Light-Mediated 3D-Printed Wound Dressings Based on Natural Polymers with Improved Adhesion and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Functionalization of the Natural Polymers
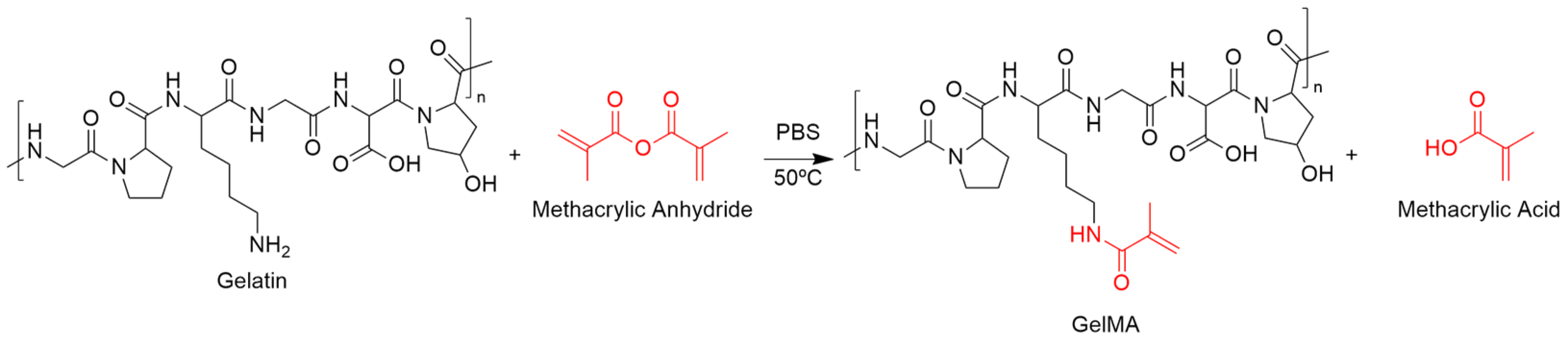
Functionalization of Gelatin with Methacrylic Anhydride (GelMA)
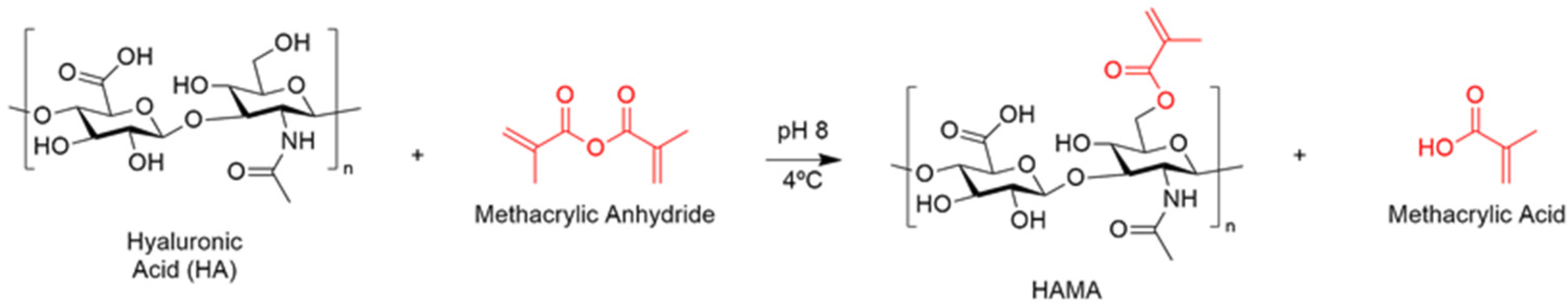
Functionalization of Hyaluronic Acid with Methacrylic Anhydride (HAMA)
Functionalization of Microcrystalline Cellulose with Allyl Glycidyl Ether (MCCA)
2.2.2. Characterization of the Polymeric Precursors
1H NMR Spectroscopy
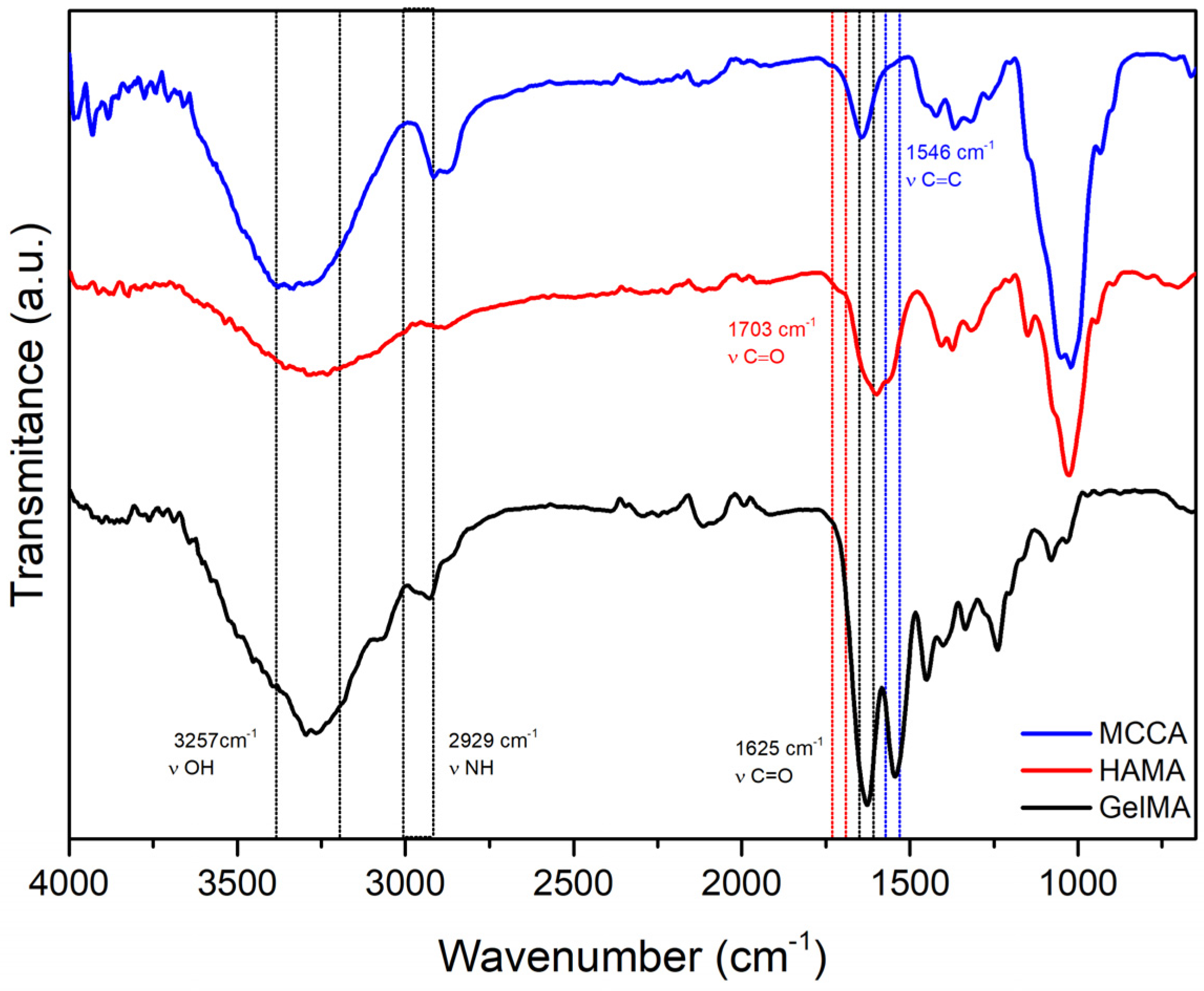
ATR-FTIR Spectroscopy
2.2.3. Preparation and Characterization of the DLP Formulations
Photorheology
2.2.4. 3D Printing Process
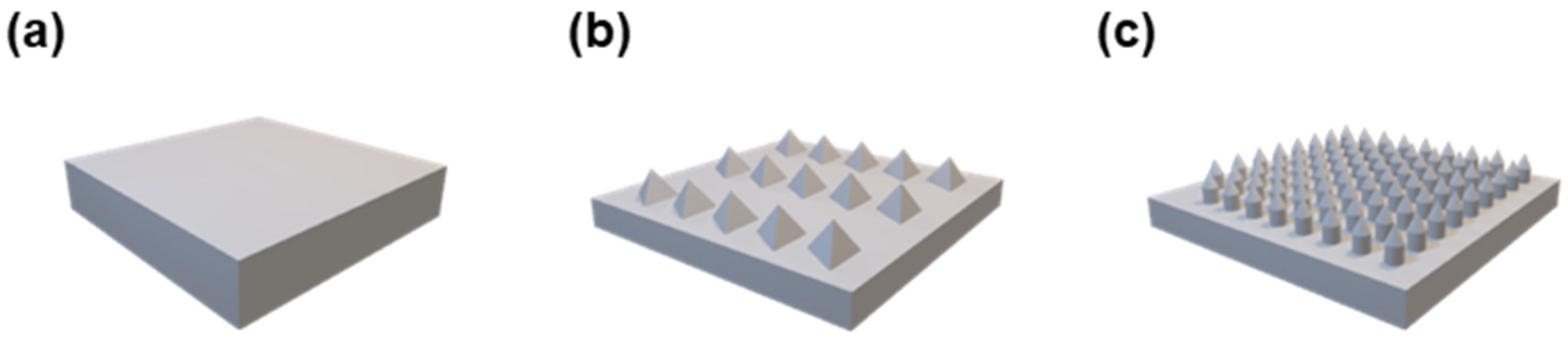
3D Models
Printing Parameters
2.2.5. Characterization of the Printed Constructs
Gel Content
Hydrolytic Degradation
Surface Morphology
2.2.6. Functionalization of the Printed Constructs with Gallic Acid (GA)
2.2.7. Antioxidant Capacity of the Printed Constructs
2.2.8. Adhesion Capacity of the Printed Constructs
2.2.9. In Vitro Cytocompatibility of 3D-Printed Hydrogels
Indirect Method
Direct Method
Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of the Polymeric Precursors
Functionalization of the Polymeric Precursors
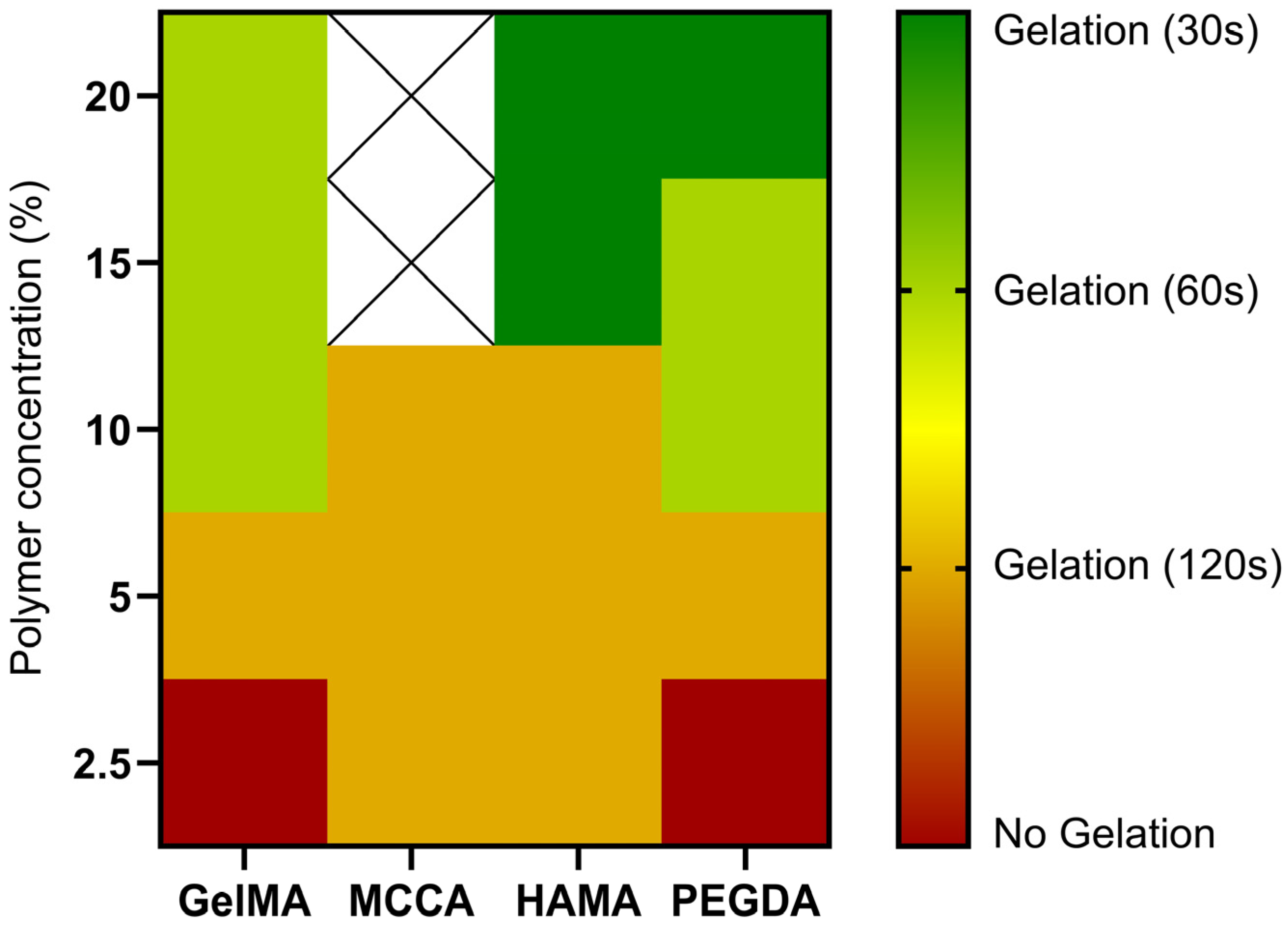
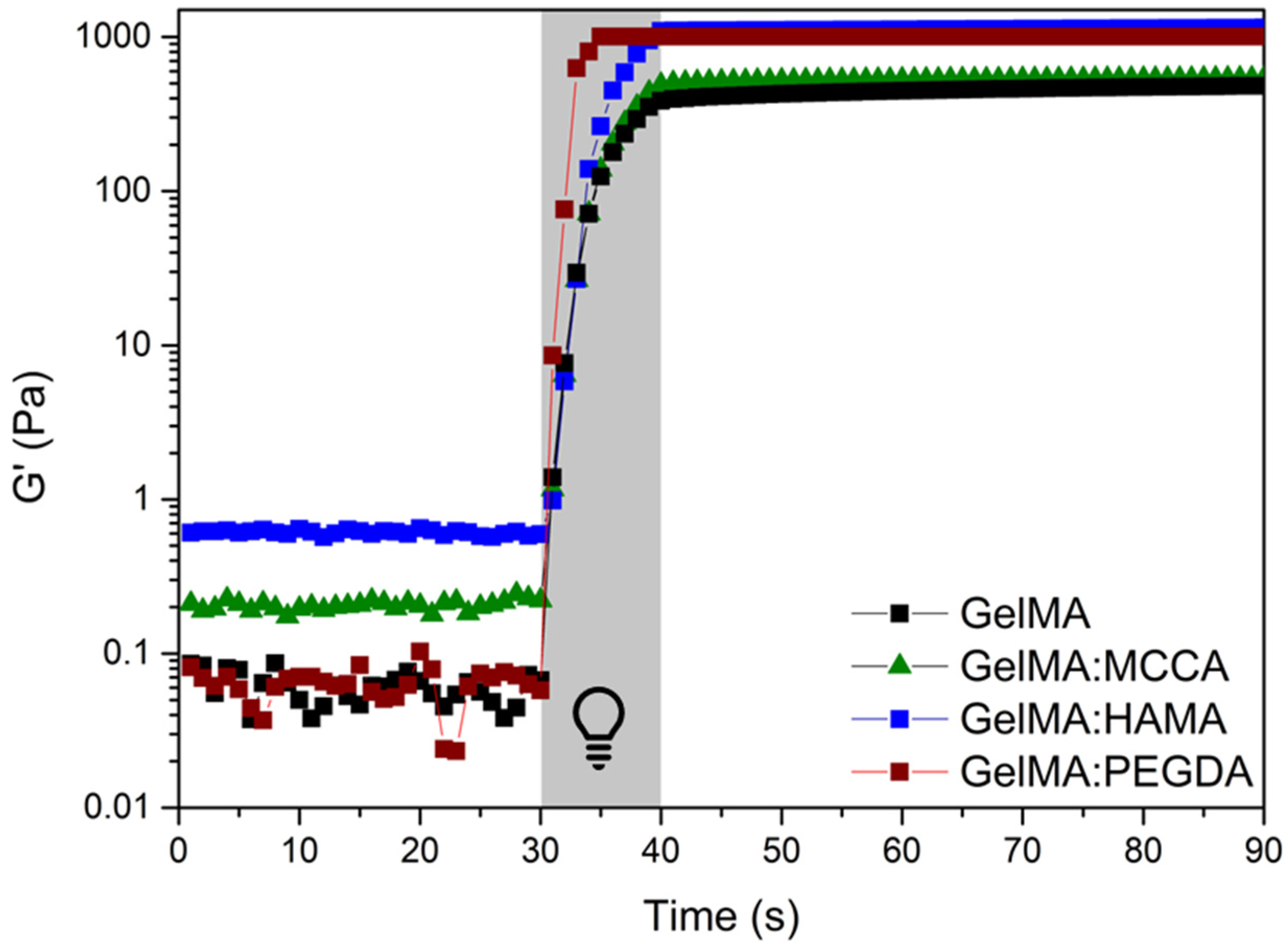
3.2. Assessment of the Photoreactivity of the Precursors Toward the Acquisition of the Hydrogels
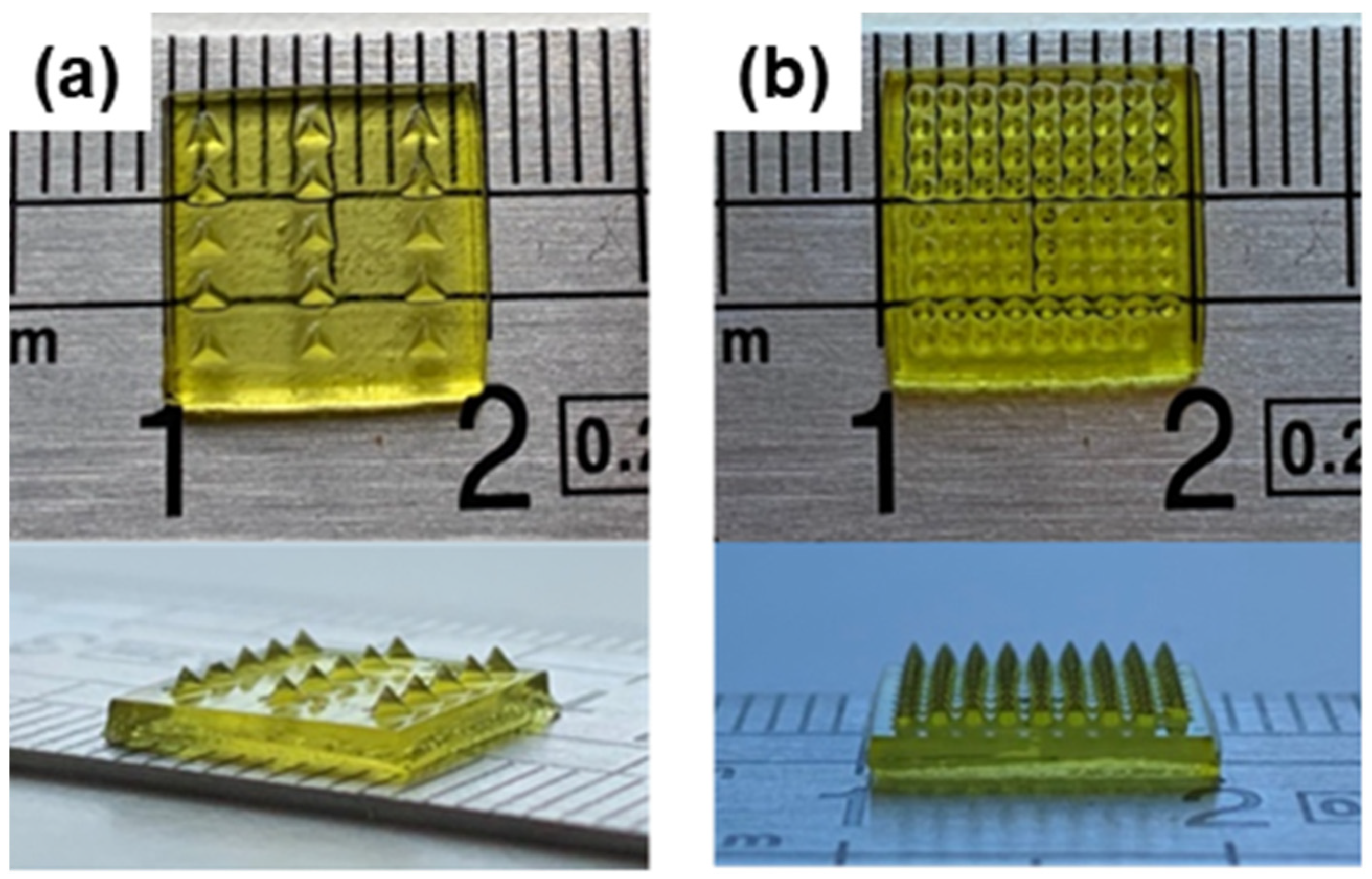
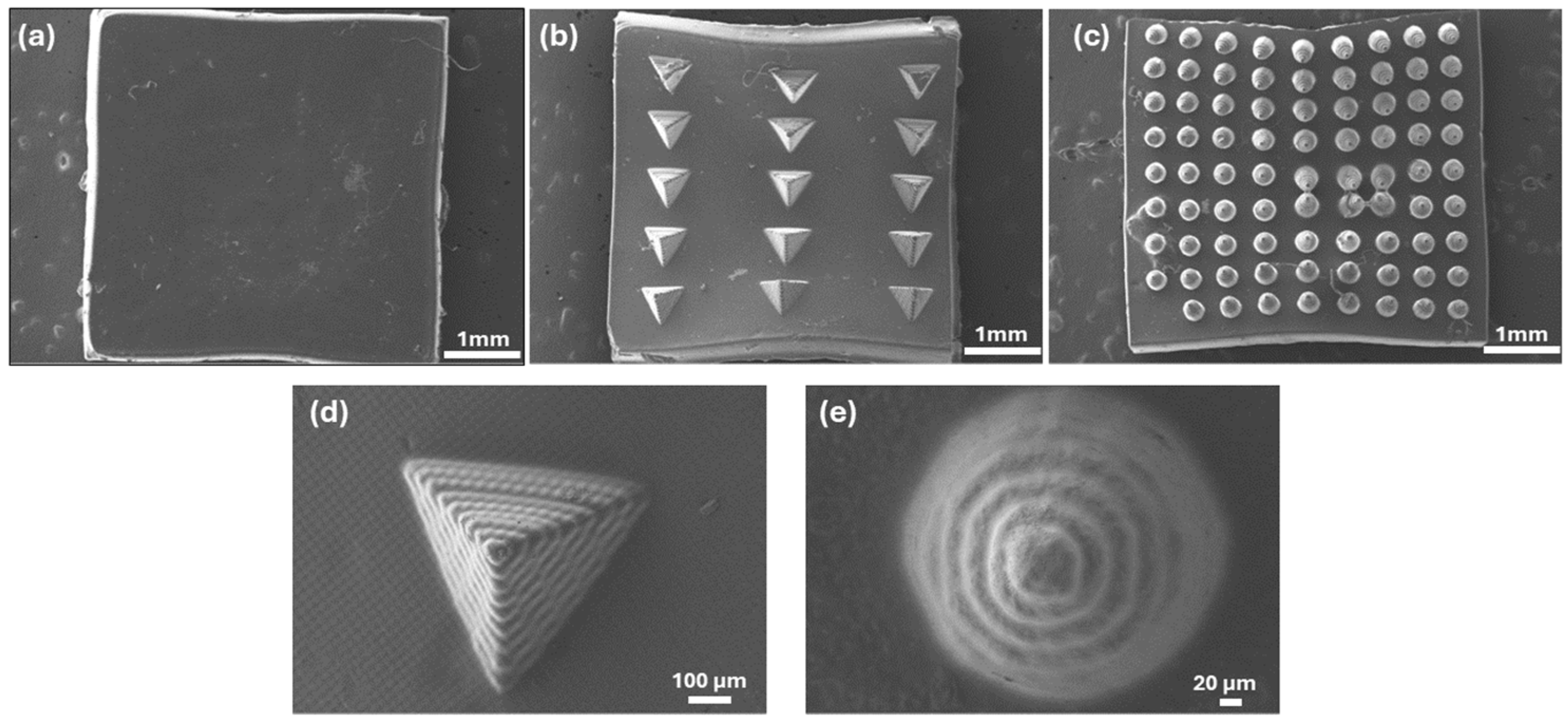
3.3. Processing of the Formulations by DLP and Characterization of the 3D Constructs
3.3.1. Surface Morphology
3.3.2. Gel Content
3.3.3. Hydrolytic Degradation
3.4. Functionalization of the 3D Constructs with Gallic Acid (GA) to Provide Antioxidant Properties
Antioxidant Properties
3.5. Adhesion Capacity of the 3D Constructs
3.6. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.-Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel Wound Dressings Accelerating Healing Process of Wounds in Movable Parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shirzaei Sani, E.; Shih, C.-D.; Lim, C.T.; Wang, J.; Armstrong, D.G.; Gao, W. Wound Management Materials and Technologies from Bench to Bedside and Beyond. Nat. Rev. Mater. 2024, 9, 550–566. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Mo, X.; Ouyang, L.; Xiong, Z.; Zhang, T. Advances in Digital Light Processing of Hydrogels. Biomed. Mater. 2022, 17, 042002. [Google Scholar] [CrossRef]
- Kammona, O.; Tsanaktsidou, E.; Kiparissides, C. Recent Developments in 3D-(Bio)Printed Hydrogels as Wound Dressings. Gels 2024, 10, 147. [Google Scholar] [CrossRef]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C.H. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 31, 2105932. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Sierra-Sánchez, Á.; Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria-de-la-Torre, R.; Martinez-Lopez, A.; Arias-Santiago, S. Mesenchymal Stromal Cell-Conditioned Medium for Skin Diseases: A Systematic Review. Front. Cell Dev. Biol. 2021, 9, 654210. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Alavi, S.Z.; Nisa, M.U.; Koohi, M.; Raza, A.; Ebrahimi Shahmabadi, H. Revolutionizing Wound Healing: Exploring Scarless Solutions through Drug Delivery Innovations. Mol. Pharm. 2024, 21, 1056–1076. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.C.M.; Rebelo, R.C.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Process Development for Flexible Films of Industrial Cellulose Pulp Using Superbase Ionic Liquids. Polymers 2021, 13, 1767. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.; Tian, J.; Lu, F.; He, M. Highly Stretchable and Compressible Cellulose Ionic Hydrogels for Flexible Strain Sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Dehghani, P.; Akbari, A.; Saadatkish, M.; Varshosaz, J.; Kouhi, M.; Bodaghi, M. Acceleration of Wound Healing in Rats by Modified Lignocellulose Based Sponge Containing Pentoxifylline Loaded Lecithin/Chitosan Nanoparticles. Gels 2022, 8, 658. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguié-Béghin, V. Langmuir–Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef]
- Weian, W.; Yunxin, Y.; Ziyan, W.; Qianzhou, J.; Lvhua, G. Gallic Acid: Design of a Pyrogallol-Containing Hydrogel and Its Biomedical Applications. Biomater. Sci. 2024, 12, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Trinh, X.T.; Long, N.V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.Y.; Heo, C.Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Guo, L.; Qiang, T.; Ma, Y.; Ren, L.; Zhu, C. Biodegradable Anti-Ultraviolet Film from Modified Gallic Acid Cross-Linked Gelatin. ACS Sustain. Chem. Eng. 2021, 9, 8393–8401. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical Tailoring of Gelatin to Adjust Its Chemical and Physical Properties for Functional Bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Nixon, E.J.; Wang, H.Y.; Dhawan, U.; Huang, Y.W.; Huang, S.H.; Jiang, C.P.; Kuo, Y.J.; Chung, R.J. Photocrosslinked Gelatin Methacryloyl (GelMA)/Hyaluronic Acid Methacryloyl (HAMA) Composite Scaffold Using Anthocyanidin as a Photoinitiator for Bone Tissue Regeneration. ACS Appl. Polym. Mater. 2023, 5, 6012–6021. [Google Scholar] [CrossRef]
- Silva, R.; Rebelo, R.C.; Paula, C.T.B.; Pereira, P.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J. All-Cellulose Resin for 3D Printing Hydrogels via Digital Light Processing (DLP). Int. J. Biol. Macromol. 2025, 306, 141389. [Google Scholar] [CrossRef]
- Hu, H.; You, J.; Gan, W.; Zhou, J.; Zhang, L. Synthesis of Allyl Cellulose in NaOH/Urea Aqueous Solutions and Its Thiol-Ene Click Reactions. Polym. Chem. 2015, 6, 3543–3548. [Google Scholar] [CrossRef]
- Yacob, N.; Hashim, K. Morphological Effect on Swelling Behaviour of Hydrogel. In Proceedings of the AIP Conference Proceedings, Kuala Lumpur, Malaysia, 30 September–2 October 2013; American Institute of Physics Inc.: Washington, DC, USA, 2014; Volume 1584, pp. 153–159. [Google Scholar]
- Rebelo, R.C.; Ribeiro, D.C.M.; Pereira, P.; De Bon, F.; Coelho, J.F.J.; Serra, A.C. Cellulose-Based Films with Internal Plasticization with Epoxidized Soybean Oil. Cellulose 2023, 30, 1823–1840. [Google Scholar] [CrossRef]
- Paula, C.T.B.; Saraiva, S.; Pereira, P.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. ROS-Responsive Electrospun Poly(Amide Thioketal) Mats for Wound Dressing Applications. Polymer 2024, 294, 126697. [Google Scholar] [CrossRef]
- Muzikova, J.; Snejdrova, E.; Martiska, J.; Doubkova, B.; Veris, A. A Study of Compressibility, Compactability and Mucoadhesivity of Tableting Materials for Matrix Systems Based on Chitosan. Polymers 2021, 13, 3636. [Google Scholar] [CrossRef]
- ISO Standard No. 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Atturu, P.; Mudigonda, S.; Wang, C.Z.; Wu, S.C.; Chen, J.W.; Forgia, M.F.F.; Dahms, H.U.; Wang, C.K. Adipose-Derived Stem Cells Loaded Photocurable and Bioprintable Bioinks Composed of GelMA, HAMA and PEGDA Crosslinker to Differentiate into Smooth Muscle Phenotype. Int. J. Biol. Macromol. 2024, 265, 130710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, Z.; Ma, L.; Wang, D.; Gao, C. Cell-Free HA-MA/PLGA Scaffolds with Radially Oriented Pores for In Situ Inductive Regeneration of Full Thickness Cartilage Defects. Macromol. Biosci. 2016, 16, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, H.; Ge, S.; Huang, L.; Chen, L.; Connor, C.; Guo, Z.; Jiang, Y.; Xu, B.B.; Peng, W. A Cellulose/Chitosan Dual Cross-Linked Multifunctional and Resilient Hydrogel for Emergent Open Wound Management. Adv. Healthc. Mater. 2024, 13, 2304676. [Google Scholar] [CrossRef] [PubMed]
- Simões, B.; Rebelo, R.C.; Ledesma, S.; Pereira, P.; Moreira, R.; Ferreira, B.C.; Coelho, J.F.J.; Serra, A.C. Development of Polyampholyte Cellulose-Based Hydrogels for Diapers with Improved Biocompatibility. Gels 2025, 11, 282. [Google Scholar] [CrossRef]
- Paula, C.T.B.; Rebelo, R.C.; Coelho, J.; Serra, A.C. The Impact of the Introduction of Hydrolyzed Cellulose on the Thermal and Mechanical Properties of LDPE Composites. Eur. J. Wood Wood Prod. 2019, 77, 1095–1106. [Google Scholar] [CrossRef]
- Rebelo, R.C.; Báguena, B.V.; Pereira, P.; Moreira, R.; Coelho, J.F.J.; Serra, A.C. Biocompatible Cellulose-Based Superabsorbents for Personal Care Products. J. Polym. Environ. 2024, 32, 5179–5194. [Google Scholar] [CrossRef]
- Caballero Aguilar, L.M.; Kapsa, R.M.; O’Connell, C.D.; McArthur, S.L.; Stoddart, P.R.; Moulton, S.E. Controlled Release from PCL-Alginate Microspheres: Via Secondary Encapsulation Using GelMA/HAMA Hydrogel Scaffolds. Soft Matter 2019, 15, 3779–3787. [Google Scholar] [CrossRef]
- Long, L.; Liu, W.; Hu, C.; Yang, L.; Wang, Y. Construction of Multifunctional Wound Dressings with Their Application in Chronic Wound Treatment. Biomater. Sci. 2022, 10, 4058–4076. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable Hydrogels: Design Mechanisms and Versatile Applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory Hydrogel Dressings and Skin Wound Healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, M.F.; de Oliveira, C.R.; Cardoso, J.C.; Bordignon, N.C.T.; Gondak, R.; Severino, P.; Souto, E.B.; de Albuquerque Júnior, R.L.C. UV-Polymerizable Methacrylated Gelatin (GelMA)-Based Hydrogel Containing Tannic Acids for Wound Healing. Drug Deliv. Transl. Res. 2023, 13, 3223–3238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent Progress of Highly Adhesive Hydrogels as Wound Dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of Chitosan, Graphene Oxide, and Cerium Oxide Composite Blended Films: Structural, Physical, and Functional Properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Lee, G.M.; Kim, S.-J.; Kim, E.M.; Kim, E.; Lee, S.; Lee, E.; Park, H.H.; Shin, H. Free Radical-Scavenging Composite Gelatin Methacryloyl Hydrogels for Cell Encapsulation. Acta Biomater. 2022, 149, 96–110. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, T.; Joshi, A.; Gugulothu, S.B.; Choudhury, S.; Singh, N. Light-Mediated 3D Printing of Micro-Pyramid-Decorated Tailorable Wound Dressings with Endogenous Growth Factor Sequestration for Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 15, 327–337. [Google Scholar] [CrossRef]
- Goh, M.C.; Du, M.; Peng, W.R.; Saw, P.E.; Chen, Z. Advancing Burn Wound Treatment: Exploring Hydrogel as a Transdermal Drug Delivery System. Drug Deliv. 2024, 31, 2300945. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering Tough, Injectable, Naturally Derived, Bioadhesive Composite Hydrogels. Adv. Healthc. Mater. 2020, 9, e1901722. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Li, Y.C.E.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue Adhesives: From Research to Clinical Translation. Nano Today 2021, 36, 101049. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cheng, H. Insights into the Photochemical Mechanism of Goethite: Roles of Different Types of Surface Hydroxyl Groups in Reactive Oxygen Species Generation and Fe(III) Reduction. Environ. Sci. Technol. 2024, 58, 14812–14822. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, S.; Yu, D.; Yang, J.; Huang, L.; Chen, L.; Li, J.; Wu, H. Cellulose-Based Dual-Crosslinked Hydrogels with Strong Wet Adhesion and Benign on-Demand Detachment. Cellulose 2024, 31, 9791–9809. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Wu, M.; Wang, X.; Zhao, Y.; Zhong, S.; Gao, Y.; Cui, X. Fe3+-Induced Coordination Cross-Linking Gallic Acid-Carboxymethyl Cellulose Self-Healing Hydrogel. Int. J. Biol. Macromol. 2024, 267, 131626. [Google Scholar] [CrossRef]



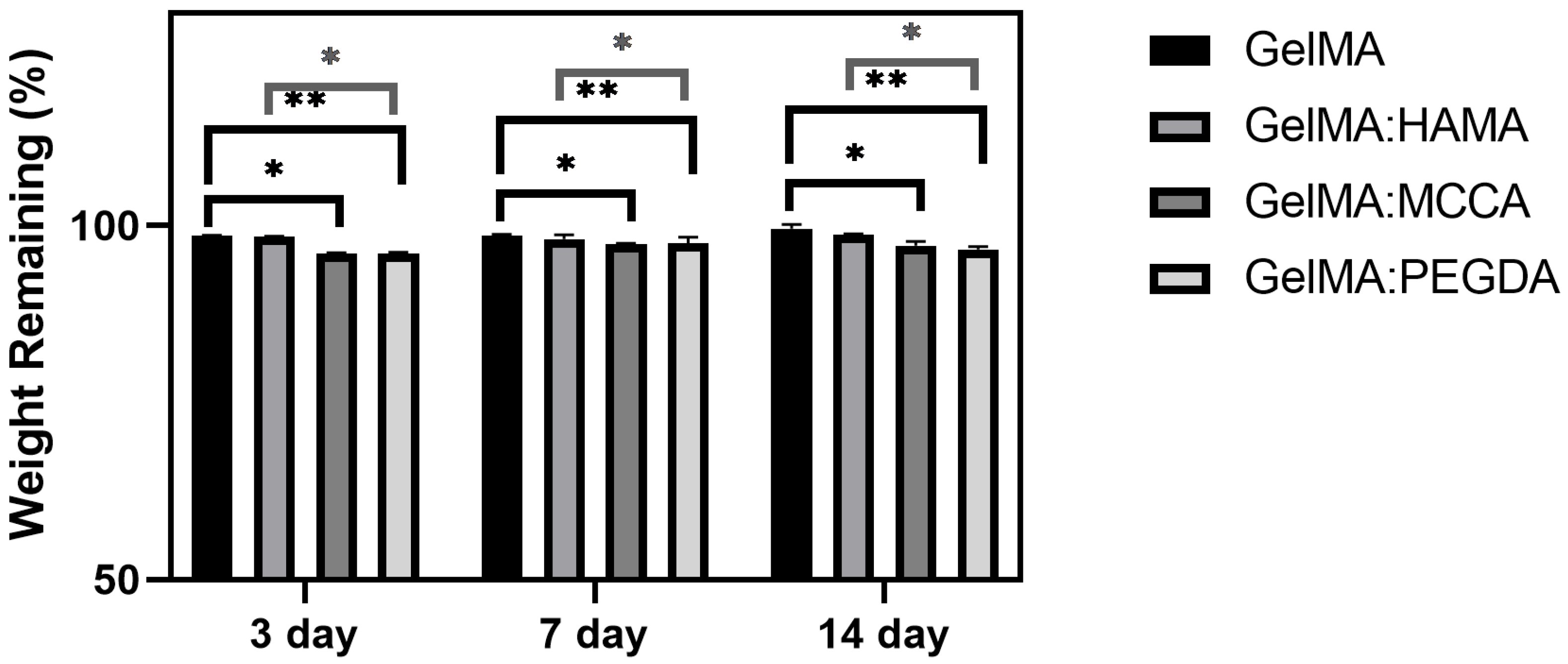

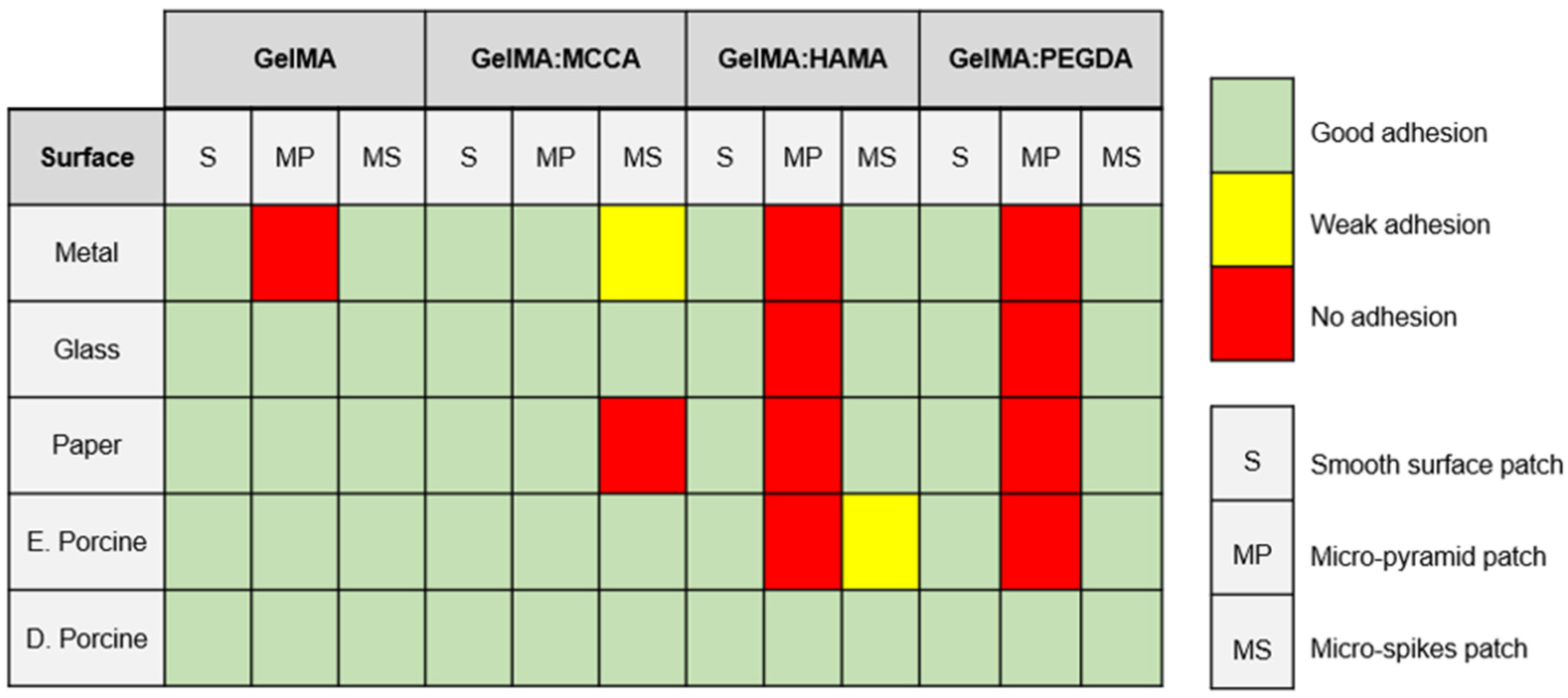
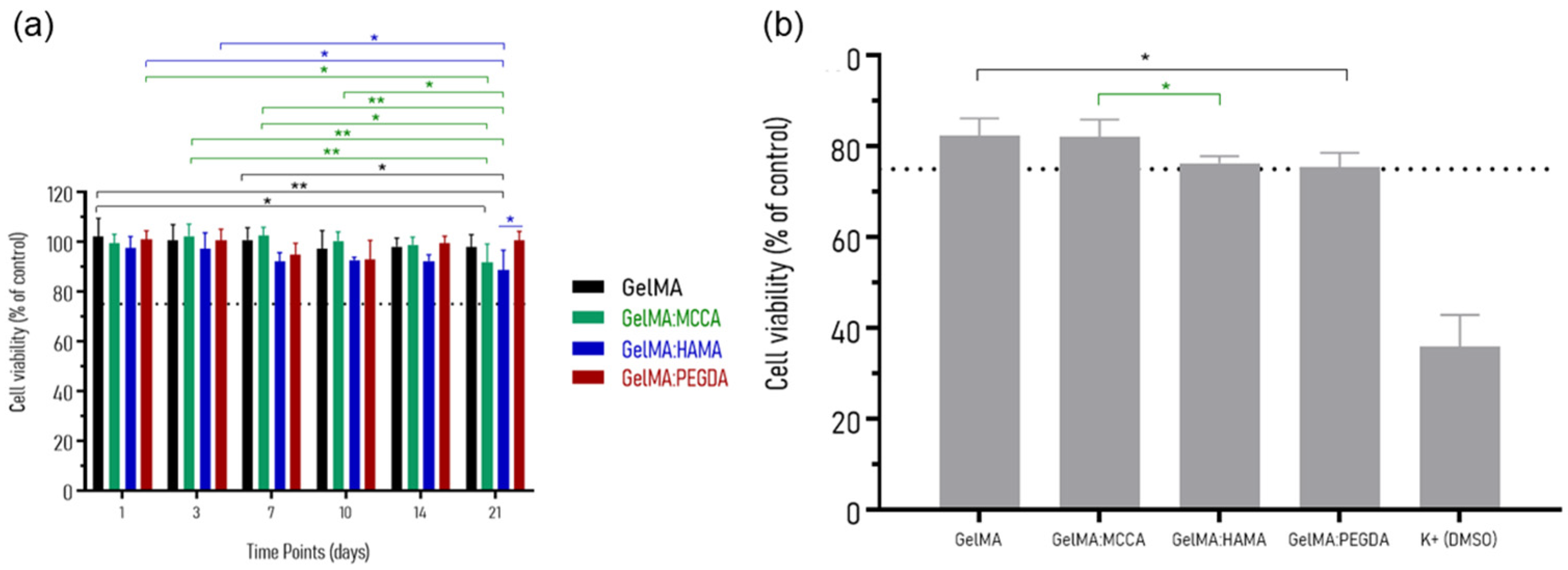
| DLP Formulation | Precursor (wt.%) | PI (wt.%) | PA (wt.%) |
|---|---|---|---|
| GelMA | 12.5% GelMA | 0.50 | 0.05 |
| GelMA:MCCA | 10% GelMA + 2.5% MCCA | ||
| GelMA:HAMA | 10% GelMA + 2.5% HAMA | ||
| GelMA:PEGDA | 10% GelMA + 2.5% PEGDA |
| Hydrogel | DS (%) |
|---|---|
| GelMA | 51.5 |
| HAMA | 37.3 |
| MCCA | 27.1 |
| Polymeric Precursor | Polymer Concentration % (w/v) | Curing Time (s) | Obs. |
|---|---|---|---|
| GelMA | 2.5 | No gelation | - |
| 5 | 120 | Weak and sticky hydrogel | |
| 10 | 60 | Soft and sticky hydrogel | |
| 15 | 60 | Stiff and sticky hydrogel | |
| 20 | 60 | Stiff hydrogel | |
| HAMA | 2.5 | 120 | Soft hydrogel |
| 5 | 120 | Soft hydrogel | |
| 10 | 120 | Elastic hydrogel | |
| 15 | 30 | Stiff hydrogel | |
| 20 | 30 | Stiff hydrogel | |
| MCCA | 2.5 | 120 | Elastic hydrogel |
| 5 | 120 | Elastic hydrogel | |
| 10 | 120 | Stiff hydrogel | |
| 15 | - | No dissolution of the precursor | |
| 20 | - | No dissolution of the precursor | |
| PEGDA | 2.5 | No gelation | - |
| 5 | 120 | Soft and elastic hydrogel | |
| 10 | 60 | Elastic hydrogel | |
| 15 | 60 | Stiff hydrogel | |
| 20 | 30 | Stiff hydrogel |
| Hydrogel | Gel Content (%) |
|---|---|
| GelMA | 87.51 ± 1.50 |
| GelMA:MCCA | 81.22 ± 0.84 |
| GelMA:HAMA | 92.30 ± 0.40 |
| GelMA:PEGDA | 94.89 ± 0.30 |
| Hydrogel | Adhesiveness (N.s) |
|---|---|
| GelMA | 2.02 ± 0.86 |
| GelMA + GA | 4.53 ± 0.46 |
| GelMA + GA + Fe | 5.21 ± 0.47 |
| GelMA:MCCA | 2.68 ± 0.07 |
| GelMA:MCCA + GA | 4.69 ± 0.14 |
| GelMA:MCCA + GA + Fe | 5.57 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.; Medeiros, M.; Paula, C.T.B.; Saraiva, S.; Rebelo, R.C.; Pereira, P.; Coelho, J.F.J.; Serra, A.C.; Fonseca, A.C. Light-Mediated 3D-Printed Wound Dressings Based on Natural Polymers with Improved Adhesion and Antioxidant Properties. Polymers 2025, 17, 1114. https://doi.org/10.3390/polym17081114
Silva R, Medeiros M, Paula CTB, Saraiva S, Rebelo RC, Pereira P, Coelho JFJ, Serra AC, Fonseca AC. Light-Mediated 3D-Printed Wound Dressings Based on Natural Polymers with Improved Adhesion and Antioxidant Properties. Polymers. 2025; 17(8):1114. https://doi.org/10.3390/polym17081114
Chicago/Turabian StyleSilva, Rute, Matilde Medeiros, Carlos T. B. Paula, Sofia Saraiva, Rafael C. Rebelo, Patrícia Pereira, Jorge F. J. Coelho, Arménio C. Serra, and Ana C. Fonseca. 2025. "Light-Mediated 3D-Printed Wound Dressings Based on Natural Polymers with Improved Adhesion and Antioxidant Properties" Polymers 17, no. 8: 1114. https://doi.org/10.3390/polym17081114
APA StyleSilva, R., Medeiros, M., Paula, C. T. B., Saraiva, S., Rebelo, R. C., Pereira, P., Coelho, J. F. J., Serra, A. C., & Fonseca, A. C. (2025). Light-Mediated 3D-Printed Wound Dressings Based on Natural Polymers with Improved Adhesion and Antioxidant Properties. Polymers, 17(8), 1114. https://doi.org/10.3390/polym17081114











