Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers
Abstract
1. Introduction
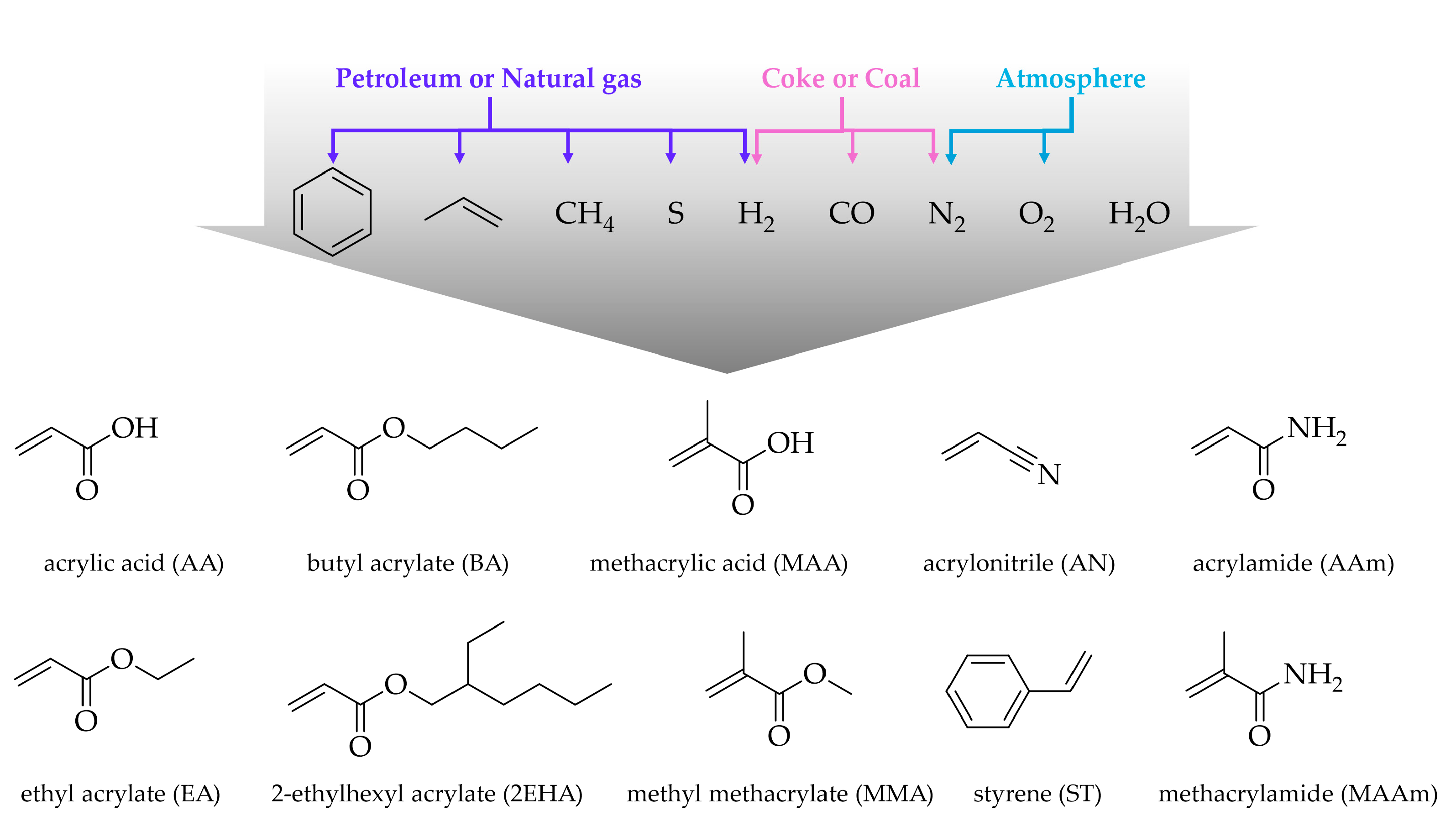
2. Waterborne Acrylic Latexes
3. Dispersed-Phase Polymerization
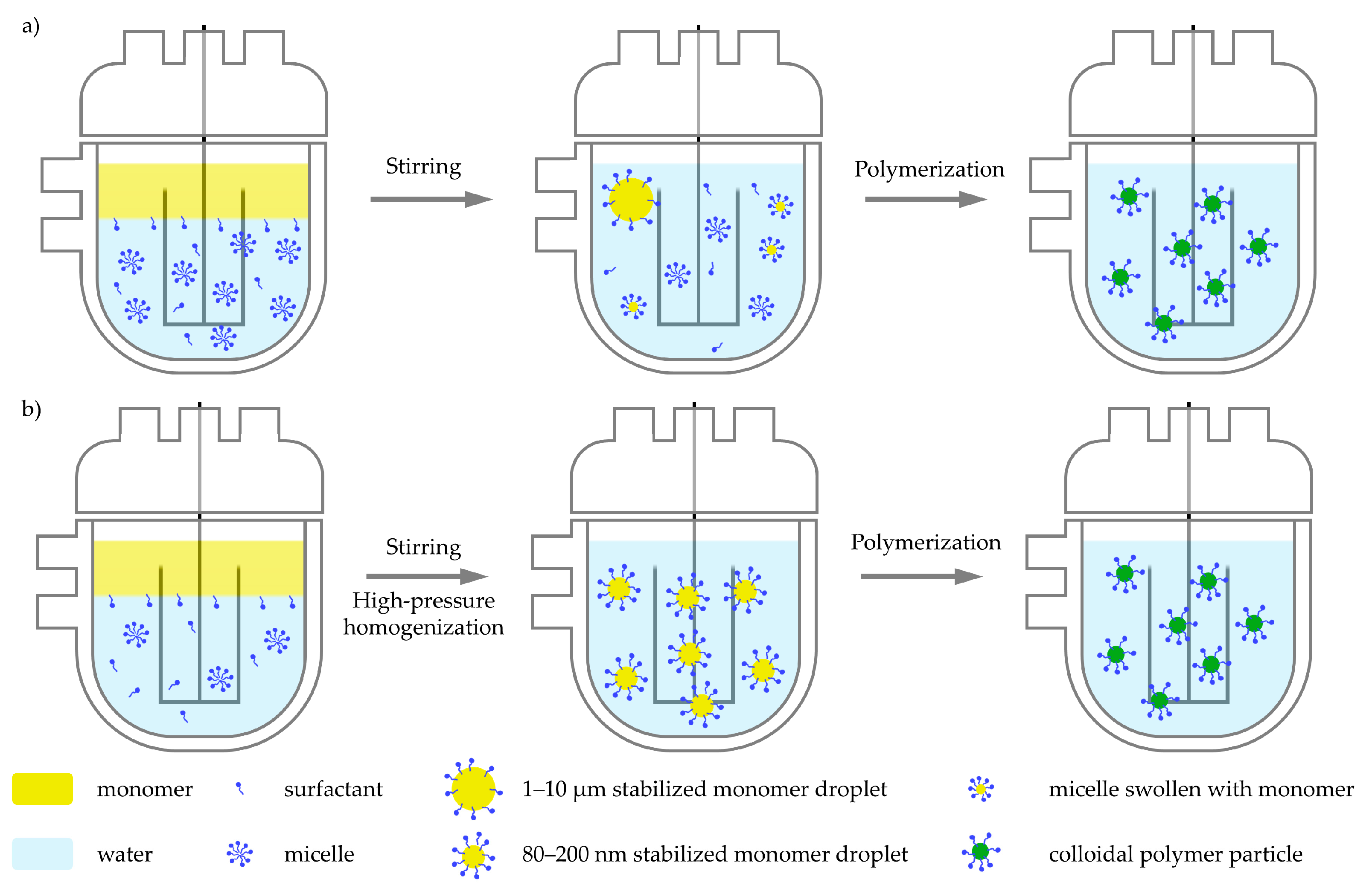
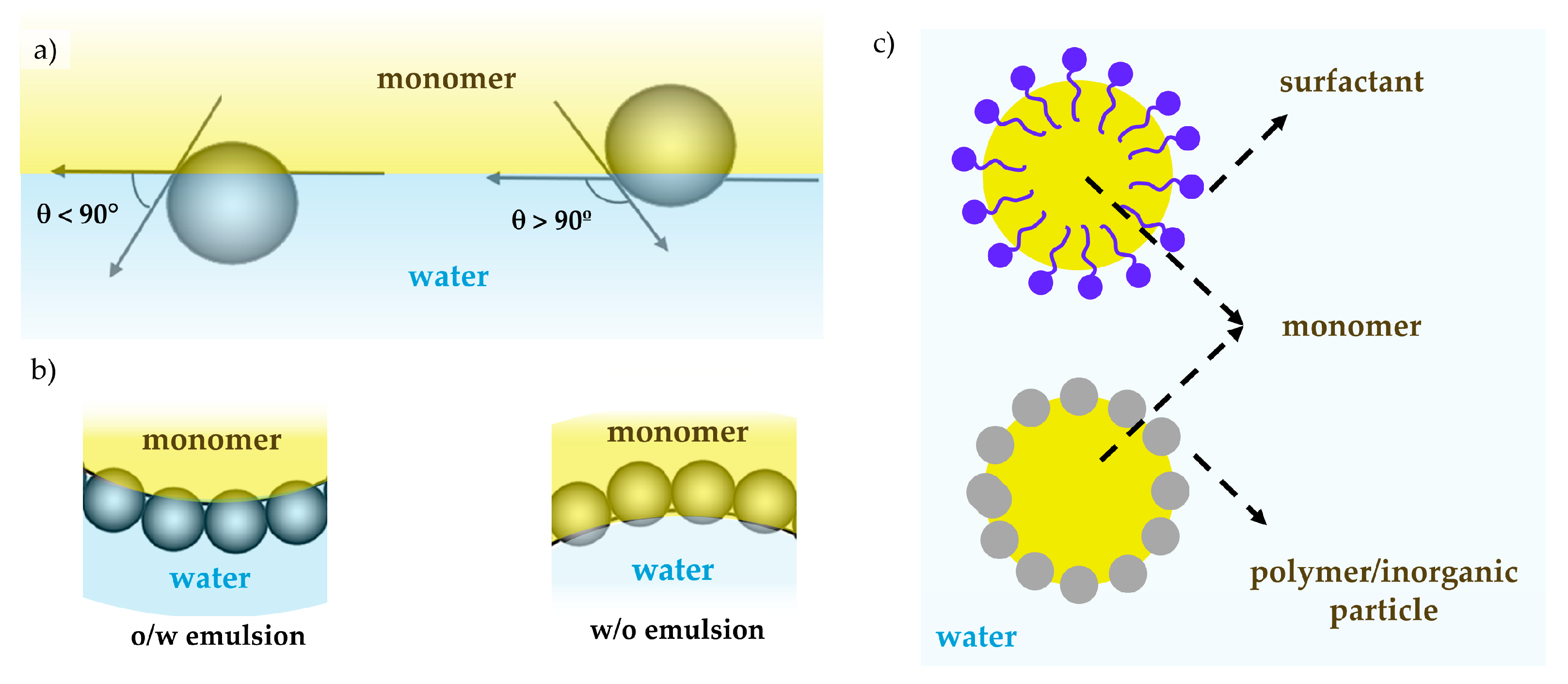
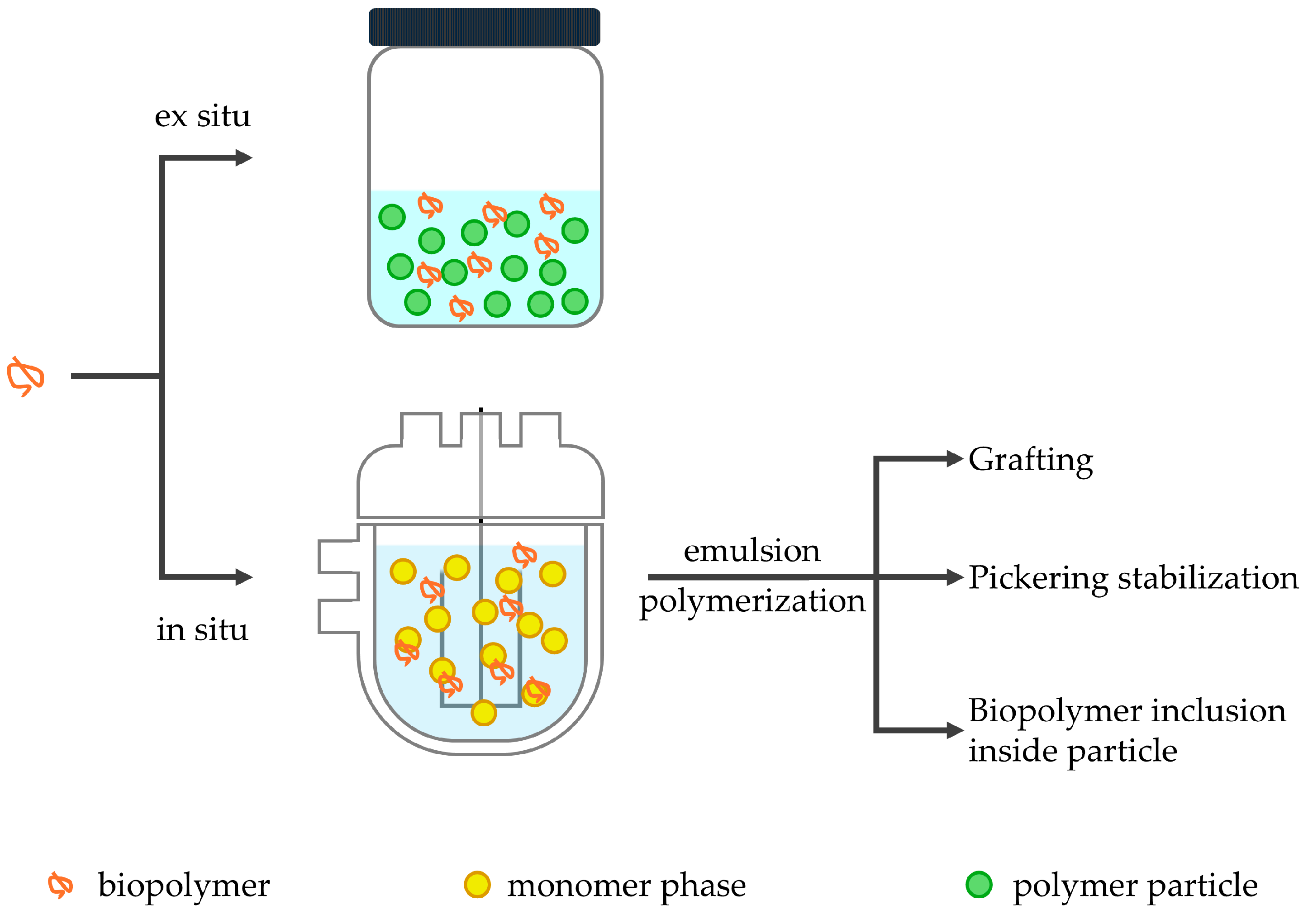
3.1. Emulsion Polymerization
3.2. Miniemulsion Polymerization
3.3. Pickering Emulsion Polymerization
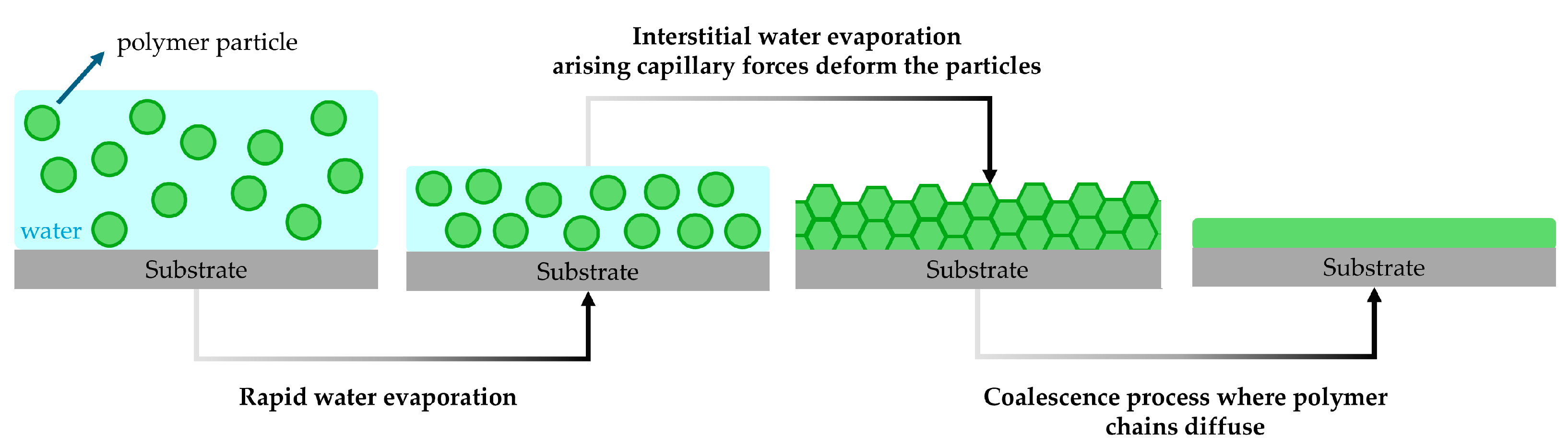
3.4. Film Formation
4. Biopolymer–Acrylic Hybrids
4.1. Proteins
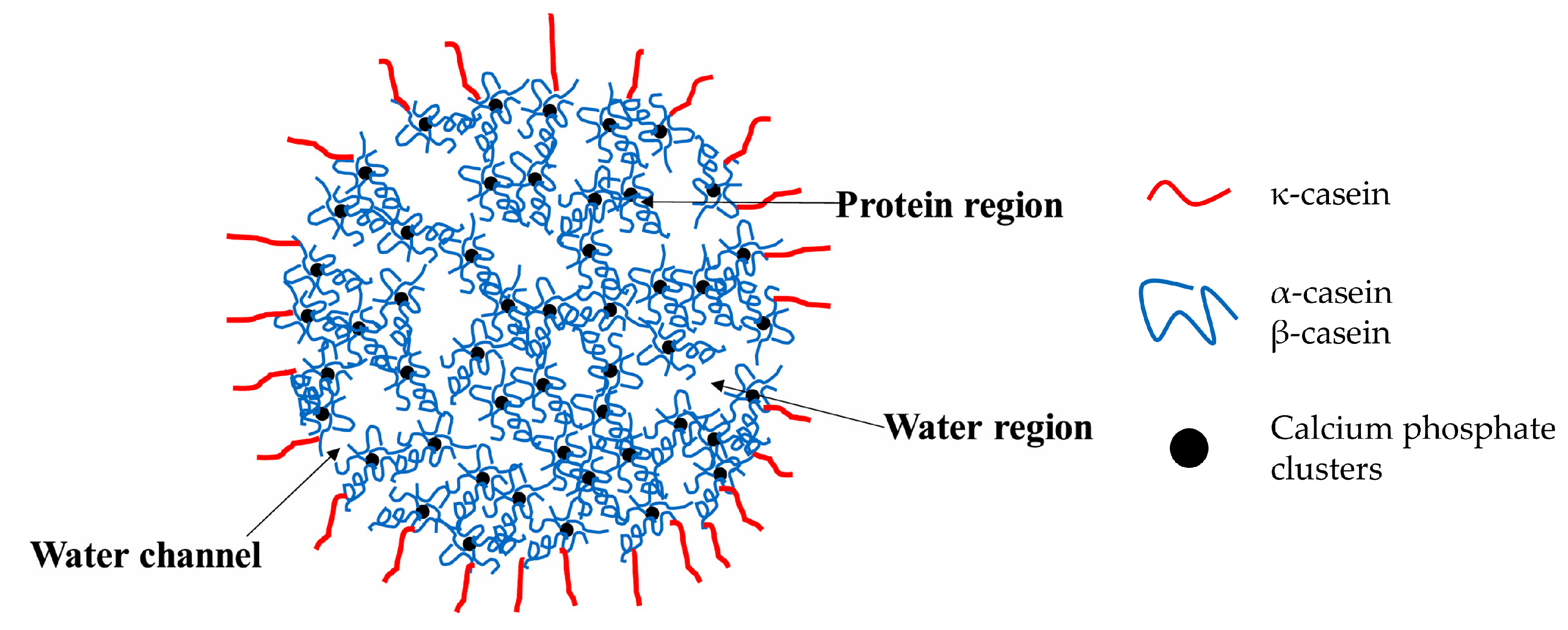
4.1.1. Casein
Ex Situ Addition
In Situ Addition
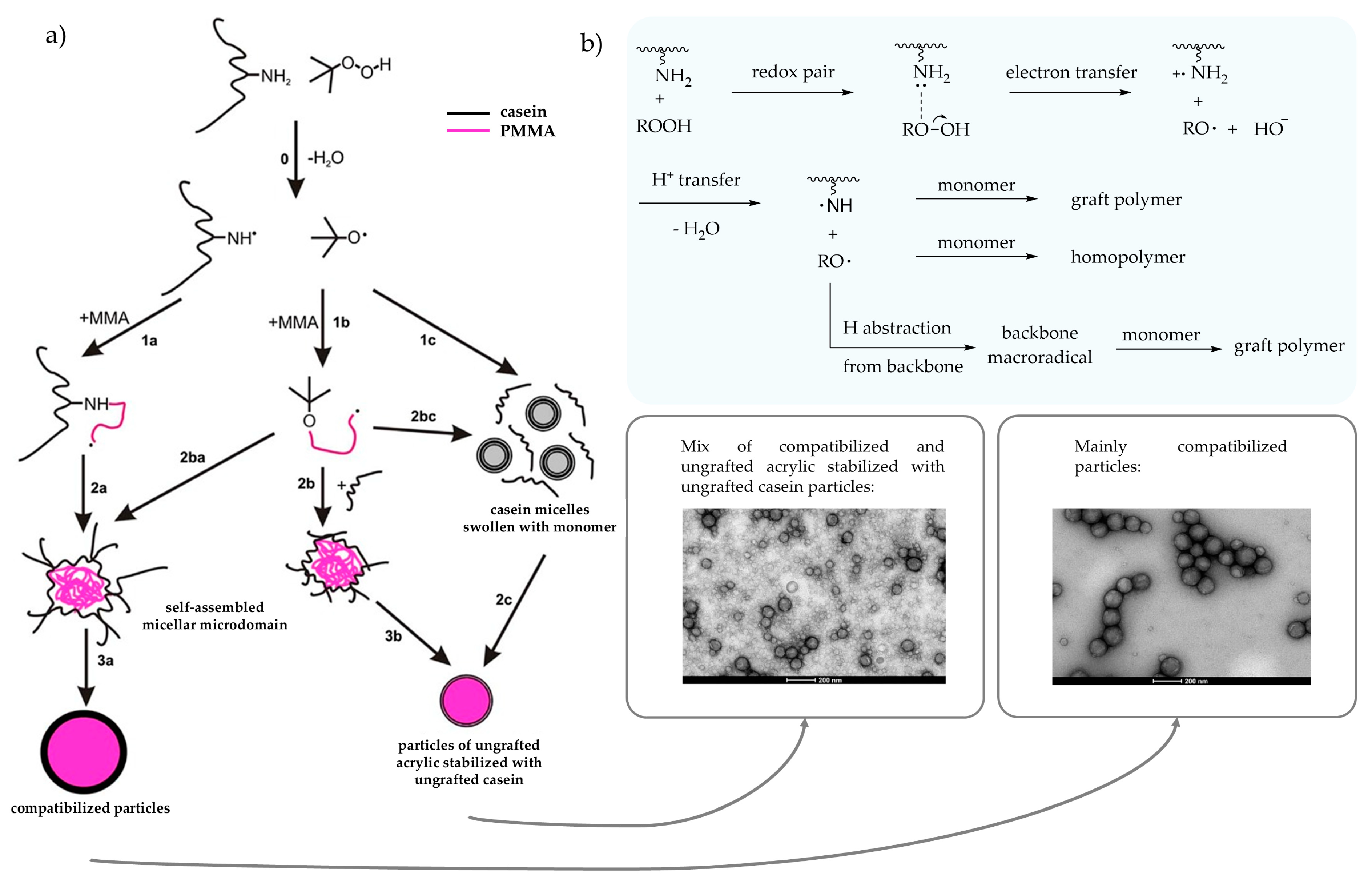
Use of Radical Initiators
Redox Pair with Amino Group from Casein
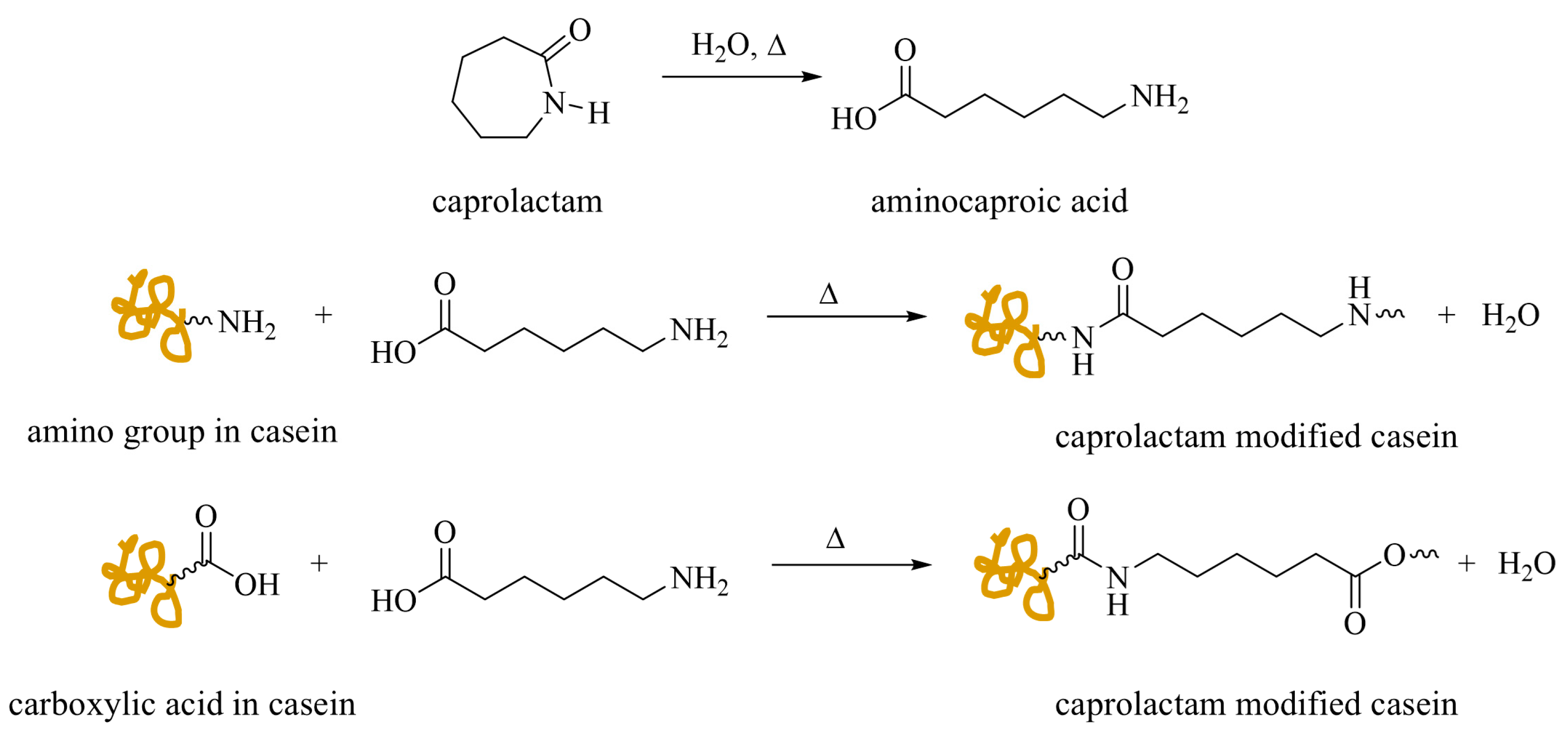
Casein–Caprolactam
Methacrylated Casein
Properties and Applications
4.1.2. Soy Protein
Ex Situ Addition
In Situ Addition
Properties and Applications
4.1.3. Collagen
Ex Situ Addition
In Situ Addition
Properties and Applications
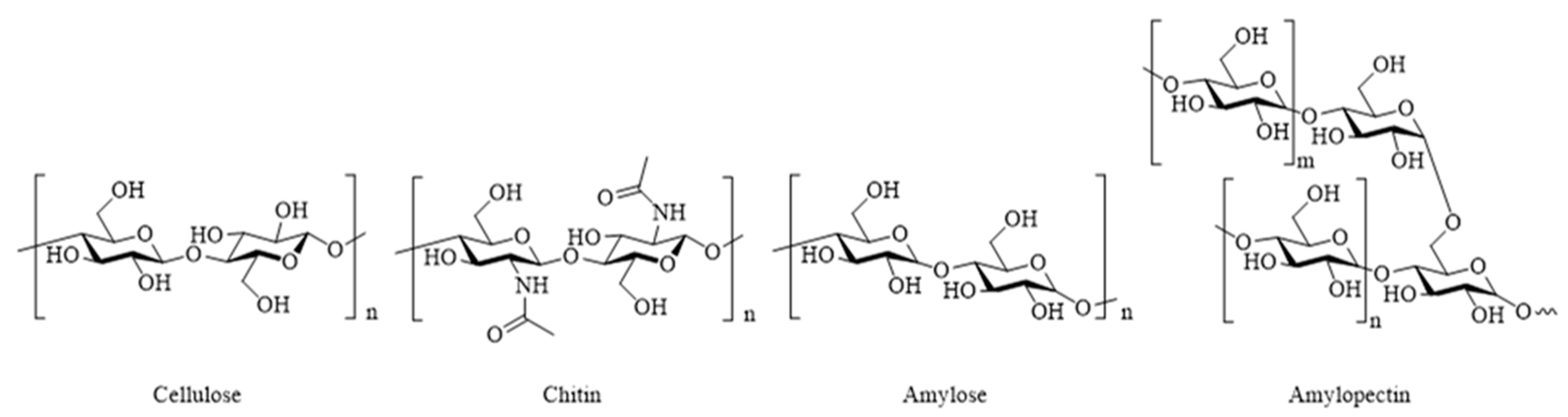
4.2. Polysaccharides
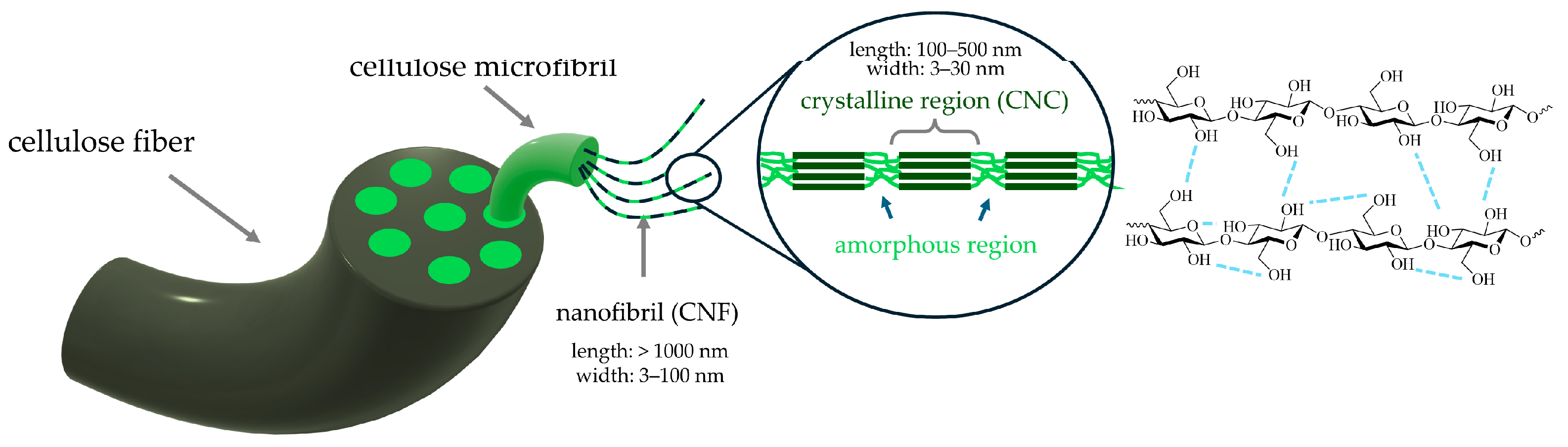
4.2.1. Cellulose and Nanocellulose
Ex Situ Addition
In Situ Addition
Properties and Applications
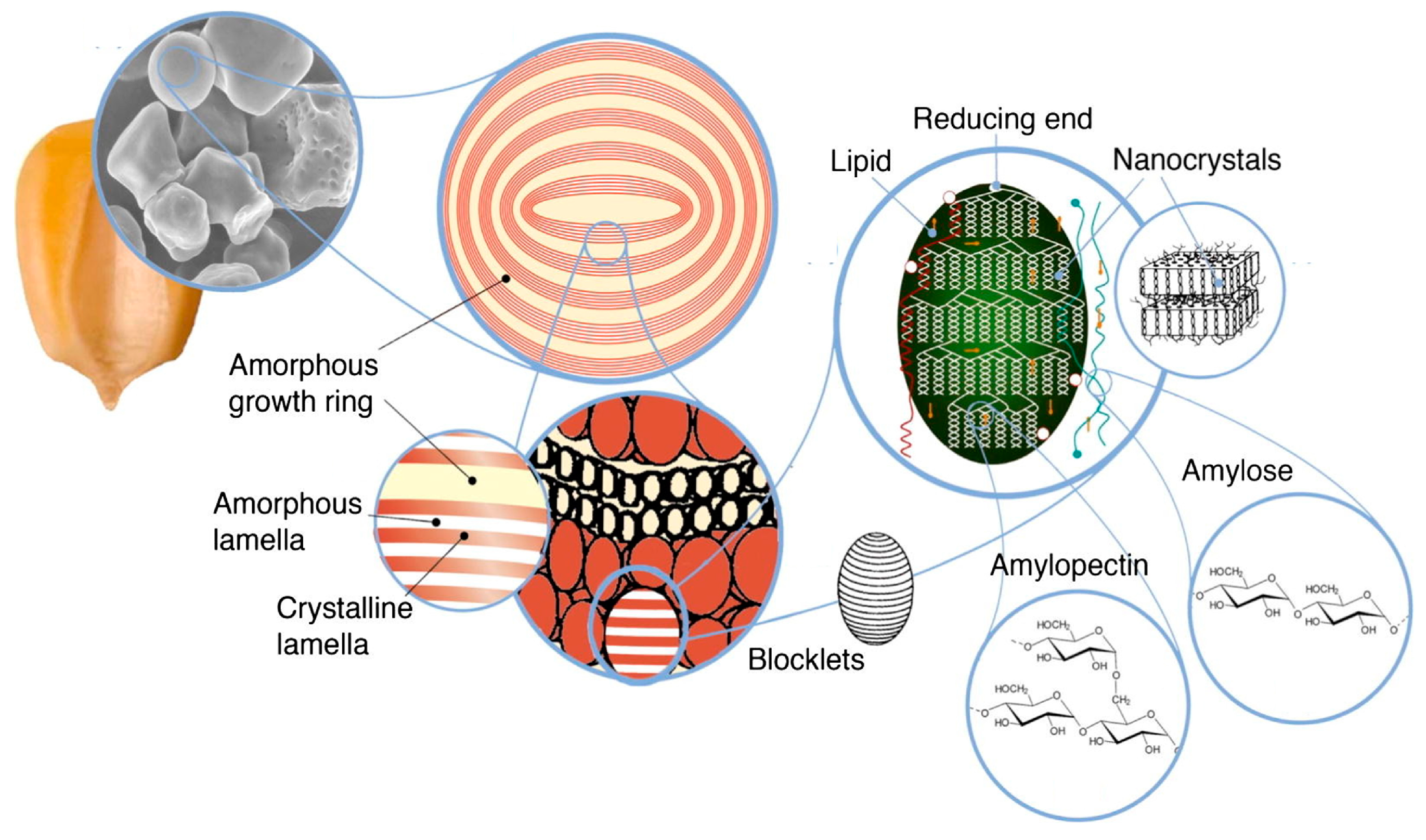
4.2.2. Starch
Ex Situ Addition
In Situ Addition
Properties and Applications
4.2.3. Chitin and Chitosan
Ex Situ Addition
In Situ Addition
Properties and Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2EHA | 2-ethylhexyl acrylate |
| 2OA | 2-octyl acrylate |
| AA | acrylic acid |
| AAEM | acetoacetoxyethyl methacrylate |
| AAm | acrylamide |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ACPA | 4,4′-azobis(4-cyanovaleric acid) |
| AFM | atomic force microscopy |
| AIBA | 2,2′-azobis(2-methylpropionamidine) dihydrochloride |
| AIBN | 2,2′-azobis(2-methylpropionitrile) |
| APS | ammonium persulfate |
| BA | butyl acrylate |
| BMA | butyl methacrylate |
| BPO | benzoyl peroxide |
| BPs | bioparticles |
| CAB | cellulose acetate butyrate |
| ChNC | chitin nanocrystal |
| ChNF | chitin nanofibril |
| CHP | cumene hydroperoxide |
| CMA | 7-(2-methacryloyloxyethoxy)-4-methylcoumarin |
| CMC | carboxy methyl cellulose |
| CMPP | carboxymethyl chitosan–melamine polyphosphate complex |
| CNC | cellulose nanocrystal |
| CNF | cellulose nanofibril |
| CPL | caprolactam |
| DAAM | diacetone acrylamide |
| DFMA | dodecafluoroheptyl methacrylate |
| EA | ethyl acrylate |
| EF-EP | Emulsifier-free emulsion polymerization |
| EP | emulsion polymerization |
| GMA | glycidyl methacrylate |
| HBP | 4-hydroxybenzophenone |
| HC | hydrolyzed collagen |
| HEMA | 2-hydroxyehtyl methacrylate |
| HFBA | hexafluorobutyl acrylate |
| iBoA | isobornyl acrylate |
| IBOMA | isobornyl methacrylate |
| ItA | itaconic acid |
| KPS | potassium persulfate |
| LMA | lauryl methacrylate |
| LPO | dilauroyl peroxide |
| LS | sodium lignosulfonate |
| MAA | methacrylic acid |
| MCC | micellar casein concentrate |
| MEP | miniemulsion polymerization |
| MFFT | minimum film formation temperature |
| MHC | Methacrylated hydrolyzed collagen |
| MMA | methyl methacrylate |
| NaPS | sodium persulfate |
| nOA | n-octyl acrylate |
| o/w | oil-in-water |
| PSA | pressure sensitive adhesive |
| SNC | starch nanocrystal |
| SNP | starch nanoparticle |
| SPI | soy protein isolate |
| ST | styrene |
| TA | tannic acid |
| tBHP | tert-butyl hydroperoxide |
| TBP | tert-butyl peroxide |
| UPyMA | 2-Ureido-4[1H]-pyrimidinone methyl methacrylate |
| VA-044 | 2,2′-azobis [2-(2-imidazolin-2-yl)propane] dihydrochloride |
| VAc | vinyl acetate |
| Vi-PDMS | polydimethylsiloxanes containing vinyl groups |
| VOCs | volatile organic compounds |
References
- Penzel, E.; Ballard, N.; Asua, J.M. Polyacrylates. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–20. ISBN 978-3-527-30673-2. [Google Scholar]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N.; Guo, Z. Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications. ACS Omega 2021, 6, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Topçuoğlu, Ö.; Altinkaya, S.A.; Balköse, D. Characterization of Waterborne Acrylic Based Paint Films and Measurement of Their Water Vapor Permeabilities. Prog. Org. Coat. 2006, 56, 269–278. [Google Scholar] [CrossRef]
- Arjmandi, A.; Bi, H.; Nielsen, S.U.; Dam-Johansen, K. From Wet to Protective: Film Formation in Waterborne Coatings. ACS Appl. Mater. Interfaces 2024, 16, 58006–58028. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Egan, L. Applications in the Adhesives and Construction Industries. In Polymer Dispersions and Their Industrial Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 191–252. ISBN 978-3-527-60058-8. [Google Scholar]
- Ballard, N. Designing Acrylic Latexes for Pressure-Sensitive Adhesives: A Review. Polym. Int. 2024, 73, 75–87. [Google Scholar] [CrossRef]
- Podgornik, B.B.; Šandrić, S.; Kert, M. Microencapsulation for Functional Textile Coatings with Emphasis on Biodegradability—A Systematic Review. Coatings 2021, 11, 1371. [Google Scholar] [CrossRef]
- Ramos-Fernández, J.M.; Guillem, C.; Lopez-Buendía, A.; Paulis, M.; Asua, J.M. Synthesis of Poly-(BA-Co-MMA) Latexes Filled with SiO2 for Coating in Construction Applications. Prog. Org. Coat. 2011, 72, 438–442. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile Chemical Products Emerging as Largest Petrochemical Source of Urban Organic Emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Gao, S.; Zhang, S.; Che, X.; Wang, Q.; Jiao, Z. Update on Volatile Organic Compound (VOC) Source Profiles and Ozone Formation Potential in Synthetic Resins Industry in China. Environ. Pollut. 2021, 291, 118253. [Google Scholar] [CrossRef]
- Waterborne Acrylic Resin Market Size, Share & Forecast 2034. Available online: https://www.gminsights.com/industry-analysis/waterborne-acrylic-resin-market (accessed on 24 February 2025).
- Lovell, P.A.; Schork, F.J. Fundamentals of Emulsion Polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- The Global Acrylic Market Is Accelerating Its Transfer to China—ECHEMI.Com. Available online: https://www.echemi.com/cms/603798.html (accessed on 8 April 2024).
- Gogin, L.L.; Zhizhina, E.G.; Pai, Z.P. Production of Methacrylic Acid and Metacrylates. Catal. Ind. 2021, 13, 125–131. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Fouilloux, H.; Thomas, C.M. Production and Polymerization of Biobased Acrylates and Analogs. Macromol. Rapid Commun. 2021, 42, 2000530. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre Di Crescenzo, M.; Zendri, E.; Sánchez-Pons, M.; Fuster-López, L.; Yusá-Marco, D.J. The Use of Waterborne Paints in Contemporary Murals: Comparing the Stability of Vinyl, Acrylic and Styrene-Acrylic Formulations to Outdoor Weathering Conditions. Polym. Degrad. Stab. 2014, 107, 285–293. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Influence of Acrylonitrile Content on the Adhesive Properties of Water-Based Acrylic Pressure-Sensitive Adhesives. Polymers 2022, 14, 909. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Vineeth, S.K. Synthesis and Characterization of Starch Stabilized Polyvinyl Acetate-Acrylic Acid Copolymer-Based Wood Adhesive. Polym. Bull. 2023, 80, 10335–10354. [Google Scholar] [CrossRef]
- Akarsu Dülgar, C.; Serhatlı, İ.E. Synthesis of Poly(BA-Co-MMA) Dispersions Having AA/MAA/AAm/MAAm Comonomers and the Comparison of Their Effect on Adhesive Performance. Polym. Bull. 2018, 75, 877–890. [Google Scholar] [CrossRef]
- Barandiaran, M.J.; de la Cal, J.C.; Asua, J.M. Emulsion Polymerization. In Polymer Reaction Engineering; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 233–272. ISBN 1405144424. [Google Scholar]
- Tomovska, R.; de la Cal, J.C.; Asua, J.M. Reactions in Heterogeneous Media. In Monitoring Polymerization Reactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 59–77. ISBN 978-1-118-73381-3. [Google Scholar]
- Distler, D. Emulsion Polymerization. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 2769–2774. ISBN 978-0-08-043152-9. [Google Scholar]
- Manea, M.; Chemtob, A.; Paulis, M.; de la Cal, J.C.; Barandiaran, M.J.; Asua, J.M. Miniemulsification in High-Pressure Homogenizers. AIChE J. 2008, 54, 289–297. [Google Scholar] [CrossRef]
- Ugelstad, J.; Kaggerud, K.H.; Hansen, F.K.; Berge, A. Absorption of Low Molecular Weight Compounds in Aqueous Dispersions of Polymer-Oligomer Particles, 2. A Two Step Swelling Process of Polymer Particles Giving an Enormous Increase in Absorption Capacity. Die Makromol. Chem. 1979, 180, 737–744. [Google Scholar] [CrossRef]
- Asua, J.M. Ostwald Ripening of Reactive Costabilizers in Miniemulsion Polymerization. Eur. Polym. J. 2018, 106, 30–41. [Google Scholar] [CrossRef]
- Ugelstad, J.; El-Aasser, M.S.; Vanderhoff, J.W. Emulsion Polymerization: Initiation of Polymerization in Monomer Droplets. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 503–513. [Google Scholar] [CrossRef]
- Capek, I.; Chern, C.-S. Radical Polymerization in Direct Mini-Emulsion Systems. In New Polymerization Techniques and Synthetic Methodologies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 101–165. ISBN 978-3-540-44473-2. [Google Scholar]
- Asua, J.M. Miniemulsion Polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Schork, F.J. In Support of Commercial Applications of Miniemulsion Polymerization. Ind. Eng. Chem. Res. 2023, 62, 2329–2335. [Google Scholar] [CrossRef]
- Lotierzo, A.; Bon, S.A.F. A Mechanistic Investigation of Pickering Emulsion Polymerization. Polym. Chem. 2017, 8, 5100–5111. [Google Scholar] [CrossRef]
- Colver, P.J.; Colard, C.A.L.; Bon, S.A.F. Multilayered Nanocomposite Polymer Colloids Using Emulsion Polymerization Stabilized by Solid Particles. J. Am. Chem. Soc. 2008, 130, 16850–16851. [Google Scholar] [CrossRef]
- Cauvin, S.; Colver, P.J.; Bon, S.A.F. Pickering Stabilized Miniemulsion Polymerization: Preparation of Clay Armored Latexes. Macromolecules 2005, 38, 7887–7889. [Google Scholar] [CrossRef]
- Brunier, B.; Sheibat-Othman, N.; Chniguir, M.; Chevalier, Y.; Bourgeat-Lami, E. Investigation of Four Different Laponite Clays as Stabilizers in Pickering Emulsion Polymerization. Langmuir 2016, 32, 6046–6057. [Google Scholar] [CrossRef]
- Fielding, L.A.; Tonnar, J.; Armes, S.P. All-Acrylic Film-Forming Colloidal Polymer/Silica Nanocomposite Particles Prepared by Aqueous Emulsion Polymerization. Langmuir 2011, 27, 11129–11144. [Google Scholar] [CrossRef]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodríguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef]
- Ballard, N.; Urrutia, J.; Eizagirre, S.; Schäfer, T.; Diaconu, G.; de la Cal, J.C.; Asua, J.M. Surfactant Kinetics and Their Importance in Nucleation Events in (Mini)Emulsion Polymerization Revealed by Quartz Crystal Microbalance with Dissipation Monitoring. Langmuir 2014, 30, 9053–9062. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-Stabilized Pickering Emulsions and Their Applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef]
- Bon, S.A.F. The phenomenon of pickering stabilization: A basic introduction. In Particle-Stabilized Emulsions and Colloids: Formation and Applications; Royal Society of Chemistry Publishing: Cambridge, UK, 2014; pp. 1–7. ISBN 978-1-78262-014-3. [Google Scholar]
- Binks, B.P. Particles as Surfactants—Similarities and Differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Steward, P.A.; Hearn, J.; Wilkinson, M.C. An Overview of Polymer Latex Film Formation and Properties. Adv. Colloid Interface Sci. 2000, 86, 195–267. [Google Scholar] [CrossRef] [PubMed]
- Keddie, J.L. Film Formation of Latex. Mater. Sci. Eng. R Rep. 1997, 21, 101–170. [Google Scholar] [CrossRef]
- Felton, L.A. Mechanisms of Polymeric Film Formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Craig, D.Q.M. Monitoring Film Coalescence from Aqueous Polymeric Dispersions Using Atomic Force Microscopy: Surface Topographic and Nano-Adhesion Studies. Asian J. Pharm. Sci. 2020, 15, 104–111. [Google Scholar] [CrossRef]
- Lee, W.P.; Routh, A.F. Why Do Drying Films Crack? Langmuir 2004, 20, 9885–9888. [Google Scholar] [CrossRef]
- Liu, L.; Liang, W.; Zhang, Y.; Fu, Q. Nanoencapsulation in Polymeric Materials: Weaving Magical Coats for Microorganisms. Nano Today 2023, 52, 101973. [Google Scholar] [CrossRef]
- Getahun, M.J.; Kassie, B.B.; Alemu, T.S. Recent Advances in Biopolymer Synthesis, Properties, & Commercial Applications: A Review. Process Biochem. 2024, 145, 261–287. [Google Scholar] [CrossRef]
- Viora, L.; Tichané, T.; Nottelet, B.; Mouton, J.; Garric, X.; Van Den Berghe, H.; Coudane, J. Casein-Based Conjugates and Graft Copolymers. Synthesis, Properties, and Applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13306. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening Improvement to a Soybean Meal-Based Bioadhesive Using an Interpenetrating Acrylic Emulsion Network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- Meganaharshini, M.; Sudhakar, V.; Dhivya Bharathi, N.; Deepak, S. Review on Recent Trends in the Application of Protein Concentrates and Isolates—A Food Industry Perspective. Food Humanit. 2023, 1, 308–325. [Google Scholar] [CrossRef]
- Southward, C.R. Casein and Caseinates|Uses in the Food Industry. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 948–958. ISBN 978-0-12-227055-0. [Google Scholar]
- Research, M.K. Cognitive Market Casein Protein Market Report 2025 (Global Edition). Available online: https://www.cognitivemarketresearch.com/casein-protein-market-report (accessed on 10 January 2025).
- Casein Market Size, Growth Report and Forecast to 2033. Available online: https://www.imarcgroup.com/casein-market (accessed on 10 January 2025).
- Casein (HS: 3501) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs/casein (accessed on 10 January 2025).
- Gelatin: Market Volume Worldwide. Available online: https://www.statista.com/statistics/712171/gelatin-market-volume-worldwide/ (accessed on 10 March 2025).
- Soy Protein Market Size|Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/global-soy-protein-market (accessed on 23 February 2025).
- Soy Protein Isolate Market Size, Share, Growth & Forecast. Available online: https://www.chemanalyst.com/industry-report/soy-protein-isolate-market-3119 (accessed on 23 February 2025).
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Chapter 2—Soy Protein: Impacts, Production, and Applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 23–45. ISBN 978-0-12-802778-3. [Google Scholar]
- Cencha, L.G.; Allasia, M.; Ronco, L.I.; Luque, G.C.; Picchio, M.L.; Minari, R.J.; Gugliotta, L.M. Proteins as Promising Biobased Building Blocks for Preparing Functional Hybrid Protein/Synthetic Polymer Nanoparticles. Ind. Eng. Chem. Res. 2021, 60, 4745–4765. [Google Scholar] [CrossRef]
- Braun, K.; Hanewald, A.; Vilgis, T.A. Milk Emulsions: Structure and Stability. Foods 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 676–682. ISBN 978-0-12-384953-3. [Google Scholar]
- Rennet: What Is and How Is It Made? Read the Caglificio Clerici’s Guide|Caglificio Clerici 2022. Available online: https://www.caglificioclerici.com/en/rennet-what-is-how-is-it-made-caglificio-clerici-guide/ (accessed on 31 January 2025).
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation and Potential Applications of Casein–Polysaccharide Conjugates: A Review. J. Sci. Food Agric. 2020, 100, 1852–1859. [Google Scholar] [CrossRef]
- Hammam, A.R.A.; Martínez-Monteagudo, S.I.; Metzger, L.E. Progress in Micellar Casein Concentrate: Production and Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4426–4449. [Google Scholar] [CrossRef]
- Carter, B.G.; Cheng, N.; Kapoor, R.; Meletharayil, G.H.; Drake, M.A. Invited Review: Microfiltration-Derived Casein and Whey Proteins from Milk. J. Dairy Sci. 2021, 104, 2465–2479. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the Structural Models of Bovine Casein Micelles—Review and Possible Improvements. Soft Matter. 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Improved Encapsulation Capacity of Casein Micelles with Modified Structure. J. Food Eng. 2022, 333, 111138. [Google Scholar] [CrossRef]
- Melnychyn, P.; Wolcott, J.M. Simple Procedure for Isolation of Alphas-Casein1. J. Dairy Sci. 1967, 50, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Zittle, C.A.; Custer, J.H. Purification and Some of the Properties of αs-Casein and κ-Casein. J. Dairy Sci. 1963, 46, 1183–1188. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-Casein: Isolation, Properties and Functionality. A Review. Int. Dairy J. 2017, 66, 115–125. [Google Scholar] [CrossRef]
- Huppertz, T.; Hennebel, J.-B.; Considine, T.; Shakeel-Ur-Rehman; Kelly, A.L.; Fox, P.F. A Method for the Large-Scale Isolation of β-Casein. Food Chem. 2006, 99, 45–50. [Google Scholar] [CrossRef]
- Qiang, X.H.; Xue, Q.; Zhang, H.; Yan, Z.; Li, M.; Xu, W.; Wang, Y.J. Preparation and Characterization of Acrylic Resin/Protein Composite Crosslinked Films. J. Coat Technol. Res. 2014, 11, 923–931. [Google Scholar] [CrossRef]
- Liu, M.; Damodaran, S. Effect of Transglutaminase-Catalyzed Polymerization of β-Casein on Its Emulsifying Properties. J. Agric. Food Chem. 1999, 47, 1514–1519. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.-M.; Haertlé, T. Improvement of Solubility and of Emulsifying Properties of Milk Proteins at Acid pHs by Esterification. Food/Nahrung 2001, 45, 87–93. [Google Scholar] [CrossRef]
- Mohan, D.; Radhakrishnan, G.; Nagabhushanam, T. Synthesis of Casein–g–Poly(Butyl Acrylate). J. Appl. Polym. Sci. 1980, 25, 1799–1806. [Google Scholar] [CrossRef]
- Mohan, D.; Radhakrishnan, G.; Rajadurai, S. Synthesis of Casein-g-Poly(n-Butyl Methacrylate). J. Macromol. Sci. Part A-Chem. 1985, 22, 75–83. [Google Scholar] [CrossRef]
- Mohan, D.; Radhakrishnan, G.; Rajadurai, S. Synthesis of Casein-g-Poly(Methyl Acrylate). J. Macromol. Sci. Part A-Chem. 1983, 20, 201–212. [Google Scholar] [CrossRef]
- Mohan, D.; Radhakrishnan, G.; Rajadurai, S. Synthesis of Casein-g-Poly(Methyl Acrylate). II. J. Appl. Polym. Sci. 1990, 39, 1507–1518. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P. Synthesis and Characterization of Poly(Methyl Methacrylate)/Casein Nanoparticles with a Well-Defined Core-Shell Structure. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3346–3353. [Google Scholar] [CrossRef]
- Li, P.; Zhu, J.; Sunintaboon, P.; Harris, F.W. New Route to Amphiphilic Core−Shell Polymer Nanospheres: Graft Copolymerization of Methyl Methacrylate from Water-Soluble Polymer Chains Containing Amino Groups. Langmuir 2002, 18, 8641–8646. [Google Scholar] [CrossRef]
- Picchio, M.L.; Minari, R.J.; Gonzalez, V.D.G.; Passeggi, M.C.G., Jr.; Vega, J.R.; Barandiaran, M.J.; Gugliotta, L.M. Waterborne Acrylic-Casein Nanoparticles. Nucleation and Grafting. Macromol. Symp. 2014, 344, 76–85. [Google Scholar] [CrossRef]
- Picchio, M.L.; Ronco, L.I.; Passeggi, M.C.G.; Minari, R.J.; Gugliotta, L.M. Poly(n-Butyl Acrylate)–Casein Nanocomposites as Promising Candidates for Packaging Films. J. Polym. Environ. 2018, 26, 2579–2587. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, J.; Zhou, J.; Wang, Y.; Zhang, J. Bio-Based Core–Shell Casein-Based Silica Nano-Composite Latex by Double-in Situ Polymerization: Synthesis, Characterization and Mechanism. Chem. Eng. J. 2013, 228, 281–289. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Q.; Zhou, J.; Gao, D.; Zhang, J.; Chen, L. Nano-Scale Core–Shell Structural Casein Based Coating Latex: Synthesis, Characterization and Its Biodegradability. Prog. Org. Coat. 2013, 76, 1346–1355. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, F.; Qiao, Y.; Xu, Q.; Zhou, J.; Zhang, J. Vi-PDMS Incorporated with Protein-Based Coatings Designed for Permeability-Enhanced Applications. J. Appl. Polym. Sci. 2018, 135, 46501. [Google Scholar] [CrossRef]
- Ma, J.; Gan, C.; Xu, Q.; Zhou, J.; Zhang, J. Amphiphilic Copolymer Stabilized Core–Shell Structural Casein-Based Emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 65–72. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Q.; Gao, D.; Zhou, J.; Zhang, J. Blend Composites of Caprolactam-Modified Casein and Waterborne Polyurethane for Film-Forming Binder: Miscibility, Morphology and Properties. Polym. Degrad. Stab. 2012, 97, 1545–1552. [Google Scholar] [CrossRef]
- Picchio, M.L.; Passeggi, M.C.G.; Barandiaran, M.J.; Gugliotta, L.M.; Minari, R.J. Acrylic/Casein Latexes with Controlled Degree of Grafting and Improved Coating Performance. Prog. Org. Coat. 2016, 101, 587–596. [Google Scholar] [CrossRef]
- Allasia, M.; Aguirre, M.; Gugliotta, L.M.; Minari, R.J.; Leiza, J.R. High Biobased Content Waterborne Latexes Stabilized with Casein. Prog. Org. Coat. 2022, 168, 106870. [Google Scholar] [CrossRef]
- Cencha, L.G.; Allasia, M.; Passeggi, M.C.G.; Gugliotta, L.M.; Minari, R.J. Formulation of Self-Crosslinkable Hybrid Acrylic/Casein Latex by Tannic Acid. Prog. Org. Coat. 2021, 159, 106413. [Google Scholar] [CrossRef]
- Picchio, M.L.; Bohórquez, S.J.; van den Berg, P.G.C.A.; Barandiaran, M.J.; Gugliotta, L.M.; Minari, R.J. Waterborne Casein-Based Latexes with High Solids Content and Their High-Throughput Coating Optimization. Ind. Eng. Chem. Res. 2016, 55, 10271–10277. [Google Scholar] [CrossRef]
- Picchio, M.L.; Passeggi, M.C.G.; Barandiaran, M.J.; Gugliotta, L.M.; Minari, R.J. Waterborne Acrylic–Casein Latexes as Eco-Friendly Binders for Coatings. Prog. Org. Coat. 2015, 88, 8–16. [Google Scholar] [CrossRef]
- Liu, J.; Tian, X.; Sun, J.; Yuan, Y. Preparation of Poly(Methyl Methacrylate-Co-Butyl Methacrylate) Nanoparticles and Their Reinforcing Effect on Natural Rubber. J. Appl. Polym. Sci. 2016, 133, 43843. [Google Scholar] [CrossRef]
- Allasia, M.; Mancilla, A.; Ronco, L.I.; Passeggi, M.C.G., Jr.; Gugliotta, L.M.; Minari, R.J. Efficient Incorporation of Protein into Waterborne Hybrid Acrylic Based Nanoparticles. Prog. Org. Coat. 2024, 188, 108171. [Google Scholar] [CrossRef]
- Kisku, S.K.; Swain, S.K. Poly(Methyl Methacrylate)/Soy Protein Green Composites as Gas Barrier Materials. Chin. J. Polym. Sci. 2012, 30, 397–404. [Google Scholar] [CrossRef]
- Lee, K.H.; Ryu, H.S.; Rhee, K.C. Protein Solubility Characteristics of Commercial Soy Protein Products. J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of Eco-Friendly Soy Protein Isolate Films with High Mechanical Properties through HNTs, PVA, and PTGE Synergism Effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; de la Caba, K. Mechanical and Thermal Properties of Soy Protein Films Processed by Casting and Compression. J. Food Eng. 2010, 100, 145–151. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Chu, F.; Wang, C.; Jin, C.; Wang, S.; Pang, J. Combinations of Soy Protein and Polyacrylate Emulsions as Wood Adhesives. Int. J. Adhes. Adhes. 2018, 82, 160–165. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Wang, C.; Chu, F.; Liu, X.; Pang, J. Fabrication of Soybean Protein-Acrylate Composite Mini-Emulsion toward Wood Adhesive. Eur. J. Wood Prod. 2018, 76, 305–313. [Google Scholar] [CrossRef]
- Su, J.; Cai, M.; Zhu, H.; Li, W.; Kang, P.; Xue, J.; Hu, W.; Li, D.; Wei, S.; Gao, Z. Self-Initiated UV-Curable Polyacrylate Using Soybean Isolate as Hydrogen Donor. Prog. Org. Coat. 2023, 174, 107238. [Google Scholar] [CrossRef]
- Feng, B.; Wang, D.; Li, Y.; Qian, J.; Yu, C.; Wang, M.; Luo, D.; Wei, S. Mechanical Properties of a Soy Protein Isolate–Grafted–Acrylate (SGA) Copolymer Used for Wood Coatings. Polymers 2020, 12, 1137. [Google Scholar] [CrossRef]
- Zhang, S.C.; Dong, L.N.; Pang, J.Y.; Sun, C. The Research of Modified-Soy-Protein—Acrylate Hybrid Emulsion. Adv. Mater. Res. 2010, 113–116, 1818–1823. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Al Hajj, W.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. Hydrolyzed Collagen: Exploring Its Applications in the Food and Beverage Industries and Assessing Its Impact on Human Health—A Comprehensive Review. Heliyon 2024, 10, e36433. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Li, Y.; Pan, J.; Liu, F.; Dai, H.; Fu, Y.; Huang, T.; Farooq, S.; Zhang, H. Collagen and Gelatin: Structure, Properties, and Applications in Food Industry. Int. J. Biol. Macromol. 2024, 254, 128037. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, D.; Chatterji, P.R. Characteristics of Heterophase Graft Copolymerizations. J. Macromol. Sci. —Pure Appl. Chem. 1992, 29, 123–135. [Google Scholar] [CrossRef]
- Wang, X.C.; Hao, X.L.; Qiang, T.T. Preparation and Sizing Performance of the Modified Collagen Surface Sizing Agent. Appl. Mech. Mater. 2012, 271–272, 367–371. [Google Scholar] [CrossRef]
- Wang, X.; Shang, Y.; Ren, L.; Zhang, S.; Guo, P. Preparation and Surface Sizing Application of Sizing Agent Based on Collagen from Leather Waste. BioResources 2015, 10, 7220–7231. [Google Scholar] [CrossRef]
- Luque, G.C.; Stürtz, R.; Passeggi, M.C.G.; Gugliotta, L.M.; Gonzalez, V.D.G.; Minari, R.J. New Hybrid Acrylic/Collagen Nanocomposites and Their Potential Use as Bio-Adhesives. Int. J. Adhes. Adhes. 2020, 100, 102624. [Google Scholar] [CrossRef]
- Luque, G.C.; Garcia, V.S.; Fontana, D.; Garay, E.; Rossini, L.; Passeggi, M.C.G.; Gugliotta, L.M.; Gonzalez, V.D.G.; Minari, R.J. Hybrid Acrylic-Modified Collagen Dispersions and Their Application as Bio-Adhesive with Acexamic Acid Release Capability. Int. J. Adhes. Adhes. 2024, 130, 103644. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, X.; You, X.; Zhang, H.J. Degradable Gelatin-Based Supramolecular Coating for Green Paper Sizing. ACS Appl. Mater. Interfaces 2021, 13, 1367–1376. [Google Scholar] [CrossRef]
- Global Wood Pulp Production 2022. Available online: https://www.statista.com/statistics/240570/consumption-and-production-of-fibrous-material-worldwide/ (accessed on 23 February 2025).
- C.M.I.P. Ltd. Cotton (Linter) Pulp Market Size, Trends and Forecast to 2030. Available online: https://www.coherentmarketinsights.com/market-insight/cotton-linter-pulp-market-5844 (accessed on 23 February 2025).
- Cellulose Market Size, Share, Growth|Industry Report 2032. Available online: https://www.fortunebusinessinsights.com/cellulose-market-102062 (accessed on 23 February 2025).
- Vilpoux, O.F.; Santos Silveira Junior, J.F. Chapter 3—Global Production and Use of Starch. In Starchy Crops Morphology, Extraction, Properties and Applications; Pascoli Cereda, M., François Vilpoux, O., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 43–66. ISBN 978-0-323-90058-4. [Google Scholar]
- Industrial Starch Market Size, Share & Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/industrial-starch-market-report (accessed on 20 January 2025).
- Chitin Market Size, Industry Share & Forecast to 2033. Available online: https://www.futuremarketinsights.com/reports/chitin-market (accessed on 21 February 2025).
- Weng, J.; Durand, A.; Desobry, S. Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics 2023, 15, 1455. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from Sources to Nanocellulose and an Overview of Synthesis and Properties of Nanocellulose/Zinc Oxide Nanocomposite Materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of Citrus Waste by Switching in the Production of Nanocellulose. IET Nanobiotechnol. 2016, 10, 395–399. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Zhou, Y.; Reshmy, R.; Philip, E.; Thomas, D.; Sindhu, R.; Bhargava, P.C.; Tiwari, A.; Ruiz, H.A.; Madhavan, A.; Pandey, A.; et al. Bacterial Nanocellulose: Optimized Synthesis and Biomedical Applications. Ind. Crops Prod. 2023, 205, 117589. [Google Scholar] [CrossRef]
- Varshney, V.K.; Naithani, S. Chemical Functionalization of Cellulose Derived from Nonconventional Sources. In Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. ISBN 978-3-642-17370-7. [Google Scholar]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent Advances in Nanocellulose Processing, Functionalization and Applications: A Review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Gabriel, V.A.; Dubé, M.A.; Cranston, E.D. Nanocellulose in Emulsions and Heterogeneous Water-Based Polymer Systems: A Review. Adv. Mater. 2021, 33, 2002404. [Google Scholar] [CrossRef]
- Chiromito, E.M.S.; Trovatti, E.; Carvalho, A.J.F. Thermoformable Fiberboards of Wood Pulp and Nanofibrillated Cellulose. Ind. Crops Prod. 2022, 187, 115433. [Google Scholar] [CrossRef]
- Yin, H.; Zhan, Y.; Bai, Y.; He, J.; Cheng, F.; Gao, H. Preparation of Acrylate Emulsions without Small Molecule Emulsifier Precipitation and Its Application in Battery Separators. Prog. Org. Coat. 2024, 195, 108669. [Google Scholar] [CrossRef]
- Bi, Y.; Pei, J.; Chen, Z.; Zhang, L.; Li, R.; Hu, D. Preparation and Characterization of Luminescent Road-Marking Paint. Int. J. Pavement Res. Technol. 2021, 14, 252–258. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Mehrpajouh, A.; Pishvaei, M. Compressive Strength of Acrylic Polymer-Stabilized Kaolinite Clay Modified with Different Additives. ACS Omega 2022, 7, 19204–19215. [Google Scholar] [CrossRef] [PubMed]
- Chruściel, J.J.; Olczyk, J.; Kudzin, M.H.; Kaczmarek, P.; Król, P.; Tarzyńska, N. Antibacterial and Antifungal Properties of Polyester, Polylactide, and Cotton Nonwovens and Fabrics, by Means of Stable Aqueous Dispersions Containing Copper Silicate and Some Metal Oxides. Materials 2023, 16, 5647. [Google Scholar] [CrossRef] [PubMed]
- Kroon, G. Associative Behavior of Hydrophobically Modified Hydroxyethyl Celluloses (HMHECs) in Waterborne Coatings. Prog. Org. Coat. 1993, 22, 245–260. [Google Scholar] [CrossRef]
- Movafagh, M.; Meek, K.M.; Bayat, P.; Cranston, E.D.; Cunningham, M.; Champagne, P.; Morse, T.; Kiriakou, M.; George, S.; Dubé, M.A. Improved Pressure-Sensitive Adhesive Performance Using Carboxylated Cellulose Nanocrystals via Blending. Polym. Eng. Sci. 2024, 64, 798–816. [Google Scholar] [CrossRef]
- Limousin, E.; Rafaniello, I.; Schäfer, T.; Ballard, N.; Asua, J.M. Linking Film Structure and Mechanical Properties in Nanocomposite Films Formed from Dispersions of Cellulose Nanocrystals and Acrylic Latexes. Langmuir 2020, 36, 2052–2062. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Al-nami, S.Y.; Alkhamis, K.; Al-Ahmed, Z.A.; Binyaseen, A.M.; Khalifa, M.E.; El-Metwaly, N.M. Simple Preparation of Long-Persistent Luminescent Paint with Superhydrophobic Anticorrosion Efficiency from Cellulose Nanocrystals and an Acrylic Emulsion. Ceram. Int. 2022, 48, 6363–6371. [Google Scholar] [CrossRef]
- Shankar, A.; AK, A.M.; Narayan, R.; Chakrabarty, A. Emulsion Polymerized Styrene Acrylic/Nanocellulose Composite Coating to Improve the Strength and Hydrophobicity of Kraft Paper. Prog. Org. Coat. 2023, 182, 107634. [Google Scholar] [CrossRef]
- Neelambaram, P.; Thounchiyath, T.; Narayan, R.; Chakrabarty, A. Cellulose Nanofiber-Incorporated High-Solid Siloxane Acrylic Latex by Mini-Emulsion Polymerization for Hydrophobic Coating and Wood Adhesive. Polym. Adv. Technol. 2023, 34, 3859–3869. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Liu, J.; Yang, K. Silane Modification of Nanofibrillated Cellulose and Its Effect on the Properties of Waterborne Acrylic Resin. J. Appl. Polym. Sci. 2023, 140, e54543. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Zhang, T.; Chen, Q.; Yang, D.; Qiu, F. Preparation of Vinyl Acetate/Acrylate Emulsion Modified with Carboxymethyl Cellulose and Fluorine for Paper Relic Protection. J. Dispers. Sci. Technol. 2022, 43, 804–813. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; Mahmoud, M.; Nada, A.A. Fabrication of Cellulose-Based Adhesive Composite as an Active Packaging Material to Extend the Shelf Life of Cheese. Int. J. Biol. Macromol. 2020, 160, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Moghbeli, M.R. Crosslinkable Latex-Based Acrylic Adhesives Containing Functionalized Cellulose Nanocrystals (fCNCs). Int. J. Adhes. Adhes. 2024, 132, 103700. [Google Scholar] [CrossRef]
- Chemtob, A.; Asua, J.M. Poly(ε-Caprolactone) and Cellulose Ester Hybrid Nanoparticles via Miniemulsion Polymerization. Colloid Polym. Sci. 2013, 291, 2503–2514. [Google Scholar] [CrossRef]
- Pakdel, A.S.; Cranston, E.D.; Dubé, M.A. Incorporating Hydrophobic Cellulose Nanocrystals inside Latex Particles via Mini-Emulsion Polymerization. Macromol. React. Eng. 2021, 15, 2100023. [Google Scholar] [CrossRef]
- Chen, R.; Chu, F.; Gauthier, C.; Chazeau, L.; Chaduc, I.; Bourgeat-Lami, E.; Lansalot, M. New Ethyl Cellulose/Acrylic Hybrid Latexes and Coatings via Miniemulsion Polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2329–2339. [Google Scholar] [CrossRef]
- Gabriel, V.A.; Champagne, P.; Cunningham, M.F.; Dubé, M.A. In-Situ Addition of Carboxylated Cellulose Nanocrystals in Seeded Semi-Batch Emulsion Polymerization. Can. J. Chem. Eng. 2022, 100, 767–779. [Google Scholar] [CrossRef]
- Saelices, C.J.; Save, M.; Capron, I. Synthesis of Latex Stabilized by Unmodified Cellulose Nanocrystals: The Effect of Monomers on Particle Size. Polym. Chem. 2019, 10, 727–737. [Google Scholar] [CrossRef]
- Errezma, M.; Mabrouk, A.B.; Magnin, A.; Dufresne, A.; Boufi, S. Surfactant-Free Emulsion Pickering Polymerization Stabilized by Aldehyde-Functionalized Cellulose Nanocrystals. Carbohydr. Polym. 2018, 202, 621–630. [Google Scholar] [CrossRef]
- Limousin, E.; Ballard, N.; Asua, J.M. Synthesis of Cellulose Nanocrystal Armored Latex Particles for Mechanically Strong Nanocomposite Films. Polym. Chem. 2019, 10, 1823–1831. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Wang, X. Photo-Responsive Cellulose Nanocrystal Modified Fluorinated Polyacrylate Based on Coumarin Chemistry. J. Appl. Polym. Sci. 2023, 140, e53757. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, S.; Zhou, M.; Lu, K.; Yu, J. Preparation of Light-Responsive Block Copolymer Modified Cellulose Nanocrystal@polydopamine Particle and Its Application in Fluorinated Polyacrylate Film. J. Appl. Polym. Sci. 2024, 141, e55272. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, T.; Li, Y.; Yuan, J. Self-Healing Cellulose Nanocrystals/Fluorinated Polyacrylate with Double Dynamic Interactions of Coumarin Groups and Multiple Hydrogen Bonds. Int. J. Biol. Macromol. 2024, 278, 134984. [Google Scholar] [CrossRef] [PubMed]
- Dogan-Guner, E.M.; Schork, F.J.; Brownell, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Encapsulation of Cellulose Nanocrystals into Acrylic Latex Particles via Miniemulsion Polymerization. Polymer 2022, 240, 124488. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Yao, H.; Li, H.; Li, X. Synthesis of Star-Shaped Nanocrystalline Cellulose/Fluorinated Polyacrylate via RAFT-Mediated Emulsion Polymerization and Its Application on Fabric Finishing. Fibers Polym. 2023, 24, 823–833. [Google Scholar] [CrossRef]
- Antoniw, J.M.; Gabriel, V.A.; Kiriakou, M.V.; Dubé, M.A.; Cunningham, M.F.; Cranston, E.D. Influence of Cellulose Nanocrystal Surface Chemistry and Dispersion Quality on Latex Nanocomposite Stability, Film Formation and Adhesive Properties. RSC Appl. Polym. 2024, 2, 262–274. [Google Scholar] [CrossRef]
- Bayat, P.; Meek, K.M.; Movafagh, M.; Cranston, E.D.; Cunningham, M.F.; Champagne, P.; Morse, T.; Kiriakou, M.V.; George, S.R.; Dubé, M.A. The Effect of Cellulose Nanocrystal Reassembly on Latex-Based Pressure-Sensitive Adhesive Performance. Biomacromolecules 2024, 25, 3018–3032. [Google Scholar] [CrossRef]
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch Production and Industrial Use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A Review of Starch, a Unique Biopolymer—Structure, Metabolism and in Planta Modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A Comprehensive Review on Starch: Structure, Modification, and Applications in Slow/Controlled-Release Fertilizers in Agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, Structure, Physicochemical and Functional Properties of Maize, Cassava, Wheat, Potato and Rice Starches. Starch Stärke 2015, 67, 14–29. [Google Scholar] [CrossRef]
- Haaj, S.B.; Magnin, A.; Boufi, S. Starch Nanoparticles Produced via Ultrasonication as a Sustainable Stabilizer in Pickering Emulsion Polymerization. RSC Adv. 2014, 4, 42638–42646. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, W.; Wu, Y. Optimization of Synthesis and Characterization of Oxidized Starch-Graft-Poly(Styrene-Butyl Acrylate) Latex for Paper Coating. Starch Stärke 2015, 67, 493–501. [Google Scholar] [CrossRef]
- Haaj, S.B.; Thielemans, W.; Magnin, A.; Boufi, S. Starch Nanocrystal Stabilized Pickering Emulsion Polymerization for Nanocomposites with Improved Performance. ACS Appl. Mater. Interfaces 2014, 6, 8263–8273. [Google Scholar] [CrossRef]
- Zhang, Y.; Cunningham, M.F.; Smeets, N.M.B.; Dubé, M.A. Starch Nanoparticle Incorporation in Latex-Based Adhesives. Eur. Polym. J. 2018, 106, 128–138. [Google Scholar] [CrossRef]
- Ben Ayed, E.; Magnin, A.; Putaux, J.-L.; Boufi, S. Vinyltriethoxysilane-Functionalized Starch Nanocrystals as Pickering Stabilizer in Emulsion Polymerization of Acrylic Monomers. Application in Nanocomposites and Pressure-Sensitive Adhesives. J. Colloid Interface Sci. 2020, 578, 533–546. [Google Scholar] [CrossRef]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch Modification for Non-Food, Industrial Applications: Market Intelligence and Critical Review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of Starch by Graft Copolymerization. Starch Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Cummings, S.; Zhang, Y.; Smeets, N.; Cunningham, M.; Dubé, M.A. On the Use of Starch in Emulsion Polymerizations. Processes 2019, 7, 140. [Google Scholar] [CrossRef]
- Mou, J.; Li, X.; Wang, H.; Fei, G.; Liu, Q. Preparation, Characterization, and Water Resistance of Cationic Acetylated Starch-g-Poly(Styrene-Butyl Acrylate) Surfactant-Free Emulsion. Starch Stärke 2012, 64, 826–834. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z.; Wu, M.; Zhao, P. Effect of Reaction Conditions on Grafting Ratio and Properties of Starch Nanocrystals-g-Polystyrene. J. Appl. Polym. Sci. 2014, 131, 40571. [Google Scholar] [CrossRef]
- Wang, R.-M.; Wang, X.-W.; Guo, J.-F.; He, Y.-F.; Jiang, M.-L. Crosslinkable Potato Starch-Based Graft Copolymer Emulsion for Humidity Controlling Coatings. Mat. Res. 2013, 16, 1246–1253. [Google Scholar] [CrossRef]
- Zhang, Y.; Cunningham, M.F.; Smeets, N.M.B.; Dubé, M.A. Increasing Starch Nanoparticle Content in Emulsion Polymer Latexes. Ind. Eng. Chem. Res. 2019, 58, 20987–20995. [Google Scholar] [CrossRef]
- Cabrera, S.F.; Ronco, L.I.; Passeggi, M.C.G., Jr.; Gugliotta, L.M.; Minari, R.J. The Role of Starch Incorporation into Waterborne Acrylic-Hybrid Nanoparticles for Film-Forming Applications. Biomacromolecules 2024, 25, 6591–6601. [Google Scholar] [CrossRef]
- Gabriel, V.A.; Dubé, M.A. Toward a Fully Biobased Pressure-Sensitive Adhesive. Ind. Eng. Chem. Res. 2023, 62, 478–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Cunningham, M.F.; Dubé, M.A. Modification of Adhesive and Latex Properties for Starch Nanoparticle-Based Pressure Sensitive Adhesives. Macromol. React. Eng. 2020, 14, 1900023. [Google Scholar] [CrossRef]
- Xu, J.; Long, L.; Hu, H. Preparation of Starch-Based Styrene Acrylate Emulsion Used as Surface-Treatment Agent for Decorative Base Paper. J. Polym. Eng. 2013, 33, 323–330. [Google Scholar] [CrossRef]
- Rajabi-Abhari, A.; Shen, Z.; Oh, K.; Im, W.; Kwon, S.; Lee, S.; Lee, H.L. Development and Application of Nanosized Polymer-Stabilized Cobinders and Their Effect on the Viscoelastic Properties and Foaming Tendencies of Coating Colors. ACS Omega 2020, 5, 9291–9300. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Liu, J.; Li, H.; Qu, W.; Cheng, J.; Wang, D. Synthesis of Cationic Starch Compound Emulsion by Graft Copolymerization with Tea Polyphenols and AEM5700. Starch Stärke 2024, 76, 2200200. [Google Scholar] [CrossRef]
- Cabrera, S.F.; Pighin, A.; Chiana, M.L.; Passeggi, M.C.G., Jr.; Ruano, G.D.; Gugliotta, L.M.; Ronco, L.I.; Minari, R.J. Synergistic Combination between Starch and Proteins in the Synthesis of New Acrylic/Biopolymers Hybrid Latexes. J. Polym. Sci. 2022, 60, 3420–3434. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Muñoz-Núñez, C.; Fernández-García, M.; Muñoz-Bonilla, A. Chitin Nanocrystals: Environmentally Friendly Materials for the Development of Bioactive Films. Coatings 2022, 12, 144. [Google Scholar] [CrossRef]
- Pandey, R.; Mathur, G. Current Trends in Chitosan Functionalization Methods and Their Applications. Starch Stärke 2025, 77, 2300248. [Google Scholar] [CrossRef]
- Abiraman, T.; Kavitha, G.; Rengasamy, R.; Balasubramanian, S. Antifouling Behavior of Chitosan Adorned Zinc Oxide Nanorods. RSC Adv. 2016, 6, 69206–69217. [Google Scholar] [CrossRef]
- Xia, J.L.; Wang, C.; Nie, Z.Y.; Peng, A.A.; Guan, X. Structure, Properties and Application to Water-Soluble Coatings of Complex Antimicrobial Agent Ag-Carboxymethyl Chitosan-Thiabendazole. J. Cent. South Univ. Technol. 2005, 12, 526–530. [Google Scholar] [CrossRef]
- Abiraman, T.; Balasubramanian, S. Synthesis and Characterization of Large-Scale (<2 Nm) Chitosan-Decorated Copper Nanoparticles and Their Application in Antifouling Coating. Ind. Eng. Chem. Res. 2017, 56, 1498–1508. [Google Scholar] [CrossRef]
- Abiraman, T.; Ramanathan, E.; Kavitha, G.; Rengasamy, R.; Balasubramanian, S. Synthesis of Chitosan Capped Copper Oxide Nanoleaves Using High Intensity (30kHz) Ultrasound Sonication and Their Application in Antifouling Coatings. Ultrason. Sonochem. 2017, 34, 781–791. [Google Scholar] [CrossRef]
- Lai, M.; Wang, Y.; Li, F.; Zhao, J. Sodium Lignosulfonate Improve the Flame Retardancy of Carboxymethyl Chitosan @ Melamine Polyphosphate-Containing Silicone Acrylic Emulsion-Based Coatings. J. Appl. Polym. Sci. 2024, 141, e55989. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Yu, K.; Li, F.; Xie, J.; Zhao, J. Silane Coupling Agent and Carboxymethyl Chitosan Co-Modified Boron Nitride for Preparing Silicone Acrylic Emulsion-Based Flame-Retarding Coatings. J. Appl. Polym. Sci. 2023, 140, e54286. [Google Scholar] [CrossRef]
- Torabi, S.; Mahdavian, A.R.; Sanei, M.; Abdollahi, A. Chitosan and Functionalized Acrylic Nanoparticles as the Precursor of New Generation of Bio-Based Antibacterial Films. Mater. Sci. Eng. C 2016, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Promlok, D.; Sonongbua, K.; Wilepsuwan, M.; Suteewong, T.; Tangboriboonrat, P. Hollow Natural Rubber Latex Particles as Bio-Based Alternative White Pigment for Coating Applications. Ind. Crops Prod. 2022, 188, 115593. [Google Scholar] [CrossRef]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Wang, J. Developing Chitin Nanocrystals for Flexible Packaging Coatings. Carbohydr. Polym. 2019, 226, 115276. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.; Wang, L. Cellulose or Chitin Nanofibril-Stabilized Latex for Medical Adhesion via Tailoring Colloidal Interactions. Carbohydr. Polym. 2022, 278, 118916. [Google Scholar] [CrossRef]
- Wada, T.; Yasuda, M.; Yako, H.; Matoba, Y.; Uragami, T. Preparation and Characterization of Hybrid Quaternized Chitosan/Acrylic Resin Emulsions and Their Films. Macromol. Mater. Eng. 2007, 292, 147–154. [Google Scholar] [CrossRef]
- Wada, T.; Uragami, T.; Matoba, Y. Chitosan-Hybridized Acrylic Resins Prepared in Emulsion Polymerizations and Their Application as Interior Finishing Coatings. J. Coat. Technol. Res. 2005, 2, 577–592. [Google Scholar] [CrossRef]
- Wada, T.; Uragami, T. Preparation and Characterization of Hybrid Chitosan/Acrylic Resin Emulsions and Their Films. Macromol. Mater. Eng. 2006, 291, 809–819. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Chitosan-Based Pickering Emulsion: A Comprehensive Review on Their Stabilizers, Bioavailability, Applications and Regulations. Carbohydr. Polym. 2023, 304, 120491. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Sahoo, P.K. Synthesis and Study of Thermal, Mechanical and Biodegradation Properties of Chitosan-g-PMMA with Chicken Egg Shell (Nano-CaO) as a Novel Bio-Filler. Mater. Sci. Eng. C 2017, 80, 149–155. [Google Scholar] [CrossRef]
- Shalbafan, A.; Hassannejad, H.; Rahmaninia, M. Formaldehyde Adsorption Capacity of Chitosan Derivatives as Bio-Adsorbents for Wood-Based Panels. Int. J. Adhes. Adhes. 2020, 102, 102669. [Google Scholar] [CrossRef]
- Le Roux, I.; Krieg, H.M.; Yeates, C.A.; Breytenbach, J.C. Use of Chitosan as an Antifouling Agent in a Membrane Bioreactor. J. Membr. Sci. 2005, 248, 127–136. [Google Scholar] [CrossRef]

| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| Ex situ | Casein Gelatin | 14.5–40 * | MMA/BA/AA/AAEM | Physical mixing | - | Crosslink agent | Coatings | [74] |
| In situ | Casein | 3–16 × 10−4 * mol casein/mol monomer | BA MA EMA | Batch EF-EP | KPS KPS/Ascorbic acid | Colloidal stabilizer Additive | Leather treatment | [77,78,79,80] |
| In situ | Casein Gelatin Chitosan | 25 * | MMA | Batch EF-EP | tBHP | Colloidal stabilizer | Nanoparticle design | [82] |
| In situ | Casein | 17–100 * | MMA | Batch EF-EP | tBHP, KPS, AAPH, AIBN, BPO, TBP, CHP | Colloidal stabilizer | Nanoparticle design | [81] |
| In situ | Casein | 3–50 | MMA | Batch EF-EP | tBHP | Colloidal stabilizer | Coatings | [83] |
| In situ | Casein | 3–50 | BA/MMA | Batch EF-EP | tBHP | Colloidal stabilizer Additive Improve biodegradability | Binder | [94] |
| In situ | Casein | 20 * | MMA/BMA | Batch EF-EP | tBHP | Colloidal stabilizer Phase compatibilizer | Dipped products | [95] |
| In situ | Casein | 3–50 | BA | Batch EF-EP | tBHP | Colloidal stabilizer Additive Improve biodegradability | Food packaging | [84] |
| In situ | Casein-CPL | 67 * | BA/MMA | EF-EP | APS | Colloidal stabilizer | Coatings | [85] |
| In situ | Casein-CPL | 70 * | BA | Batch EF-EP | APS KPS APS/NaHSO3 KPS/NaHSO3 | Colloidal stabilizer Improve biodegradability | Binder Leather treatment | [86] |
| In situ | Casein-CPL | 67 * | BA/MMA/VAc | Batch EF-EP | APS | Colloidal stabilizer | Ink binder | [88] |
| In situ | Casein-CPL | 140 * | BA/MMA/Vi-PDMS | Semi-batch EF-EP | APS | Colloidal stabilizer | Adhesive | [87] |
| Textile | ||||||||
| Food packaging | ||||||||
| In situ | Methacrylated casein | 25 | BA/MMA | Batch EF-EP | tBHP | Colloidal stabilizer Additive | Coatings | [90] |
| In situ | Methacrylated casein | 25–50 | BA/MMA | Batch EF-EP | tBHP | Colloidal stabilizer Additive | Paints | [93] |
| In situ | Casein Methacrylated casein | 25 | IBOMA/2OA/MMA/BA | Batch EF-EP | tBHP | Colloidal stabilizer | Coatings | [91] |
| In situ | Methacrylated casein | 25 | BA/MMA | EF-EP | KPS | Colloidal stabilizer Additive | Coatings | [92] |
| In situ | Casein/zein | 20–60 | BA/MMA | Batch EF-EP Semi-batch EF-EP | KPS | Colloidal stabilizer Additive | Coatings | [96] |
| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| Ex situ | Soy meal | 600–3000 * | Commercial acrylic emulsion | Physical mixing | - | Structural component | Adhesive (wood) | [49] |
| Ex situ | Modified SPI | 0–900 * | BA/MMA | Physical mixing | - | Structural component | Adhesive (plywood) | [101] |
| In situ | Soy protein | 1–5 | PMMA | Batch EF-EP | KPS | Oxygen barrier Flame retardant Improve biodegradability | Packaging | [97] |
| In situ | Modified soy protein | 1.8 * | BA/VAc/MMA/AA | EP | APS | Reinforcement | Adhesive (wood) | [105] |
| In situ | Soy protein Modified soy protein | 1.3–6.3 * | BA/MMA | Batch MEP | APS | Additive Protective colloid | Adhesive (plywood) | [102] |
| In situ | SPI | 4–5.5 * | BA/MMA/AAEM | Semi-batch EP | APS | Additive UV curing agent | UV curing coatings (wood) | [103] |
| In situ | SPI | 2.6–15.4 * | BA/MMA/AAEM | Semi-batch EP | APS | Reinforcement | Wood coatings | [104] |
| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| Ex situ | Casein Gelatin | 14.5–40 * | MMA/BA/AA/AAEM | Physical mixing | - | Crosslink agent | Coatings | [74] |
| In situ | Gelatin | 25–350 * | AA, AAm, MMA and VAc | EF-EP | KPS | Colloidal stabilizer | Not specified | [111] |
| In situ | Gelatin | 33.3–133.3 * | MA/VAc | Batch EF-EP | APS | Colloidal stabilizer Sizing agent | Paper | [112] |
| In situ | Gelatin | 166.7 * | MAA | Batch EF-EP | UV photoinitiator | Colloidal stabilizer Sizing agent Improve biodegradability | Paper | [116] |
| In situ | Collagen | 150 * | EA/ST | Batch EF-EP | APS | Colloidal stabilizer Sizing agent | Paper | [113] |
| In situ | HC | 15–50 | BA/AA | Batch EF-EP | H2O2 | Colloidal stabilizer Additive | Adhesives | [114] |
| In situ | Methacrylated HC | 25 | BA/AA | Batch EF-EP | KPS | Colloidal Stabilizer Additive | Adhesives | [115] |
| Linkage | Reaction | Derivatives |
|---|---|---|
| Ethers | Reaction with chloroalkanes (e.g., methyl chloride, monochloroacetic acid) previously treated in alkali media | methyl cellulose (MC) carboxymethyl cellulose (CMC) |
| Reaction with ethylene and propylene oxide previously treated in alkali media | hydroxyethyl cellulose (HEC) | |
| Reaction with epoxides | ||
| Esters | Acetylation by acetic anhydride in acetic acid media and catalyzed by sulfuric acid or perchloric acid | Cellulose acetate (CA) |
| Cellulose treated with nitric acid | Nitrocellulose | |
| Cellulose treated with reagents such as sulfuric acid, chlorosulfonic acid, or sulfur trioxide | Cellulose sulfate | |
| Reaction with acid anhydrides | ||
| Oxidized | TEMPO-mediated hypochlorite oxidation to introduce carboxylic groups | Oxidized cellulose |
| Silylated | Reaction with chlorosilanes | Silylated cellulose |
| Urethane | Reaction with isocyanates | Urethane cellulose |
| Grafting | Formation of polymer chains on cellulose backbone through free radical, reversible addition fragmentation chain transfer or atom-transfer radical polymerization route |
| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| Ex situ | Cellulose fiber CNF | 33.3–300 * | MMA | Physical mixing | - | Structural component | Fiberboards | [135] |
| Ex situ | CMC | Added as 0.5 wt% solution | BA/AA/ST | Physical mixing | - | Co-solvent Thickening agent | Adhesive/binder for battery separators | [136] |
| Ex situ | CMC | 0.5 wt% in formula | Commercial acrylic emulsion | Physical mixing | - | Anti-sediment agent | Paints (road marks) | [137] |
| Ex situ | CMC | 1–5 wt% in formula | BA/MMA | Physical mixing | - | Thickening agent | Soil treatment | [138] |
| Ex situ | HEC | 0.8–1.5 wt% in formula | Commercial acrylic latex | Physical mixing | - | Thickening agent | Textile | [139] |
| Ex Situ | Modified HEC | 0.2–0.8 wt% in formula | Commercial acrylic latex | Physical mixing | - | Thickening agent | Paints | [140] |
| Ex situ | CNC | 0.3–1 | Acrylic latex | Physical mixing | - | Reinforcement | Adhesives | [141] |
| Ex situ | CNC | 2.5–20 | BA/MMA | Physical mixing | - | Reinforcement | Coatings | [142] |
| Ex situ | CNC | 0.5 wt% in formula | Commercial acrylic latex | Physical mixing | - | Crosslinking and anticorrosion agent | Paint | [143] |
| Ex situ In situ | CNF | 0.5–1 0.25–1 | 2EHA/AA/ST | Physical mixing MEP | APS | Reinforcement | Paper packaging | [144] |
| Ex situ In situ | CNF | 0.5–1 0.5–1 | BA/MMA/AA | Physical mixing MEP | KPS | Reinforcement | Wood adhesives Fabric coatings | [145] |
| Ex situ | Modified CNF | 0.3 wt% based on latex | Commercial acrylic latex | Physical mixing | - | Reinforcement | Coatings | [146] |
| In situ | CMC | 7–9 * | BA/MMA/DFMA/VAc | Semi-batch EP | KPS | Reinforcement | Paper | [147] |
| In situ | HEC | - | AA/MBA | Batch EP | KPS | Reinforcement | Adhesive | [148] |
| In situ | CAB | 20–100 * | BA/MMA/AA | Batch MEP | KPS | Reinforcement Colloidal stabilizer | Nanoparticles design | [150] |
| In situ | EC | 5–20 | BA/MMA | Batch MEP | APS LPO | Reinforcement | Coatings | [152] |
| In situ | CNC | 1 | BA/MMA | Semi-batch EP | KPS/AIBN | Reinforcement | Adhesive | [153] |
| In situ | CNC | 10–30 | MMA/BA | Batch EF-EP | AIBA | Colloidal stabilizer Reinforcement | Coatings | [156] |
| In situ | CNC | 0.2–2.3 | iBoA, MMA, BMA, LMA, ST | PEP | AIBN ACPA | Colloidal stabilizer | Not specified | [154] |
| In situ | CNC | 1 | BA/MMA | Semi-batch EP | KPS | Reinforcement | Adhesive | [162] |
| In situ | CNC | 0–1.5 | 2EHA/MMA/ST | Semi-batch EP | NaPS | Reinforcement | Adhesive | [163] |
| In situ | Modified CNC | 0.5–1.5 | BA/MMA/HEMA | Batch EP | KPS | Reinforcement | Adhesives | [149] |
| In situ | Modified CNC | 0.5–1.5 | BA/AA/VAc | Batch MEP | KPS | Additive | Adhesive | [151] |
| In situ | Modified CNC | 0.5–10 | BMA | PEP | KPS/Na2S2O5 | Colloidal stabilizer Reinforcement | Binder Adhesive | [155] |
| In situ | Modified CNC | 0.4–1 * | BA/MMA/HFBA/CMA | PEP | KPS | Colloidal stabilizer photo-responsive agent Reinforcement | Textile | [157] |
| In situ | Modified CNC | 0.6–1.4 * | BA/SA/HFBA/MMA/UPyMA/CMA | PEP | APS | Colloidal stabilizer Reinforcement Self-healing agent | Textile Self-healing coatings | [158,159] |
| In situ | CNC Modified CNC | 0.5–1 | BA/MMA | Batch MEP | KPS | Reinforcement | Coatings | [160] |
| In situ | Modified CNC | 17 * | BA/MMA/HFBA | Semi-batch EF-EP | APS | Colloidal stabilizer Reinforcement | Textile | [161] |
| Type | Process | Modification |
|---|---|---|
| Chemical | Hydrolysis | The hydrolysis of glycosidic bonds occurs preferentially in amorphous regions, which are more susceptible than crystalline regions. This process is commonly carried out using mineral acids. |
| Oxidation | Oxidation of hydroxyl groups to form carbonyl and carboxyl groups using oxidizing agents. | |
| Crosslinking | Formation of covalent bonds between different starch chains by targeting hydroxyl groups. | |
| Esterification | Esterification of hydroxyl groups through treatment with organic and inorganic acids. | |
| Etherification | The formation of ether linkages from hydroxyl groups. Depending on the introduced functional group, cationic, anionic, amphoteric, or non-ionic starches can be obtained. | |
| Grafting | Formation of polymer chain covalent bonds attached to starch chains. There are several strategies that have previously been reviewed [175]. | |
| Physical | Thermal | Involves disrupting the starch intermolecular bonds in water and heat to produce pre-gelatinized and granular cold-water-swelling starches. |
| Non-thermal | Process such as milling, ultrasounds, microwave, pulsed electric field, freezing and thawing, and high pressure, which rearrange starch granules, altering their functional properties. |
| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| In situ | Acetylated cationic starch | 59–111 * | BA/ST | Semi-batch EF-EP | FeSO4/H2O2 | Colloidal stabilizer Structural component | Paper | [177] |
| In situ | Starch | 20–50 | BA/MMA/DAAM | Semi-batch EF-EP | KPS | Colloidal stabilizer Absorbent agent | Indoor coatings (humidity control) Paints | [179] |
| In situ | Starch | 2.8 * | AA/VAc | Semi-batch EP | APS | Filler (Increased bio-content) Crosslinkable functionality | Adhesive (wood) | [19] |
| In situ | Starch | 7–11 wt% based on formula | MMA/ST/cationic acrylate MMA/ST/BA MMA/ST/EA MMA/ST/2EHA | Semi-batch EF-EP | APS | Colloidal stabilizer | Paper | [184] |
| In situ | Starch | 50 * | BA/AA/ST | Semi-batch EF-EP | AAPH | Colloidal stabilizer | Paints | [185] |
| In situ | Starch | 67 * | BA/ST | Semi-batch EF-EP | FeSO4/H2O2 | Colloidal stabilizer Antibacterial | Antibacterial coatings | [186] |
| In situ | SNP | 2–10* | BMA | PEP | KPS | Colloidal stabilizer Reinforcement | Coatings Adhesives | [169] |
| In situ | Modified SNP | 15–60 wt% total mass of solids | BA/MMA/AA | Semi-batch EP | KPS | Filler (Increased bio-content) | Adhesive | [180] |
| In situ | Modified SNP CNC | 6–12 0.5–1 | nOA/AA/ST | Semi-batch EP | KPS | Filler (Increased bio-content) | Adhesives | [182] |
| In situ | Modified SNP | 15 | BA/MMA/AA | Semi-batch EP | KPS | Filler (Increased bio-content) | Adhesive | [183] |
| Ex situ In situ | SNC | 2–12 * | BMA | PEP | KPS | Colloidal stabilizer Reinforcement | Coatings Adhesives | [171] |
| In situ | SNC | 4–10 | BMA BMA/EHA | PEP | Citric acid/H2O2 | Colloidal stabilizer Reinforcement | Adhesives | [173] |
| In situ | Starch/zein | 25 * | BA/MMA | Semi-batch EP | KPS | Reinforcement | Binder | [181] |
| In situ | Casein Starch/zein | 5 11–25 * | BA/MMA | Batch EF-EP Semi-batch EF-EP | KPS | Colloidal stabilizer Reinforcement | Coatings Adhesives | [187] |
| Process | Modification |
|---|---|
| Deacetylation | Alkaline hydrolysis, generally, using sodium hydroxide at high temperatures [191]. |
| Carboxymethyl chitosan | O- and N-carboxymethylation of chitosan by sodium monochloracetate in presence of sodium hydroxide [189]. |
| Aminoethyl chitosan | Reaction with ethylene oxide. |
| Quaternized chitosan | Quaternary ammonium groups (-NR3+) added to the chitosan backbone, generally, by quaternary ammonium salts. |
| Thiolated chitosan | Thiol groups (-SH) are incorporated into the chitosan backbone, typically by thiolating agents. |
| Approach | Biopolymer | Acrylic Composition | Synthesis | Initiator | Role of Biopolymer | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Type | wt% Based on Monomer | |||||||
| Ex situ | Chitosan | 0.06 * wt% in formula | Commercial acrylic paint | Physical mixing | - | Capping agent | Antifouling coatings | [192,194,195] |
| Ex situ | Chitosan | 11.1–66.7 * | BA/MMA/GMA | Physical mixing | - | Antibacterial agent | Antibacterial films | [198] |
| Ex situ | Chitosan | 4.1 wt% based on total polymer | Natural rubber/acrylic hybrid latex | Physical mixing | - | Formaldehyde absorption | Paints | [199] |
| Ex situ | Carboxymethyl chitosan | 0.1 wt% in formula | Acrylic emulsion | Physical mixing | - | Chelating agent | Antibacterial and antifouling paints | [193] |
| Ex situ | Carboxymethyl chitosan | 0.5 * wt% in formula | Silicone acrylic emulsion | Physical mixing | - | Flame retardant | Coatings (wood) Paints (wood) | [196,197] |
| In situ | Quaterrnized chitosan | 5 wt% in solids | MMA/2EHA/AA DAAM | Semi-batch EP | AAPH | Formaldehyde absorption Antibacterial Crosslinkable functionality | Coatings | [202] |
| In situ | Chitosan | 3–10 wt% in solids | MMA/2EHA/AA MMA/2EHA/ItA MMA/2EHA/MA | Semi-batch EP | AAPH | Formaldehyde absorption | Interior finishing coatings | [203,204] |
| In situ | Chitosan | - | PMMA | Batch EP | APS/CuSO4:Glycine (1:1) | Reinforcement Biocompatible matrix | Bioadhesive | [206] |
| In situ | ChNF CNF | 1.8 * | 2EHA/MMA | PEP | VA-044 KPS | Colloidal Stabilizer Reinforcement Antibacterial | Adhesive | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solera-Sendra, J.; Ballard, N.; del Valle, L.J.; Franco, L. Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers. Polymers 2025, 17, 1027. https://doi.org/10.3390/polym17081027
Solera-Sendra J, Ballard N, del Valle LJ, Franco L. Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers. Polymers. 2025; 17(8):1027. https://doi.org/10.3390/polym17081027
Chicago/Turabian StyleSolera-Sendra, Jordi, Nicholas Ballard, Luis J. del Valle, and Lourdes Franco. 2025. "Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers" Polymers 17, no. 8: 1027. https://doi.org/10.3390/polym17081027
APA StyleSolera-Sendra, J., Ballard, N., del Valle, L. J., & Franco, L. (2025). Recent Advances in Combining Waterborne Acrylic Dispersions with Biopolymers. Polymers, 17(8), 1027. https://doi.org/10.3390/polym17081027











