Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review
Abstract
1. Introduction
2. Conventional Scaffold Fabrication
3. Additive Manufacturing Techniques in Tissue Engineering
3.1. Additive Manufacturing in Tissue Engineering
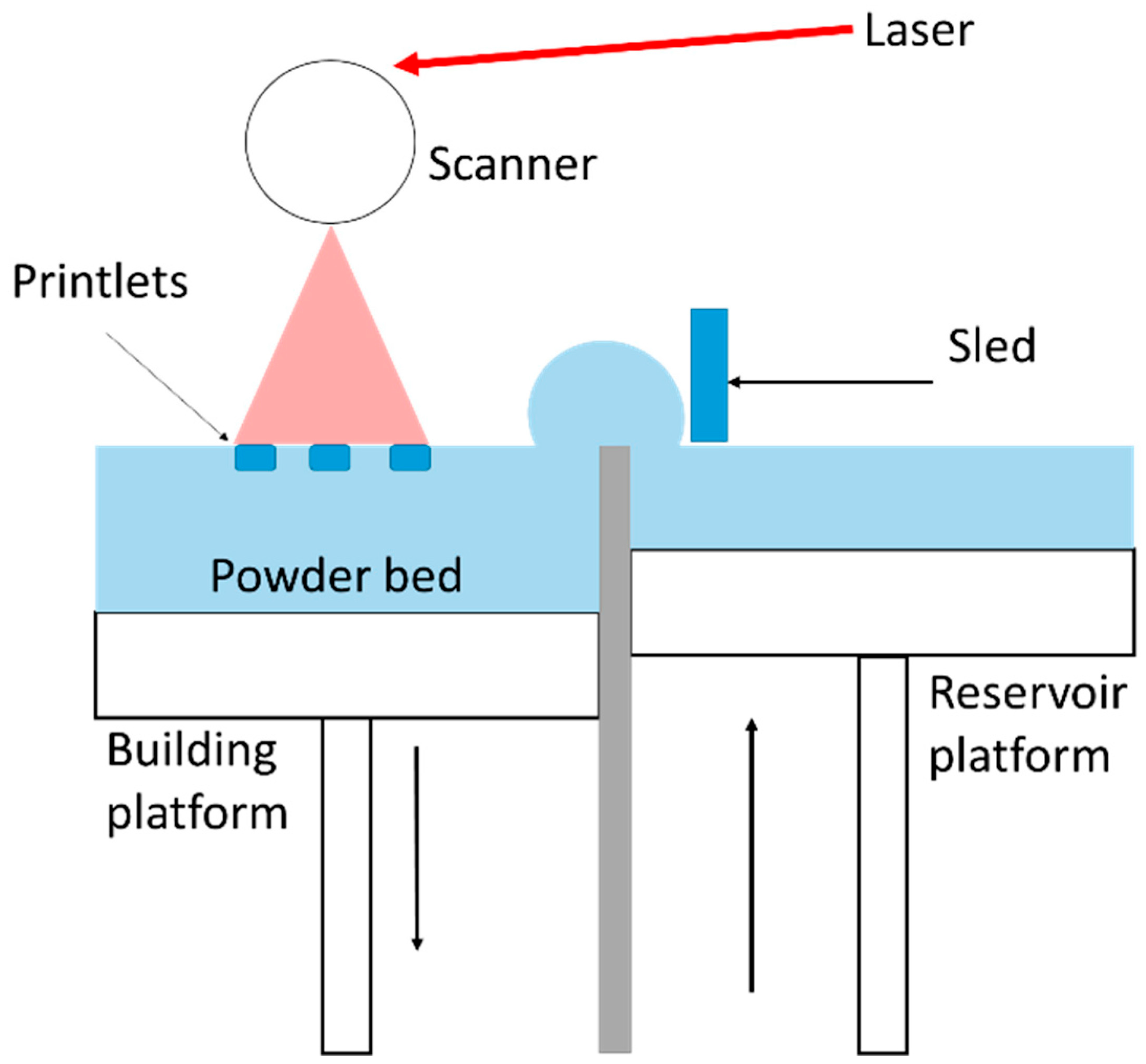
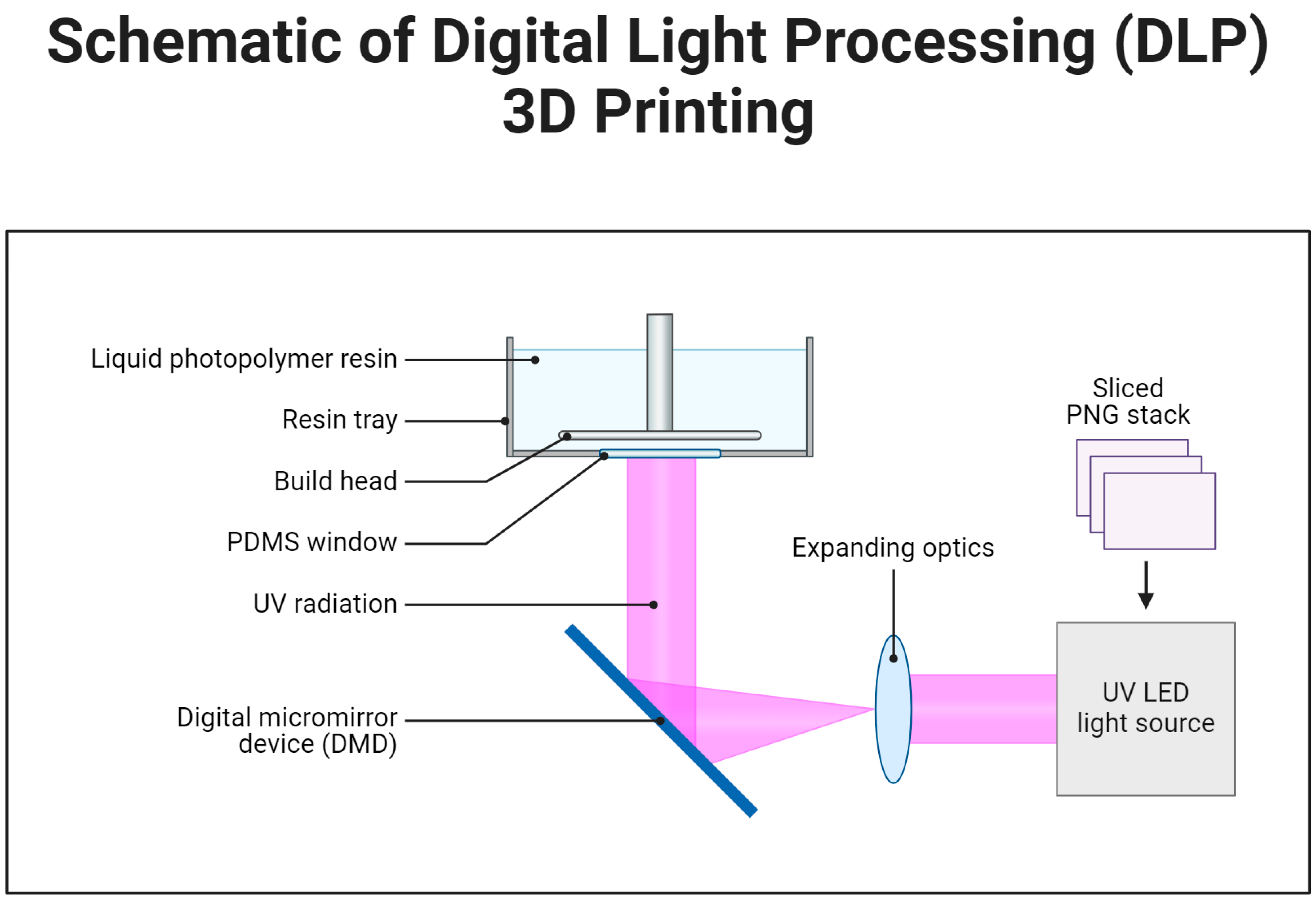
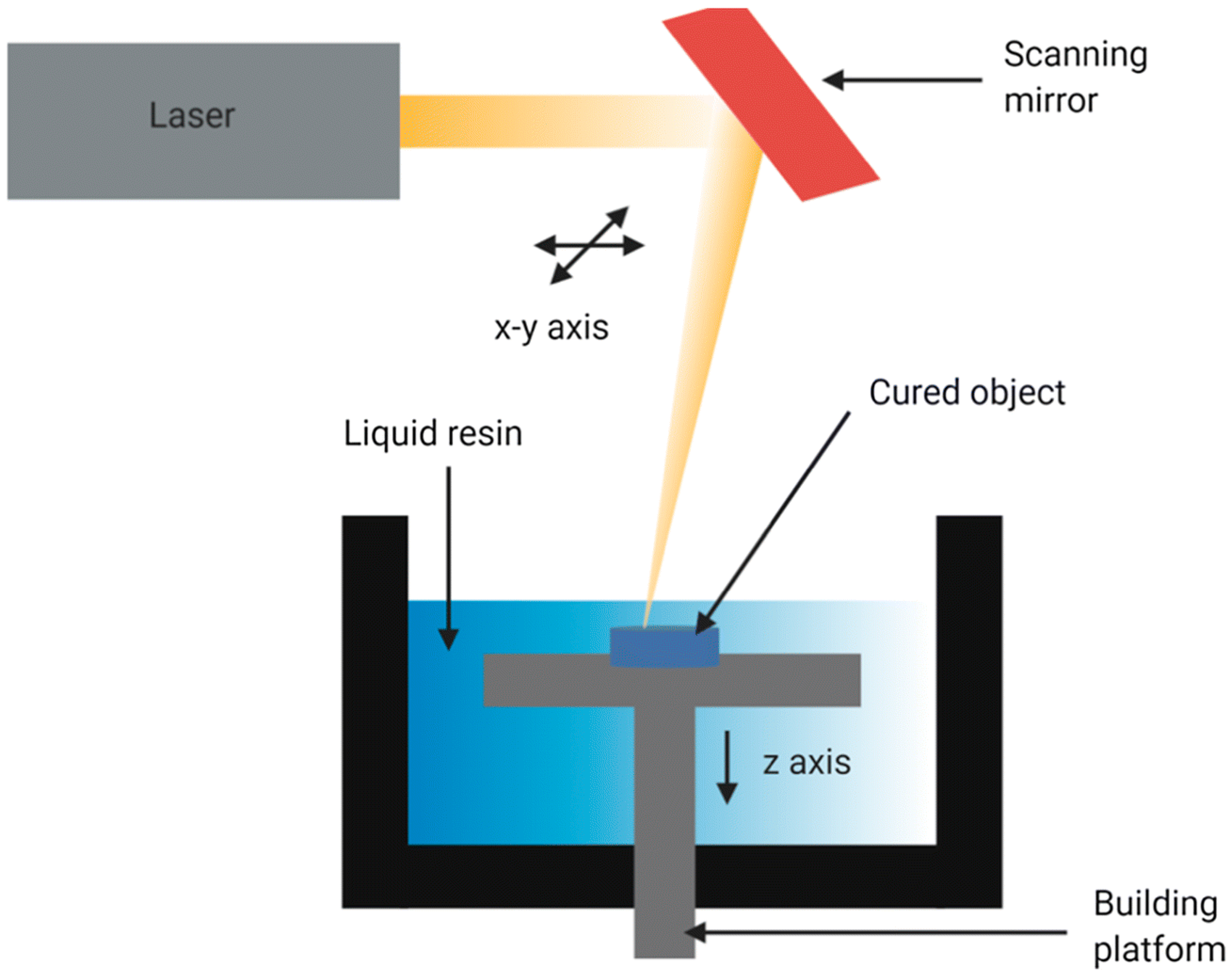
3.1.1. Laser-Based Methods
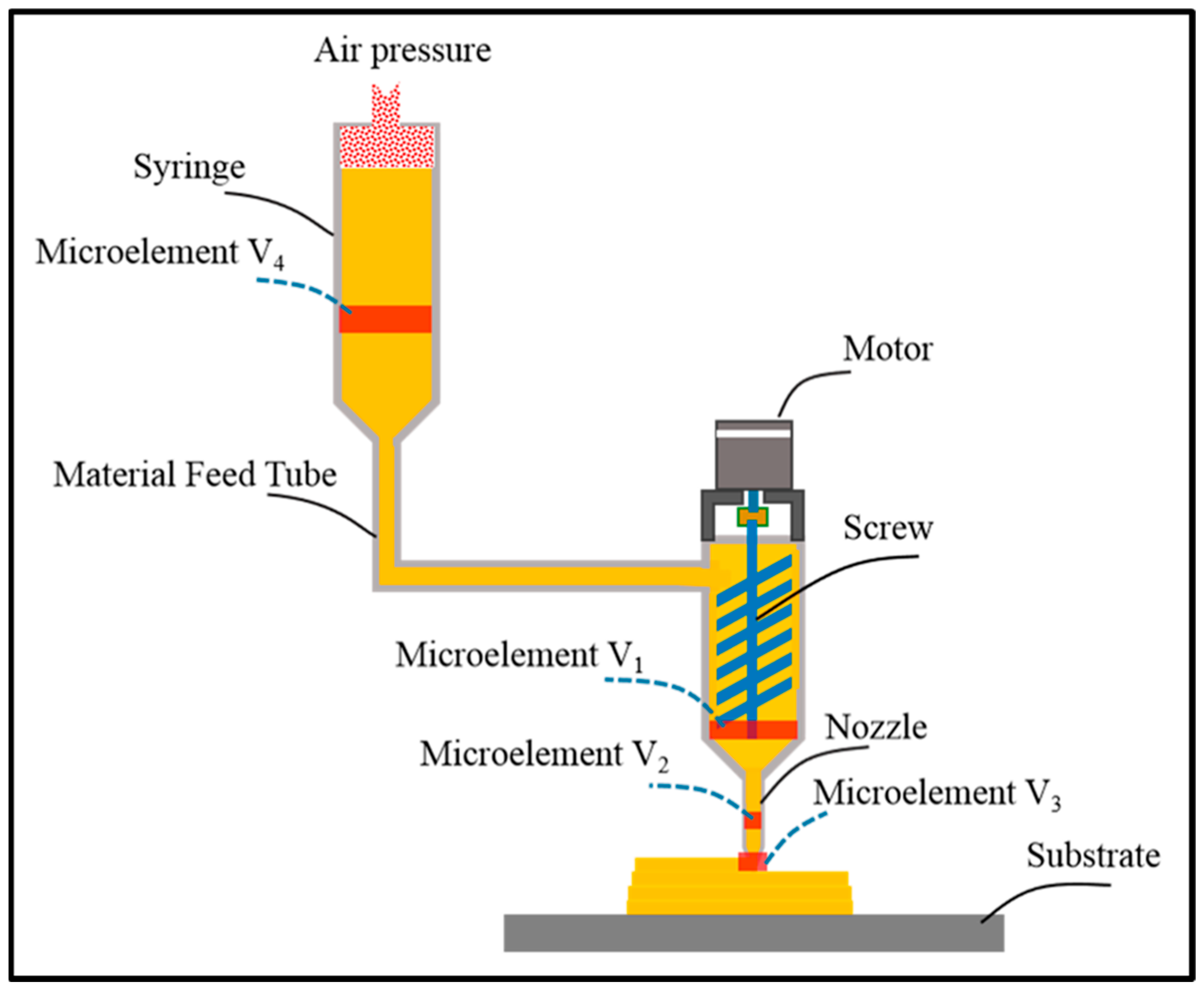
3.1.2. Non-Laser-Based Methods
3.1.3. Heat Based Techniques
4. Biomaterials Used in Additive Manufacturing
4.1. Natural Polymers
4.2. Synthetic Polymers
4.3. Ceramics
4.4. Metals
5. Design Principles for Scaffold Fabrication
6. Characterization Techniques of Scaffolds
6.1. Mechanical Characterization
6.2. Physico-Chemical Characterization
6.3. Biological Characterization
7. Applications of Additive Manufactured Scaffolds in Tissue Engineering
7.1. Bone Tissue Engineering
7.2. Cartilage Regeneration
7.3. Skin Replacement
7.4. Vascular Tissue Engineering
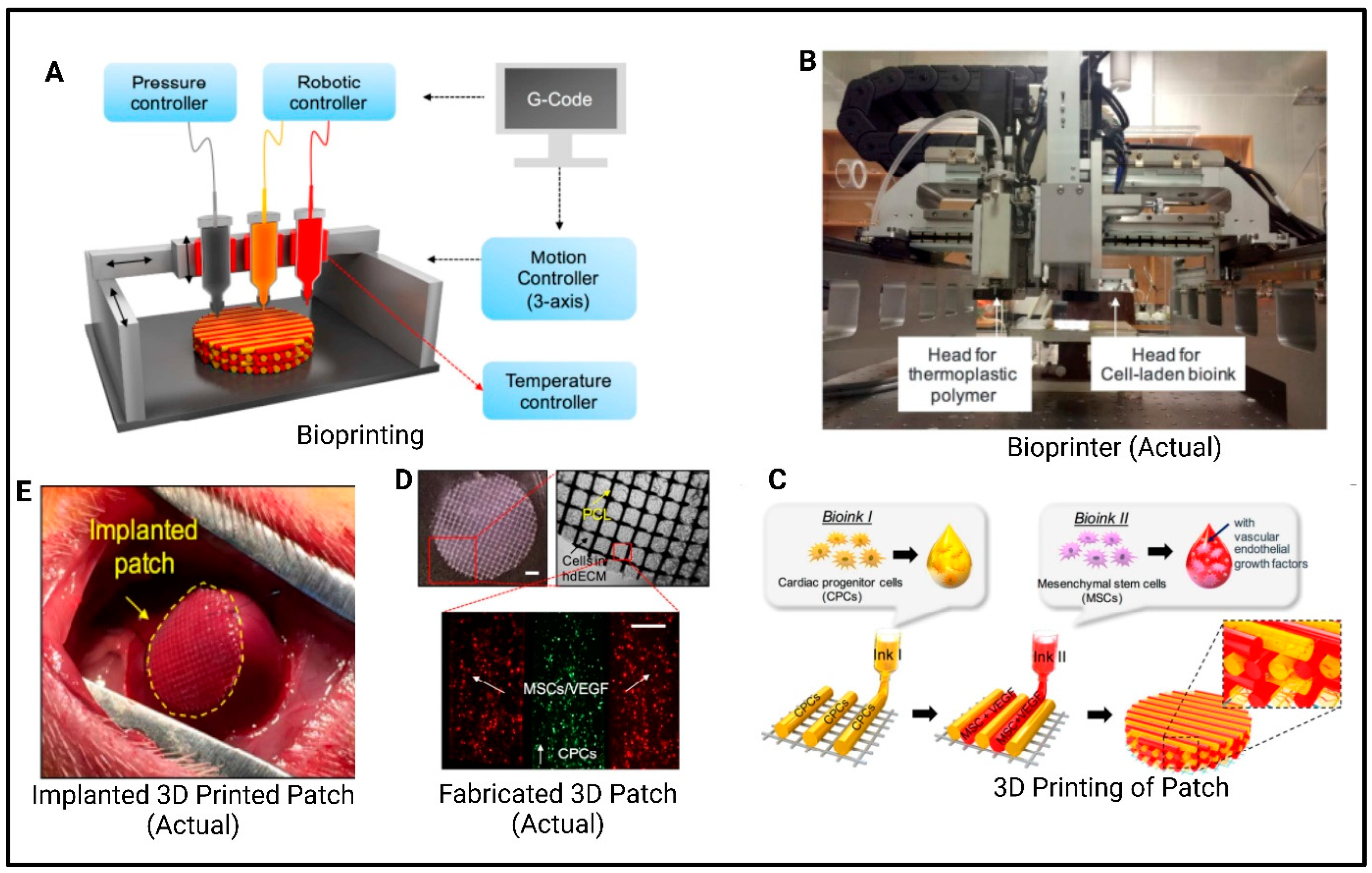
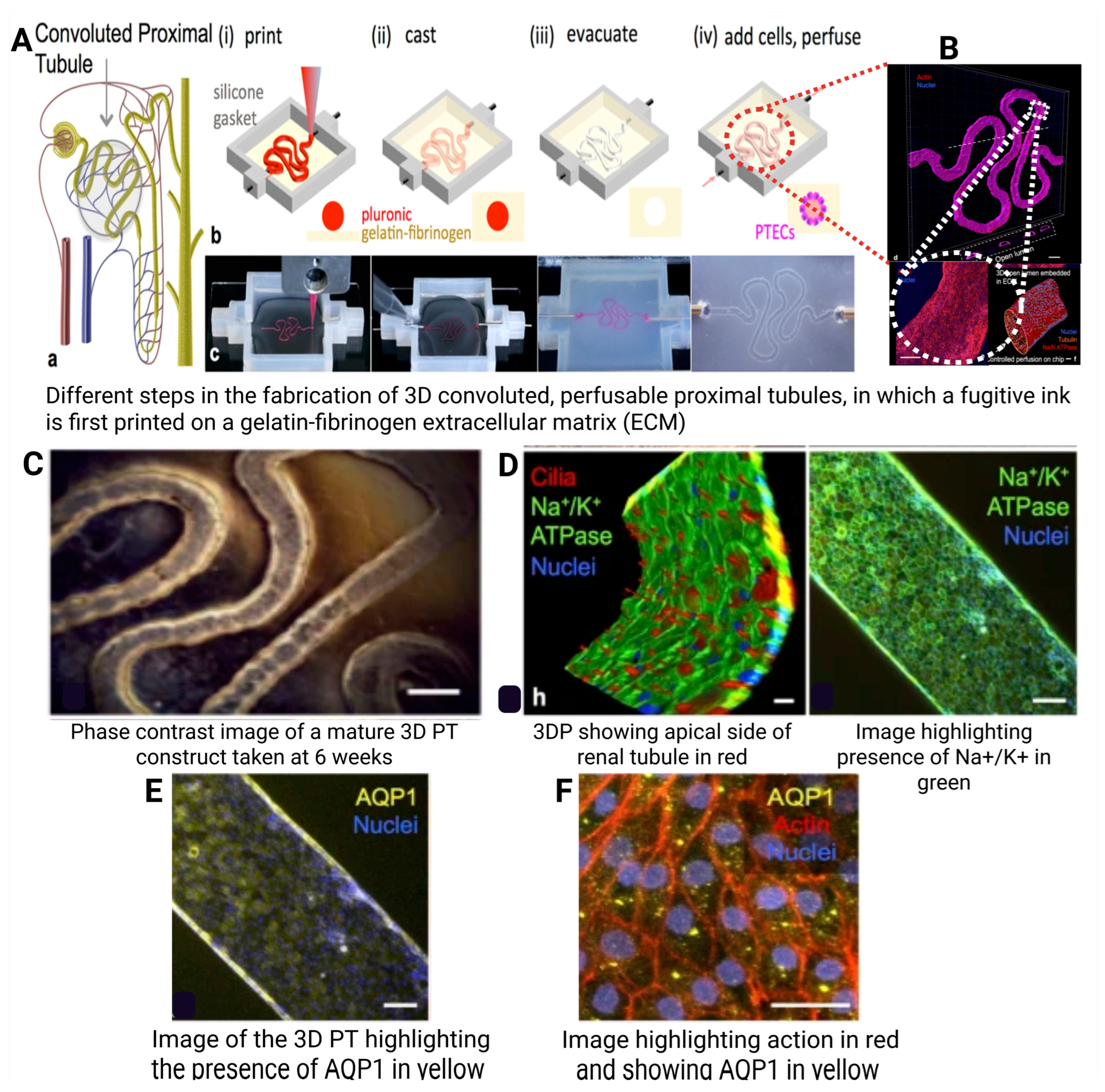
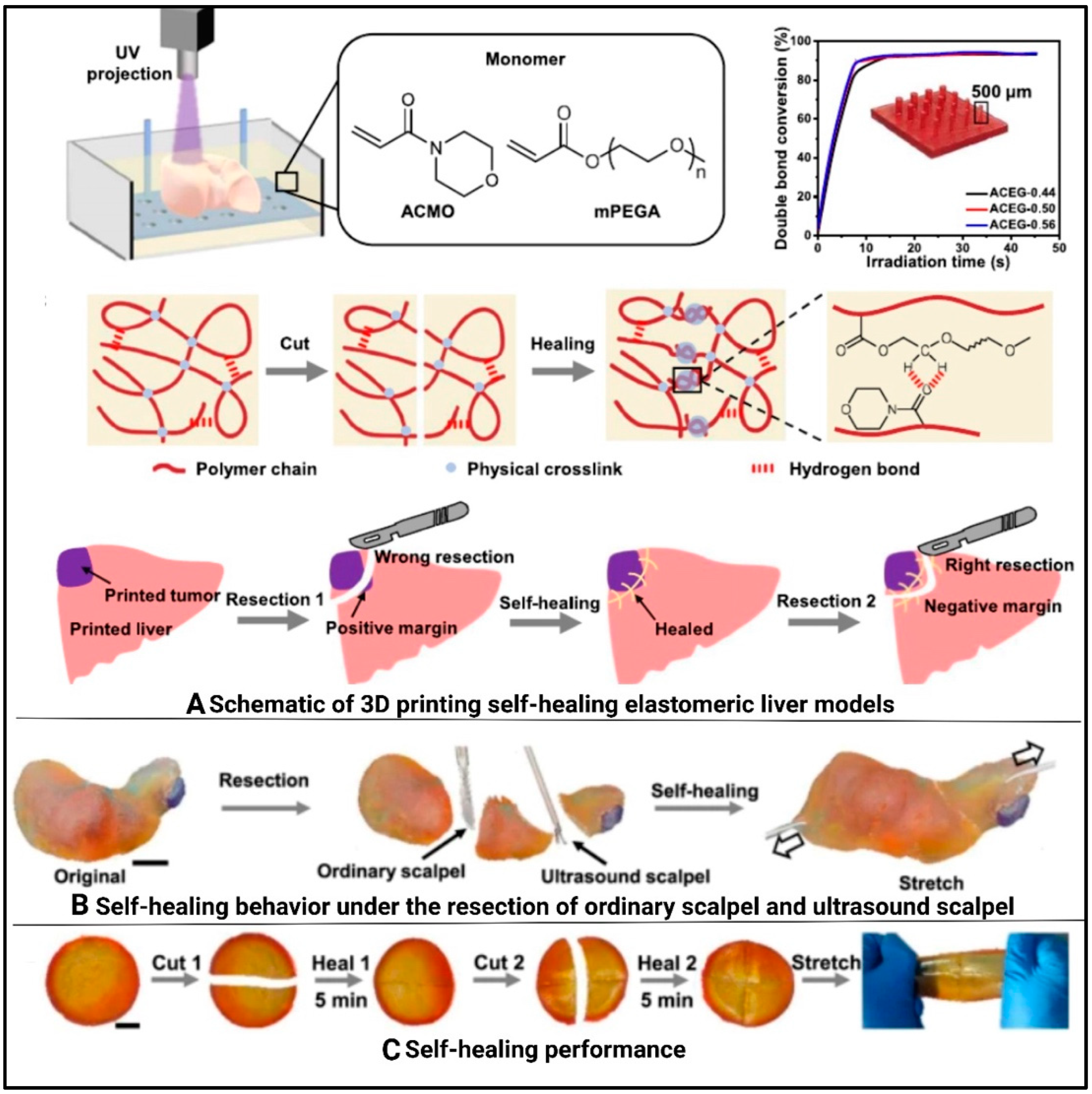
7.5. Organs
8. Challenges and Future Prospectus
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lanza, R.; Langer, R.; Vacanti, J. Principles of Tissue Engineering, 3rd ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H., Jr. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Vyas, J.; Raytthatha, N.; Singh, S.; Prajapati, B. Next-Generation Computational Automation-Based Additive Manufacturing of Pharmaceuticals: An Approach to Fabricate Precise Medicine. In Handbook of 3D Printing in Pharmaceutics; CRC Press: Boca Raton, FL, USA, 2024; pp. 153–175. [Google Scholar]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z. Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Pharmacogn. Rev. 2016, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Chirasatitsin, S.; Ngamkham, K.; Engler, A.J.; Battaglia, G. Cell instructive microporous scaffolds through interface engineering. J. Am. Chem. Soc. 2012, 134, 20103–20109. [Google Scholar] [CrossRef]
- Edelman, E.R.; Mathiowitz, E.; Langer, R.; Klagsbrun, M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials 1991, 12, 619–626. [Google Scholar] [CrossRef]
- Li, Z.; Xie, M.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent Progress in Tissue Engineering and Regenerative Medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Babu, P.J. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Le, T.Y.L.; Daly, S.; Wang, Y.; Maitz, P.K.; Schindeler, A.; Dehghani, F. Formation of porous biodegradable scaffolds based on poly(propylene carbonate) using gas foaming technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 824–830. [Google Scholar] [CrossRef]
- Raucci, M.; Guarino, V.; Ambrosio, L. Hybrid composite scaffolds prepared by sol–gel method for bone regeneration. Compos. Sci. Technol. 2010, 70, 1861–1868. [Google Scholar] [CrossRef]
- Basu, B.; Dutta, A.; Ash, D.; Garala, K.; Singh, S.; Prajapati, B.G. Recent Advancements in the Diagnosis and Management of Cancer Using Biomaterials-Fabricated Nanofibers: A Review. Curr. Med. Chem. 2024, 31, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Baino, F.; Tallia, F.; Gervasio, C.; Verné, E. Bioactive glass-derived trabecular coating: A smart solution for enhancing osteointegration of prosthetic elements. J. Mater. Sci. Mater. Med. 2012, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J. Biomed. Mater. research. Part A 2011, 97, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Hsu, H.C.; Hsiao, S.H.; Ho, W.F. Preparation of porous 45S5 Bioglass®-derived glass-ceramic scaffolds by using rice husk as a porogen additive. J. Mater. Sci. Mater. Med. 2009, 20, 1229–1236. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, W.; Rahaman, M.N.; Day, D.E. Bone regeneration in rat calvarial defects implanted with fibrous scaffolds composed of a mixture of silicate and borate bioactive glasses. Acta Biomater. 2013, 9, 9126–9136. [Google Scholar] [CrossRef]
- Boateng, J.; Mani, J.; Kianfar, F. Improving drug loading of mucosal solvent cast films using a combination of hydrophilic polymers with amoxicillin and paracetamol as model drugs. BioMed Res. Int. 2013, 2013, 198137. [Google Scholar] [CrossRef]
- Di Muzio, L.; Simonetti, P.; Carriero, V.C.; Brandelli, C.; Trilli, J.; Sergi, C.; Tirillò, J.; Cairone, F.; Cesa, S.; Radocchia, G.; et al. Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings. Molecules 2022, 27, 2959. [Google Scholar] [CrossRef]
- Panraksa, P.; Tipduangta, P.; Jantanasakulwong, K.; Jantrawut, P. Formulation of Orally Disintegrating Films as an Amorphous Solid Solution of a Poorly Water-Soluble Drug. Membranes 2020, 10, 376. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release Profile of Gentamicin Sulfate from Polylactide-co-Polycaprolactone Electrospun Nanofiber Matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef]
- Romeo, A.; Musumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic Acid-Loaded Polymeric Nanoparticles for Potential Ocular Delivery. Pharmaceutics 2021, 13, 687. [Google Scholar] [CrossRef]
- Jakubowska, E.; Bielejewski, M.; Milanowski, B.; Lulek, J. Freeze-drying of drug nanosuspension– study of formulation and processing factors for the optimization and characterization of redispersible cilostazol nanocrystals. J. Drug Deliv. Sci. Technol. 2022, 74, 103528. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Ghaffari, S.; Dabirsiaghi, A.; Haghighat, S. Freeze-drying of ampicillin solid lipid nanoparticles using mannitol as cryoprotectant. Braz. J. Pharm. Sci. 2015, 51, 797–802. [Google Scholar] [CrossRef]
- Nogueira, J.C.F.; Paliashvili, K.; Bradford, A.; Di Maggio, F.; Richards, D.A.; Day, R.M.; Chudasama, V. Functionalised thermally induced phase separation (TIPS) microparticles enabled for “click” chemistry. Org. Biomol. Chem. 2020, 18, 2215–2218. [Google Scholar] [CrossRef]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008, 4, 264–272. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Pan, Z.; Li, B.; Mo, X.; Li, Y.; Wang, J.; Wang, Y.; Wei, Z.; Chen, Y.; et al. Three-dimensional gas-foamed scaffolds decorated with metal phenolic networks for cartilage regeneration. Mater. Today Bio 2024, 29, 101249. [Google Scholar] [CrossRef]
- Bak, T.-Y.; Kook, M.-S.; Jung, S.-C.; Kim, B.-H. Biological Effect of Gas Plasma Treatment on CO2 Gas Foaming/Salt Leaching Fabricated Porous Polycaprolactone Scaffolds in Bone Tissue Engineering. J. Nanomater. 2014, 2014, 657542. [Google Scholar] [CrossRef]
- Yap, J.X.; Leo, C.; Mohd Yasin, N.H.; Show, P.L.; Chu, D.-T.; Singh, V.; Derek, C.J.B. Recent advances of natural biopolymeric culture scaffold: Synthesis and modification. Bioengineered 2022, 13, 2226–2247. [Google Scholar] [CrossRef]
- Pisani, S.; Mauri, V.; Negrello, E.; Friuli, V.; Genta, I.; Dorati, R.; Bruni, G.; Marconi, S.; Auricchio, F.; Pietrabissa, A.; et al. Hybrid 3D-Printed and Electrospun Scaffolds Loaded with Dexamethasone for Soft Tissue Applications. Pharmaceutics 2023, 15, 2478. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Potential of electrospun core—Shell structured gelatin—Chitosan nanofibers for biomedical applications. Carbohydr. Polym. 2016, 136, 1098–1107. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, F.; Polley, P.; Giri, J. Fabrication and Characterization of Core-Shell Nanofibers Using a Next-Generation Airbrush for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 41924–41934. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Znaidi, L.; Touam, T.; Vrel, D.; Souded, N.; Yahia, S.; Brinza, O.; Fischer, A.; Boudrioua, A. ZnO Thin Films Synthesized by Sol-Gel Process for Photonic Applications. Acta Phys. Pol. Ser. A 2012, 121, 165–168. [Google Scholar] [CrossRef]
- Rizki, K.; Pranowo, D.; Raharjo, T. Immobilization of lipase in silica gel from rice husk ash and its activity assay to hydrolyze palm oil. BIO Web Conf. 2020, 28, 03005. [Google Scholar] [CrossRef]
- Tabard, L.; Garnier, V.; Prud’Homme, E.; Courtial, E.J.; Meille, S.; Adrien, J.; Jorand, Y.; Gremillard, L. Robocasting of highly porous ceramics scaffolds with hierarchized porosity. Addit. Manuf. 2021, 38, 101776. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Mo, X.M.; Teoh, H.; Hutmacher, D. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.I.; Brown, T.E.; Killgore, J.P. Digital light processing in a hybrid atomic force microscope: In Situ, nanoscale characterization of the printing process. Addit. Manuf. 2021, 38, 101744. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.; Klee, D.; Möller, M. Electrospinning of polymer melts: Phenomenological observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital light processing (DLP)-based (bio)printing strategies for tissue modeling and regeneration. Aggregate 2023, 4, e270. [Google Scholar] [CrossRef]
- Magalhães, L.; Santos, F.E.P.; Elias, C.M.V.; Afewerki, S.; Sousa, G.F.; Furtado, A.S.A.; Marciano, F.R.; Lobo, A.O. Printing 3D Hydrogel Structures Employing Low-Cost Stereolithography Technology. J. Funct. Biomater. 2020, 11, 12. [Google Scholar] [CrossRef]
- Balc, N.; Yankov, E.; Nikolova, M.P. Comparison of the Accuracy of 3D Printed Prototypes Using the Stereolithography (SLA) Method with the Digital CAD Models. MATEC Web Conf. 2017, 137, 02014. [Google Scholar]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 1–20. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Damle, M.N.; Chaudhari, L.; Tardalkar, K.; Bhamare, N.; Jagdale, S.; Gaikwad, V.; Chhabra, D.; Kumar, B.; Manuja, A.; Joshi, M.G. A biologically functional bioink based on extracellular matrix derived collagen for 3D printing of skin. Int. J. Biol. Macromol. 2024, 258, 128851. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Cui, X.; Qin, Z.; Su, C.; Zhang, G.; Tang, J.-N.; Li, J.-A.; Zhang, J.-Y. Three-dimensional bioprinting of artificial blood vessel: Process, bioinks, and challenges. Int. J. Bioprinting 2023, 9, 740. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K. Inkjet 3D printing of microfluidic structures—On the selection of the printer towards printing your own microfluidic chips. J. Micromech. Microeng. 2015, 25, 085013. [Google Scholar] [CrossRef]
- Hu, F.; Mikolajczyk, T.; Pimenov, D.Y.; Gupta, M.K. Extrusion-Based 3D Printing of Ceramic Pastes: Mathematical Modeling and In Situ Shaping Retention Approach. Materials 2021, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gao, Z.; Lu, B.; Bao, C.; Zheng, B.; Wang, L. Performance optimization of complicated structural SiC/Si composite ceramics prepared by selective laser sintering. Ceram. Int. 2019, 46, 568–575. [Google Scholar] [CrossRef]
- Zhu, L.; Bai, M.; Xiao, S.; Liu, Y.; Zhu, Q.; Wang, Z.; Zhao, J.; Zhang, W.; Chen, D. In-situ monitoring of cellular H(2)O(2) within 3D cell clusters using conductive scaffolds. Talanta 2024, 279, 126559. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Kang, S.; Sun, D.; Liu, Y.; Wang, X.; Lu, L. Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds. Front. Bioeng. Biotechnol. 2024, 12, 1345163. [Google Scholar] [CrossRef]
- Polak, J.M.; Mantalaris, S. Stem Cells Bioprocessing: An Important Milestone to Move Regenerative Medicine Research Into the Clinical Arena. Pediatr. Res. 2008, 63, 461–466. [Google Scholar] [CrossRef]
- Xing, F.; Xiang, Z.; Rommens, P.M.; Ritz, U. 3D Bioprinting for Vascularized Tissue-Engineered Bone Fabrication. Materials 2020, 13, 2278. [Google Scholar] [CrossRef]
- Lou, X.; Chirila, T.V. Swelling behavior and mechanical properties of chemically cross-linked gelatin gels for biomedical use. J. Biomater. Appl. 1999, 14, 184–191. [Google Scholar] [CrossRef]
- Dobaj Štiglic, A.; Lackner, F.; Nagaraj, C.; Beaumont, M.; Bračič, M.; Duarte, I.; Kononenko, V.; Drobne, D.; Madhan, B.; Finšgar, M.; et al. 3D-Printed Collagen–Nanocellulose Hybrid Bioscaffolds with Tailored Properties for Tissue Engineering Applications. ACS Appl. Bio Mater. 2023, 6, 5596–5608. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Cai, H.; Hu, H.; Hu, K.; Sun, Z.; Liu, R.; Wei, Y.; Han, L. A dual-crosslinking electroactive hydrogel based on gelatin methacrylate and dibenzaldehyde-terminated telechelic polyethylene glycol for 3D bio-printing. Sci. Rep. 2024, 14, 4118. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Patel, G.K.; Patel, N.; Singh, S.; Chittasupho, C. Alginate Nanoparticles: A Potential Drug Carrier in Tuberculosis Treatment. In Tubercular Drug Delivery Systems: Advances in Treatment of Infectious Diseases; Shegokar, R., Pathak, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 207–234. [Google Scholar]
- Ketabat, F.; Maris, T.; Duan, X.; Yazdanpanah, Z.; Kelly, M.E.; Badea, I.; Chen, X. Optimization of 3D printing and in vitro characterization of alginate/gelatin lattice and angular scaffolds for potential cardiac tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1161804. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karati, D.; Singh, S.; Prajapati, B.G. Chitosan-based nanomedicine in the management of age-related macular degeneration: A review. Curr. Nanomed. (Former. Recent Pat. Nanomed.) 2024, 14, 13–27. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen-Based Bioink for 3D Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef]
- Shah, S.R.; Modi, C.D.; Singh, S.; Mori, D.D.; Soniwala, M.M.; Prajapati, B.G. Recent Advances in Additive Manufacturing of Polycaprolactone-Based Scaffolds for Tissue Engineering Applications: A Comprehensive Review. Regen. Eng. Transl. Med. 2024, 11, 112–131. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Wong, Y.S.; Tay, C.Y.; Wen, F.; Venkatraman, S.; Tan, L.P. Engineered Polymeric Biomaterials for Tissue Engineering. Curr. Tissue Eng. 2012, 1, 41–53. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Bakaic, E.; Smeets, N.M.B.; Hoare, T. Injectable hydrogels based on poly(ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015, 5, 35469–35486. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Durbal, R.; Leach, J.B. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010, 6, 3404–3414. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Awaid, M.; Cacciotti, I. Bioactive Glasses with Antibacterial Properties: Mechanisms, Compositions, and Applications. In Bioactive Glasses and Glass-Ceramics: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 581–614. [Google Scholar]
- Vyas, J.; Raytthatha, N.; Singh, S.; Prajapati, B.G.; Mohite, P.; Munde, S. Sustainable sources of raw materials as substituting biomaterials for additive manufacturing of dental implants: A review. Periodontal Implant. Res. 2024, 8, 3. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Magdalena Musuc, A.; Paiva Santos, A.C.; Paszkiewicz, S.; Igwe Idumah, C.; Singh, S.; Varma, R.S.; Berrada, M. Nano-hydroxyapatite (nHAp) scaffolds for bone regeneration: Preparation, characterization and biological applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105601. [Google Scholar] [CrossRef]
- Dragosloveanu, Ş.; Dragosloveanu, C.D.Μ.; Stanca, H.T.; Cotor, D.C.; Andrei, A.C.; Dragosloveanu, C.I.; Stoica, C.I. Tricalcium phosphate and hydroxyapatite treatment for benign cavitary bone lesions: A prospective clinical trial. Exp. Ther. Med. 2020, 20, 215. [Google Scholar] [CrossRef]
- Roohani, I.; No, Y.J.; Zuo, B.; Xiang, S.D.; Lu, Z.; Liu, H.; Plebanski, M.; Zreiqat, H. Low-Temperature Synthesis of Hollow β-Tricalcium Phosphate Particles for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2022, 8, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Ridi, F. An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. J. Funct. Biomater. 2023, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Eugen, G.; Claus, M.; Anna-Maria, S.; Niklas, D.; Philipp, S.; Andrea, E.; Andrea, M.-L.; Elke, V. Degradation of 3D-printed magnesium phosphate ceramics in vitro and a prognosis on their bone regeneration potential. Bioact. Mater. 2023, 19, 376–391. [Google Scholar] [CrossRef]
- Xiao, R.; Feng, X.; Fan, R.; Chen, S.; Song, J.; Gao, L.; Lu, Y. 3D printing of titanium-coated gradient composite lattices for lightweight mandibular prosthesis. Compos. Part B Eng. 2020, 193, 108057. [Google Scholar] [CrossRef]
- Wang, X.; Han, G.; Ye, J.; Zhang, C. Biocompatibility of 3D-printed Titanium Alloy Porous Scaffold using Osteoblasts. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation (ICMA), Tianjin, China, 4–7 August 2019; pp. 1382–1386. [Google Scholar]
- Ma, C.; de Barros, N.R.; Zheng, T.; Gomez, A.; Doyle, M.; Zhu, J.; Nanda, H.S.; Li, X.; Khademhosseini, A.; Li, B. 3D Printing and Surface Engineering of Ti6Al4V Scaffolds for Enhanced Osseointegration in an In Vitro Study. Biomimetics 2024, 9, 423. [Google Scholar] [CrossRef]
- Choi, J.-W.; Ahn, J.-S. 3D printed titanium implant for the skull reconstruction: A preliminary case study. J. Int. Soc. Simul. Surg. 2014, 1, 99–102. [Google Scholar] [CrossRef]
- Vyas, J.; Shah, I.; Singh, S.; Prajapati, B.G. Biomaterials-based additive manufacturing for customized bioengineering in management of otolaryngology: A comprehensive review. Front. Bioeng. Biotechnol. 2023, 11, 1234340. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D.; Cai, B. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Q.; Zhu, Z.; Shi, S.; Xu, C.; Xie, R.; Li, Y. Hyaluronic Acid-Based Dynamic Hydrogels for Cartilage Repair and Regeneration. Gels 2024, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nwabor, O.F.; Sukri, D.M.; Wunnoo, S.; Dumjun, K.; Lethongkam, S.; Kusolphat, P.; Hemtanon, N.; Klinprathum, K.; Sunghan, J.; et al. Poly (vinyl alcohol) copolymerized with xanthan gum/hypromellose/sodium carboxymethyl cellulose dermal dressings functionalized with biogenic nanostructured materials for antibacterial and wound healing application. Int. J. Biol. Macromol. 2022, 216, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: Cellular responses to fiber parameters. NPJ Regen. Med. 2019, 4, 15. [Google Scholar] [CrossRef]
- Shah, D.D.; Raghani, N.R.; Chorawala, M.R.; Singh, S.; Prajapati, B.G. Harnessing three-dimensional (3D) cell culture models for pulmonary infections: State of the art and future directions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2861–2880. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D printing method for bone tissue engineering scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Kim, M.H.; Singh, Y.P.; Celik, N.; Yeo, M.; Rizk, E.; Hayes, D.J.; Ozbolat, I.T. High-throughput bioprinting of spheroids for scalable tissue fabrication. Nat. Commun. 2024, 15, 10083. [Google Scholar] [CrossRef]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug. Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef]
- Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. [Google Scholar] [CrossRef]
- Breuls, R.G.; Jiya, T.U.; Smit, T.H. Scaffold stiffness influences cell behavior: Opportunities for skeletal tissue engineering. Open Orthop. J. 2008, 2, 103–109. [Google Scholar] [CrossRef]
- Imere, A.; Foster, N.C.; Hajiali, H.; Okur, K.E.; Wright, A.L.; Barroso, I.A.; Haj, A.J.E. Enhanced chondrogenic potential in GelMA-based 3D cartilage model via Wnt3a surface immobilization. Sci. Rep. 2024, 14, 15022. [Google Scholar] [CrossRef]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4+ T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Holzberg, T.R.; Lim, C.G.; Gao, F.; Gargava, A.; Trachtenberg, J.E.; Mikos, A.G.; Fisher, J.P. 3D printing PLGA: A quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 2017, 9, 024101. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110939. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Nagaraj, A.; Etxeberria, A.E.; Naffa, R.; Zidan, G.; Seyfoddin, A. 3D-Printed Hybrid Collagen/GelMA Hydrogels for Tissue Engineering Applications. Biology 2022, 11, 1561. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs. 2023, 21, 189. [Google Scholar] [CrossRef]
- Soltani Khaboushan, A.; Sadeghmousavi, S.; Kajbafzadeh, A.-M. Emerging Stem Cell Therapy and Tissue Engineering-Based Approaches in Neurodegenerative Diseases. In Handbook of Stem Cell Applications; Haider, K.H., Ed.; Springer Nature: Singapore, 2023; pp. 1–49. [Google Scholar]
- Zhou, Y.; Pereira, G.; Tang, Y.; James, M.; Zhang, M. 3D Porous Scaffold-Based High-Throughput Platform for Cancer Drug Screening. Pharmaceutics 2023, 15, 1691. [Google Scholar] [CrossRef]
- Orr, A.; Kalantarnia, F.; Nazir, S.; Bolandi, B.; Alderson, D.; O’Grady, K.; Hoorfar, M.; Julian, L.M.; Willerth, S.M. Recent advances in 3D bioprinted neural models: A systematic review on the applications to drug discovery. Adv. Drug Deliv. Rev. 2025, 218, 115524. [Google Scholar] [CrossRef]
- Nystuen, K.L.; McNamee, S.M.; Akula, M.; Holton, K.M.; DeAngelis, M.M.; Haider, N.B. Alzheimer’s Disease: Models and Molecular Mechanisms Informing Disease and Treatments. Bioengineering 2024, 11, 45. [Google Scholar] [CrossRef]
- Prakash, J.; Shenoy, M.; Alhasmi, A.; Al Saleh, A.A.; Shivakumar, S.C.; Shivakumar, S. Biocompatibility of 3D-Printed Dental Resins: A Systematic Review. Cureus 2024, 16, e51721. [Google Scholar] [CrossRef] [PubMed]
- Arunprasath, K.; Vijayakumar, M.; Ramarao, M.; Arul, T.G.; Peniel Pauldoss, S.; Selwin, M.; Radhakrishnan, B.; Manikandan, V. Dynamic mechanical analysis performance of pure 3D printed polylactic acid (PLA) and acrylonitrile butadiene styrene (ABS). Mater. Today Proc. 2022, 50, 1559–1562. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, V.; Arzt, E.; Federle, W.; Hensel, R. Strong Wet and Dry Adhesion by Cupped Microstructures. ACS Appl. Mater. Interfaces 2019, 11, 26483–26490. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT—a digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef]
- Van Cleynenbreugel, T.; Schrooten, J.; Van Oosterwyck, H.; Vander Sloten, J. Micro-CT-based screening of biomechanical and structural properties of bone tissue engineering scaffolds. Med. Biol. Eng. Comput. 2006, 44, 517–525. [Google Scholar] [CrossRef]
- Askari, E.; Cengiz, I.F.; Alves, J.L.; Henriques, B.; Flores, P.; Fredel, M.C.; Reis, R.L.; Oliveira, J.M.; Silva, F.S.; Mesquita-Guimarães, J. Micro-CT based finite element modelling and experimental characterization of the compressive mechanical properties of 3-D zirconia scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 102, 103516. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Álvarez-Echazú, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef]
- Ledda, M.; Merco, M.; Sciortino, A.; Scatena, E.; Convertino, A.; Lisi, A.; Del Gaudio, C. Biological Response to Bioinspired Microporous 3D-Printed Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5383. [Google Scholar] [CrossRef]
- Criscenti, G.; De Maria, C.; Vozzi, G.; Moroni, L. Characterization of Additive Manufactured Scaffolds. In 3D Printing and Biofabrication; Ovsianikov, A., Yoo, J., Mironov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–78. [Google Scholar]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineering and Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Jang, J.-W.; Min, K.-E.; Kim, C.; Shin, J.; Lee, J.; Yi, S. Review: Scaffold Characteristics, Fabrication Methods, and Biomaterials for the Bone Tissue Engineering. Int. J. Precis. Eng. Manuf. 2023, 24, 511–529. [Google Scholar] [CrossRef]
- Wu, R.; Li, Y.; Shen, M.; Yang, X.; Zhang, L.; Ke, X.; Yang, G.; Gao, C.; Gou, Z.; Xu, S. Bone tissue regeneration: The role of finely tuned pore architecture of bioactive scaffolds before clinical translation. Bioact. Mater 2021, 6, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Dellavia, C.; Canciani, E.; Pellegrini, G.; Tommasato, G.; Graziano, D.; Chiapasco, M. Histological assessment of mandibular bone tissue after guided bone regeneration with customized computer-aided design/computer-assisted manufacture titanium mesh in humans: A cohort study. Clin. Implant. Dent. Relat. Res. 2021, 23, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Engstrand, T.; Kihlström Burenstam Linder, L.; Åberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, H.; Ji Roh, E.; Bae An, S.; Han, I.-B.; Hyung Kim, G. A multicellular bioprinted cell construct for vascularized bone tissue regeneration. Chem. Eng. J. 2022, 431, 133882. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Cui, D.; Liu, Y.; Zou, Q.; Xu, S.; Luo, S.; Ye, C. Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Mater. Des. 2022, 213, 110349. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; She, H.; Wang, R.; Bai, F.; Xiang, B. A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects. Regen. Ther. 2022, 21, 307–321. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Jin, X.-Y.; Ma, Y.-H.; Cai, W.-J.; Xiao, W.-Y.; Li, Z.-W.; Qi, X.; Ding, J. Injectable stress relaxation gelatin-based hydrogels with positive surface charge for adsorption of aggrecan and facile cartilage tissue regeneration. J. Nanobiotechnol. 2021, 19, 214. [Google Scholar] [CrossRef]
- Li, Y.; Xun, X.; Xu, Y.; Zhan, A.; Gao, E.; Yu, F.; Wang, Y.; Luo, H.; Yang, C. Hierarchical porous bacterial cellulose scaffolds with natural biomimetic nanofibrous structure and a cartilage tissue-specific microenvironment for cartilage regeneration and repair. Carbohydr. Polym. 2022, 276, 118790. [Google Scholar] [CrossRef]
- Haghighi, P.; Shamloo, A. Fabrication of a novel 3D scaffold for cartilage tissue repair: In-vitro and in-vivo study. Mater. Sci. Eng. C 2021, 128, 112285. [Google Scholar] [CrossRef] [PubMed]
- Alves da Silva, M.L.; Martins, A.; Costa-Pinto, A.R.; Costa, P.; Faria, S.; Gomes, M.; Reis, R.L.; Neves, N.M. Cartilage Tissue Engineering Using Electrospun PCL Nanofiber Meshes and MSCs. Biomacromolecules 2010, 11, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Heirani-Tabasi, A.; Hosseinzadeh, S.; Rabbani, S.; Ahmadi Tafti, S.H.; Jamshidi, K.; Soufizomorrod, M.; Soleimani, M. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan–hyaluronic acid hydrogel. Biomed. Mater. 2021, 16, 055003. [Google Scholar] [CrossRef] [PubMed]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Möbius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1801501. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. 3D bioprinting applications for the printing of skin: A brief study. Sens. Int. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Ghidini, T. Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation. J. Thorac. Dis. 2018, 10, S2363–S2375. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials Based on Marine Resources for 3D Bioprinting Applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef]
- Haleem, A.; Gupta, P.; Bahl, S.; Javaid, M.; Kumar, L. 3D scanning of a carburetor body using COMET 3D scanner supported by COLIN 3D software: Issues and solutions. Mater. Today Proc. 2021, 39, 331–337. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of porous substrates: A review of processes using pore forming agents in the biomaterial field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Dave, B.; Chorawala, M.R.; Prajapati, B.G.; Singh, S.; Elossaily, G.M.; Ansari, M.N.; Ali, N. An Insight on Microfluidic Organ-on-a-Chip Models for PM2. 5-Induced Pulmonary Complications. ACS Omega 2024, 9, 13534–13555. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef]
- Neff, E.P. Printing cures: Organovo advances with 3D-printed liver tissue. Lab Anim. 2017, 46, 57. [Google Scholar] [CrossRef]
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D bioprinting for lungs and hollow organs. Transl. Res. J. Lab. Clin. Med. 2019, 211, 19–34. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969. [Google Scholar] [CrossRef]
- Mehrotra, S.; de Melo, B.A.G.; Hirano, M.; Keung, W.; Li, R.A.; Mandal, B.B.; Shin, S.R. Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Funct. Mater. 2020, 30, 1907436. [Google Scholar] [CrossRef]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications-A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Bosi, S.; Ballerini, L.; Prato, M. Carbon Nanotubes in Tissue Engineering. In Making and Exploiting Fullerenes, Graphene, and Carbon Nanotubes; Marcaccio, M., Paolucci, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 181–204. [Google Scholar]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, Y.; Cai, Y.; Li, X.; Wan, S.; Xu, G. Use 3D printing technology to enhance stone free rate in single tract percutaneous nephrolithotomy for the treatment of staghorn stones. Urolithiasis 2020, 48, 509–516. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Han, F.; Zhao, Q.; Xie, T.; Wu, J.; Zhang, Y. 3D printing of self-healing personalized liver models for surgical training and preoperative planning. Nat. Commun. 2023, 14, 8447. [Google Scholar] [CrossRef]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplant. 2013, 19, 1304–1310. [Google Scholar] [CrossRef]
- Baimakhanov, Z.; Soyama, A.; Takatsuki, M.; Hidaka, M.; Hirayama, T.; Kinoshita, A.; Natsuda, K.; Kuroki, T.; Eguchi, S. Preoperative simulation with a 3-dimensional printed solid model for one-step reconstruction of multiple hepatic veins during living donor liver transplantation. Liver Transplant. 2015, 21, 266–268. [Google Scholar] [CrossRef]
- Horváth, L.; Umehara, Y.; Jud, C.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci. Rep. 2015, 5, 7974. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Eskinazi-Budge, A.; Manickavasagam, D.; Czech, T.; Novak, K.; Kunzler, J.; Oyewumi, M.O. Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. Drug Dev. Ind. Pharm. 2018, 44, 1583–1590. [Google Scholar] [CrossRef]






| Method | Materials Used | Key Findings | Shortcomings | References |
|---|---|---|---|---|
| Solvent casting | Paracetamol, amoxicillin carboxymethyl cellulose, sodium alginate, carrageenan | Improved drug loading, enhanced mucoadhesion, improved drug retention | Potential brittleness and mechanical properties need optimization | [18] |
| silver and gellan gum methacrylate film dressings | Developed nanocomposite films containing silver nanoparticles | Requires UV exposure; incomplete curing | [19] | |
| Phenytoin, polyvinyl alcohol, and high methoxyl pectin | Improved dissolution and rapid disintegration. | Traces of residual solvents; require careful solvent selection | [20] | |
| Gentamicin sulfate-loaded polycaprolactone (PCL) matrices | Enhanced drug distribution and improved anti-bacterial efficacy | Requires use of organic solvents; potential toxicity concerns | [21] | |
| Freeze Drying | Ferulic Acid-loaded PLA and PLGA Polymeric Nanoparticles | Ocular drug delivery with enhanced antioxidant stability | Challenges in maintaining nanoparticle integrity | [22] |
| Cilostazol, trehalose, maltodextrin and PEG 1500 | Improving stability and bioavailability | Need to remove traces of DMSO | [23] | |
| Insulin- PLGA nanoparticles, Trehalose, glucose, sucrose, fructose, and sorbitol | Preserved insulin activity for 6 months | Need to optimize cryoprotectants to prevent aggregation | [24] | |
| Ampicillin solid lipid nanoparticles | Reduced aggregation and improved stability | Not reported | [25] | |
| TIPS | Functionalized TIPS Microparticles | Developed functionalized TIPS microparticles for “click” conjugation, enhancing surface functionalization. | Limited exploration in surface functionalization. | [26] |
| Protein-loaded PLGA TIPS microspheres | Introduced rapid formation of monodisperse porous microspheres using TIPS. | Specific to certain polymer–solvent systems only | [27] | |
| Rhodamine B | Rapid formation; customizable porosity | Not reported | ||
| Recombinant Human Growth Hormone (rhGH) | Controlled release preparation suitable for tissue engineering | Requires precise control over fabrication parameters | ||
| Gas foaming | Poly(L-lactide-co-ε-caprolactone)/Silk Fibroin (PLCL/SF) with Strontium-EGCG MPNs (metal phenolic networks) | Simultaneous inflammation mitigation and cartilage matrix remodeling; enhanced cell infiltration | Requires precise control of MPN composition | [28] |
| Poly (propylene carbonate) (PPC), starch, bioglass particles | Benign degradation byproducts; tunable porosity and mechanical properties | Potential challenges in achieving uniform pore size distribution | [11] | |
| Polycaprolactone (PCL) | Highly porous structure; well-interconnected pores; improved hydrophilicity and biocompatibility after plasma treatment | Requires post-treatment to enhance biocompatibility | [29] | |
| Electrospinning | Mycophenolic Acid and collagen | Targeted drug delivery, protection from degradation, and stability in biological fluids | Complexity in fabrication leads to poor scalability | [30] |
| Dexamethasone, PLA, PCL | Controlled drug release, uniform sized nanometric fiber, enhanced cell adhesion and proliferation | Potential challenges in optimizing scaffold properties for specific tissues | [31] | |
| Nanofibers using gelatin (core) and chitosan (shell) | Biocompatible and biodegradable scaffolds for TE and wound healing, supports cell growth and regeneration | Not reported | [32] | |
| Electrospinning combined with Gas Foaming | Poly(L-lactide-co-ε-caprolactone)/Silk Fibroin (PLCL/SF) | Improved cell proliferation and maintenance of chondrocyte phenotype | Complex fabrication process | [33] |
| Electrospinning with co-axial needles | Shell (PCL/PEO) needles, forming core–shell nanofibers. | Tunable sustained release behavior, improved mechanical strength | Use of organic solvents | [34] |
| Sol–gel method | Titanium dioxide nanoparticles, ethanol, acetic acid, water | Enhanced photocatalytic activity due to anatase phase formation | Requires precise control of synthesis parameters | [35] |
| Zinc acetate dihydrate thin films, ethanol, monoethanolamine | Uniform and transparent thin films suitable for photonic applications | Sensitivity to annealing conditions affecting film quality | [36] | |
| Silica sol, lipase enzyme, | Effective immobilization and preserving enzyme activity | Limitations in diffusion | [37] | |
| Starch consolidation | Corn starch and ceramic powders | high porosity, suitable for bone TE applications | Potential brittleness due to high porosity | [38] |
| Starch-based polymer scaffolds via 3D printing | Cornstarch, dextran and gelatin | Scaffolds with interconnected pores for TE | Insufficient mechanical properties may be for load-bearing applications | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyas, J.; Raytthatha, N.; Vyas, P.; Prajapati, B.G.; Uttayarat, P.; Singh, S.; Chittasupho, C. Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review. Polymers 2025, 17, 1090. https://doi.org/10.3390/polym17081090
Vyas J, Raytthatha N, Vyas P, Prajapati BG, Uttayarat P, Singh S, Chittasupho C. Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review. Polymers. 2025; 17(8):1090. https://doi.org/10.3390/polym17081090
Chicago/Turabian StyleVyas, Jigar, Nensi Raytthatha, Puja Vyas, Bhupendra G. Prajapati, Pimpon Uttayarat, Sudarshan Singh, and Chuda Chittasupho. 2025. "Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review" Polymers 17, no. 8: 1090. https://doi.org/10.3390/polym17081090
APA StyleVyas, J., Raytthatha, N., Vyas, P., Prajapati, B. G., Uttayarat, P., Singh, S., & Chittasupho, C. (2025). Biomaterial-Based Additive Manufactured Composite/Scaffolds for Tissue Engineering and Regenerative Medicine: A Comprehensive Review. Polymers, 17(8), 1090. https://doi.org/10.3390/polym17081090










