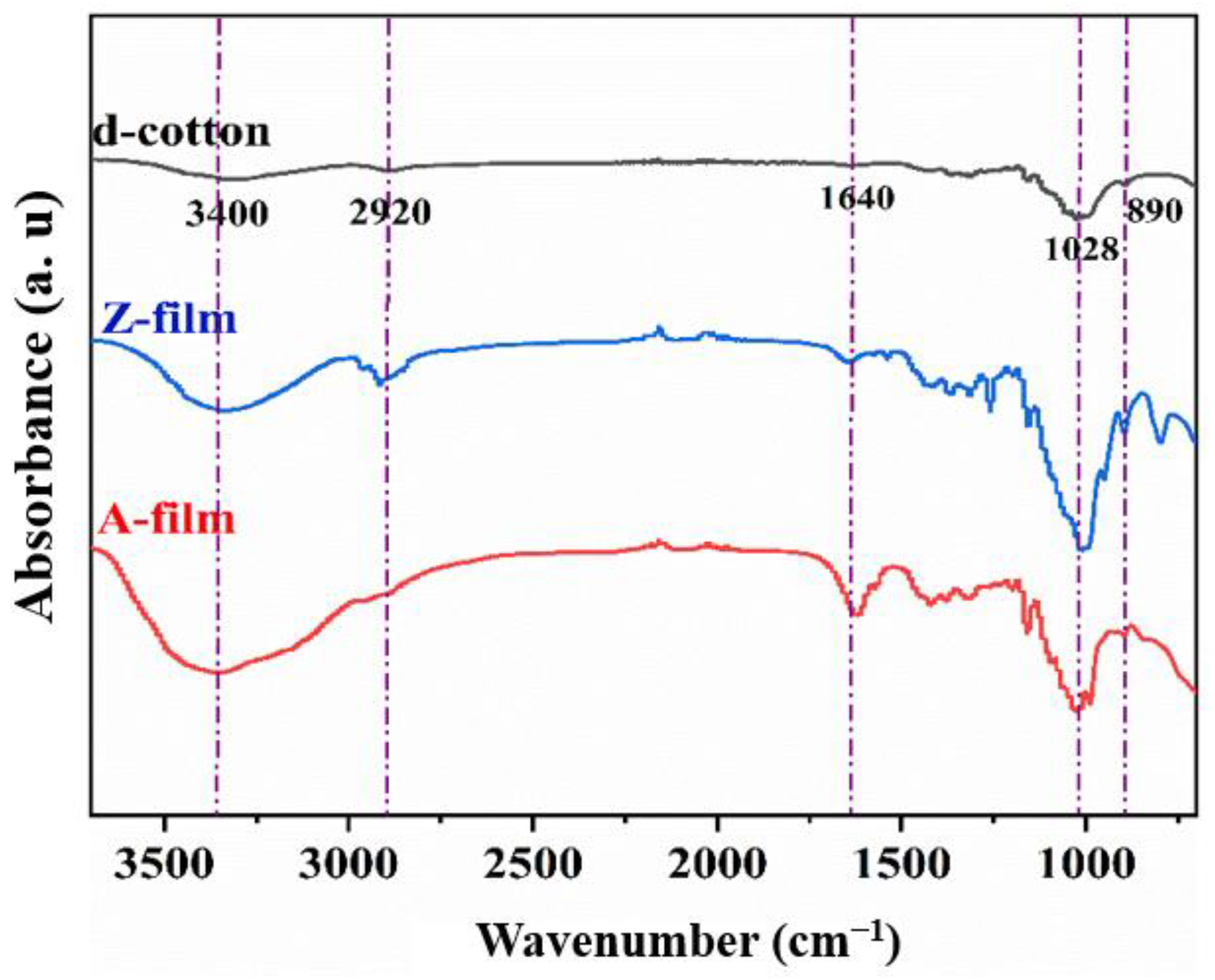
3.3.1. FT-IR Analysis
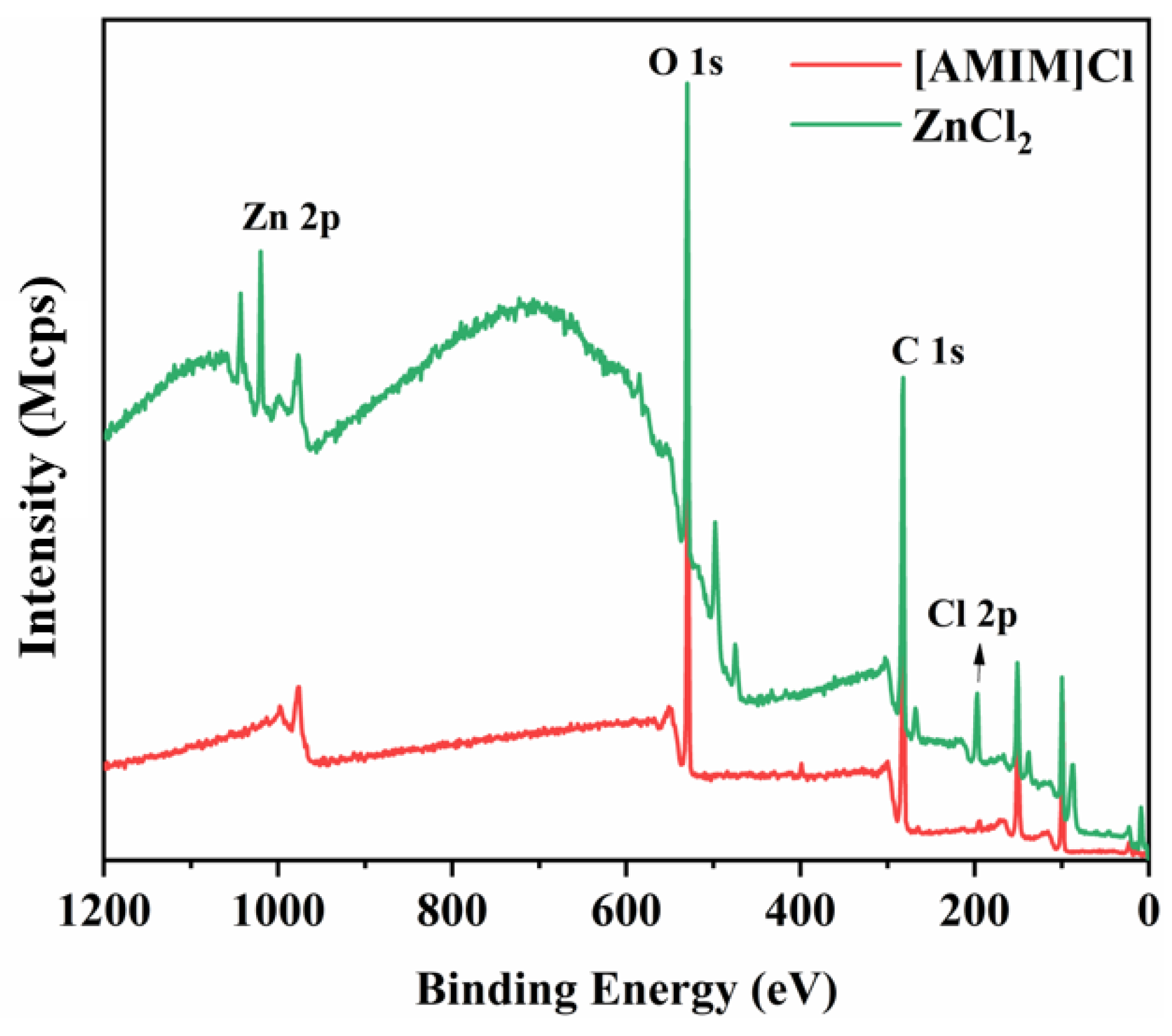
3.3.2. XPS Analysis
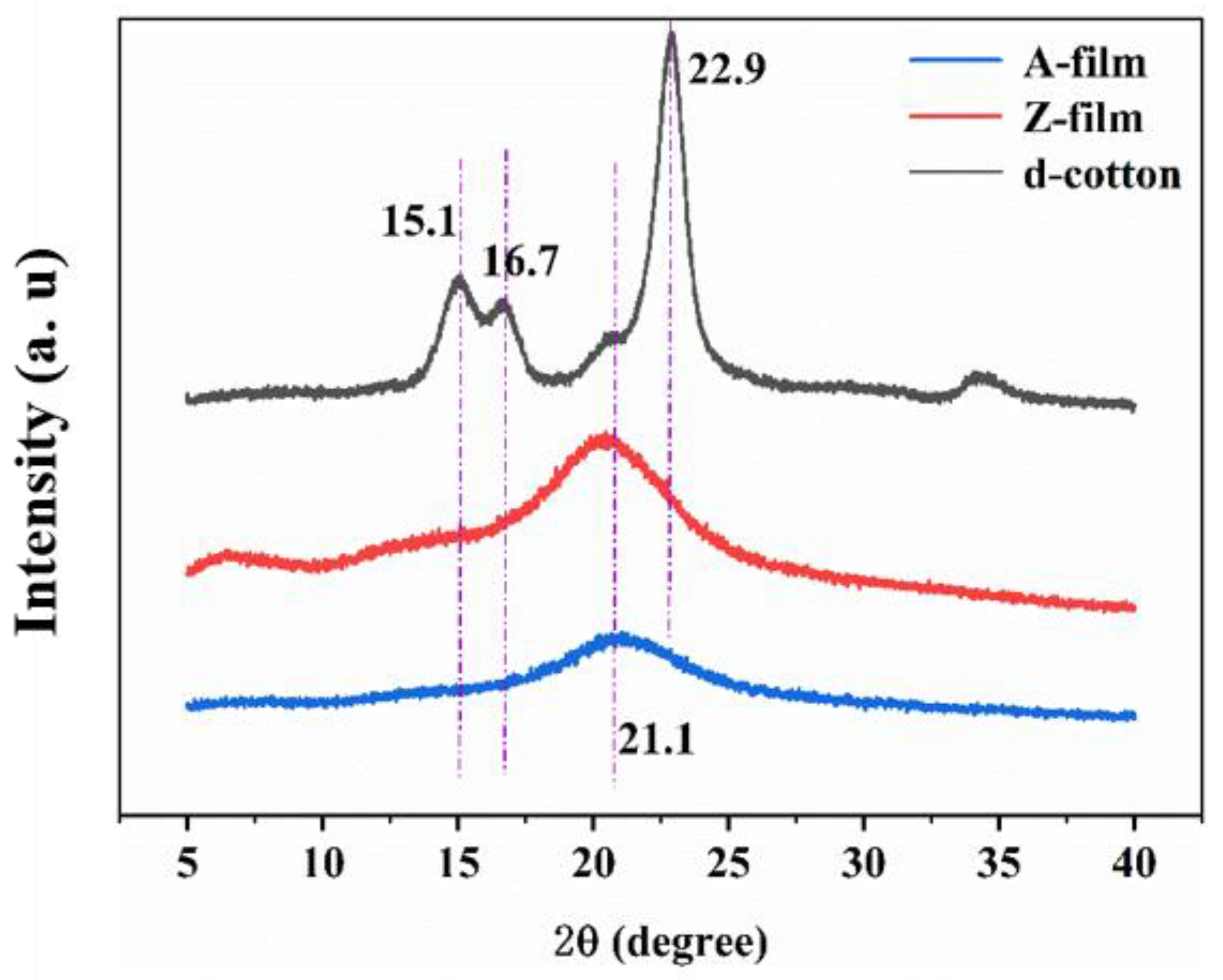
3.3.3. XRD Analysis
3.3.4. SEM Analysis
4. Conclusions
- The iodine equilibrium adsorption value of the new pure cotton fabric is slightly higher than that of the waste fabric, indicating enhanced accessibility. However, the crystallinity of the waste fabric remains unchanged and even exhibits a higher XRD intensity compared to that of the new dyed fabric. Notably, there is no direct linear relationship between cellulose crystallinity and fabric strength. BET test results reveal no correlation between the specific surface area of long-term used fabrics and that of newly produced fabrics.
- In an ionic liquid system with a composition ratio of [AMIM]Cl: DMSO = 1:1, optimal dissolution conditions are achieved at 110 °C for 120 min; ideal forming conditions include a film solution concentration of 6%, solidification time of 3 min, and a solidification bath temperature maintained at 0 °C.
- Under optimal processing conditions, the Z-film demonstrates lower mechanical properties (91.0 MPa) and transparency (89.3%) in comparison to the A-film (139.6 MPa) and (93.0%). Surface morphology analysis indicates that the Z-film possesses a defective epidermal layer, whereas the A-film features a smooth surface with uniform density throughout its cross-section. Deformation vibration absorption peaks for CH2 and C–O–H appear at 890 cm−1 for both films; however, the Z-film shows slightly higher crystallinity (29.2%) than the A-film (21.3%).
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, I.S.F.; Prates, A.; Evtuguin, D.V. Production of rayon fibres from cellulosic pulps: State of the art and current developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [PubMed]
- Liu, H.; Fan, W.; Miao, Y.; Dou, H.; Shi, Y.; Wang, S.; Zhang, X.; Hou, L.; Yu, X.; Lam, S.S.; et al. Closed-loop recycling of colored regenerated cellulose fibers from the dyed cotton textile waste. Cellulose 2022, 30, 2597–2610. [Google Scholar]
- Qiao, T.; Yang, C.; Zhao, L.; Feng, Y.; Feng, X.; Mao, Z.; Wang, B. High tensile regenerated cellulose fibers via cyclic freeze-thawing enabled dissolution in phosphoric acid for textile-to-textile recycling of waste cotton fabrics. Int. J. Biol. Macromol. 2024, 277, 133911. [Google Scholar] [PubMed]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero-waste milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar]
- Kahoush, M. Towards sustainable textile sector: Fractionation and separation of cotton/polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar]
- Huang, X.; Tan, Y.; Huang, J.; Zhu, G.; Yin, R.; Tao, X.; Tian, X. Industrialization of open- and closed-loop waste textile recycling towards sustainability: A review. J. Clean. Prod. 2024, 436, 140676. [Google Scholar]
- Nasri-Nasrabadi, B.; Byrne, N. Converting waste textiles into highly effective sorbent materials. RSC Adv. 2020, 10, 37596–37599. [Google Scholar]
- Rissanen, M.; Schlapp-Hackl, I.; Sawada, D.; Raiskio, S.; Ojha, K.; Smith, E.; Sixta, H. Chemical recycling of hemp waste textiles via the ionic liquid based dry-jet-wet spinning technology. Text. Res. J. 2022, 93, 2545–2557. [Google Scholar]
- Ji, L. Review of the Dissolved Pulp Market in 2023 and Outlook for 2024. China Pap. Newsl. 2024, 2, 35–42. [Google Scholar]
- Zhai, H.; Liu, Z.; Xu, L.; Liu, T.; Fan, Y.; Jin, L.; Dong, R.; Yi, Y.; Li, Y. Waste Textile Reutilization Via a Scalable Dyeing Technology: A Strategy to Enhance Dyestuffs Degradation Efficiency. Adv. Fiber Mater. 2022, 4, 1595–1608. [Google Scholar]
- Nakamura, Y.; Ono, Y.; Saito, T.; Isogai, A. Characterization of cellulose microfibrils, cellulose molecules, and hemicelluloses in buckwheat and rice husks. Cellulose 2019, 26, 6529–6541. [Google Scholar]
- Wu, Y.; Che, Y.; Wei, X.; Hu, Q.; Xu, J.; Guo, B.; Niu, Z. Nondestructive Recovery of Cotton from Waste Polycotton Textiles by Catalytic Hydrolysis. ACS Sustain. Chem. Eng. 2024, 12, 10446–10454. [Google Scholar]
- Feng, L.; Chen, Z. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar]
- Wang, Y. Fiber and Textile Waste Utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar]
- Chang, C.; Kao, Y.; Chen, J.; Zhang, H.; Wu, S. Sustainable waste textile upcycling by selective dye decoloration using ionic liquid and Bi11VO19 photocatalyst. Ind. Eng. Chem. 2023, 124, 412–421. [Google Scholar]
- Iurii, B.; Charlotte, J.; Unnikrishnan, K.; Simon, F.; Ian, M.; Bradley, W. Dissolution of Cellulose: Are Ionic Liquids Innocent or Noninnocent Solvents? ACS Sustain. Chem. Eng. 2020, 8, 10142–10150. [Google Scholar]
- Ren, Q.; Zhang, J. Synthesis of 1-allyl, 3-methylimidazole ionic liquids at room temperature and preliminary study on their solubility to cellulose. Acta Polym. Sin. 2003, 3, 448–451. [Google Scholar]
- Kallidanthiyil, C.; Sigvart, E.; Vishwesh, V.; Syed, N.; Anne, F. Highly efficient cellulose dissolution by alkaline ionic liquids. Carbohydr. Polym. 2020, 229, 115594. [Google Scholar]
- Asaadi, S.; Hummel, M.; Hellsten, S.; Härkäsalmi, T.; Ma, Y.; Michud, A.; Sixta, H. Renewable High-Performance Fibers from the Chemical Recycling of Cotton Waste Utilizing an Ionic Liquid. ChemSusChem 2016, 9, 3250–3258. [Google Scholar]
- Lv, F.; Wang, C.; Zhu, P.; Zhang, C. Isolation and recovery of cellulose from waste nylon/cotton blended fabrics by 1-allyl-3-methylimidazolium chloride. Carbohydr. Polym. 2015, 123, 424–431. [Google Scholar]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. [Google Scholar] [PubMed]
- Letters, K. Viskosimetrische Untersuchungen über die Reaktion von Zellulose mit konzentrierten Chlorzinklösungen. Kolloid-Zeitschrift 1932, 58, 229–239. [Google Scholar]
- Yang, G.; Chong, Z.; Yifeng, X.; Dejia, S. Experimental parameters of Modal fiber and cotton fiber mixture by formic acid/zinc chloride process. Prog. Text. Sci. Technol. 2021, 8, 19–23. [Google Scholar]
- Ma, W.; Li, X.; Zhang, L.; Zheng, Y.; Xi, Y.; Ma, J.; Wang, Z. Novel insights on room temperature-induced cellulose dissolution mechanism via ZnCl2 aqueous solution: Migration, penetration, interaction, and dispersion. Int. J. Biol. Macromol. 2024, 272, 132912. [Google Scholar]
- GBT1548-2016; Determination of Intrinsic Viscosity in Pulp Copper Ethylenediamine (CED) Solution. SAC: Beijing, China, 2016.
- ISO 5351:2010; Pulps—Determination of Limiting Viscosity Number in Cupri-Ethylenediamine (CED) Solution. ISO: Geneva, Switzerland, 2010.
- Isogai, A. Modifications of cellulose and their applications to pulp and paper. J. Mol. Liq. 2006, 49, 5–11. [Google Scholar]
- Zhang, K.; Fang, X.; Ma, Z.; Zhou, Z.; Yu, J.; Gao, C.; Zhou, R.; Chen, Y. Stress–Strain Characteristics of Intergranular Liquid Film in High-Silicon Aluminum Alloys. Metall. Mater. Trans. A 2024, 55, 2596–2601. [Google Scholar]
- Xu, Y.; German, G.K.; Mertz, A.F.; Dufresne, E.R. Imaging stress and strain in the fracture of drying colloidal films. Soft Matter 2013, 9, 3735–3740. [Google Scholar]
- Chen, M.; Ren, M.; Zhu, M.; Zhang, H.; Chen, T.; Zhang, Y.; Yang, S. Effect of degree of polymerization on regenerated cellulose ultrafiltration membrane performance through ZnCl2/AlCl3 aqueous solvent system. Carbohydr. Polym. 2024, 345, 122557. [Google Scholar]
- De Silva, R.; Byrne, N. Utilization of cotton waste for regenerated cellulose fibres: Influence of degree of polymerization on mechanical properties. Carbohydr. Polym. 2017, 174, 89–94. [Google Scholar]
- Li, Y.; Li, Y.; Zhao, Q.; Li, L.; Chen, R.; He, C. Cotton fiber functionalized with 2D covalent organic frameworks for iodine capture. Cellulose 2020, 27, 1517–1529. [Google Scholar]
- Liu, C.; Shan, H.; Chen, X.; Si, Y.; Yin, X.; Yu, J.; Ding, B. Novel Inorganic-Based N-Halamine Nanofibrous Membranes As Highly Effective Antibacterial Agent for Water Disinfection. ACS Appl. Mater. Interfaces 2018, 10, 44209–44215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pang, G.; Shen, W.; Tong, X.; Jia, M. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019, 207, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Pandiyarasan, V.; Archana, J.; Pavithra, A.; Ashwin, V.; Navaneethan, M.; Hayakawa, Y.; Ikeda, H. Hydrothermal growth of reduced graphene oxide on cotton fabric for enhanced ultraviolet protection applications. Mater. Lett. 2017, 188, 123–126. [Google Scholar] [CrossRef]
- Saini, A.; Rauert, C.; Simpson, M.J.; Harrad, S.; Diamond, M.L. Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Sci. Total Environ. 2016, 563–564, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Li, J.; Wang, F.; Chang, G.; Fu, T.; Zhou, W. Effects of temperature on cellulose hydrogen bonds during dissolution in ionic liquid. Carbohydr. Polym. 2018, 201, 387–391. [Google Scholar] [CrossRef]
- Roos, E.; Gradaus, C.; Sebastiani, D. A force field for the solubility of cellulose in DMSO/Ionic liquids. Cellulose 2024, 31, 4793–4815. [Google Scholar] [CrossRef]
- Minjia, Z.; Chunyan, D.; Wanzhen, Y.; Yazhi, W.; Sun, B.; Lanhui, R. Research progress on dissolution and regeneration of wood fiber raw materials in ionic liquids. Chem. Prod. Technol. 2021, 27, 36–41. [Google Scholar]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, C.; Huang, F.; Jia, H.; Wei, P. Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohydr. Polym. 2013, 94, 38–45. [Google Scholar] [CrossRef]
- Ray, U.; Zhu, S.; Pang, Z.; Li, T. Mechanics Design in Cellulose-Enabled High-Performance Functional Materials. Adv. Mater. 2021, 33, 2002504. [Google Scholar]
- Vo, L.T.T.; Široká, B.; Manian, A.P.; Bechtold, T. Functionalisation of cellulosic substrates by a facile solventless method of introducing carbamate groups. Carbohydr. Polym. 2010, 82, 1191–1197. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of solvent exchange on the supramolecular structure, the molecular mobility and the dissolution behavior of cellulose in LiCl/DMAc. Carbohydr. Res. 2008, 343, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, C.; Liu, N.; Teng, Y.; Yin, C. Preparation of transparent anti-pollution cellulose carbamate regenerated cellulose membrane with high separation ability. Int. J. Biol. Macromol. 2019, 139, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ansell, M.P.; Mwaikambo, L.Y. The structure of cotton and other plant fibres. In Handbook of Textile Fibre Structure; Eichhorn, S.J., Hearle, J.W.S., Jaffe, M., Kikutani, T., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 62–94. [Google Scholar]
- Zhang, Y.; Zhao, L.; Liu, Y.; Dong, C.; Zhang, K. Production of flame-retardant phosphorylated cellulose nanofibrils by choline chloride based reactive deep eutectic solvent. Carbohydr. Polym. 2025, 348, 122931. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Wan, J.; Xia, G.; Yu, J.; He, J.; Zhang, J. Directly Converting Agricultural Straw into All-Biomass Nanocomposite Films Reinforced with Additional in Situ-Retained Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2017, 5, 5127–5133. [Google Scholar]
- Zhang, L.Y.; Tao, L.; Zhao, C.; Dong, Y.; Liu, K.; Zhang, H. Liimatainen, Fabrication of flame-retardant and water-resistant nanopapers through electrostatic complexation of phosphorylated cellulose nanofibers and chitin nanocrystals. J. Colloid Interface Sci. 2024, 676, 61–71. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, W.; Xing, W.; Xu, Y.; Zhang, G. Efficient Recovery of Waste Cotton Fabrics Using Ionic Liquid Methods. Polymers 2025, 17, 900. https://doi.org/10.3390/polym17070900
Zhang X, Zhou W, Xing W, Xu Y, Zhang G. Efficient Recovery of Waste Cotton Fabrics Using Ionic Liquid Methods. Polymers. 2025; 17(7):900. https://doi.org/10.3390/polym17070900
Chicago/Turabian StyleZhang, Xiaozheng, Wenhao Zhou, Wenhao Xing, Yingjun Xu, and Gangqiang Zhang. 2025. "Efficient Recovery of Waste Cotton Fabrics Using Ionic Liquid Methods" Polymers 17, no. 7: 900. https://doi.org/10.3390/polym17070900
APA StyleZhang, X., Zhou, W., Xing, W., Xu, Y., & Zhang, G. (2025). Efficient Recovery of Waste Cotton Fabrics Using Ionic Liquid Methods. Polymers, 17(7), 900. https://doi.org/10.3390/polym17070900





