Thin-Film Composite Polyamide Membranes Modified with HKUST-1 for Water Treatment: Characterization and Nanofiltration Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Film Membrane Preparation
2.2.1. Porous Substrate Preparation
2.2.2. Formation of PA Layer on a Porous Substrate
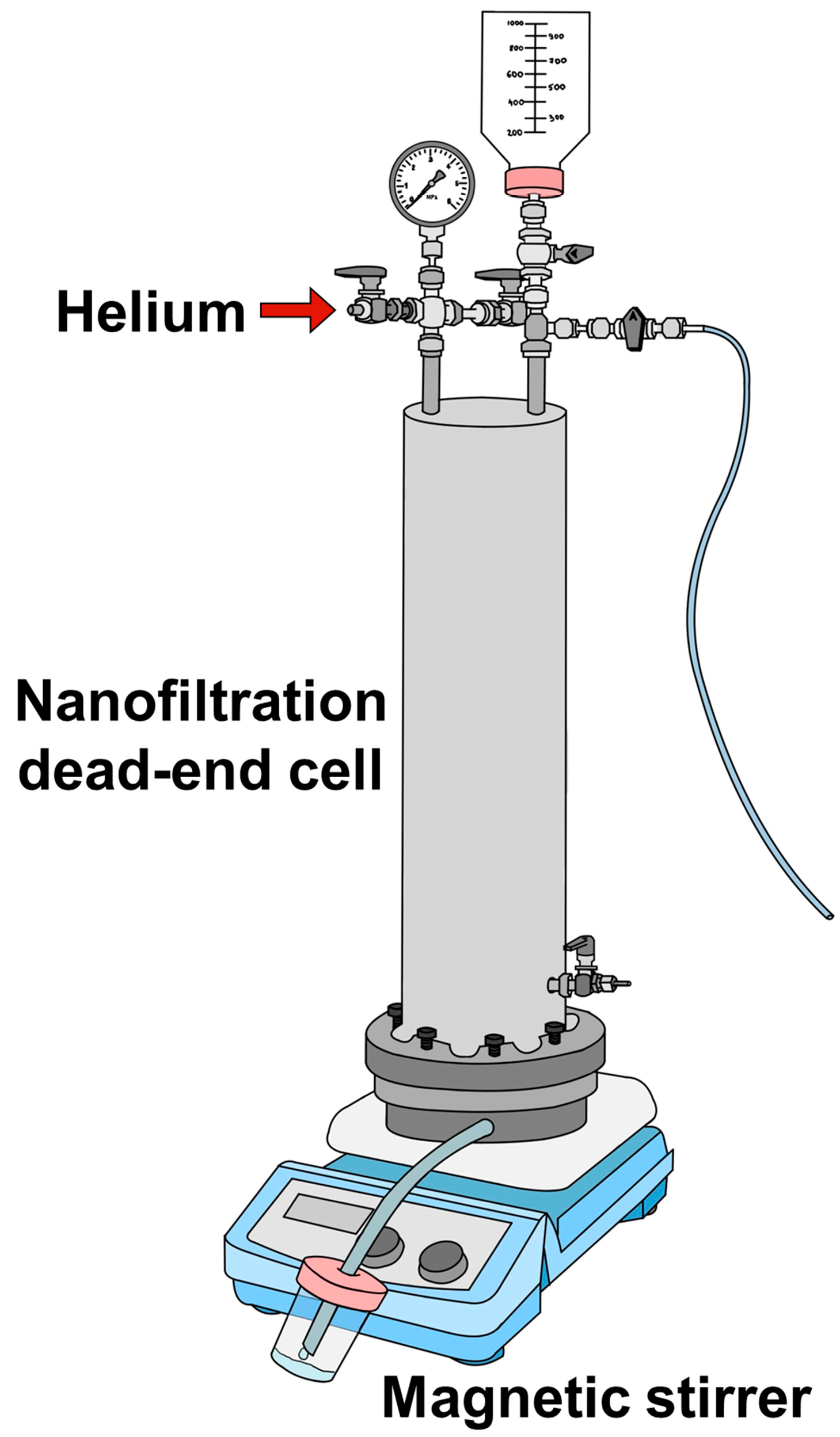
2.3. Membrane Performance in Nanofiltration
2.4. Fourier-Transform Infrared Spectroscopy
2.5. Scanning Electron Microscopy
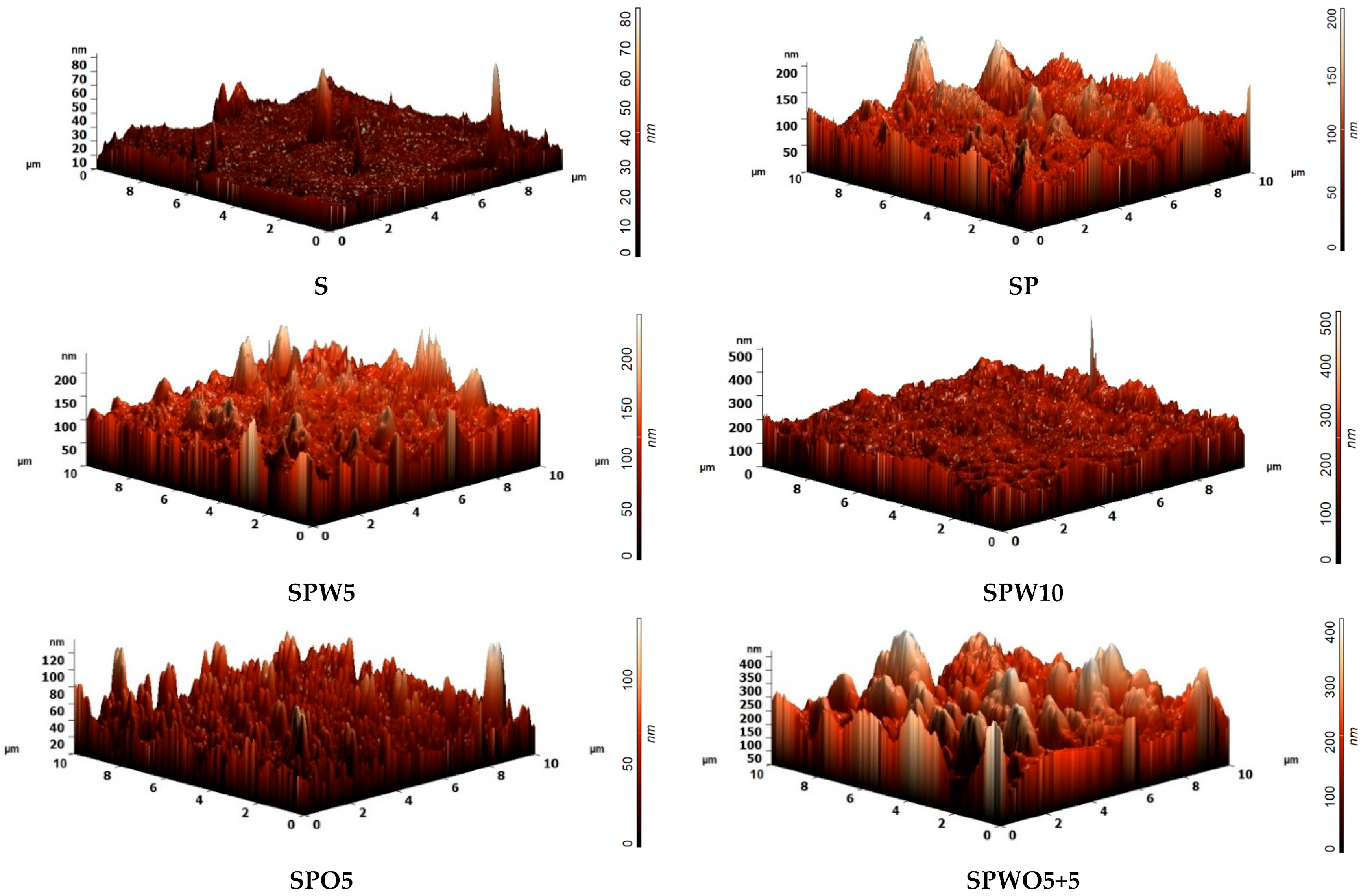
2.6. Atomic Force Microscopy
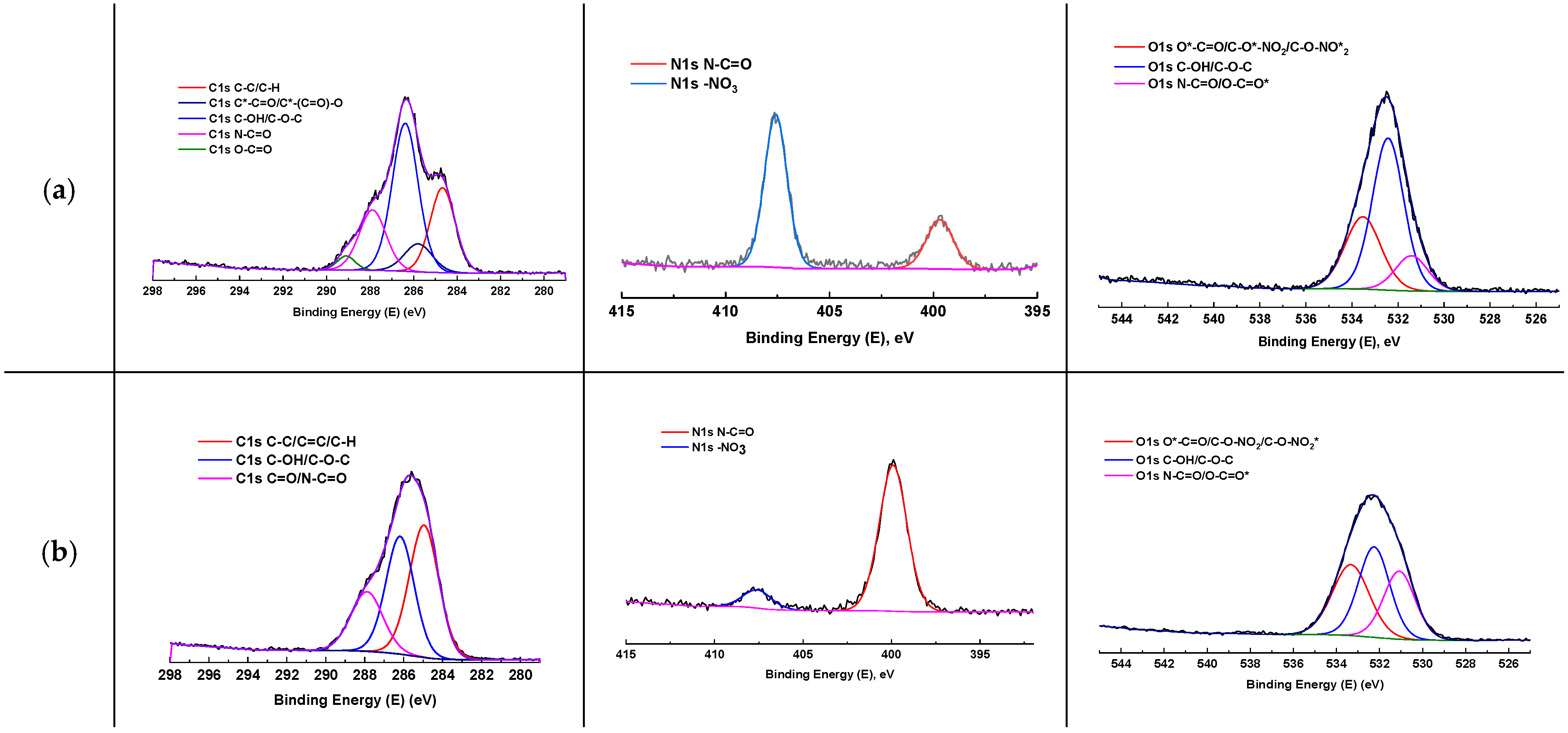
2.7. X-Ray Photoelectron Spectroscopy
2.8. Measurement of Contact Angle
3. Results and Discussions
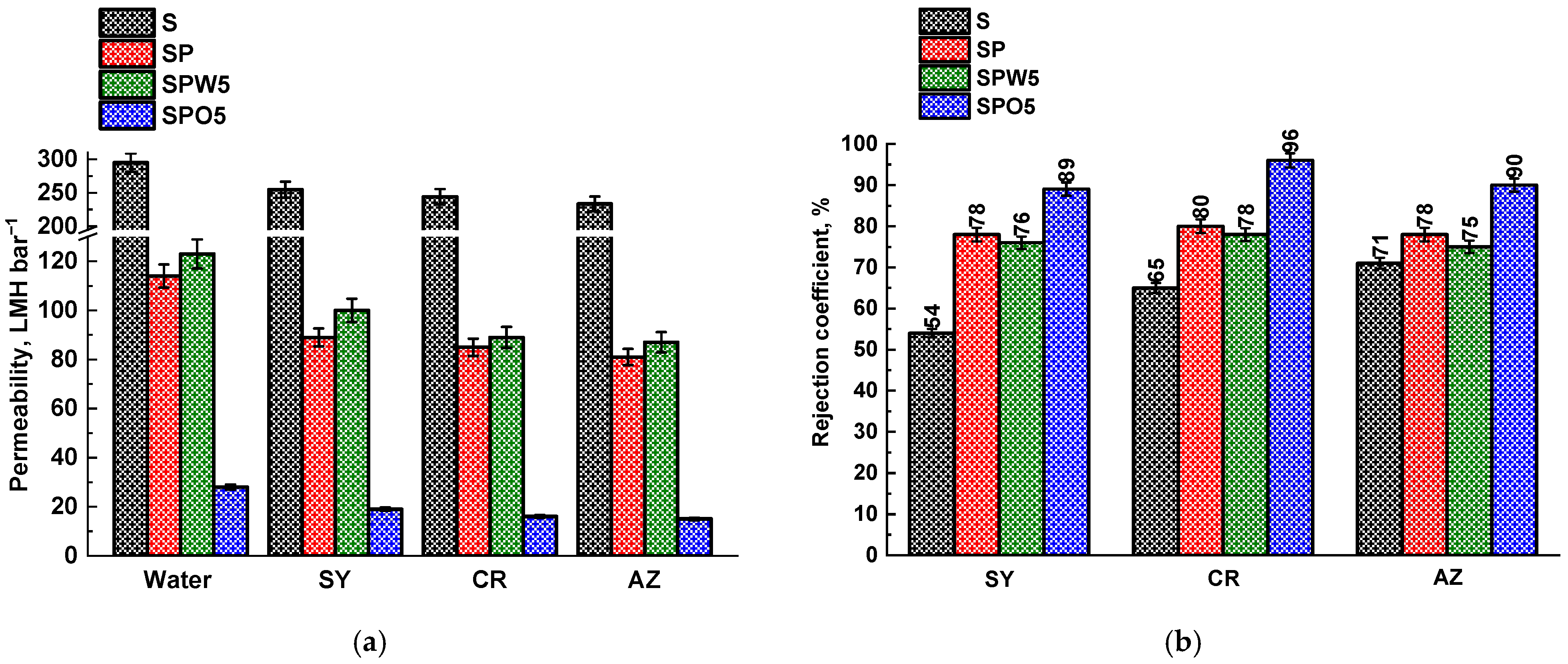
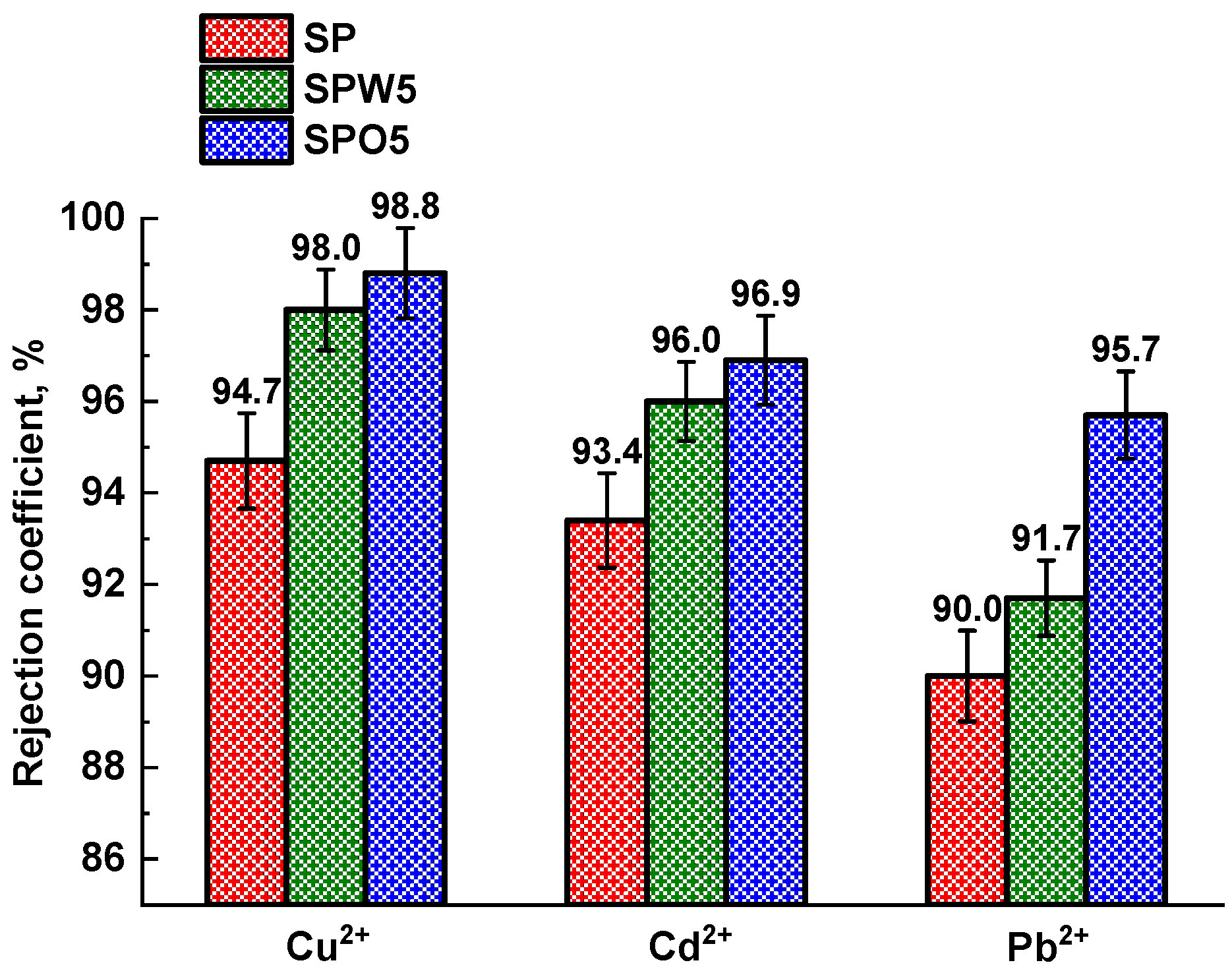
3.1. TFC Membrane Performance
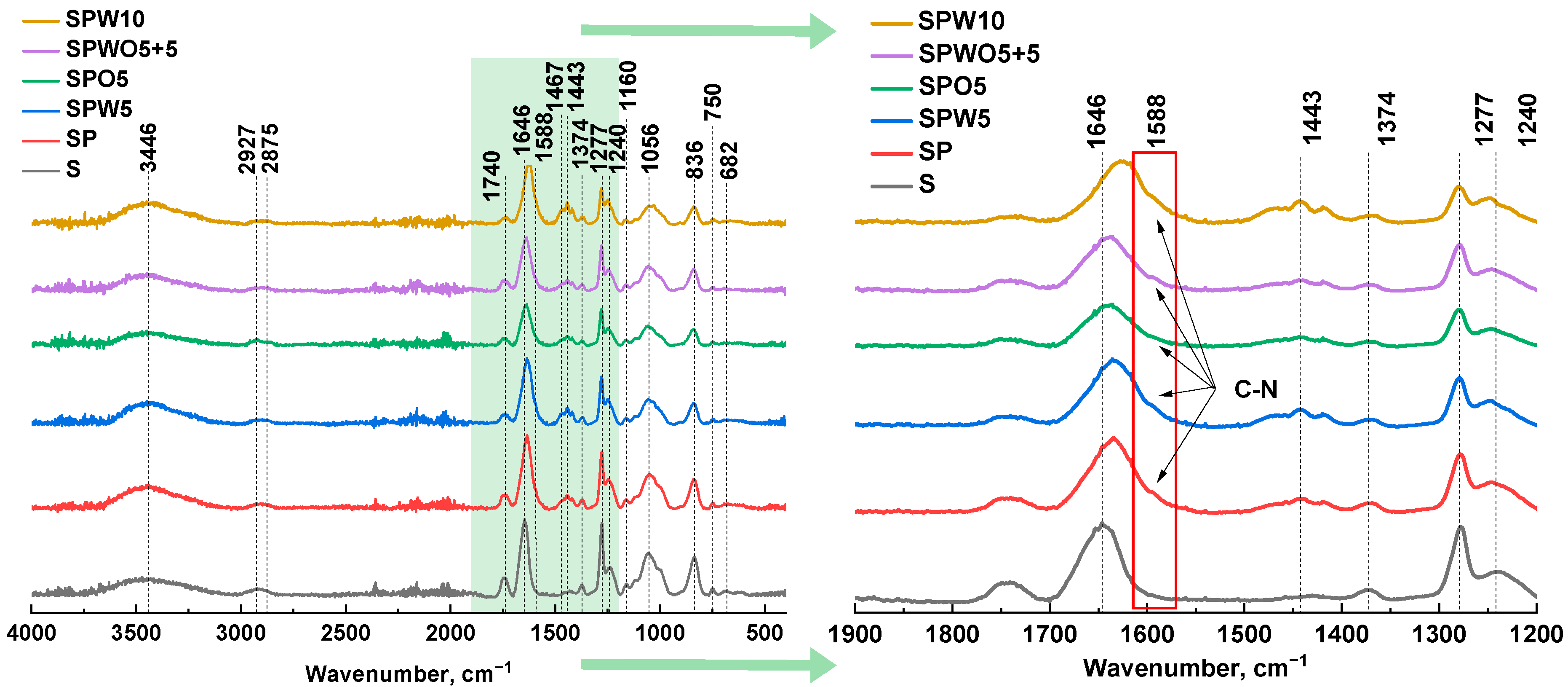
3.2. Structure and Physicochemical Properties
3.3. Membrane Comparison and Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Nam, S.-N.; Jung, B.; Min Park, C.; Jang, M.; Park, C.; Chae, S.; Huang, Y.; Jun, B.-M.; Yoon, Y. Removal of Contaminants of Emerging Concerns and Dyes by MXene-Based Membranes in Water: A Review. Sep. Purif. Technol. 2024, 351, 128125. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of Two Cationic Dyes Removal from Aqueous Environments Using CNT/MgO/CuFe2O4 Magnetic Composite Powder: A Comparative Study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Dalvi, A.; Hubale, V.; Sawant, V. Two Dimensional Co-Based Metal Organic Framework for Selective Adsorption of Congo Red from Wastewater. Sep. Purif. Technol. 2024, 348, 127712. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; El Messaoudi, N.; Miyah, Y.; Georgin, J.; Franco, D.S.P.; Benjelloun, M.; Şenol, Z.M.; Kazan-Kaya, E.S.; Temur Ergan, B. Recent Advances in the Removal of Sunset Yellow Dye from Wastewater: A Review. Sustain. Mater. Technol. 2024, 42, e01187. [Google Scholar] [CrossRef]
- Vega, E.N.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Barros, L.; Morales, P. Natural Sources of Food Colorants as Potential Substitutes for Artificial Additives. Foods 2023, 12, 4102. [Google Scholar] [CrossRef] [PubMed]
- Olgun, M.; Sivrikaya Özak, S.; Dalmaz, A. Spectrophotometric Determination for Green Hydrophobic Deep Eutectic Solvent-Based Microextraction of Brilliant Blue FCF (E133) from Beverages. J. Chromatogr. A 2024, 1736, 465374. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A Review on Agro-Industrial Waste (AIW) Derived Adsorbents for Water and Wastewater Treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Dong, M.; Guo, J.; Wang, Y.; Gai, X.; Xiong, X.; Zeng, J.; Wang, Y.; Wu, Y. Humic Acid Non-Covalent Functionalized Multi-Walled Carbon Nanotubes Composite Membrane and Its Application for the Removal of Organic Dyes. J. Environ. Chem. Eng. 2022, 10, 107320. [Google Scholar] [CrossRef]
- Demissie, H.; An, G.; Jiao, R.; Ritigala, T.; Lu, S.; Wang, D. Modification of High Content Nanocluster-Based Coagulation for Rapid Removal of Dye from Water and the Mechanism. Sep. Purif. Technol. 2021, 259, 117845. [Google Scholar] [CrossRef]
- Melo, R.P.F.; Barros Neto, E.L.; Nunes, S.K.S.; Castro Dantas, T.N.; Dantas Neto, A.A. Removal of Reactive Blue 14 Dye Using Micellar Solubilization Followed by Ionic Flocculation of Surfactants. Sep. Purif. Technol. 2018, 191, 161–166. [Google Scholar] [CrossRef]
- Feng, L.; Liu, J.; Guo, Z.; Pan, T.; Wu, J.; Li, X.; Liu, B.; Zheng, H. Reactive Black 5 Dyeing Wastewater Treatment by Electrolysis-Ce (IV) Electrochemical Oxidation Technology: Influencing Factors, Synergy and Enhancement Mechanisms. Sep. Purif. Technol. 2022, 285, 120314. [Google Scholar] [CrossRef]
- Jin, K.; Hou, K.; Wang, J.; Zhai, S.; Fan, Z.; Zhao, Y.; Xie, K.; Cai, Z.; Feng, X. Composite Membranes with Multifunctionalities for Processing Textile Wastewater: Simultaneous Oil/Water Separation and Dye Adsorption/Degradation. Sep. Purif. Technol. 2023, 320, 124176. [Google Scholar] [CrossRef]
- Vatanpour, V.; Ardic, R.; Esenli, B.; Eryildiz-Yesir, B.; Yaqubnezhad Pazoki, P.; Jarahiyan, A.; Matloubi Moghaddam, F.; Castro-Muñoz, R.; Koyuncu, I. Defected Ag/Cu-MOF as a Modifier of Polyethersulfone Membranes for Enhancing Permeability, Antifouling Properties and Heavy Metal and Dye Pollutant Removal. Sep. Purif. Technol. 2024, 345, 127336. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Xu, D.; Zhao, R.; Liu, Y.; Li, G.; Gao, Q.; Zhang, X.; Volodine, A.; Van der Bruggen, B. Facile Fabrication of a Positively Charged Nanofiltration Membrane for Heavy Metal and Dye Removal. Sep. Purif. Technol. 2022, 282, 120155. [Google Scholar] [CrossRef]
- Athinarayanan, B.; Jeong, D.-Y.; Kang, J.-H.; Koo, B.-H. Fabrication of Nanoporous Aluminum-Oxide Composite Membranes. J. Korean Phys. Soc. 2015, 67, 1970–1976. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic Nanocomposite Membranes and Membrane Fouling: A Review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for Next-Generation Desalination and Water Purification Membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, S.; Wang, F.; Pan, X.; Yang, T.; Hu, S.; Tang, S.; Jia, Q. Solid Membrane-Based Aqueous Lithium Extraction and Adsorption: Advances, Challenges, and Prospects. Chem. Eng. J. 2025, 510, 161748. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Makarava, M.; Krasnova, M.; Penkova, A.; Ermakov, S.; Grigoryev, E.; Komolkin, A.; Bildyukevich, A. The Effect of PEG-Content and Molecular Weight of PEG-PPG-PEG Block Copolymers on the Structure and Performance of Polyphenylsulfone Ultra- and Nanofiltration Membranes. J. Memb. Sci. 2024, 704, 122869. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Zhang, Z.; Zhou, X.; Pang, R. A Review on Antifouling Polyamide Reverse Osmosis Membrane for Seawater Desalination. Environ. Res. 2025, 274, 121305. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Studer, R.M.; Lee, M.; Rodriguez, K.M.; Teesdale, J.J.; Smith, Z.P. Post-Synthetic Modification of MOFs to Enhance Interfacial Compatibility and Selectivity of Thin-Film Nanocomposite (TFN) Membranes for Water Purification. J. Memb. Sci. 2023, 666, 121133. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-Matrix Nanocomposite Membranes for Water Treatment. J. Memb. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y. The Upper Bound of Thin-Film Composite (TFC) Polyamide Membranes for Desalination. J. Memb. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Kumar, A.; Alghazali, T.; Ganesan, S.; Shankhyan, A.; Sharma, G.C.; Satyam Naidu, K.; Rahbari-Sisakht, M. Recent Advances in Thin Film Composite (TFC) Membrane Development: Materials and Modification Methods. Environ. Sci. 2025. [Google Scholar] [CrossRef]
- Mohammad Gheimasi, M.H.; Lorestani, B.; Kiani Sadr, M.; Cheraghi, M.; Emadzadeh, D. Synthesis of Novel Hybrid NF/FO Nanocomposite Membrane by Incorporating Black TiO2 Nanoparticles for Highly Efficient Heavy Metals Removal. Int. J. Environ. Res. 2021, 15, 475–485. [Google Scholar] [CrossRef]
- Bakhodaye Dehghanpour, S.; Parvizian, F.; Vatanpour, V. The Role of CuO/TS-1, ZnO/TS-1, and Fe2O3/TS-1 on the Desalination Performance and Antifouling Properties of Thin-Film Nanocomposite Reverse Osmosis Membranes. Sep. Purif. Technol. 2022, 302, 122083. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.L.; Chung, T.-S. Na+ Functionalized Carbon Quantum Dot Incorporated Thin-Film Nanocomposite Membranes for Selenium and Arsenic Removal. J. Memb. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.-S. Applications of Carbon Quantum Dots (CQDs) in Membrane Technologies: A Review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef]
- Li, J.; Cheng, L.; Song, W.; Xu, Y.; Liu, F.; Wang, Z. In-Situ Sol-Gel Generation of SiO2 Nanoparticles inside Polyamide Membrane for Enhanced Nanofiltration. Desalination 2022, 540, 115981. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Xue, W.; Zhao, G.; Ding, W.; Zhang, K.; Wang, S.; Li, Y. Effect of Carbon Nanotube Nanochannel on the Separation Performance of Thin-Film Nanocomposite (TFN) Membranes. Desalination 2023, 546, 116216. [Google Scholar] [CrossRef]
- He, H.; Xu, P.; Wang, S.; Wang, X.; Ma, S.; Peng, H.; Lv, Y.; Zhou, H.; Chen, C. Inorganic Salt-Conditioning Preparation of a Copper (II) Ions-Doped Thin Film Composite Membrane with Ridge-Valley Morphology for Efficient Organic Solvent Nanofiltration. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131114. [Google Scholar] [CrossRef]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Ismail, A.F. Greener Synthesis of Functionalized-GO Incorporated TFN NF Membrane for Potential Recovery of Saline Water from Salt/Dye Mixed Solution. Desalination 2022, 523, 115403. [Google Scholar] [CrossRef]
- Liu, Y.; Ban, Y.; Yang, W. Microstructural Engineering and Architectural Design of Metal–Organic Framework Membranes. Adv. Mater. 2017, 29, 1606949. [Google Scholar] [CrossRef]
- Li, Q.; Liao, Z.; Xie, J.; Ni, L.; Wang, C.; Qi, J.; Sun, X.; Wang, L.; Li, J. Enhancing Nanofiltration Performance by Incorporating Tannic Acid Modified Metal-Organic Frameworks into Thin-Film Nanocomposite Membrane. Environ. Res. 2020, 191, 110215. [Google Scholar] [CrossRef]
- Van Goethem, C.; Verbeke, R.; Pfanmöller, M.; Koschine, T.; Dickmann, M.; Timpel-Lindner, T.; Egger, W.; Bals, S.; Vankelecom, I.F.J. The Role of MOFs in Thin-Film Nanocomposite (TFN) Membranes. J. Memb. Sci. 2018, 563, 938–948. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Wang, X.; Wang, Q.; Ji, Z.; Wang, X.; Wu, T.; Gao, C. Highly and Stably Water Permeable Thin Film Nanocomposite Membranes Doped with MIL-101 (Cr) Nanoparticles for Reverse Osmosis Application. Materials 2016, 9, 870. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.S.; Ali, F.A.A.; Mishra, U.; Hamid, A.A. Thin-Film Nanocomposite Membrane Incorporated with Porous Zn-Based Metal–Organic Frameworks: Toward Enhancement of Desalination Performance and Chlorine Resistance. ACS Appl. Mater. Interfaces 2021, 13, 28818–28831. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Xie, Z.; Zhang, K. Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration. Membranes 2022, 12, 639. [Google Scholar] [CrossRef]
- Gul, S.; Latafat, K.R.; Asma, M.; Ahmad, M.; Kilic, Z.; Zafar, M.; Ding, Y.; Malik, A. Microscopic Techniques for Fabrication of Polyethersulfone Thin-film Nanocomposite Membranes Intercalated with UiO-66-SO3H for Heavy Metal Ions Removal from Water. Microsc. Res. Tech. 2022, 85, 1289–1299. [Google Scholar] [CrossRef]
- Misdan, N.; Ramlee, N.; Hairom, N.H.H.; Ikhsan, S.N.W.; Yusof, N.; Lau, W.J.; Ismail, A.F.; Nordin, N.A.H.M. CuBTC Metal Organic Framework Incorporation for Enhancing Separation and Antifouling Properties of Nanofiltration Membrane. Chem. Eng. Res. Des. 2019, 148, 227–239. [Google Scholar] [CrossRef]
- Mandal, J.R.; Mondal, M. Removal of Arsenic from Water Using a CuBTC Incorporated Thin Film Nanocomposite Hollow Fiber Membrane by Interfacial Polymerization on Lumen Side. J. Water Process Eng. 2023, 52, 103587. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Wang, R. Thin Film Nanocomposite Hollow Fiber Membranes Incorporated with Surface Functionalized HKUST-1 for Highly-Efficient Reverses Osmosis Desalination Process. J. Memb. Sci. 2019, 589, 117249. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, K.; Zhang, M.; Li, Z. Polydopamine-Modified HKUST-1 as Nanofiller of PPS@PA Membrane with Well Improved Desalination Performance. Polymer 2022, 253, 124988. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Téllez, C.; Coronas, J. HKUST-1 MOF: A Matrix to Synthesize CuO and CuO–CeO2 Nanoparticle Catalysts for CO Oxidation. Chem. Eng. J. 2012, 195–196, 180–187. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3] n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The Effect of Water Adsorption on the Structure of the Carboxylate Containing Metal–Organic Frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A Mater. 2013, 1, 11922. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High Flux Thin Film Nanocomposite Membranes Based on Metal–Organic Frameworks for Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Worrall, S.D.; Bissett, M.A.; Hill, P.I.; Rooney, A.P.; Haigh, S.J.; Attfield, M.P.; Dryfe, R.A.W. Metal-Organic Framework Templated Electrodeposition of Functional Gold Nanostructures. Electrochim. Acta 2016, 222, 361–369. [Google Scholar] [CrossRef]
- Rickhoff, T.A.; Sullivan, E.; Werth, L.K.; Kissel, D.S.; Keleher, J.J. A Biomimetic Cellulose-based Composite Material That Incorporates the Antimicrobial Metal-organic Framework HKUST-1. J. Appl. Polym. Sci. 2019, 136, 46978. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, R. Effect of Metal Organic Framework on Crystallization and Ultraviolet Protection Ability of Biobased PA5,6. Mater. Today Commun. 2023, 37, 107072. [Google Scholar] [CrossRef]
- Polyakov, V.; Dmitrenko, M.; Kalmakhelidze, M.; Kuzminova, A.; Dubovenko, R.; Mukhanova, E.; Soldatov, A.; Penkova, A. Development and Characterization of PVA Membranes Modified with In(BTC) Metal–Organic Framework for Sustainable Pervaporation Separation of Isopropanol/Water. Sustainability 2024, 16, 10257. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant Roles of Substrate Properties in Forward Osmosis Membrane Performance: A Review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Szekely, G. The 12 Principles of Green Membrane Materials and Processes for Realizing the United Nations’ Sustainable Development Goals. RSC Sustain. 2024, 2, 871–880. [Google Scholar] [CrossRef]
- Ehsani, M.; Kalugin, D.; Doan, H.; Lohi, A.; Abdelrasoul, A. Bio-Sourced and Biodegradable Membranes. Appl. Sci. 2022, 12, 12837. [Google Scholar] [CrossRef]
- Morris, E.; Pulham, C.R.; Morrison, C.A. Structure and Properties of Nitrocellulose: Approaching 200 Years of Research. RSC Adv. 2023, 13, 32321–32333. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yao, Z.; Wang, X.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-Based Materials in Wastewater Treatment of Petroleum Industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Dubovenko, R.; Selyutin, A.; Wu, J.; Su, R.; Penkova, A. Novel Mixed Matrix Membranes Based on Poly(Vinylidene Fluoride): Development, Characterization, Modeling. Polymers 2023, 15, 1222. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Selyutin, A.; Ermakov, S.; Penkova, A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers 2023, 15, 1341. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Ouyang, G.; Huang, L.; Li, L.; Li, W.; Huang, W.; Li, D. Ultrathin Organic Solvent Nanofiltration Membrane with Polydopamine-HKUST-1 Interlayer for Organic Solvent Separation. J. Environ. Sci. 2024, 141, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, M.; Zhao, H.; Jiang, Y.; Liu, G.; Gao, J. Enhanced Dispersibility of Metal–Organic Frameworks (MOFs) in the Organic Phase via Surface Modification for TFN Nanofiltration Membrane Preparation. RSC Adv. 2020, 10, 4045–4057. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, Y.; Lim, T.; Wang, R.; Hu, X. A Novel Metal–Organic Framework (MOF)–Mediated Interfacial Polymerization for Direct Deposition of Polyamide Layer on Ceramic Substrates for Nanofiltration. Adv. Mater. Interfaces 2019, 6, 1900132. [Google Scholar] [CrossRef]
- Gayatri, R.; Yuliwati, E.; Jaafar, J.; Fizal, A.N.S.; Hossain, M.S.; Zulkifli, M.; Yahaya, A.N.A.; Taweepreda, W. Polymer-Based Nanocomposite Membranes for Industrial Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2024, 12, 113276. [Google Scholar] [CrossRef]
- Han, B.; Gabriel, J.-C.P. Thin-Film Nanocomposite (TFN) Membrane Technologies for the Removal of Emerging Contaminants from Wastewater. J. Clean. Prod. 2024, 480, 144043. [Google Scholar] [CrossRef]
- Dai, R.; Li, J.; Wang, Z. Constructing Interlayer to Tailor Structure and Performance of Thin-Film Composite Polyamide Membranes: A Review. Adv. Colloid. Interface Sci. 2020, 282, 102204. [Google Scholar] [CrossRef]
- Lu, X.; Nejati, S.; Choo, Y.; Osuji, C.O.; Ma, J.; Elimelech, M. Elements Provide a Clue: Nanoscale Characterization of Thin-Film Composite Polyamide Membranes. ACS Appl. Mater. Interfaces 2015, 7, 16917–16922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef]
- Seah, M.Q.; Chua, S.F.; Ang, W.L.; Lau, W.J.; Mansourizadeh, A.; Thamaraiselvan, C. Advancements in Polymeric Membranes for Challenging Water Filtration Environments: A Comprehensive Review. J. Environ. Chem. Eng. 2024, 12, 112628. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Qiu, X.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. Novel Porous Phosphoric Acid-Based Geopolymer Foams for Adsorption of Pb(II), Cd(II) and Ni(II) Mixtures: Behavior and Mechanism. Ceram. Int. 2023, 49, 7030–7039. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S.; Sha, Q.; Wang, Y.; Ma, C.; Zhang, X.; Yan, X.; Han, N. Positively Charged Thin-Film Nanocomposite Membrane Doped with Functionalized Covalent Organic Frameworks Nanosphere for Heavy Metal Ion Removal. Sep. Purif. Technol. 2025, 363, 131996. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.-H. Copper-Based Metal-Organic Framework for Highly Efficient Adsorption of Lead Ions from Aqueous Solution. Mater. Res. Express 2022, 9, 095505. [Google Scholar] [CrossRef]
- Shaheed, N.; Javanshir, S.; Esmkhani, M.; Dekamin, M.G.; Naimi-Jamal, M.R. Synthesis of Nanocellulose Aerogels and Cu-BTC/Nanocellulose Aerogel Composites for Adsorption of Organic Dyes and Heavy Metal Ions. Sci. Rep. 2021, 11, 18553. [Google Scholar] [CrossRef] [PubMed]
- Ounifi, I.; Guesmi, Y.; Ursino, C.; Castro-Muñoz, R.; Agougui, H.; Jabli, M.; Hafiane, A.; Figoli, A.; Ferjani, E. Synthesis and Characterization of a Thin-Film Composite Nanofiltration Membrane Based on Polyamide-Cellulose Acetate: Application for Water Purification. J. Polym. Environ. 2022, 30, 707–718. [Google Scholar] [CrossRef]
- Sun, B.-B.; Yao, B.-H.; He, Y.-Q.; Yang, B. Preparation and Photochromic Performance of Homogeneous Phase Nitrocellulose Membrane Grafting Spirooxazine Moieties. Coatings 2020, 10, 569. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Addagada, A. Chemistry of Proton Track Registration in Cellulose Nitrate Polymers. Radiat. Phys. Chem. 2007, 76, 691–698. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Synthesis and Characterization of Alumina Nano-Particles Polyamide Membrane with Enhanced Flux Rejection Performance. Sep. Purif. Technol. 2012, 89, 245–251. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, G.; Pan, G.; Yu, H.; Tang, G.; Luo, F.; Zhong, T.; Zhang, Y.; Liu, Y. Thin-Film Composite Nanofiltration Membrane Based on Polysulfonamide for Extremely Acidic Conditions. Sep. Purif. Technol. 2025, 356, 129918. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Gong, J.-L.; Fang, S.-Y.; Li, J.; Cao, W.-C.; Chen, Z.-P. Outstanding Anti-Bacterial Thin-Film Composite Membrane Prepared by Incorporating Silver-Based Metal–Organic Framework (Ag-MOF) for Water Treatment. Appl. Surf. Sci. 2022, 590, 153059. [Google Scholar] [CrossRef]
- Akther, N.; Yuan, Z.; Chen, Y.; Lim, S.; Phuntsho, S.; Ghaffour, N.; Matsuyama, H.; Shon, H. Influence of Graphene Oxide Lateral Size on the Properties and Performances of Forward Osmosis Membrane. Desalination 2020, 484, 114421. [Google Scholar] [CrossRef]
- Namvar-Mahboub, M.; Pakizeh, M.; Davari, S. Preparation and Characterization of UZM-5/Polyamide Thin Film Nanocomposite Membrane for Dewaxing Solvent Recovery. J. Memb. Sci. 2014, 459, 22–32. [Google Scholar] [CrossRef]
- Jia, Y.; Huo, X.; Gao, L.; Shao, W.; Chang, N. Controllable Design of Polyamide Composite Membrane Separation Layer Structures via Metal–Organic Frameworks: A Review. Membranes 2024, 14, 201. [Google Scholar] [CrossRef]
- Jia, M.-M.; Feng, J.-H.; Shao, W.; Chen, Z.; Yu, J.-R.; Sun, J.-J.; Wu, Q.-Y.; Li, Y.; Xue, M.; Chen, X.-M. In-Situ Interfacial Synthesis of Metal-Organic Framework/Polyamide Thin-Film Nanocomposite Membranes with Elevated Nanofiltration Performances. J. Memb. Sci. 2024, 694, 122418. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Zhao, S.; Chai, M.; Hou, J.; Lin, R. Recent Advances in the Interfacial Engineering of MOF-Based Mixed Matrix Membranes for Gas Separation. Nanoscale 2024, 16, 7716–7733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Wang, X.; Huang, X.; Xie, Y.F. Impacts of Metal–Organic Frameworks on Structure and Performance of Polyamide Thin-Film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2019, 11, 13724–13734. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-K.; Mo, J.-W.; Jin, X.-G.; Ma, X.-H.; Xu, Z.-L. Positively Charged Nanofiltration Membranes with Gradient Structures for Enhancing Separation Properties. Desalination 2024, 584, 117750. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-Performance Polyamide Thin-Film-Nanocomposite Reverse Osmosis Membranes Containing Hydrophobic Zeolitic Imidazolate Framework-8. J. Memb. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database (Beamson, G.; Briggs, D.). J. Chem. Educ. 1993, 70, A25. [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, Z.; Ren, J.; Geng, Z.; Luan, J.; Wang, G. Novel Sulfonated Thin-Film Composite Nanofiltration Membranes with Improved Water Flux for Treatment of Dye Solutions. J. Memb. Sci. 2012, 394–395, 218–229. [Google Scholar] [CrossRef]
- Ma, T.; Su, Y.; Li, Y.; Zhang, R.; Liu, Y.; He, M.; Li, Y.; Dong, N.; Wu, H.; Jiang, Z. Fabrication of Electro-Neutral Nanofiltration Membranes at Neutral PH with Antifouling Surface via Interfacial Polymerization from a Novel Zwitterionic Amine Monomer. J. Memb. Sci. 2016, 503, 101–109. [Google Scholar] [CrossRef]
- Shao, L.-L.; An, Q.-F.; Ji, Y.-L.; Zhao, Q.; Wang, X.-S.; Zhu, B.-K.; Gao, C.-J. Preparation and Characterization of Sulfated Carboxymethyl Cellulose Nanofiltration Membranes with Improved Water Permeability. Desalination 2014, 338, 74–83. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Sun, C.; Yu, S.; Liu, M.; Gao, C. Thin-Film Composite Membranes Formed by Interfacial Polymerization with Natural Material Sericin and Trimesoyl Chloride for Nanofiltration. J. Memb. Sci. 2014, 471, 381–391. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, C.; Dong, B.; Wu, Z.; Wang, L.; Yu, S.; Gao, C. Enhancing the Permselectivity of Thin-Film Composite Poly(Vinyl Alcohol) (PVA) Nanofiltration Membrane by Incorporating Poly(Sodium-p-Styrene-Sulfonate) (PSSNa). J. Memb. Sci. 2014, 463, 173–182. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Zeng, H.; Yang, H.; Shen, J.; Darvishmanesh, S.; Luis, P.; Sotto, A.; Van der Bruggen, B. Fractionation of Direct Dyes and Salts in Aqueous Solution Using Loose Nanofiltration Membranes. J. Memb. Sci. 2015, 477, 183–193. [Google Scholar] [CrossRef]
- Ye, C.-C.; Zhao, F.-Y.; Wu, J.-K.; Weng, X.-D.; Zheng, P.-Y.; Mi, Y.-F.; An, Q.-F.; Gao, C.-J. Sulfated Polyelectrolyte Complex Nanoparticles Structured Nanoflitration Membrane for Dye Desalination. Chem. Eng. J. 2017, 307, 526–536. [Google Scholar] [CrossRef]
- Tang, C.; Chen, V. Nanofiltration of Textile Wastewater for Water Reuse. Desalination 2002, 143, 11–20. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Tian, M.; Liu, J. Fabrication of a Mixed Matrix Membrane with in Situ Synthesized Quaternized Polyethylenimine Nanoparticles for Dye Purification and Reuse. ACS Sustain. Chem. Eng. 2015, 3, 690–701. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, H.; Liu, J. Development of a Molecular Separation Membrane for Efficient Separation of Low-Molecular-Weight Organics and Salts. Desalination 2015, 359, 176–185. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Dubovenko, R.; Puzikova, M.; Mikulan, A.; Korovina, A.; Koroleva, A.; Selyutin, A.; Semenov, K.; Su, R.; et al. Development and Study of Novel Ultrafiltration Membranes Based on Cellulose Acetate. Polymers 2024, 16, 1236. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Narendra, B.; Chen, X.; Feng, S.; Wan, Y.; Luo, J. Advancing High-Performance Nanofiltration Membranes: Tailoring Monomer Molecular Design to Enhance Diffusion-Reaction Synergy in Interfacial Polymerization. Desalination 2025, 598, 118415. [Google Scholar] [CrossRef]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-Scale Production of Metal–Organic Frameworks. Adv. Funct. Mater. 2024, 34, 2309089. [Google Scholar] [CrossRef]
- Sayed Moghaieb, H.; Khalil, S.; Ganguly, A.; Maguire, P.; Mariotti, D.; Chakrabarti, S. Metal-Organic Framework (MOF) Dispersion Based Fluids for Solar-Thermal Energy Conversion. Solar Energy 2024, 273, 112542. [Google Scholar] [CrossRef]
- El Fadil, A.; Verbeke, R.; Kyburz, M.; Aerts, P.E.M.; Vankelecom, I.F.J. From Academia to Industry: Success Criteria for Upscaling Nanofiltration Membranes for Water and Solvent Applications. J. Memb. Sci. 2023, 675, 121393. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and Characterization of Porous HKUST-1 Metal Organic Frameworks for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Gentile, F.S.; Pannico, M.; Causà, M.; Mensitieri, G.; Di Palma, G.; Scherillo, G.; Musto, P. Metal Defects in HKUST-1 MOF Revealed by Vibrational Spectroscopy: A Combined Quantum Mechanical and Experimental Study. J. Mater. Chem. A Mater. 2020, 8, 10796–10812. [Google Scholar] [CrossRef]


| Dyes | Molecular Formula | Structure | Absorption Maximum, nm | Molar Mass, g mol−1 |
|---|---|---|---|---|
| SY | C16H10N2Na2O7S2 | 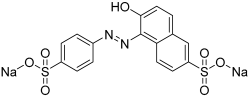 | 483 | 429 |
| CR | C32H22N6Na2O6S2 |  | 505 | 697 |
| AZ | C37H34Na2N2O9S3 | 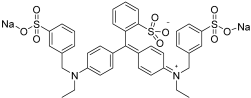 | 628 | 793 |
| Membrane | CA/CN-20 (1:3) Substrate | PA Layer | HKUST-1 Phase | HKUST-1 Concentration, wt.% |
|---|---|---|---|---|
| S | + | − | − | 0 |
| SP | + | + | − | 0 |
| SPO5 | + | + | Organic | 0.05 |
| SPW5 | + | + | Water | 0.05 |
| SPW10 | + | + | Water | 0.1 |
| SPWO5+5 | + | + | Organic + water | 0.05 + 0.05 |
| Membrane | Ra, nm | Contact Angle, ° |
|---|---|---|
| S | 1.9 | 45 ± 2 |
| SP | 16.9 | 26 ± 2 |
| SPW5 | 14.9 | 19 ± 2 |
| SPO5 | 9.4 | 25 ± 2 |
| SPWO5+5 | 50.7 | 17 ± 2 |
| SPW10 | 21.0 | 18 ± 2 |
| Membrane | Cu, % | C, % | O, % | N, % | C/N | O/N |
|---|---|---|---|---|---|---|
| S | 0 | 56.51 | 36.11 | 7.38 | 7.66 | 4.89 |
| SPW5 | 0.13 | 64.54 | 24.99 | 10.34 | 6.24 | 2.42 |
| NF Membranes * | L, LMH bar−1 | Dye * | MM, g mol−1 | R, % | Reference |
|---|---|---|---|---|---|
| SPW5 | 123 | SY | 429 | 76 | This study |
| SPO5 | 28 | SY | 429 | 89 | This study |
| TFC-(6FAPBS/6FBABDS+PIP+TMC)/PPSU | 9 | Methyl Orange | 327 | 76 | [90] |
| TFC-PEI-g-SBMA | 13.2 | Orange GII | 452 | 90 | [91] |
| TFC-PPSU/PEG-PPG-PEG | 4.8 | Indigo carmine | 466 | 90 | [20] |
| Sulfated CMC | 6.6 | Xylenol orange | 673 | 90 | [92] |
| SPW5 | 123 | CR | 697 | 78 | This study |
| SPO5 | 28 | CR | 697 | 96 | This study |
| TFC-(sericin-TMC) | 11.9 | CR | 697 | >99 | [93] |
| TFC-PSSNa/PVA–PSF | 8.3 | CR | 697 | >99 | [94] |
| Sepro NF 2A | 10.5 | CR | 697 | >99 | [95] |
| mTFC-mZIF | 14.9 | RB2 | 774 | 99 | [69] |
| SPW5 | 123 | AZ | 793 | 75 | This study |
| SPO5 | 28 | AZ | 793 | 90 | This study |
| SPECMs | 6.7 | Methyl blue | 800 | >99 | [96] |
| mTFC-mZIF | 14.9 | RB5 | 992 | 99 | [69] |
| TFC-SR2 | 11.8 | RB5 | 992 | 99 | [97] |
| QPEI-PES | 12.6 | RB5 | 992 | 97 | [98] |
| HNTs-PIL/PES | 11.6 | RB5 | 992 | 95 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubovenko, R.; Dmitrenko, M.; Mikulan, A.; Puzikova, M.; Dzhakashov, I.; Rakovskaya, N.; Kuzminova, A.; Mikhailovskaya, O.; Su, R.; Penkova, A. Thin-Film Composite Polyamide Membranes Modified with HKUST-1 for Water Treatment: Characterization and Nanofiltration Performance. Polymers 2025, 17, 1137. https://doi.org/10.3390/polym17091137
Dubovenko R, Dmitrenko M, Mikulan A, Puzikova M, Dzhakashov I, Rakovskaya N, Kuzminova A, Mikhailovskaya O, Su R, Penkova A. Thin-Film Composite Polyamide Membranes Modified with HKUST-1 for Water Treatment: Characterization and Nanofiltration Performance. Polymers. 2025; 17(9):1137. https://doi.org/10.3390/polym17091137
Chicago/Turabian StyleDubovenko, Roman, Mariia Dmitrenko, Anna Mikulan, Margarita Puzikova, Ilnur Dzhakashov, Nadezhda Rakovskaya, Anna Kuzminova, Olga Mikhailovskaya, Rongxin Su, and Anastasia Penkova. 2025. "Thin-Film Composite Polyamide Membranes Modified with HKUST-1 for Water Treatment: Characterization and Nanofiltration Performance" Polymers 17, no. 9: 1137. https://doi.org/10.3390/polym17091137
APA StyleDubovenko, R., Dmitrenko, M., Mikulan, A., Puzikova, M., Dzhakashov, I., Rakovskaya, N., Kuzminova, A., Mikhailovskaya, O., Su, R., & Penkova, A. (2025). Thin-Film Composite Polyamide Membranes Modified with HKUST-1 for Water Treatment: Characterization and Nanofiltration Performance. Polymers, 17(9), 1137. https://doi.org/10.3390/polym17091137








