Direct In Situ Conversion of Both Lignin and Hemicellulose into Single Functional Biopolymers via Biomass Fractionation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
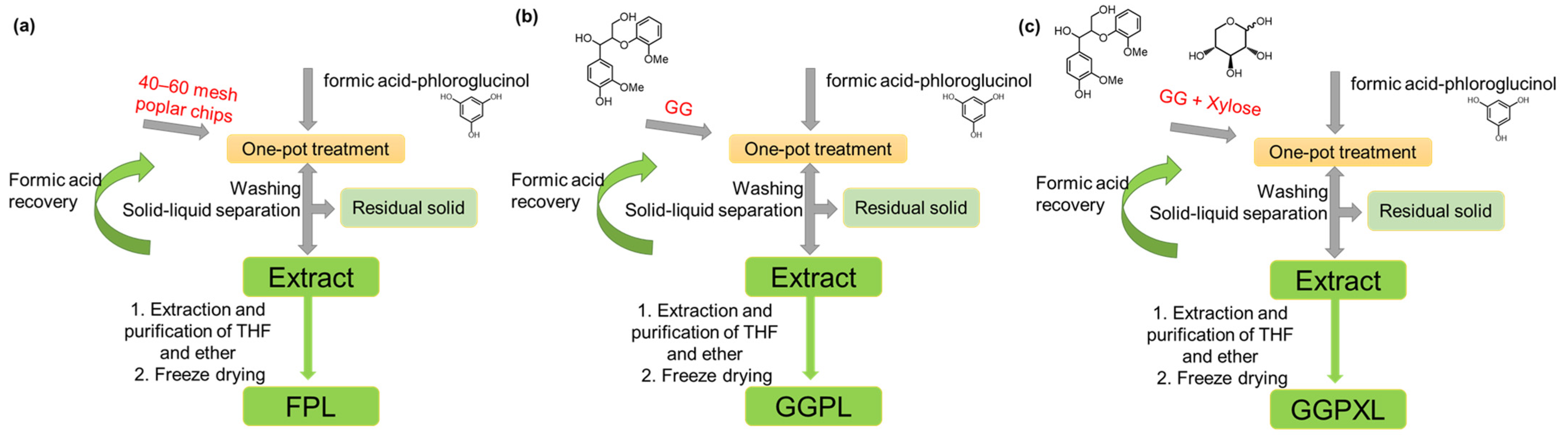
2.2. Formic Acid–Phloroglucinol Treatment
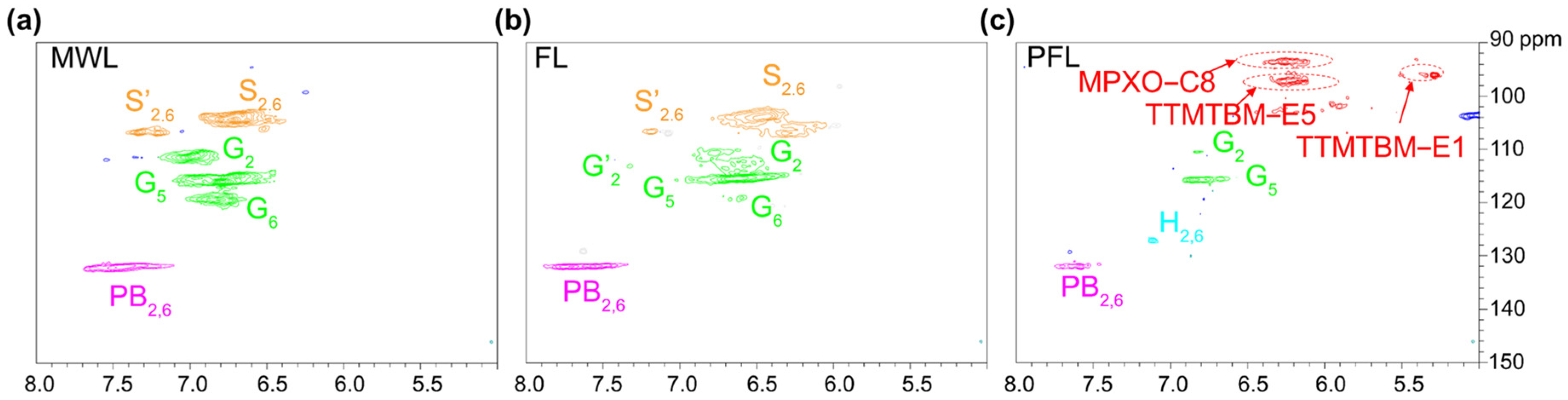
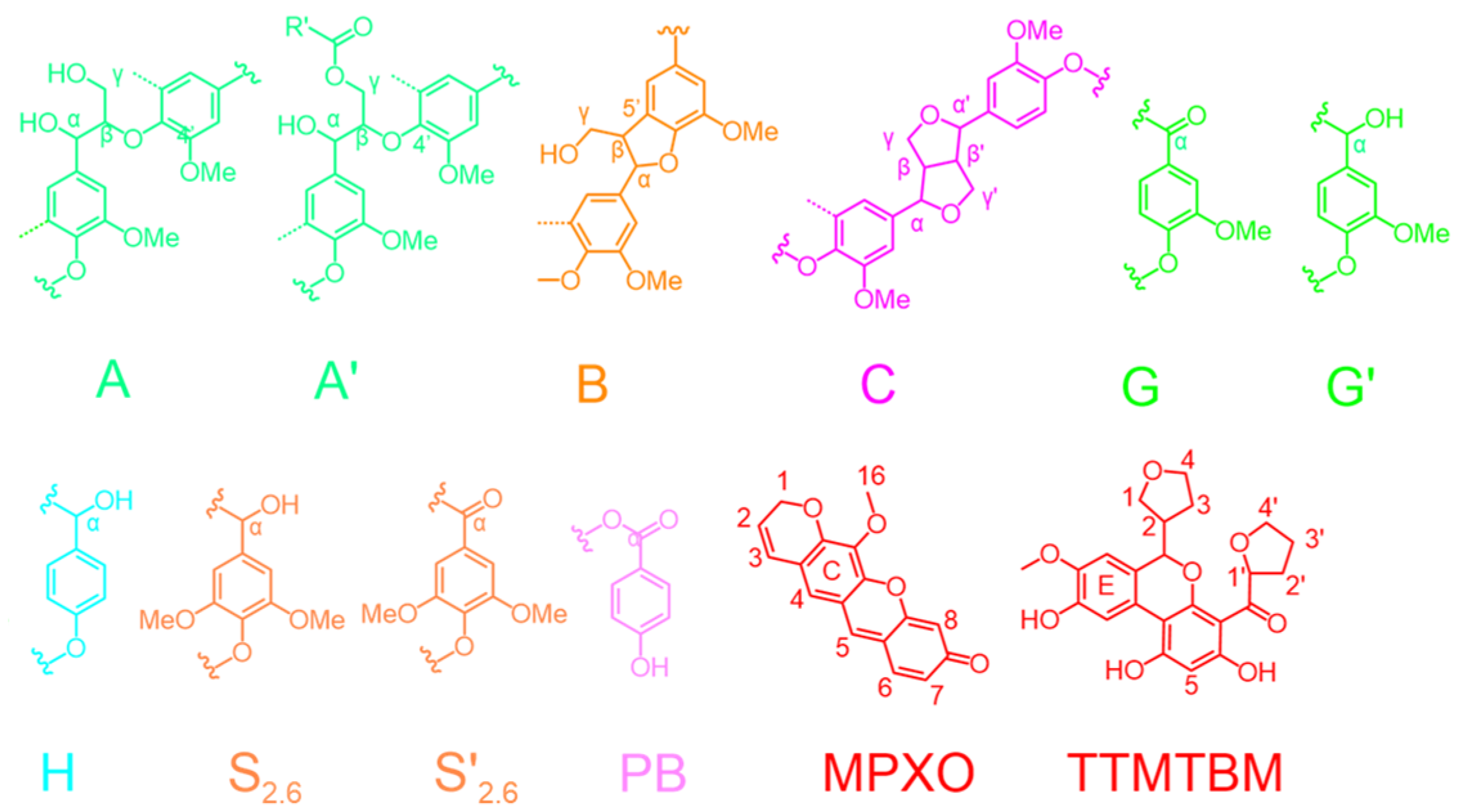
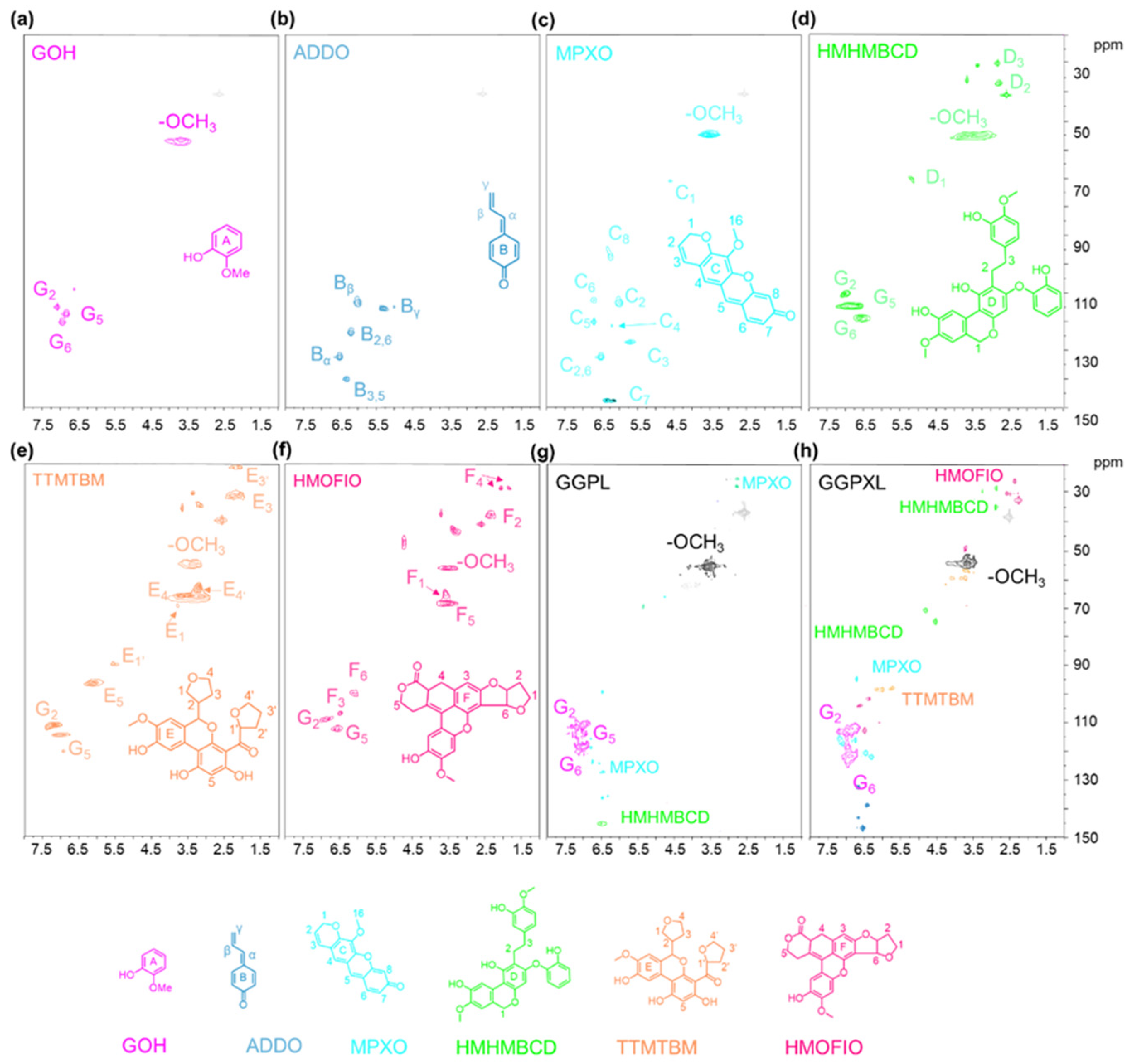
2.3. Characterization of Extracted Products
2.4. Preparation of FPL\Fe3+\Fabric
2.5. Characterization of Hydrophobic Properties of Modified Fabric
3. Results and Discussion
3.1. The Analysis of Formic Acid–Phloroglucinol Treatment
3.2. The Conversion Mechanism of the Formic Acid–Phloroglucinol System
3.3. Hydrophobic Fabrics Were Prepared by Combining Hemicellulose and Lignin with Metal Ions
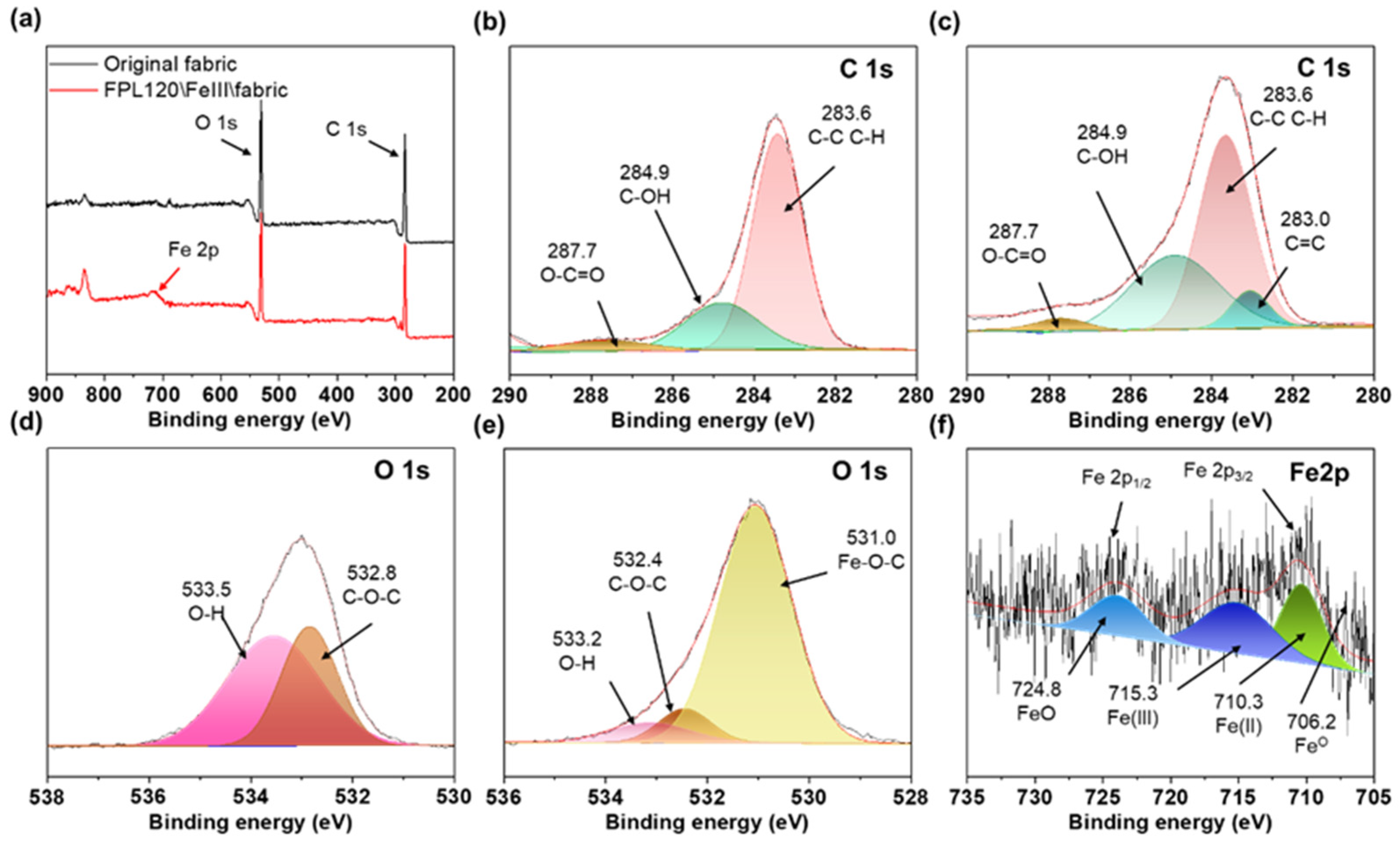
3.4. Feasibility Analysis of FPL120\/Fe3+ Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J.J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Feng, Y.; Li, G.Y.; Li, X.Y.; Zhu, N.; Xiao, B.; Li, J.; Wang, Y.J. Enhancement of biomass conversion in catalytic fast pyrolysis by microwave-assisted formic acid pretreatment. Bioresour. Technol. 2016, 214, 520–527. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, Z.; Wang, M. Self-hydrogen transfer hydrogenolysis of native lignin over Pd-PdO/TiO2. Appl. Catal. B Environ. 2022, 301, 120767. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; He, J.; Zhang, Y. Redox-neutral photocatalytic strategy for selective C–C bond cleavage of lignin and lignin models via PCET process. Sci. Bull. 2019, 64, 1658–1666. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F. Catalytic Lignin Depolymerization to Aromatic Chemicals. Acc. Chem. Res. 2020, 53, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.P.; Ralph, S.A.; Padmakshan, D.; Liu, S.; Foster, C.E. Estimation of Syringyl Units in Wood Lignins by FT-Raman Spectroscopy. J. Agric. Food Chem. 2019, 67, 4367–4374. [Google Scholar] [CrossRef]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymers: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Zhang, B.; Qiang, G.; Barta, K.; Sun, Z. Bio–based polymers from lignin. Innov. Mater. 2024, 2, 100062. [Google Scholar] [CrossRef]
- Huang, J.; Fu, S.; Gan, L. (Eds.) Chapter 2—Structure and Characteristics of Lignin. In Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–50. [Google Scholar]
- Wang, Z.; Deuss, P.J. Catalytic Hydrogenolysis of Lignin: The Influence of Minor Units and Saccharides. ChemSusChem 2021, 14, 5186–5198. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, L.; Wang, S.; Qi, L.; Lu, Z.; Chen, L.; Xu, D.; Deng, H.; Chen, C. An ultrathin nanocellulosic ion redistributor for long-life zinc anode. Innov. Mater. 2023, 1, 100029. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Qi, L.; Chen, C. Chemical modification of polysaccharides for sustainable bioplastics. Trends Chem. 2024, 6, 314–331. [Google Scholar] [CrossRef]
- Xie, J.; Xu, J.; Zhang, Z.; Wang, B.; Zhu, S.; Li, J.; Chen, K. New ternary deep eutectic solvents with cycle performance for efficient pretreated radiata pine forming to lignin containing cellulose nanofibrils. Chem. Eng. J. 2023, 451, 138591. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Fu, M.; Zhao, X.; Zhai, S.; Yan, Y.; Zhang, L.; Zhang, X. Preparation of Fe/N Double Doped Carbon Nanotubes from Lignin in Pennisetum as Oxygen Reduction Reaction Electrocatalysts for Zinc–Air Batteries. ACS Appl. Energy Mater. 2022, 5, 4340–4350. [Google Scholar] [CrossRef]
- Reshmy, R.; Athiyaman Balakumaran, P.; Divakar, K.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sirohi, R.; Binod, P.; Kumar Awasthi, M.; Sindhu, R. Microbial valorization of lignin: Prospects and challenges. Bioresour. Technol. 2022, 344, 126240. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Ni, S.Z.; Wu, R.J.; Fu, Y.J.; Qin, M.H.; Willför, S.; Xu, C.L. Green fractionation approaches for isolation of biopolymers and the critical technical challenges. Ind. Crop. Prod. 2022, 177, 114451. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Deak, N.; Wang, Z.W.; Yu, H.P.; Hameleers, L.; Jurak, E.; Deuss, P.J.; Barta, K. Tunable and functional deep eutectic solvents for lignocellulose valorization. Nat. Commun. 2021, 12, 5424. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.T.; Dick, G.R.; Questell-Santiago, Y.M.; Luterbacher, J.S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat. Protoc. 2019, 14, 921–954. [Google Scholar] [CrossRef]
- Alriols, M.G.; Tejado, A.; Blanco, M.; Mondragon, I.; Labidi, J. Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem. Eng. J. 2009, 148, 106–114. [Google Scholar] [CrossRef]
- Nadif, A.; Hunkeler, D.; Käuper, P. Sulfur-free lignins from alkaline pulping tested in mortar for use as mortar additives. Bioresour. Technol. 2002, 84, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Zhang, C.; Qi, H.; Hubbe, M.A. Holocellulosic fibers and nanofibrils using peracetic acid pulping and sulfamic acid esterification. Carbohydr. Polym. 2022, 295, 119902. [Google Scholar] [CrossRef]
- Olayo, M.G.; Alvarado, E.J.; González-Torres, M.; Gómez, L.M.; Cruz, G.J. Quantifying amines in polymers by XPS. Polym. Bull. 2023, 81, 2319–2328. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Yoo, C.G.; Yang, X.; Lin, X.; Ralph, J.; Pan, X. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef]
- Manzanares, P. The role of biorefinering research in the development of a modern bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Sun, S.-N.; Li, H.-Y.; Cao, X.-F.; Xu, F.; Sun, R.-C. Structural variation of eucalyptus lignin in a combination of hydrothermal and alkali treatments. Bioresour. Technol. 2015, 176, 296–299. [Google Scholar] [CrossRef]
- Questell-Santiago, Y.M.; Galkin, M.V.; Barta, K.; Luterbacher, J.S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020, 4, 311–330. [Google Scholar] [CrossRef]
- Questell-Santiago, Y.M.; Zambrano-Varela, R.; Amiri, M.T.; Luterbacher, J.S. Carbohydrate stabilization extends the kinetic limits of chemical polysaccharide depolymerization. Nat. Chem. 2018, 10, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Heroguel, F.; Li, Y.D.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wang, X.; Lin, Q.; Li, R.; Zhao, L.; Ren, J.; Zhang, F.J. Formic acid–hydrogen peroxide treatment of furfural residue for production of nanocellulose, lignin, and nano-scale lignin. Green Chem. 2022, 24, 6232–6240. [Google Scholar] [CrossRef]
- Zhan, B.; Zhang, L.; Deng, Y.; Yan, L.J.G.C. A multifunctional lignin-based composite ultra-adhesive for wood processing. Green Chem. 2023, 25, 10061–10071. [Google Scholar] [CrossRef]
- Qi, H.; Li, Y.; Zhou, Z.; Cao, Y.; Liu, F.; Guan, W.; Zhang, L.; Liu, X.; Li, L.; Su, Y.; et al. Synthesis of piperidines and pyridine from furfural over a surface single-atom alloy Ru1CoNP catalyst. Nat. Commun. 2023, 14, 6329. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, L.; An, Y.; Jin, X.; Li, X.; Liu, Z.; Wang, G.; Liu, R.J. Study on the removal of lignin from pre-hydrolysis liquor by laccase-induced polymerization and the conversion of xylose to furfural. Green Chem. 2022, 24, 1603–1614. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Bian, H.; Ni, S.; Li, Z.; Liu, N.; Qin, M.; Zhang, F. Highly hydrophobic cotton fabric by in-situ co-deposition of lignin/metal particles for oil/water separation. Ind. Crops Prod. 2023, 204, 117393. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Hernández-García, H.; Zamudio-Aguilar, M.A.M.; Oropeza-Guzmán, M.T.; Ochoa-Terán, A.; López-Martínez, L.M.; Martinez-Quiroz, M.; Valdez, R.; Olivas, A. Chemical issues of coffee and Tule lignins as ecofriendly materials for the effective removal of hazardous metal ions contained in metal finishing wastewater. Chem. Eng. J. 2020, 397, 125384. [Google Scholar] [CrossRef]
- Chen, F.; Shahabadi, S.I.S.; Zhou, D.; Liu, W.; Kong, J.; Xu, J.; Lu, X. Facile preparation of cross-linked lignin for efficient adsorption of dyes and heavy metal ions. React. Funct. Polym. 2019, 143, 104336. [Google Scholar] [CrossRef]
- Heo, J.W.; An, L.; Chen, J.; Kim, M.S.; Lee, S.-D.; Kim, Y.S. Application of three types of aminated lignins for efficient removal of Cd(II) and Pb(II) ions in aqueous solution. BioResources 2022, 17, 5958–5983. [Google Scholar] [CrossRef]
- Zheng, L.; Seidi, F.; Wu, H.; Huang, Y.; Wu, W.; Xiao, H. Low swelling Alginate/Lignin network gels with redox responsiveness for sustained release of agricultural fungicide and Pb2+ complexation. Eur. Polym. J. 2024, 207, 112805. [Google Scholar] [CrossRef]
- Chen, H.; Qu, X.; Liu, N.; Wang, S.; Chen, X.; Liu, S. Study of the adsorption process of heavy metals cations on Kraft lignin. Chem. Eng. Res. Des. 2018, 139, 248–258. [Google Scholar] [CrossRef]
- Gao, Z.; Duan, L.; Yang, Y.; Hu, W.; Gao, G. Mussel-inspired tough hydrogels with self-repairing and tissue adhesion. Appl. Surf. Sci. 2018, 427, 74–82. [Google Scholar] [CrossRef]
- Wu, R.; Li, Y.; Wang, X.; Fu, Y.; Qin, M.; Zhang, Y. In-situ lignin sulfonation for enhancing enzymatic hydrolysis of poplar using mild organic solvent pretreatment. Bioresour. Technol. 2023, 369, 128410. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, J.Y.; Fu, Y.; Zhang, H.; Yuan, Z.; Qin, M.; Wang, Z. Rapid flow-through fractionation of biomass to preserve labile aryl ether bonds in native lignin. Green Chem. 2019, 21, 4625–4632. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Shao, Z.; Fu, Y.; Wang, P.; Zhang, Y.; Qin, M.; Li, X.; Zhang, F. Modification of the aspen lignin structure during integrated fractionation process of autohydrolysis and formic acid delignification. Int. J. Biol. Macromol. 2020, 165, 1727–1737. [Google Scholar] [CrossRef]
- Li, N.; Yan, K.; Rukkijakan, T.; Liang, J.; Liu, Y.; Wang, Z.; Nie, H.; Muangmeesri, S.; Castiella-Ona, G.; Pan, X.; et al. Selective lignin arylation for biomass fractionation and benign bisphenols. Nature 2024, 630, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zhuang, L.; Li, M.; Liu, H.; Caruso, F.; Hao, J.; Cui, J. Interfacial Assembly of Metal–Phenolic Networks for Hair Dyeing. ACS Appl. Mater. Interfaces 2020, 12, 29826–29834. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, W.; Yang, P.; Hu, J.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Metal-phenolic network green flame retardants. Polymer 2021, 221, 123627. [Google Scholar] [CrossRef]
- Liu, X.; Ni, S.; Chen, X.; Li, Z.; Fu, Y.; Qin, M.; Zhang, F. Green fabrication of fabric by ethanol/water solvent-mediated self-assembly of homogeneous lignin for oil–water separation. Green Chem. 2024, 26, 3418–3428. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Zhang, L.; An, B.; Chen, H.; Chu, J.; Ma, J.; Fan, Y.; Wang, Z. Botryoidal nanolignin channel stabilized ultrasmall PdNP incorporating with filter membrane for enhanced removal of Cr(VI) via synergetic filtration and catalysis. Sep. Purif. Technol. 2022, 296, 121409. [Google Scholar] [CrossRef]
- Choi, J.-H.; Park, S.-Y.; Kim, J.-H.; Cho, S.-M.; Jang, S.-K.; Hong, C.; Choi, I.-G. Selective deconstruction of hemicellulose and lignin with producing derivatives by sequential pretreatment process for biorefining concept. Bioresour. Technol. 2019, 291, 121913. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Elmanama, A.A.; Amara, N.; Qodih, F.S.; Selmane, M.; Chehimi, M.M. The efficacy of surfactants in stabilizing coating of nano-structured CuO particles onto the surface of cotton fibers and their antimicrobial activity. Mater. Chem. Phys. 2018, 215, 221–228. [Google Scholar] [CrossRef]






| Hydroxyl Group (mmol/g) | ||||||
|---|---|---|---|---|---|---|
| Sample | Aliphatic-OH | S-OH | G-OH | H-OH | Condensed-OH | Total Phenolic |
| MWL | 4.49 | 0.21 | 0.70 | 0.61 | 0.01 | 1.53 |
| FPL100 | 0.58 | 026 | 0.39 | 1.92 | 0.48 | 3.05 |
| FPL120 | 0.56 | 0.3 | 0.37 | 1.62 | 0.40 | 2.70 |
| FPL140 | 0.54 | 0.51 | 0.87 | 2.05 | 0.61 | 4.04 |
| Chemical Groups (%) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | C=C | C-C C-H | C-OH | O-C=O | C-O-C | Fe-O-C | O-H |
| Original fabric | - | 69.75 | 23.08 | 7.17 | 39.45 | - | 60.45 |
| FPL120\Fe3+\fabric | 7.43 | 53.83 | 34.61 | 4.13 | 8.54 | 83.61 | 7.85 |
| Process | Esimple | Ecomplex | MI | RME (%) | Ref |
|---|---|---|---|---|---|
| FPL120\Fe3+ fabric | 0.84 | 96.54 | 98.52 | 68.4 | This work |
| FAL\Cu fabric | 0.14 | 89.69 | 85.69 | 98.93 | [40] |
| FAL\Fe fabric | 0.47 | 98.62 | 82.60 | 98.99 | [55] |
| CuO-NPs fabric | 1.10 | 115.9 | 88.95 | 101.71 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ni, S.; Wang, Z.; Fu, Y.; Qin, M.; Zhang, Y. Direct In Situ Conversion of Both Lignin and Hemicellulose into Single Functional Biopolymers via Biomass Fractionation Process. Polymers 2025, 17, 1029. https://doi.org/10.3390/polym17081029
Liu C, Ni S, Wang Z, Fu Y, Qin M, Zhang Y. Direct In Situ Conversion of Both Lignin and Hemicellulose into Single Functional Biopolymers via Biomass Fractionation Process. Polymers. 2025; 17(8):1029. https://doi.org/10.3390/polym17081029
Chicago/Turabian StyleLiu, Caiyun, Shuzhen Ni, Zhaojiang Wang, Yingjuan Fu, Menghua Qin, and Yongchao Zhang. 2025. "Direct In Situ Conversion of Both Lignin and Hemicellulose into Single Functional Biopolymers via Biomass Fractionation Process" Polymers 17, no. 8: 1029. https://doi.org/10.3390/polym17081029
APA StyleLiu, C., Ni, S., Wang, Z., Fu, Y., Qin, M., & Zhang, Y. (2025). Direct In Situ Conversion of Both Lignin and Hemicellulose into Single Functional Biopolymers via Biomass Fractionation Process. Polymers, 17(8), 1029. https://doi.org/10.3390/polym17081029




