Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
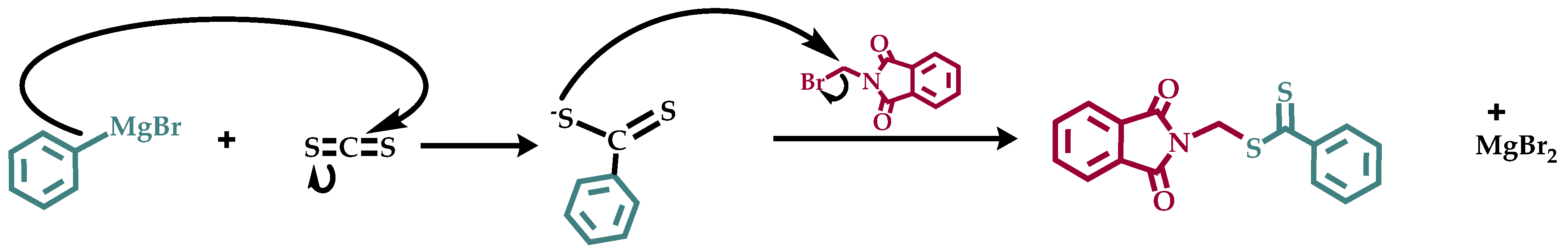
2.2. Synthesis of Phthalimidylmethyl Dithiobenzoate, CTA XM
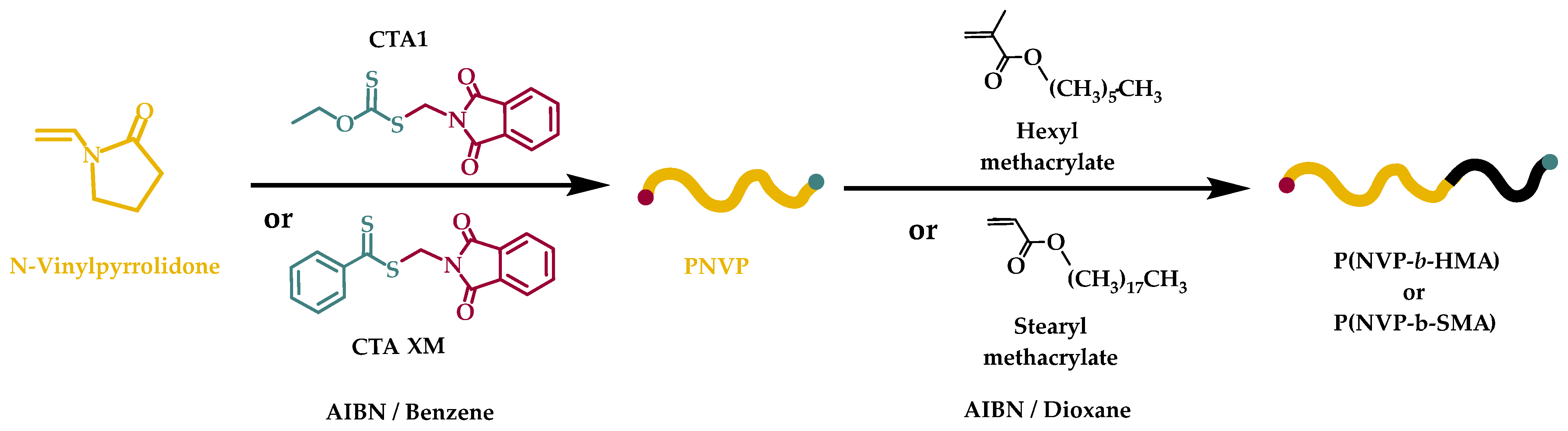
2.3. Synthesis of PNVP-b-PHMA and PNVP-b-PSMA Block Copolymers via RAFT Polymerization
2.4. Encapsulation Procedure
2.5. Characterization Techniques
3. Results and Discussion
3.1. Synthesis of Phthalimidylmethyl Dithiobenzoate, CTA XM
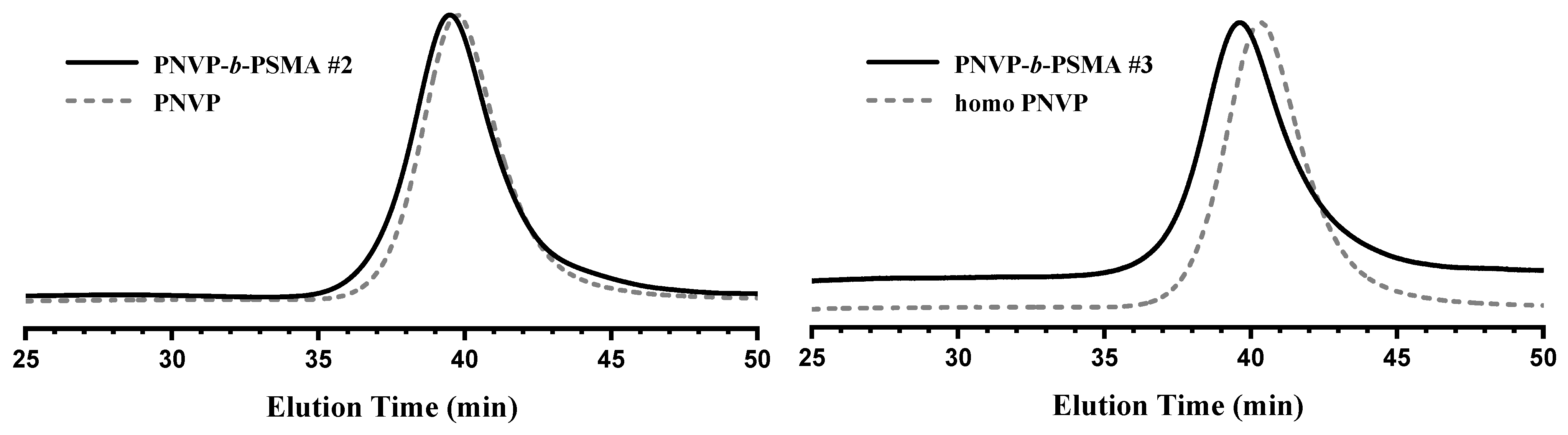
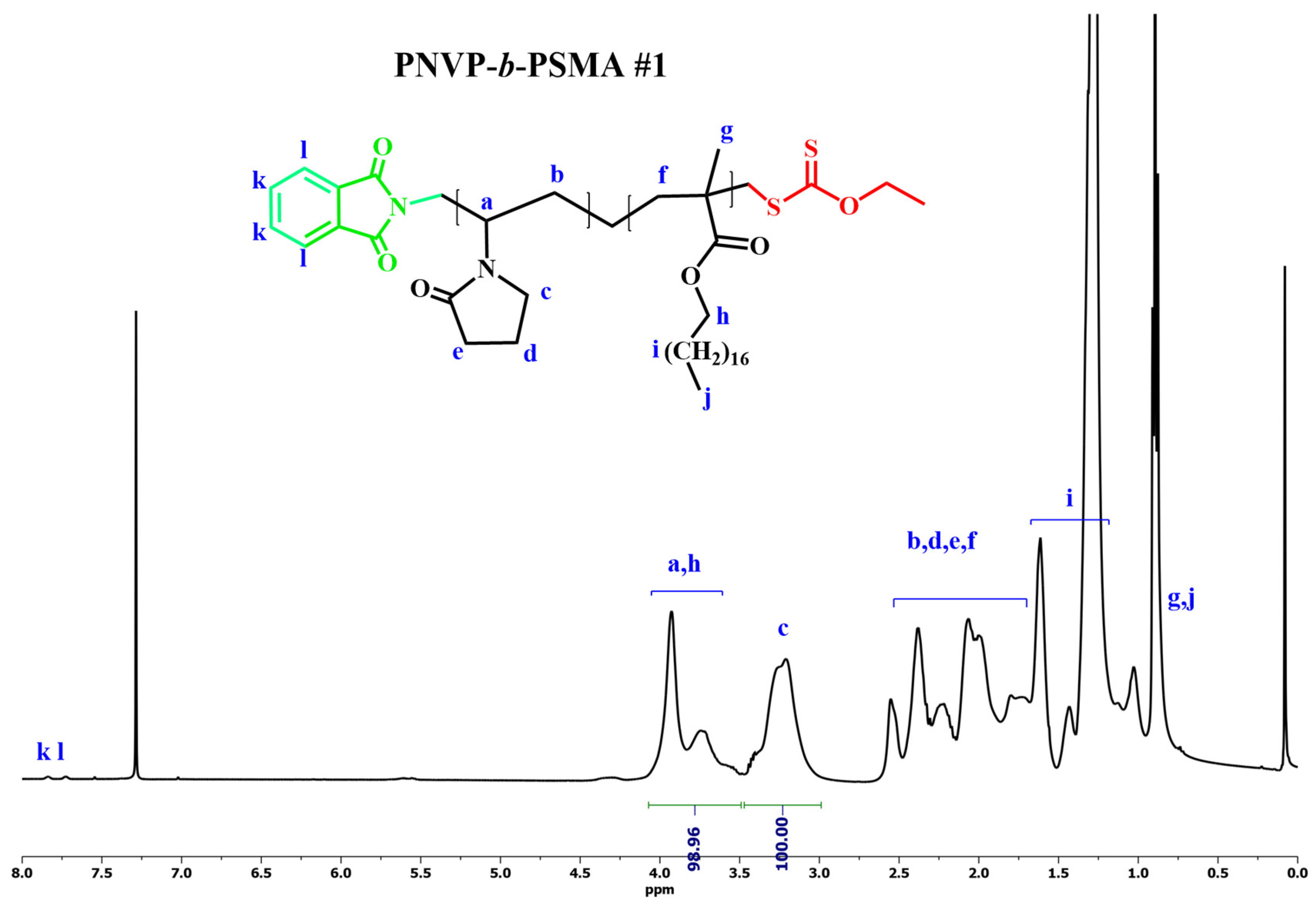
3.2. Synthesis of Block Copolymers PNVP-b-PHMA and PNVP-b-PSMA
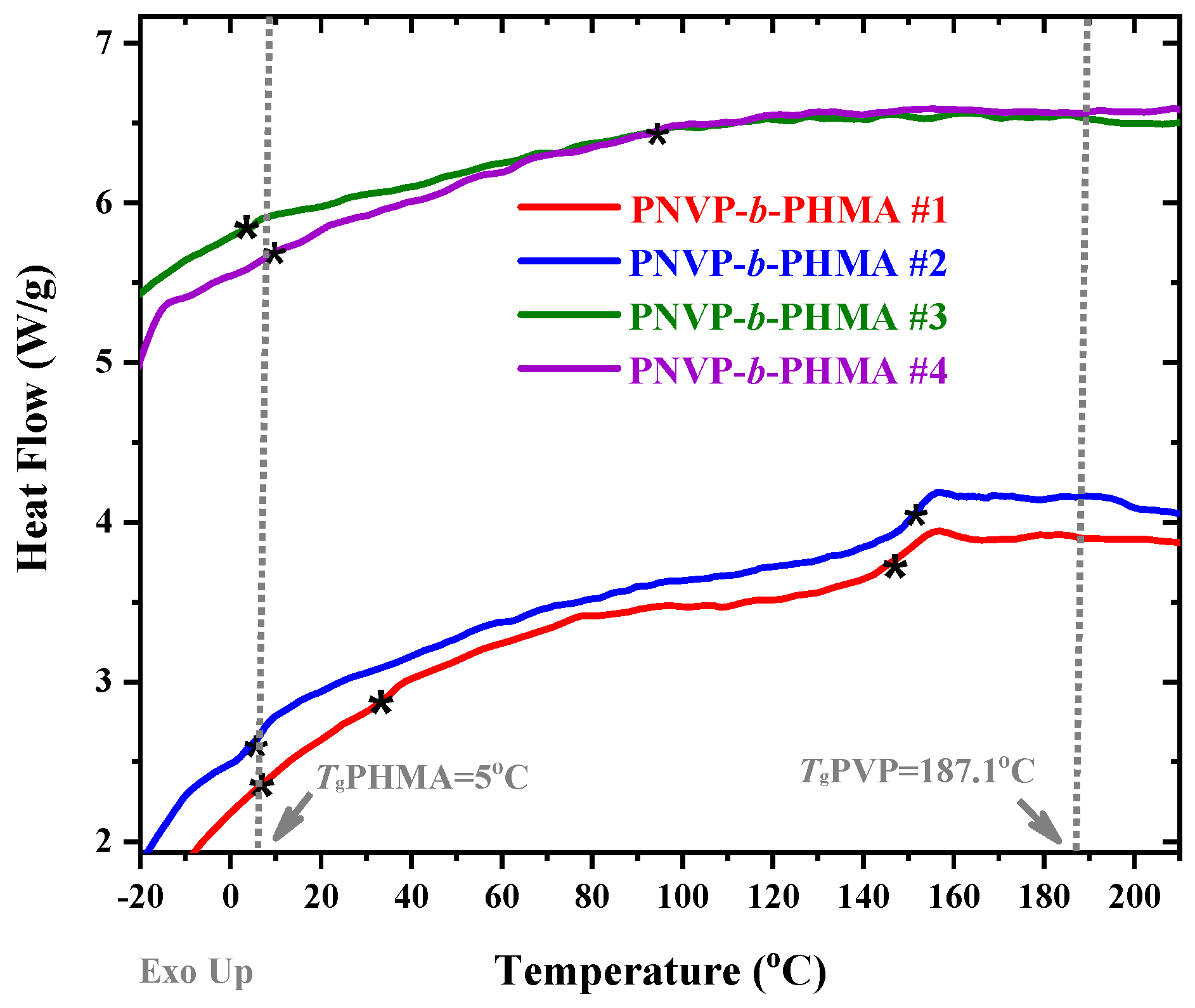
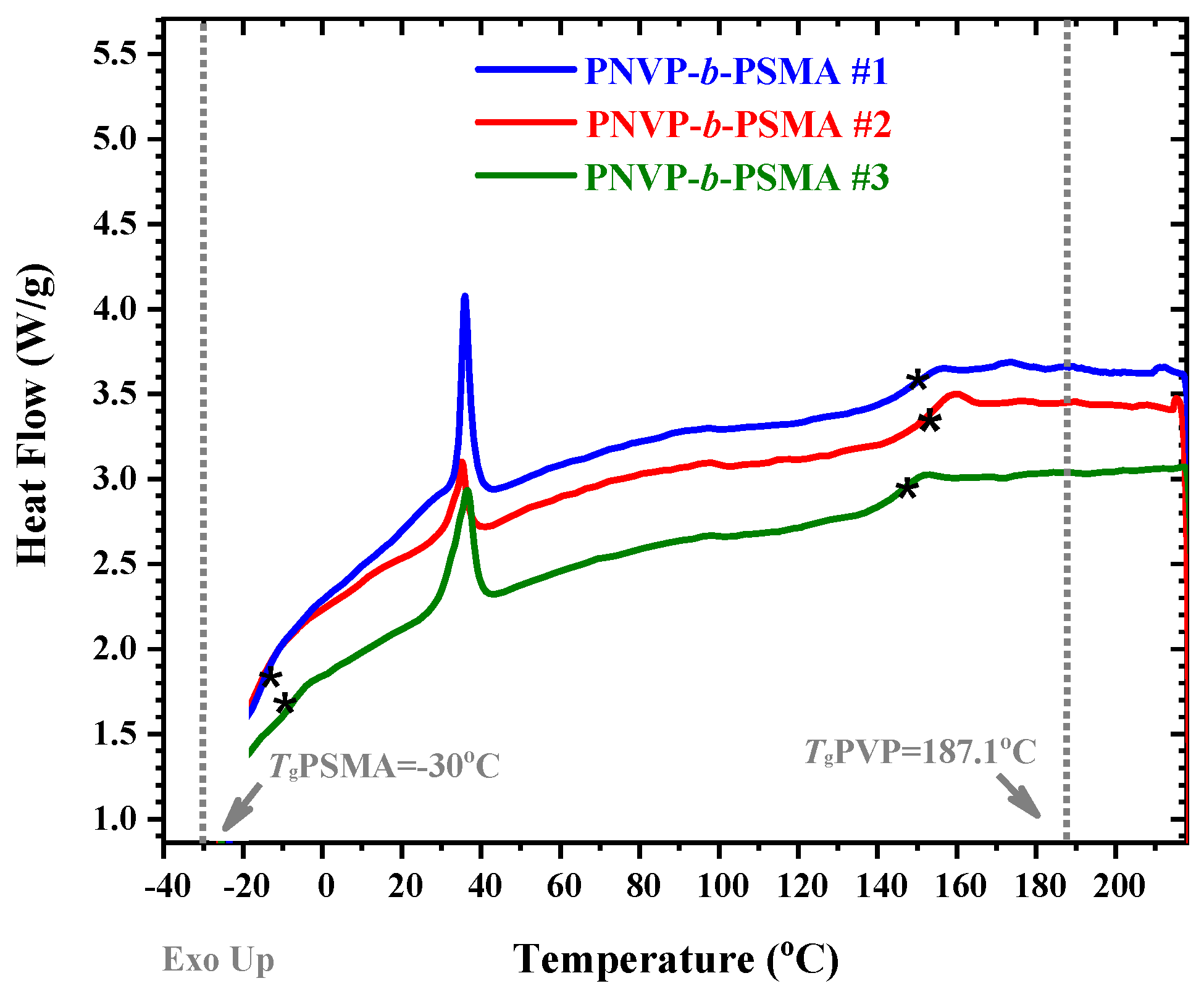
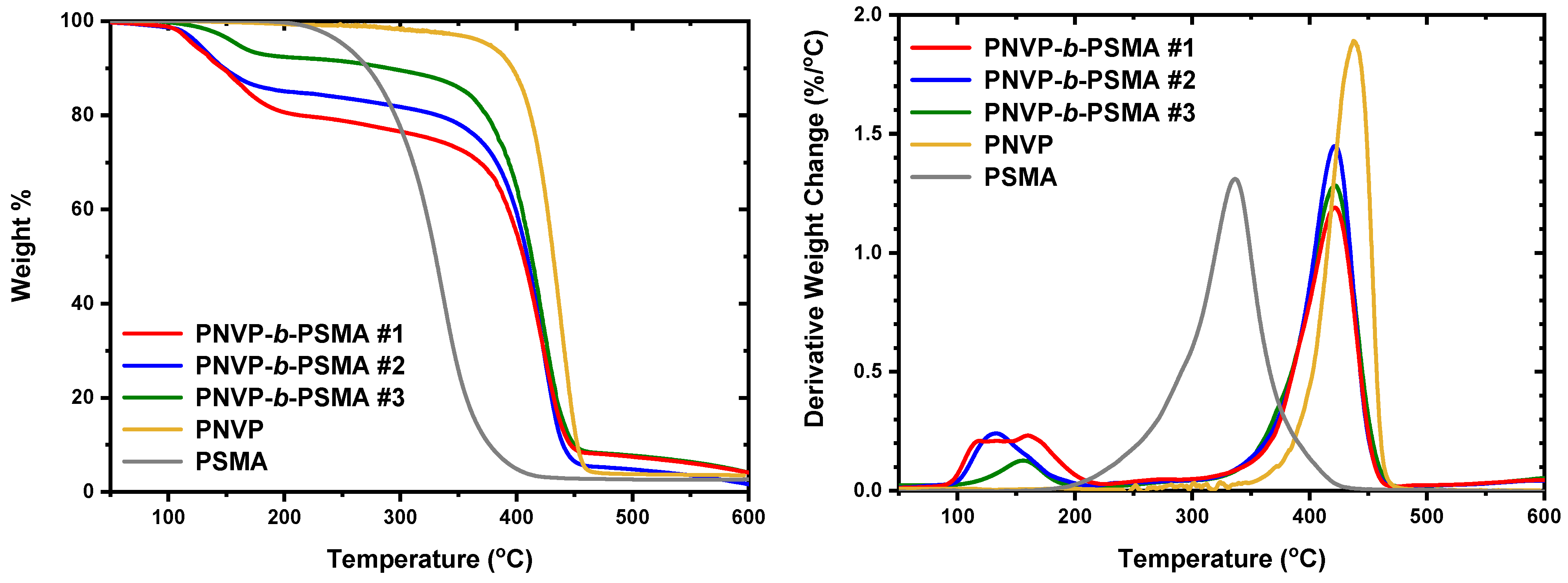
3.3. Thermal Properties
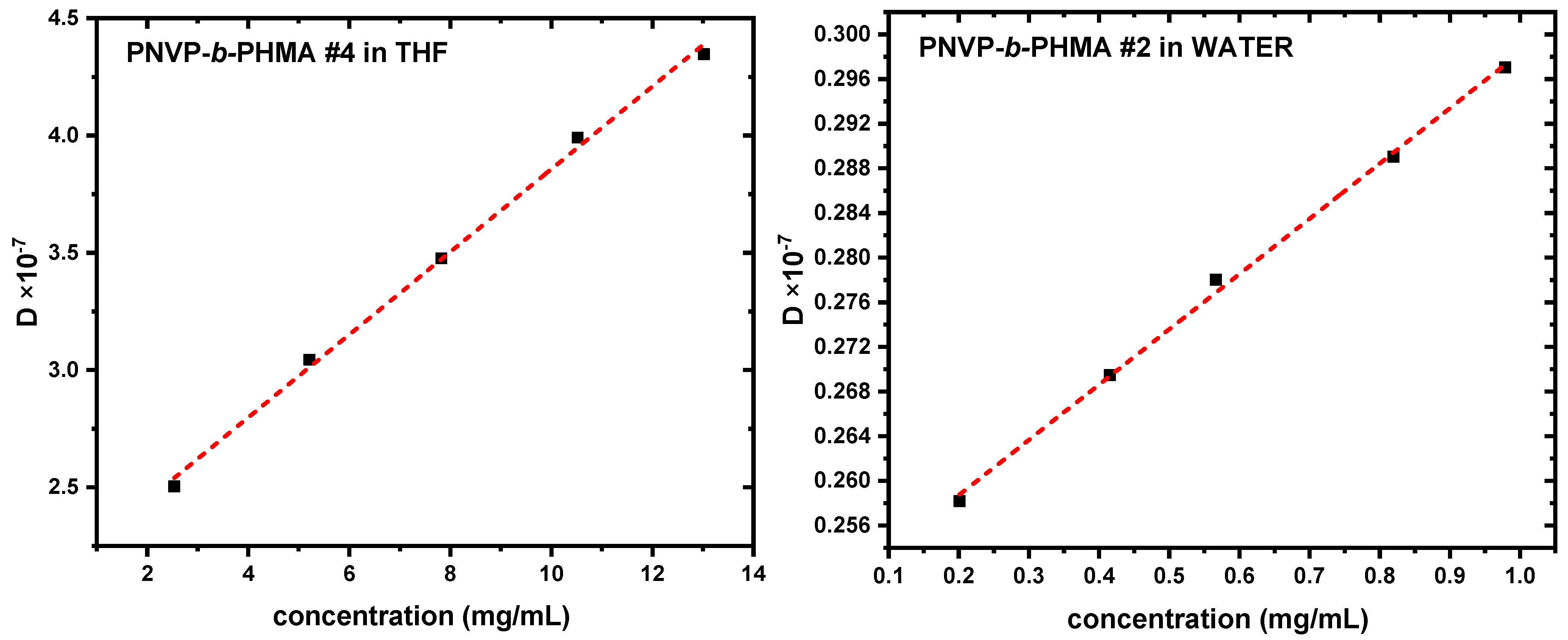
3.4. Self-Assembly Behavior of PNVP-b-PHMA Block Copolymers in THF and Aqueous Solutions
3.5. Self-Assembly Behavior of PNVP-b-PSMA Block Copolymers in THF and Aqueous Solutions
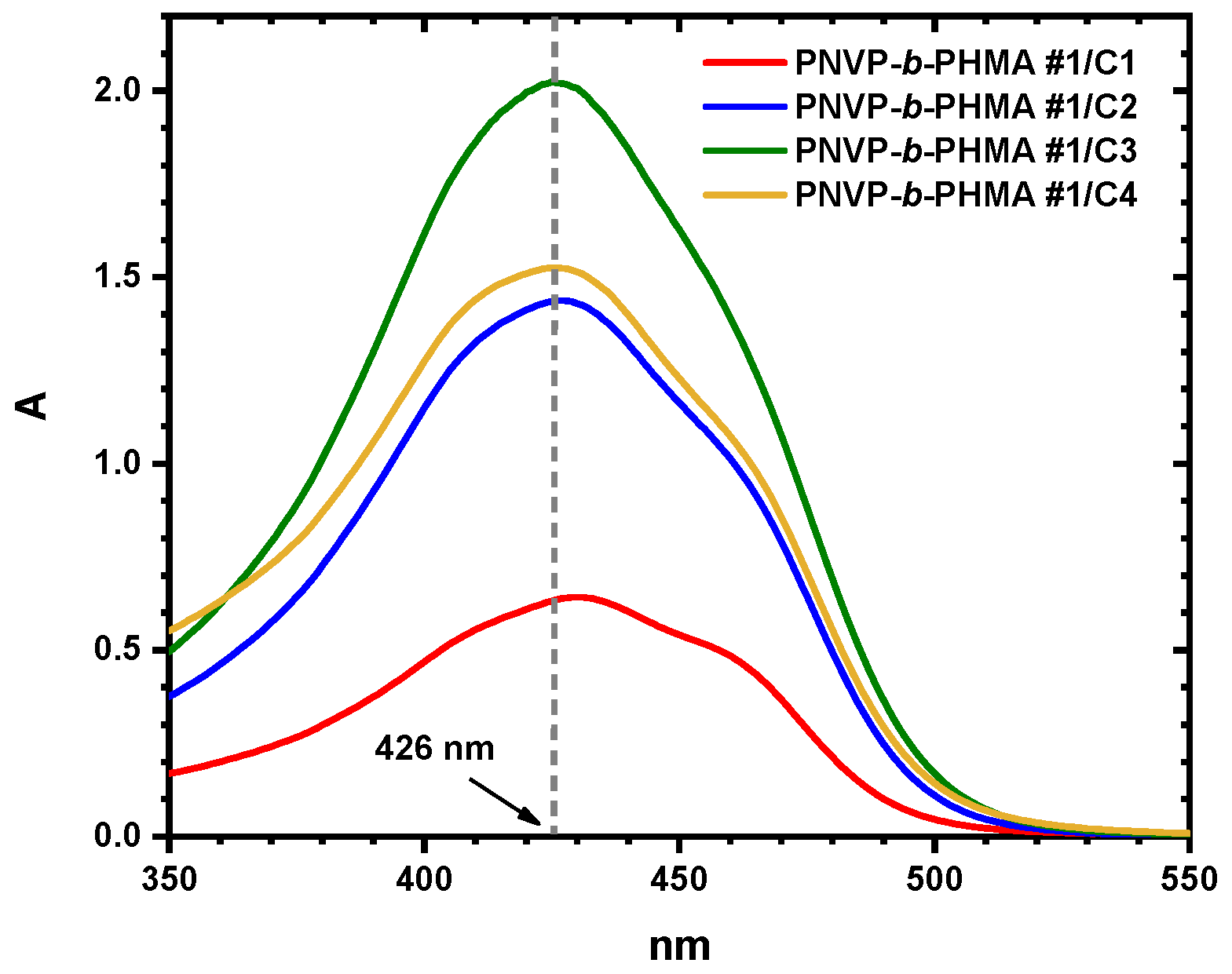
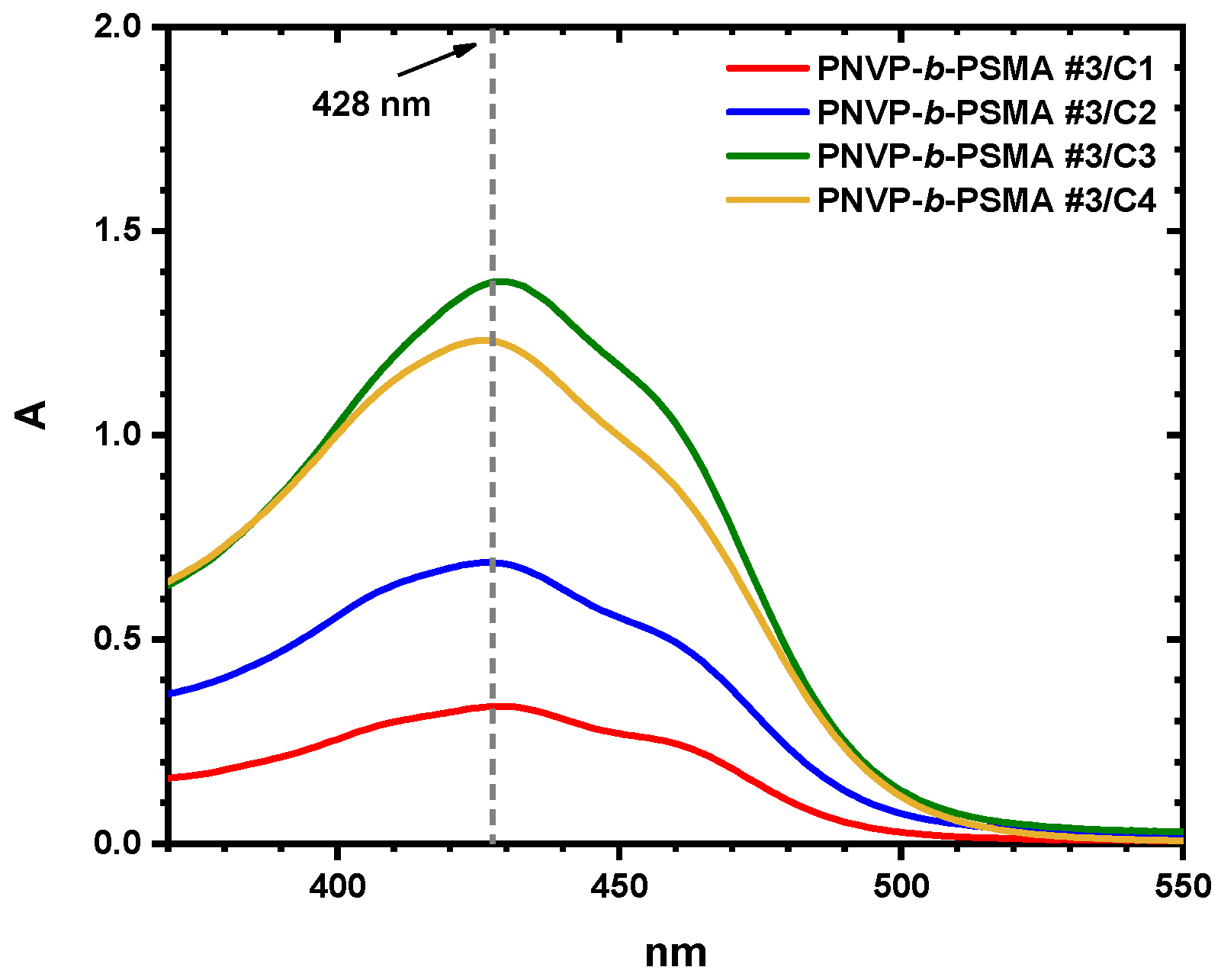
3.6. Encapsulation of Curcumin into Micellar Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Franco, F.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part. A Recovery Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Reis, C.P.; Silva, C.; Martinho, N.; Rosado, C. Drug carriers for oral delivery of peptides and proteins: Accomplishments and future Perspectives. Therap. Deliv. 2013, 4, 251–265. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Dolman, M.E.M.; Harmsen, S.; Storm, G.; Hennink, W.E.; Kok, R.J. Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug Deliv. Rev. 2010, 62, 1344–1357. [Google Scholar] [CrossRef]
- Kurakula, M.; Koteswara Rao, G.S.N. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly(N-vinylpyrrolidone)-Modified Surfaces for Biomedical Applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.-C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; et al. Recent application developments of water-soluble synthetic polymers. J. Industr. Engin. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem. Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Y.; Bae, Y.H. Polymer Architecture and Drug Delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Karanikolopoulos, N.; Pitsikalis, M.; Hadjichristidis, N.; Georgikopoulou, K.; Calogeropoulou, T.; Dunlap, J.R. pH-Responsive aggregates from double hydrophilic block copolymers carrying zwitterionic groups. Encapsulation of antiparasitic compounds for the treatment of leishmaniasis. Langmuir 2007, 23, 4214–4224. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Karanikolopoulos, N.; Zamurovic, M.; Pitsikalis, M.; Hadjichristidis, N. Poly(DL-lactide)-b-Poly(N,N-dimethylamino-2-ethyl methacrylate): Synthesis, characterization, micellization behaviour in aqueous solutions and encapsulation of the hydrophobic drug dipyridamole. Biomacromolecules 2010, 11, 430–438. [Google Scholar] [CrossRef]
- Trubetskoy, V.S. Polymeric micelles as carriers of diagnostic agents. Adv. Drug Deliv. Rev. 1999, 37, 81–88. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Kataoka, K. Block copolymer micelles for delivery of gene and related compounds. Adv. Drug Deliv. Rev. 2002, 54, 203–222. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Valetsky, P.M.; Hartmann, J.; Coelfen, H.; Schnablegger, H.; Antonietti, M. Stabilization of metal nanoparticles in aqueous medium by poly(ethylene oxide)-poly(ethylene imine) block copolymers. J. Colloid. Interface Sci. 1999, 212, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Yin, W.; Armstrong, J.L.; Webber, S.E. Adsorption of photoactive amphiphilic polymers onto hydrophobic polymer films polystyrene-b-poly(2-vinylnaphthalene)-b-poly(methacrylic acid). Langmuir 1994, 10, 1841–1847. [Google Scholar] [CrossRef]
- Spatz, J.P.; Sheiko, S.; Möller, M. Ion stabilized block copolymer micelles film formation and inter micellar interaction. Macromolecules 1996, 29, 3220–3226. [Google Scholar] [CrossRef]
- Lazzari, M.; Scalarone, D.; Hoppe, C.E.; Vazquez-Vazquez, C.; Lòpez-Quintela, M.A. Tunable polyacrylonitrile based micellar aggregates as a potential tool for the fabrication of carbon nanofibers. Chem. Mater. 2007, 19, 5818–5820. [Google Scholar] [CrossRef]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.-S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Alhan, H.B.; et al. Linear block copolymer synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J.W. Macromolecular architectures by living and controlled/living polymerizations. Progr. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Dey, A.; Hldar, U.; De, P. Block copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front. Chem. 2021, 9, 644547. [Google Scholar] [CrossRef]
- Diaz, C.; Mehrkhodavandi, P. Strategies for the synthesis of block copolymers with biodegradable polyester segments. Polym. Chem. 2021, 12, 783–806. [Google Scholar] [CrossRef]
- Theodosopoulos, G.; Pitsikalis, M. Block Copolymers: Recent Synthetic Routes and Developments. In Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Matyjaszewski, K. (Ed.) Cationic polymerization. In Mechanisms, Synthesis and Applications; Marcel Dekker Inc.: New York, NY, USA, 1996. [Google Scholar]
- Kennedy, J.P.; Iván, B. Designed Polymers by Carbocationic Macromolecular Engineering; Theory and Practice; Hanser Publishers: Munich, Germany, 1992. [Google Scholar]
- Coessens, V.; Pintauer, T.; Matyjaszewski, K. Functional polymers by atom transfer radical polymerization. Progr Polym. Sci. 2001, 26, 337–377. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- McCormick, C.J.; Lowe, A.B. Aqueous RAFT polymerization: Recent developments in synthesis of functional water-soluble (co) polymers with controlled structures. Acc. Chem. Res. 2004, 37, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R. Homogeneous metathesis polymerization by well-defined group VI and group VIII transition-metal alkylidenes: Fundamentals and applications in the preparation of advanced materials. Chem. Rev. 2000, 100, 1565–1604. [Google Scholar] [CrossRef] [PubMed]
- Ofstead, E.A.; Wagener, K.B. New Methods for Polymer Synthesis; Mijs, W.J., Ed.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Trnka, T.M.; Grubbs, R.H. The development of L2X2Ru CHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K. PEO-related block copolymer surfactants. Colloids Surf. A 2001, 183, 277–292. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Ulrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar]
- Xiong, X.Y.; Tam, K.C.; Gan, L.H. Release kinetics of hydrophobic and hydrophilic model drugs from pluronic F127/poly(lactic acid) nanoparticles. J. Control. Release 2005, 103, 73–82. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ha, J.C.; Lee, Y.M. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide)/poly(ϵ-caprolactone)(PCL) amphiphilic block copolymeric nanospheres: II. Thermo-responsive drug release behaviors. J. Control. Release 2000, 65, 345–358. [Google Scholar] [CrossRef]
- Son, K.; Ueda, M.; Taguchi, K.; Maruyama, T.; Takeoka, S.; Ito, Y. Evasion of the accelerated blood clearance phenomenon by polysarcosine coating of liposomes. J. Control. Release 2020, 322, 209–216. [Google Scholar] [CrossRef]
- Hatori, Y.; Tamaki, K.; Sakasai, S.; Ozaki, K.J.; Onishi, H. Effects of PEG anchors in PEGylated siRNA lipoplexes on in vitro gene-silencing effects and siRNA biodistribution in mice. Mol. Med. Rep. 2020, 22, 4183–4196. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science 2020, 80. [Google Scholar] [CrossRef]
- Berger, M.; Toussaint, F.; Ben Djemaa, S.; Laloy, J.; Pendeville, H.; Evrard, B.; Jerôme, C.; Lechanteur, A.; Mottet, D.; Debuigne, A.; et al. Poly(vinyl pyrrolidone) derivatives as PEG alternatives for stealth, non-toxic and less immunogenic siRNA-containing lipoplex delivery. J. Control. Release 2023, 361, 87–101. [Google Scholar] [CrossRef]
- Torchilin, V.; Levchenko, T.; Whiteman, K.; Yaroslav, A.; Tsatsakis, A.; Rizos, A.; Michailova, E.; Shtilman, M. Amphiphilic poly-N-vinylpyrrolidone synthesis, properties and liposome surface modification. Biomaterials 2001, 22, 3035–3044. [Google Scholar] [CrossRef]
- Yamskov, L.A.; Kuskov, A.N.; Babievsky, K.K.; Berezin, B.B.; Krayukhina, N.; Samoylova, A.; Tikhonov, V.E.; Shtilman, M.I. Novel liposomal forms of antifungal antibiotics modified by amphiphilic polymers. Appl. Biochem. Microbiol. 2008, 44, 624–628. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Xu, X.; Gao, N.; Liu, X. Preparation, characterization and in vivo pharmacokinetic study of PVP-modified oleanolic acid liposomes. Int. J. Pharm. 2017, 517, 1–7. [Google Scholar] [CrossRef]
- Roka, N.; Kokkorogianni, O.; Kontoes-Georgoudakis, P.; Choinopoulos, I.; Pitsikalis, M. Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-vinyl pyrrolidone) and the RAFT Polymerization Technique. Polymers 2022, 14, 701. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Pagidi, S.; Park, H.S.; Lee, D.Y.; Kim, M.S.; Lee, S.H. Nanosize-confined nematic liquid crystals at slippery interfaces of polymer composites consisting of poly(hexyl methacrylate). J. Mol. Liquids 2022, 350, 118540. [Google Scholar] [CrossRef]
- Nielsen, B.V.; Nevell, T.G.; Barbu, E.; Smith, J.R.; Rees, G.D.; Tsibouklis, J. Multifunctional poly(alkyl methacrylate) films for dental care. Biomed. Mater. 2011, 6, 015003. [Google Scholar] [CrossRef]
- Li, J.; Barrow, D.; Howell, H.; Kalachandra, S. In vitro drug release study of methacrylate polymer blend system: Effect of polymer blend composition, drug loading and solubilizing surfactants on drug release. J. Mater. Sci. Mater. Med. 2010, 21, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, C.; Salz, U.; Moszner, N.; Fiore, G.L.; Weder, C. Supramolecular Cross-Links in Poly(alkyl methacrylate) Copolymers and Their Impact on the Mechanical and Reversible Adhesive Properties. ACS Appl. Mater. Interf. 2015, 7, 13395–13404. [Google Scholar] [CrossRef] [PubMed]
- Khai, N.; Nguyen, H.; Dang, H.H.; Nguyen, L.T.; Nguyen, L.M.T.; Truong, T.T.; Nguyen, H.T.; Nguyen, T.Q.; Tran, C.D.; Nguyen, L.-T.T. Self-healing elastomers from supramolecular random copolymers of 4-vinyl pyridine. Europ. Polym. J. 2023, 199, 112474. [Google Scholar]
- Oktay, B.; Baştürk, E.; Kahraman, M.V.; Apohan, N.K. Designing Coconut Oil Encapsulated Poly(stearyl methacrylate-co-hydroxylethyl metacrylate) Based Microcapsule for Phase Change Materials. Chem. Select 2019, 4, 5110–5151. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, B.; Wang, S.; Liu, M.; Zhang, Y.; Tao, L.; Wei, Y. Aggregation-induced emission material based fluorescent organic nanoparticles: Facile PEGylation and cell imaging applications. RSC Adv. 2013, 3, 9633–9636. [Google Scholar] [CrossRef]
- Jennings, J.; Butler, M.F.; McLeod, M.; Csányi, E.; Ryan, A.J.; Mykhaylyk, O.O. Stearyl Methacrylate-Based Polymers as Crystal Habit Modifiers for Triacylglycerols. Cryst. Growth Des. 2018, 18, 7094–7105. [Google Scholar] [CrossRef]
- Pingpin, Z.; Yuanli, L.; Haiyang, Y.; Xiaoming, C. Effect of non-ideal mixed solvents on dimensions of poly(N-vinylpyrrolidone) and poly(methyl methacrylate) coils. J. Macromol. Sci. Part B Polym. Phys. 2006, 45, 1125–1134. [Google Scholar]
- Roka, N.; Pitsikalis, M. Synthesis and micellization behavior of amphiphilic block copolymers of poly(N-vinyl pyrrolidone) and poly(benzyl methacrylate): Block versus statistical copolymers. Polymers 2023, 15, 2215. [Google Scholar] [CrossRef]
- Kontoes-Georgoudakis, P.; Plachouras, N.V.; Kokkorogianni, O.; Pitsikalis, M. Amphiphilic block copolymers of poly(N-vinyl pyrrolidone) and poly(isobornyl methacrylate). Synthesis, characterization and micellization behaviour in selective solvents. Eur. Polym. J. 2024, 208, 112873. [Google Scholar] [CrossRef]
- Wan, D.; Satoh, K.; Kamigaito, M.; Okamoto, Y. Xanthate-mediated radical polymerization of N-vinylpyrrolidone in fluoroalcohols for simultaneous control of molecular weight and tacticity. Macromolecules 2005, 38, 10397–10405. [Google Scholar] [CrossRef]
- Postma, A.; Davis, T.P.; Li, G.; Moad, G.; O’Shea, M.S. RAFT polymerization with phthalimidomethyl trithiocarbonates or xanthates. On the origin of bimodal molecular weight distributions in living radical polymerization. Macromolecules 2006, 39, 5307–5318. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Experimental techniques in high-vacuum anionic polymerization. J. Polym. Sci. Part. A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Roka, N.; Pitsikalis, M. Statistical copolymers of N-vinylpyrrolidone and benzyl methacrylate via RAFT: Monomer reactivity ratios, thermal properties and kinetics of thermal decomposition. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 222–230. [Google Scholar] [CrossRef]
- Hwang, Y.; Patterson, G.D.; Stevens, J.R. Photon correlation spectroscopy of bulk poly(n-hexyl methacrylate) near the glass transition. J. Polym. Sci. Part. B Polym. Phys. Ed. 1996, 34, 2291–2305. [Google Scholar] [CrossRef]
- Hempel, E.; Beiner, M.; Huth, H.; Donth, E. Temperature modulated DSC for the multiple glass transition in poly(n-alkyl methacrylates). Thermochim. Acta 2002, 391, 219–225. [Google Scholar] [CrossRef]
- Gedde, U.W. Polymer Physics, 3rd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Alig, I.; Jarek, M.; Hellmann, G.P. Restricted segmental mobility in side-chain crystalline comblike polymers, studied by dielectric relaxation measurements. Macromolecules 1998, 31, 2245–2251. [Google Scholar] [CrossRef]
- Pitsikalis, M.; Siakali-Kioulafa, E.; Hadjichristidis, N. Block Copolymers of Styrene and Stearyl Methacrylate. Synthesis and Micellization Properties in Selective Solvents. Macromolecules 2000, 33, 5460–5469. [Google Scholar] [CrossRef]
- Ruzette, A.-V.G.; Banerjee, P.; Mayes, A.M.; Pollard, M.; Russell, T.P.; Jerome, R.; Slawecki, T.; Hjelm, R.; Thiyagarajan, P. Phase behavior of diblock copolymers between styrene and n-alkyl methacrylates. Macromolecules 1998, 31, 8509–8516. [Google Scholar] [CrossRef]
- Gikarakis, T.; Pappas, I.; Arvanitaki, P.; Pantazi, E.; Mitsoni, E.; Roka, N.; Pitsikalis, M. Thermal stability and kinetics of thermal decomposition of statistical copolymers of N-vinylpyrrolidone and alkyl methacrylates synthesized via RAFT polymerization. J. Chem. 2021, 2021, 6633052. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggrawal, B.B. Bioavailability of curcumin: Problems and promise. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Tomeh, M.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Scandalis, A.; Selianitis, D.; Pispas, S. PnBA-b-PNIPAM-b-PDMAEMA thermos-responsive triblock terpolymers and their quaternized analogs as gene and drug delivery vectors. Polymers 2021, 13, 2361. [Google Scholar] [CrossRef]




| Macro-CTA (PNVP) * | Block Copolymers * | NVP | HMA | |||
|---|---|---|---|---|---|---|
| Sample | Mn 103 (Daltons) | Ð | Mn 103 (Daltons) | Ð | % mol ** | % mol ** |
| PNVP-b-PHMA #1 | 29.8 | 1.29 | 36 | 1.26 | 87 | 13 |
| PNVP-b-PHMA #2 | 10.6 | 1.26 | 13 | 1.27 | 84 | 16 |
| PNVP-b-PHMA #3 | 27.0 | 1.60 | 32 | 1.68 | 74 | 26 |
| PNVP-b-PHMA #4 | 6.3 | 1.34 | 130 | 1.53 | 5 | 95 |
| Macro-CTA (PNVP) * | Block Copolymers * | NVP | SMA | |||
|---|---|---|---|---|---|---|
| Sample | Mn 103 (Daltons) | Ð | Mn 103 (Daltons) | Ð | % mol ** | % mol ** |
| PNVP-b-PSMA #1 | 29.8 | 1.29 | 37 | 1.31 | 67 | 33 |
| PNVP-b-PSMA #2 | 7.9 | 1.28 | 9.3 | 1.35 | 94 | 6 |
| PNVP-b-PSMA #3 | 7.9 | 1.28 | 10.8 | 1.31 | 83 | 17 |
| Sample | Tg Experimental (°C) | ||
|---|---|---|---|
| PNVP-b-PHMA #1 | 11.4 | 36.7 | 151.3 |
| PNVP-b-PHMA #2 | 7.1 | - | 152.8 |
| PNVP-b-PHMA #3 | 5.5 | - | 118.1 |
| PNVP-b-PHMA #4 | 18.5 | - | 114.6 |
| PNVP | - | - | 187.1 |
| PHMA | 5 | - | - |
| Sample | WSMA % | Tm (°C) | ΔH(j/g block) | ΔH (j/gSMA) | Xc % | Tg1 (°C) | Tg2 (°C) |
|---|---|---|---|---|---|---|---|
| PNVP-b-PSMA #1 | 59.9 | 35.9 | 31.6 | 52.7 | 69.4 | −14.8 | 147.0 |
| PNVP-b-PSMA #2 | 16.2 | 35.2 | 9.6 | 59.2 | 77.9 | −15.9 | 154.7 |
| PNVP-b-PSMA #3 | 38.4 | 36.6 | 22.3 | 58.0 | 76.4 | −7.9 | 147.2 |
| PNVP | - | - | - | - | - | - | 187.1 |
| PSMA | - | 34.0 | - | 75.9 | - | −30 | - |
| Sample | Start1 | End1 | Max1 (°C) | Start2 | End2 | Max2 (°C) |
|---|---|---|---|---|---|---|
| PNVP-b-PHMA #1 | 94.9 | 220.4 | 128.7 (broad) | 320.2 | 466.1 | 420.9 |
| PNVP-b-PHMA #2 | 94.9 | 194.5 | 127.7 | 318.9 | 463.8 | 421.0 |
| PNVP-b-PHMA #3 | 204.3 | 413.2 | 296.9 | - | - | - |
| PNVP-b-PHMA #4 | 188.3 | 333.0 | 291.5 (broad) | - | - | - |
| PNVP | - | - | - | 347.63 | 484.06 | 437.53 |
| PHMA | 190.8 | 356.6 | 299.1 (shoulder) | - | - | - |
| Sample | Start1 | End1 | Max1 (°C) | Start2 | End2 | Max2 (°C) |
|---|---|---|---|---|---|---|
| PNVP-b-PSMA #1 | 88.8 | 216.6 | 140.8 (broad) | 339.4 | 463.7 | 419.3 |
| PNVP-b-PSMA #2 | 94.3 | 209.2 | 129.1 | 312.6 | 469.2 | 423.5 |
| PNVP-b-PSMA #3 | 113.4 | 198.9 | 154.8 | 323.6 | 470.8 | 421.0 |
| PNVP | - | - | - | 347.6 | 484.0 | 437.5 |
| PSMA | 185.9 | 430.3 | 335.8 | - | - | - |
| Sample | Solvent | MW From SEC | Mw From SLS | Nw | Rg (nm) | A (cm3mol/g2) |
|---|---|---|---|---|---|---|
| PNVP-b-PHMA #1 | CHCl3 | 36 × 103 | ||||
| PNVP-b-PHMA #1 | THF | 5.59 × 104 | 1.55 | 2.20 × 10−4 | ||
| PNVP-b-PHMA #1 | WATER | 5.88 × 107 | 1633 | 59.9 | 2.96 × 10−5 | |
| PNVP-b-PHMA #2 | CHCl3 | 13 × 103 | ||||
| PNVP-b-PHMA #2 | THF | 2.84 × 104 | 2.18 | 1.75 × 10−4 | ||
| PNVP-b-PHMA #2 | WATER | 3.58 × 108 | 27,538 | 103.6 | 9.98 × 10−6 | |
| PNVP-b-PHMA #3 | CHCl3 | 32 × 103 | ||||
| PNVP-b-PHMA #3 | THF | 1.32 × 105 | 4.13 | 4.20 × 10−5 | ||
| PNVP-b-PHMA #3 | WATER | 8.06 × 108 | 25,187 | 124.0 | 1.18 × 10−5 | |
| PNVP-b-PHMA #4 | CHCl3 | 130 × 103 | ||||
| PNVP-b-PHMA #4 | THF | 8.76 × 105 | 6.74 | 65.0 | 1.01 × 10−3 |
| Sample | Solvent | Do | Kd | Rg (nm) | Rho (nm) | Rg/Rho |
|---|---|---|---|---|---|---|
| PNVP-b-PHMA #1 | CHCl3 | 6.294 × 10−7 | 23.36 | 6.41 | ||
| PNVP-b-PHMA #1 | THF | 7.635 × 10−7 | 19.04 | 6.20 | ||
| PNVP-b-PHMA #1 | WATER | 3.469 × 10−8 | 384 | 59.9 | 70.73 | 0.85 |
| PNVP-b-PHMA #2 | CHCl3 | 3.500 × 10−7 | 143 | 11.53 | ||
| PNVP-b-PHMA #2 | THF | 9.611 × 10−7 | 37.59 | 4.93 | ||
| PNVP-b-PHMA #2 | WATER | 2.488 × 10−8 | 199 | 103.6 | 98.62 | 1.05 |
| PNVP-b-PHMA #3 | CHCl3 | 2.532 × 10−7 | 160 | 15.94 | ||
| PNVP-b-PHMA #3 | THF | 5.106 × 10−7 | 33.23 | 9.28 | ||
| PNVP-b-PHMA #3 | WATER | 2.106 × 10−8 | 1023 | 124 | 116.51 | 1.06 |
| PNVP-b-PHMA #4 | CHCl3 | 1.621 × 10−7 | 103 | 24.90 | ||
| PNVP-b-PHMA #4 | THF | 2.092 × 10−7 | 84.35 | 65 | 22.64 | 2.87 |
| Sample | Solvent | Mw From SEC | Mw From SLS | Nw | Rg (nm) | A2 (cm3mol/g2) |
|---|---|---|---|---|---|---|
| PNVP-b-PSMA #1 | CHCl3 | 37 × 103 | ||||
| PNVP-b-PSMA #1 | THF | 2.53 × 105 | 6.84 | 6.00 × 10−4 | ||
| PNVP-b-PSMA #1 | WATER | 7.53 × 107 | 2035 | 48.0 | 4.53 × 10−5 | |
| PNVP-b-PSMA #2 | CHCl3 | 9.3 × 103 | ||||
| PNVP-b-PSMA #2 | THF | 4.70 × 104 | 5.05 | 3.50 × 10−4 | ||
| PNVP-b-PSMA #2 | WATER | 3.68 × 107 | 3957 | 43.8 | 7.40 × 10−5 | |
| PNVP-b-PSMA #3 | CHCl3 | 10.8 × 103 | ||||
| PNVP-b-PSMA #3 | THF | 7.20 × 104 | 6.67 | 2.50 × 10−4 | ||
| PNVP-b-PSMA #3 | WATER | 1.192 × 107 | 1104 | 36.1 | 7.30 × 10−5 |
| Sample | Solvent | Do | Kd | Rg (nm) | Rho (nm) | Rg/Rho |
|---|---|---|---|---|---|---|
| PNVP-b-PSMA #1 | CHCl3 | 3.289 × 10−7 | 158.8 | 12.27 | ||
| PNVP-b-PSMA #1 | THF | 6.295 × 10−7 | 50.05 | 7.53 | ||
| PNVP-b-PSMA #1 | WATER | 3.171 × 10−8 | 1179 | 48 | 77.38 | 0.62 |
| PNVP-b-PSMA #2 | CHCl3 | 3.116 × 10−7 | 231.2 | 12.96 | ||
| PNVP-b-PSMA #2 | THF | 9.033 × 10−7 | 28.90 | 5.24 | ||
| PNVP-b-PSMA #2 | WATER | 4.418 × 10−8 | 952.0 | 43.8 | 55.53 | 0.79 |
| PNVP-b-PSMA #3 | CHCl3 | 2.567 × 10−7 | 166.0 | 15.72 | ||
| PNVP-b-PSMA #3 | THF | 6.577 × 10−7 | 156.8 | 7.20 | ||
| PNVP-b-PSMA #3 | WATER | 6.474 × 10−8 | 462.9 | 36.1 | 37.90 | 0.95 |
| Sample | DLC% | DLE% |
|---|---|---|
| PNVP-b-PHMA #1 | ||
| 1/c1 | 0.59 | 50.54 |
| 1/c2 | 1.35 | 76.50 |
| 1/c3 | 1.92 | 67.96 |
| 1/c4 | 1.46 | 38.58 |
| PNVP-b-PHMA #2 | ||
| 2/c1 | 0.12 | 13.30 |
| 2/c2 | 0.78 | 35.75 |
| 2/c3 | 0.82 | 29.09 |
| 2/c4 | 1.01 | 29.56 |
| PNVP-b-PHMA #3 | ||
| 3/c1 | 0.32 | 34.65 |
| 3/c2 | 0.99 | 48.46 |
| 3/c3 | 1.28 | 47.33 |
| 3/c4 | 1.60 | 48.46 |
| Sample | DLC% | DLE% |
|---|---|---|
| PNVP-b-PSMA #1 | - | - |
| 1/c1 | 0.40 | 39.97 |
| 1/c2 | 0.91 | 40.99 |
| 1/c3 | 1.32 | 46.11 |
| 1/c4 | 1.68 | 47.01 |
| PNVP-b-PSMA #2 | ||
| 2/c1 | 0.14 | 11.88 |
| 2/c2 | 0.92 | 39.21 |
| 2/c3 | 0.81 | 26.74 |
| 2/c4 | 1.43 | 39.57 |
| PNVP-b-PSMA #3 | ||
| 3/c1 | 0.27 | 24.01 |
| 3/c2 | 0.61 | 26.39 |
| 3/c3 | 1.26 | 43.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roka, N.; Pitsikalis, M. Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates. Polymers 2025, 17, 1122. https://doi.org/10.3390/polym17081122
Roka N, Pitsikalis M. Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates. Polymers. 2025; 17(8):1122. https://doi.org/10.3390/polym17081122
Chicago/Turabian StyleRoka, Nikoletta, and Marinos Pitsikalis. 2025. "Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates" Polymers 17, no. 8: 1122. https://doi.org/10.3390/polym17081122
APA StyleRoka, N., & Pitsikalis, M. (2025). Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates. Polymers, 17(8), 1122. https://doi.org/10.3390/polym17081122







