Nano-Hydroxyapatite/Poly(methyl methacrylate) Composite Bone Scaffold: Surfactant Surface Effects
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of n-HA
2.3. Electrospinning of PMMA Nanofibers
2.4. Morphological Characterization
2.5. Mechanical Analysis
2.6. Cell Viability XTT Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of HA Nanoparticles
3.2. Morphological Analysis of PMMA Nanofibers
3.3. Mechanical Analysis of PMMA Nanofibers
3.4. Cell Viability XTT Analysis of PMMA Nanofibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2024, 33, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Bourne, L.E.; Wheeler-Jones, C.P.D.; Orriss, I.R. Regulation of mineralisation in bone and vascular tissue: A comparative review. J. Endocrinol. 2021, 248, R51–R65. [Google Scholar] [CrossRef]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular matrices for bone regeneration: A literature review. Tissue Eng. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef]
- Dinatha, I.K.H.; Diputra, A.H.; Wihadmadyatami, H.; Partini, J.; Yusuf, Y. Nanofibrous electrospun scaffold doped with hydroxyapatite derived from sand lobster shell (Panulirus homarus) for bone tissue engineering. RSC Adv. 2024, 14, 8222–8239. [Google Scholar] [CrossRef]
- Zhang, J.; Che, L.; Wu, Y.; Zhou, L.; Liu, L.; Yue, Y.; Song, D.; Lou, X. Osteogenesis of Human iPSC-Derived MSCs by PLLA/SF Nanofiber Scaffolds Loaded with Extracellular Matrix. J. Tissue Eng. Regen. Med. 2023, 2023, 5280613. [Google Scholar] [CrossRef]
- Dai, W.; Cheng, J.; Yan, W.; Cao, C.; Zhao, F.; Li, Q.; Hu, X.; Wang, J.; Ao, Y. Enhanced osteochondral repair with hyaline cartilage formation using an extracellular matrix-inspired natural scaffold. Sci. Bull. 2023, 68, 1904–1917. [Google Scholar] [CrossRef]
- Griffanti, G.; McKee, M.D.; Nazhat, S.N. Mineralization of Bone Extracellular Matrix-like Scaffolds Fabricated as Silk Sericin-Functionalized Dense Collagen–Fibrin Hybrid Hydrogels. Pharmaceutics 2023, 15, 1087. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Bai, H.; Zhang, J.; Wang, Z.; Li, Z.; Zhao, X.; Wang, J.; Liu, H. Functionalization of biomimetic mineralized collagen for bone tissue engineering. Mater. Today Bio 2023, 20, 100660. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Kim, J.H.; Cho, M.J.; Kim, S.H.; Xu, B.; Amirthalingam, S.; Hwang, N.S.; Lee, J.H. Bioactive magnesium-based whitlockite ceramic as bone cement additives for enhancing osseointegration and bone regeneration. Mater. Des. 2023, 229, 111914. [Google Scholar] [CrossRef]
- Kadhum Alsaedi, S.; Salih, S.; Hashim, F. Preparation and Characterization of Polymer Blend and Nano Composite Materials Based on PMMA Used for Bone Tissue Regeneration. Eng. Technol. J. 2020, 38, 501–509. [Google Scholar] [CrossRef]
- Luo, Q.; He, X.; Duan, X.; Liu, H.; Zhou, Z.; Cheng, K. A Facile Synthesis of P(VDF-TrFE)-Coated-PMMA Janus Membranes for Guided Bone Regeneration. Coatings 2022, 12, 1947. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Kołbuk, D.; Gradys, A.; Sajkiewicz, P. Development of Poly(methyl methacrylate)/nano-hydroxyapatite (PMMA/nHA) Nanofibers for Tissue Engineering Regeneration Using an Electrospinning Technique. Polymers 2024, 16, 531. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Lin, Y.C.; Thirumurugan, S.; Hu, C.C.; Duann, Y.F.; Chung, R.J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef]
- Darjanki, C.M.; Hananta, J.S.; Prahasanti, C.; Ulfah, N.; Kusumawardani, B.; Wijaksana, I.K.E.; Aljunaid, M.; Nkuba, A. Expression of VEGF and BMP-2 in Osteoblast cells exposed to a combination of poly(methyl methacrylate) (PMMA) and hydroxyapatite (HAp). J. Oral Biol. Craniofacial Res. 2023, 13, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.L.; Wu, Y.S.; Wang, C.Y.; Hong, D.W.; Chen, Z.X.; Huang, C.A.; Chu, I.M.; Lai, P.L. Incorporation of surface-modified hydroxyapatite into poly(methyl methacrylate) to improve biological activity and bone ingrowth. R. Soc. Open Sci. 2019, 6, 182060. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Yang, Y.; Yin, Q. Bone defects are repaired by enhanced osteogenic activity of the induced membrane: A case report and literature review. BMC Musculoskelet. Disord. 2021, 22, 447. [Google Scholar] [CrossRef]
- Grafe, I.A.; Baier, M.; Nöldge, G.; Weiss, C.; Da Fonseca, K.; Hillmeier, J.; Libicher, M.; Rudofsky, G.; Metzner, C.; Nawroth, P.; et al. Calcium-phosphate and poly(methyl methacrylate) cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine 2008, 33, 1284–1290. [Google Scholar] [CrossRef]
- Li, T.; Weng, X.; Bian, Y.; Zhou, L.; Cui, F.; Qiu, Z. Influence of Nano-HA coated bone collagen to acrylic (poly(methyl methacrylate)) bone cement on mechanical properties and bioactivity. PLoS ONE 2015, 10, e0129018. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Hassanzadeh Tabrizi, S.A.; Ismail, A.F.; Seifalian, A.; RamaKrishna, S.; Berto, F. Poly(methyl methacrylate) bone cement, its rise, growth, downfall and future. Polym. Int. 2021, 70, 1182–1201. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Y.; Kang, T.; Rao, M.; Li, K.; Wang, Q.; Quan, C.; Zhang, C.; Jiang, Q.; Shen, H. Synergistic effect of HA and BMP-2 mimicking peptide on the bioactivity of HA/PMMA bone cement. Colloids Surf. B Biointerfaces 2015, 131, 39–46. [Google Scholar] [CrossRef]
- Dalby, M.J.; Di Silvio, L.; Harper, E.J.; Bonfield, W. Initial interaction of osteoblasts with the surface of a hydroxyapatite-poly(methylmethacrylate) cement. Biomaterials 2001, 22, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Tithito, T.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M.; Pon-On, W. Fabrication of biocomposite scaffolds made with modified hydroxyapatite inclusion of chitosan-grafted-poly(methyl methacrylate) for bone tissue engineering. Biomed. Mater. 2019, 14, 025013. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Sneha, M.; Sundaram, N.M. Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int. J. Nanomed. 2015, 10, 99–106. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. In vitro bioactivity of 3D microstructure hydroxyapatite/collagen based-egg white as an antibacterial agent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1412–1424. [Google Scholar] [CrossRef]
- Kalidas, S.; Sumathi, S. Electrospun gelatin-polyvinyl alcohol-silk fibre/hydroxyapatite scaffolds and their physicochemical and biological studies. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 684, 133030. [Google Scholar] [CrossRef]
- Balen, R.; Da Costa, W.V.; De Lara Andrade, J.; Piai, J.F.; Muniz, E.C.; Companhoni, M.V.; Nakamura, T.U.; Lima, S.M.; Da Cunha Andrade, L.H.; Bittencourt, P.R.S.; et al. Structural, thermal, optical properties and cytotoxicity of PMMA/ZnO fibers and films: Potential application in tissue engineering. Appl. Surf. Sci. 2016, 385, 257–267. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, J.; Mo, A.; Li, Y.; Li, J.; Wang, X. Characterization and human gingival fibroblasts biocompatibility of hydroxyapatite/PMMA nanocomposites for provisional dental implant restoration. Appl. Surf. Sci. 2008, 255, 328–330. [Google Scholar] [CrossRef]
- Zhang, J.C.; Mo, A.C.; Li, J.D.; Wang, X.J.; Li, Y. Characteristics of hydroxyapatite/PMMA nanocomposites for provisional restoration and its biocompatibility with human gingival fibroblasts. J. Sichuan Univ. Med. Sci. 2014, 45, 502–505. [Google Scholar]
- Chen, L.; Niu, D.; Lee, C.H.; Yao, Y.; Lui, K.; Ho, K.M.; Li, P. Amphiphilic Core–Shell Nanocomposite Particles for Enhanced Magnetic Resonance Imaging. Part. Part. Syst. Charact. 2016, 33, 756–763. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C. Preparation of PLGA-PEG/Hydroxyapatite Composites via Simple Methodology of Film Formation and Assessment of Their Structural, Thermal, and Biological Features. J. Turkish Chem. Soc. Sect. A Chem. 2023, 10, 1123–1132. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C. Comprehensive assessment of PLGA/nHAp combined with Hemp oil bionanocomposites: Revealing the in vitro behavior and features. Nano-Struct. Nano-Objects 2024, 38, 101152. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, thermal, and mechanical properties of melt-processed poly (ε-caprolactone) (pcl)/hydroxyapatite (hap) composites. Materials 2022, 15, 104. [Google Scholar] [CrossRef]
- Noohom, W.; Jack, K.S.; Martin, D.; Trau, M. Understanding the roles of nanoparticle dispersion and polymer crystallinity in controlling the mechanical properties of HA/PHBV nanocomposites. Biomed. Mater. 2009, 4, 015003. [Google Scholar] [CrossRef]
- Xue, S.; Gao, H.; Zhang, H.; Dai, H.; Lei, S.; Wang, L. Characteristics of ox bone derived natural porous hydroxyapatite and its application in composite phase change materials. Inorg. Chem. Commun. 2023, 156, 111251. [Google Scholar] [CrossRef]
- Kim, T.R.; Goh, T.S.; Lee, J.S.; Ryu, D.; Yoon, S.Y.; Lee, C. Experimental and numerical investigations for compressive behavior of porous hydroxyapatite bone scaffold fabricated via freeze-gel casting method. Mater. Des. 2023, 231, 112080. [Google Scholar] [CrossRef]
- Jeon, I.S.; Lee, M.H.; Choi, H.H.; Lee, S.; Chon, J.W.; Chung, D.J.; Park, J.H.; Jho, J.Y. Mechanical properties and bioactivity of polyetheretherketone/hydroxyapatite/carbon fiber composite prepared by the mechanofusion process. Polymers 2021, 13, 1978. [Google Scholar] [CrossRef]
- Ko, H.S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical properties and bioactivity of poly(Lactic acid) composites containing poly(glycolic acid) fiber and hydroxyapatite particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, X.; Mao, J.; Xu, F.; Cai, Q. Hydroxyapatite-poly(l-lactide) nanohybrids via surface-initiated ATRP for improving bone-like apatite-formation abilities. Appl. Surf. Sci. 2012, 258, 6823–6830. [Google Scholar] [CrossRef]
- Lee, J.H.; Shofner, M.L. Dispersion of polymer-decorated hydroxyapatite nanoparticles in poly(ethylene oxide) at low grafting densities. Polymer 2012, 53, 5146–5154. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, S.; Dong, S.; Wei, W.; Zhang, Y. Effects of sodium dodecyl sulfate and solution chemistry on retention and transport of biogenic nano-hydroxyapatite in saturated porous media. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 661, 130956. [Google Scholar] [CrossRef]
- Sebastiammal, S.; Fathima, A.S.L.; Al-Ghanim, K.A.; Nicoletti, M.; Baskar, G.; Iyyappan, J.; Govindarajan, M. Synthesis and characterisation of magnesium-wrapped hydroxyapatite nanomaterials for biomedical applications. Surf. Interfaces 2024, 44, 103779. [Google Scholar] [CrossRef]
- Wang, L.; Weng, L.; Wang, L.; Song, S. Hydrothermal synthesis of hydroxyapatite nanoparticles with various counterions as templates. J. Ceram. Soc. Jpn. 2010, 118, 1195–1198. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Miyajima, T.; Sugawara-Narutaki, A.; Kato, K.; Nagata, F. Development of paclitaxel-loaded poly(lactic acid)/hydroxyapatite core-shell nanoparticles as a stimuli-responsive drug delivery system. R. Soc. Open Sci. 2021, 8, 202030. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, K.C.; Li, Z.Y.; Huang, S.P. Plate-like hydroxyapatite nanoparticles synthesized by the hydrothermal method. J. Phys. Chem. Solids 2009, 70, 243–248. [Google Scholar] [CrossRef]
- Çiftçi, F. Design, Characterization and in vitro Simulations of nano-HAP/GO Composite Drug Delivery System Produced by Hydrothermal Methods Loaded with Paclitaxel. Cumhur. Sci. J. 2023, 44, 302–314. [Google Scholar] [CrossRef]
- Ozder, M.N.; Ciftci, F.; Rencuzogullari, O.; Arisan, E.D.; Ustündag, C.B. In situ synthesis and cell line studies of nano-hydroxyapatite/graphene oxide composite materials for bone support applications. Ceram. Int. 2023, 49, 14791–14803. [Google Scholar] [CrossRef]
- Raj, S.S.; Michailovich, K.A.; Subramanian, K.; Sathiamoorthyi, S.; Kandasamy, K.T. Philosophy of selecting ASTM standards for mechanical characterization of polymers and polymer composites. Mater. Plast. 2021, 58, 247–256. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018.
- Ustundag, C.B. Fabrication of porous hydroxyapatite-carbon nanotubes composite. Mater. Lett. 2016, 167, 89–92. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Lin, T.; Wang, X.G. Controlling the morphologies of electrospun nanofibres. In Nanofibers and Nanotechnology in Textiles; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9781845691059. [Google Scholar] [CrossRef]
- Zhou, X.D.; Zhang, S.C.; Huebner, W.; Ownby, P.D.; Gu, H. Effect of the solvent on the particle morphology of spray dried PMMA. J. Mater. Sci. 2001, 36, 3759–3768. [Google Scholar] [CrossRef]
- Duygulu, N.E.; Ciftci, F.; Ustundag, C.B. Electrospun drug blended poly(lactic acid) (PLA) nanofibers and their antimicrobial activities. J. Polym. Res. 2020, 27, 232. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Lin, Z.Y.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Subash, A.; Basanth, A.; Kandasubramanian, B. Biodegradable polyphosphazene–hydroxyapatite composites for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1093–1111. [Google Scholar] [CrossRef]
- Saad, S.M.I.; Policova, Z.; Acosta, E.J.; Neumann, A.W. Effect of surfactant concentration, compression ratio and compression rate on the surface activity and dynamic properties of a lung surfactant. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 103–116. [Google Scholar] [CrossRef]
- Sunita, T.; Sharma, P.; Malviya, R. Influence of Concentration on Surface Tension & Viscosity of Tamarind (Tamarindus Indica) Seed Gum. Ann. Mol. Genet. Med. 2017, 1, 8–12. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Reid, J.P.; Malila, J.; Prisle, N.L. The surface tension of surfactant-containing, finite volume droplets. Proc. Natl. Acad. Sci. USA 2020, 117, 8335–8343. [Google Scholar] [CrossRef] [PubMed]
- Patra, N.; Barone, A.C.; Salerno, M. Solvent effects on the thermal and mechanical properties of poly(methyl methacrylate) casted from concentrated solutions. Adv. Polym. Technol. 2011, 30, 12–20. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Park, H.J.; Kim, J.H.; Lee, O.J.; Park, C.H. A novel approach to fabricate silk nanofibers containing hydroxyapatite nanoparticles using a three-way stopcock connector. Nanoscale Res. Lett. 2013, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M.Y.; Karakatenko, E.Y.; Yurtov, E.V. Synthesis of Hydroxyapatite Nanoparticles by Controlled Precipitation in the Presence of Sodium Dodecyl Sulfate. Colloid J. 2020, 82, 275–283. [Google Scholar] [CrossRef]
- Drdlik, D.; Slama, M.; Hadraba, H.; Cihlar, J. Hydroxyapatite/zirconia-microfibre composites with controlled microporosity and fracture properties prepared by electrophoretic deposition. Ceram. Int. 2015, 41, 11202–11212. [Google Scholar] [CrossRef]
- Zeng, L.P.; Cao, L.Y.; Huang, J.F.; Guo, S. Influence of nano-hydroxyapatite on the mechanical properties of short-carbon-fiber/poly(methyl methacrylate) bio-composites. Wuji Cailiao Xuebao/J. Inorg. Mater. 2009, 24, 475–479. [Google Scholar] [CrossRef]
- Liping, Z.; Liyun, C.; Jianfeng, H.; Shen, G. Effect of processing factors on flexural properties of Cf-HA/PMMA composites. J. Reinf. Plast. Compos. 2010, 29, 1187–1194. [Google Scholar] [CrossRef]
- Xing, Z.C.; Han, S.J.; Shin, Y.S.; Koo, T.H.; Moon, S.; Jeong, Y.; Kang, I.K. Enhanced osteoblast responses to poly(methyl methacrylate)/hydroxyapatite electrospun nanocomposites for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 61–76. [Google Scholar] [CrossRef]
- Krestou, A.; Xenidis, A.; Panias, D. Mechanism of aqueous uranium(VI) uptake by hydroxyapatite. Miner. Eng. 2004, 17, 373–381. [Google Scholar] [CrossRef]
- Bansal, G.; Gautam, R.K.; Misra, J.P.; Mishra, A. Tribological behavior of silver-doped eggshell-derived hydroxyapatite reinforcement in PMMA-based composite. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2024, 238, 2142–2157. [Google Scholar] [CrossRef]
- Kenny, S.M.; Buggy, M. Bone cements and fillers: A review. J. Mater. Sci. Mater. Med. 2003, 14, 923–938. [Google Scholar] [CrossRef]
- Kumari, S.; Mishra, R.K.; Parveen, S.; Avinashi, S.K.; Hussain, A.; Kumar, S.; Banerjee, M.; Rao, J.; Kumar, R.; Gautam, R.K.; et al. Fabrication, structural, and enhanced mechanical behavior of MgO substituted PMMA composites for dental applications. Sci. Rep. 2024, 14, 2128. [Google Scholar] [CrossRef]
- Gudapati, S.; Satish, R.K.; Kumar, V.S.; Sajjan, G.S.; Varma, K.M.; Yedida, S.H. Multifarious bone cement and its applications in endodontics—A review. Int. J. Oral Health Dent. 2022, 8, 9–13. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Shirdar, M.R.; Qi, M.L.; Taheri, M.M.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Huang, Z.; Agrawal, M.; Lu, Y.P.; et al. Novel PMMA bone cement nanocomposites containing magnesium phosphate nanosheets and hydroxyapatite nanofibers. Mater. Sci. Eng. C 2020, 109, 110497. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Portolés, M.T.; Ramírez-Santillán, C.; Vallet-Regí, M.; Serro, A.P.; Grácio, J.; Marques, P.A.A.P. Evaluation of the in vitro biocompatibility of PMMA/high-load HA/carbon nanostructures bone cement formulations. J. Mater. Sci. Mater. Med. 2013, 24, 2787–2796. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Taheri, M.M.; Qi, M.L.; Gohari, S.; Farajpour, N.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Shokuhfar, T. Optimization of the mechanical properties and the cytocompatibility for the pmma nanocomposites reinforced with the hydroxyapatite nanofibers and the magnesium phosphate nanosheets. Materials 2021, 14, 5893. [Google Scholar] [CrossRef]
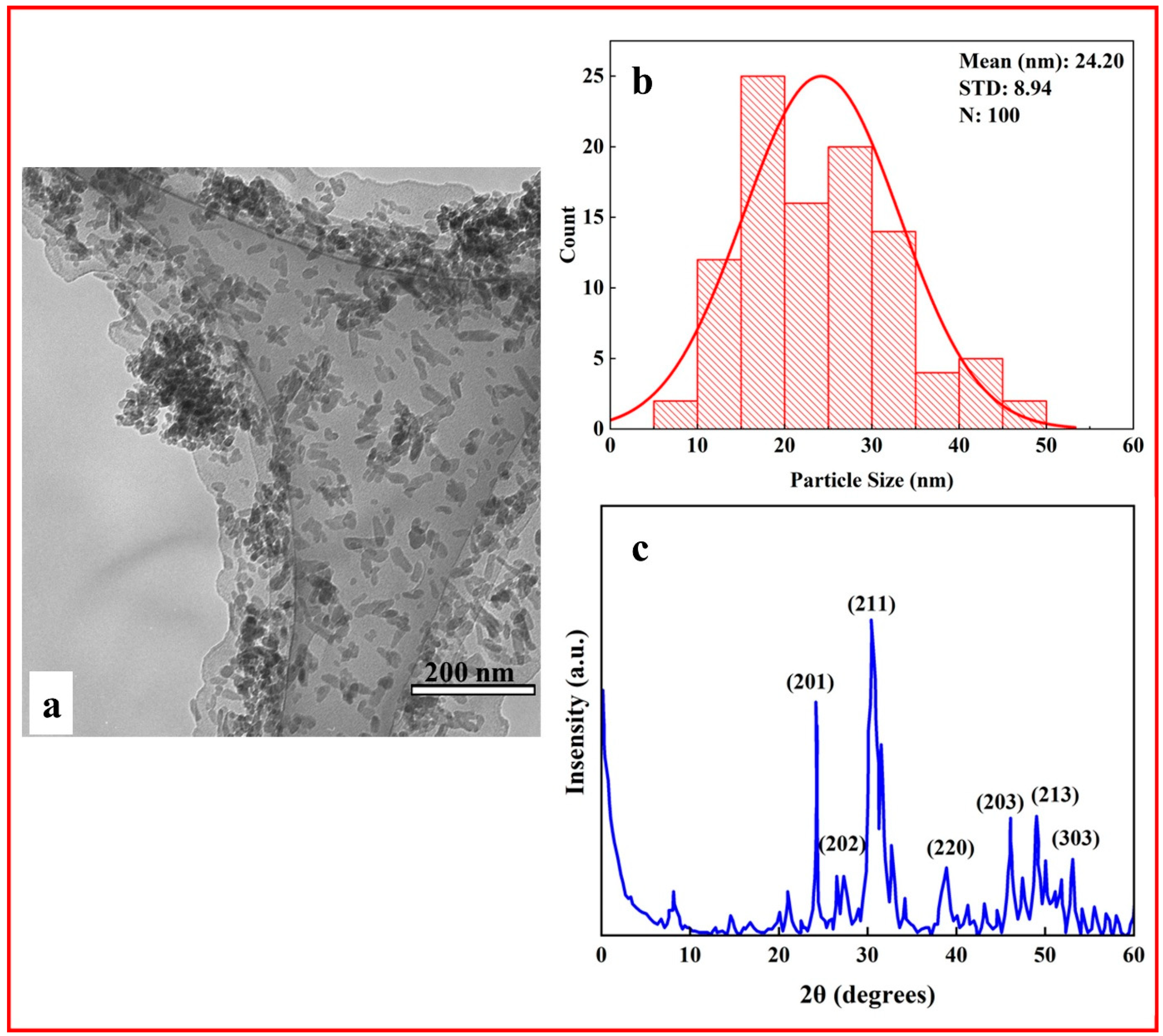


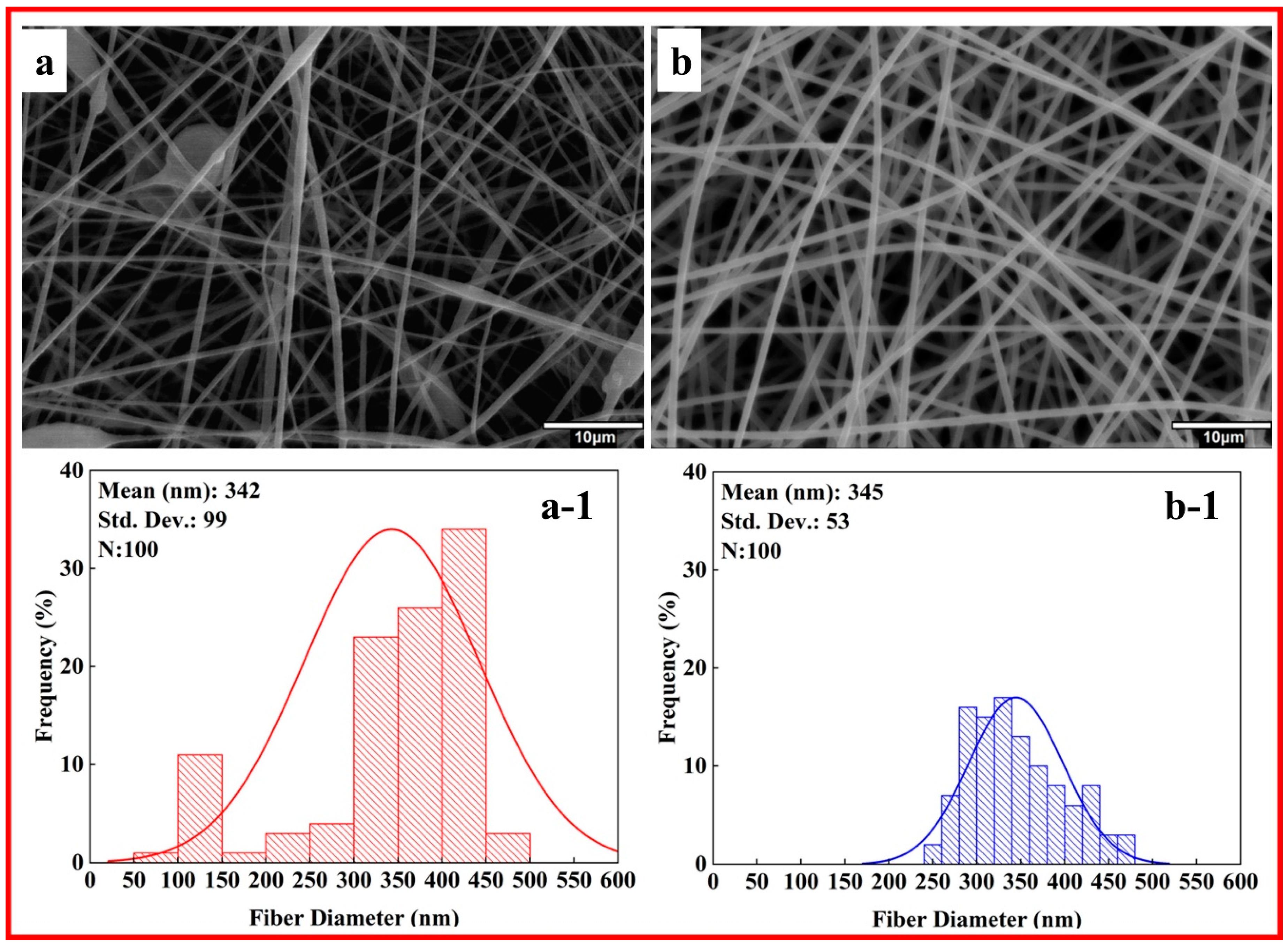
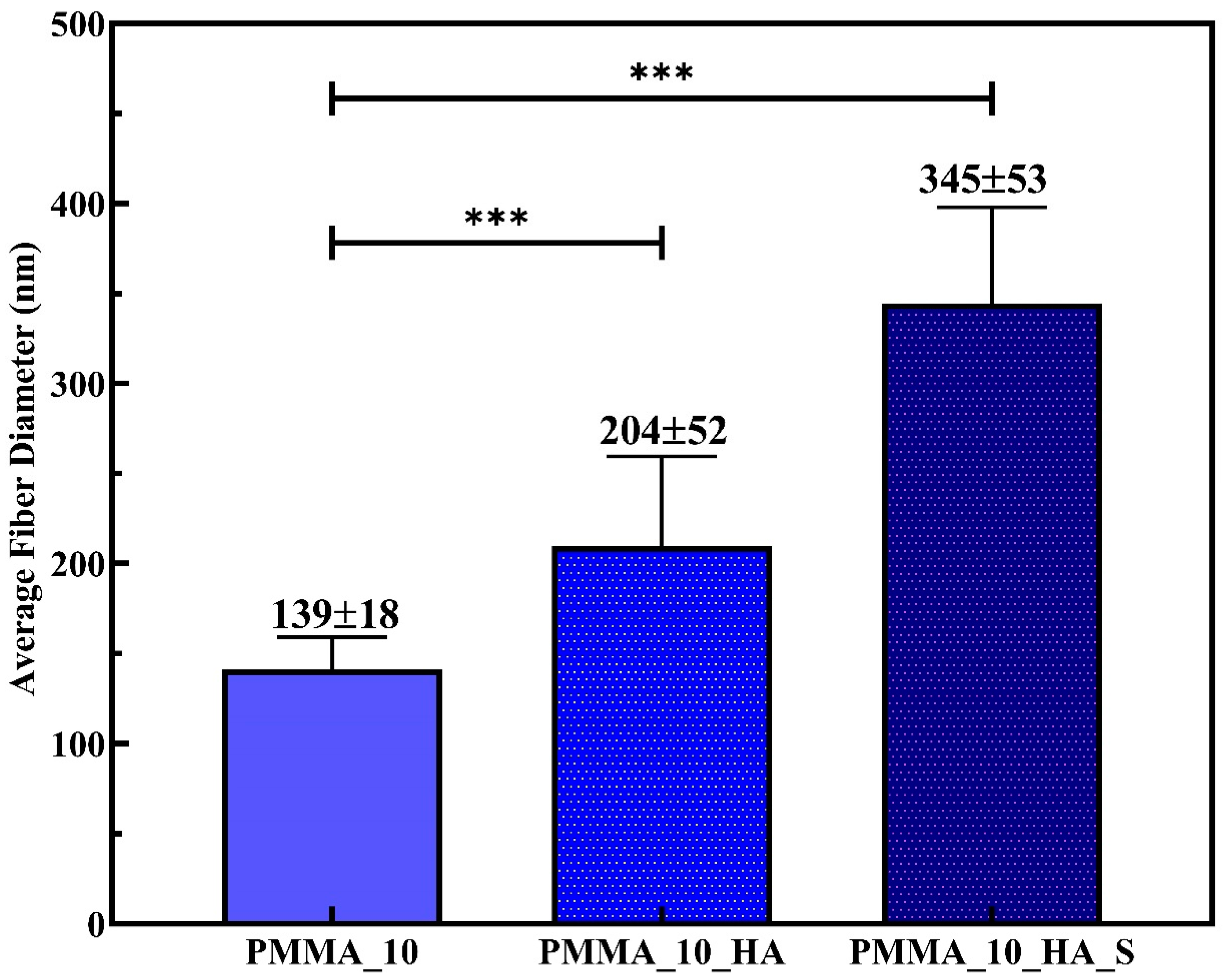


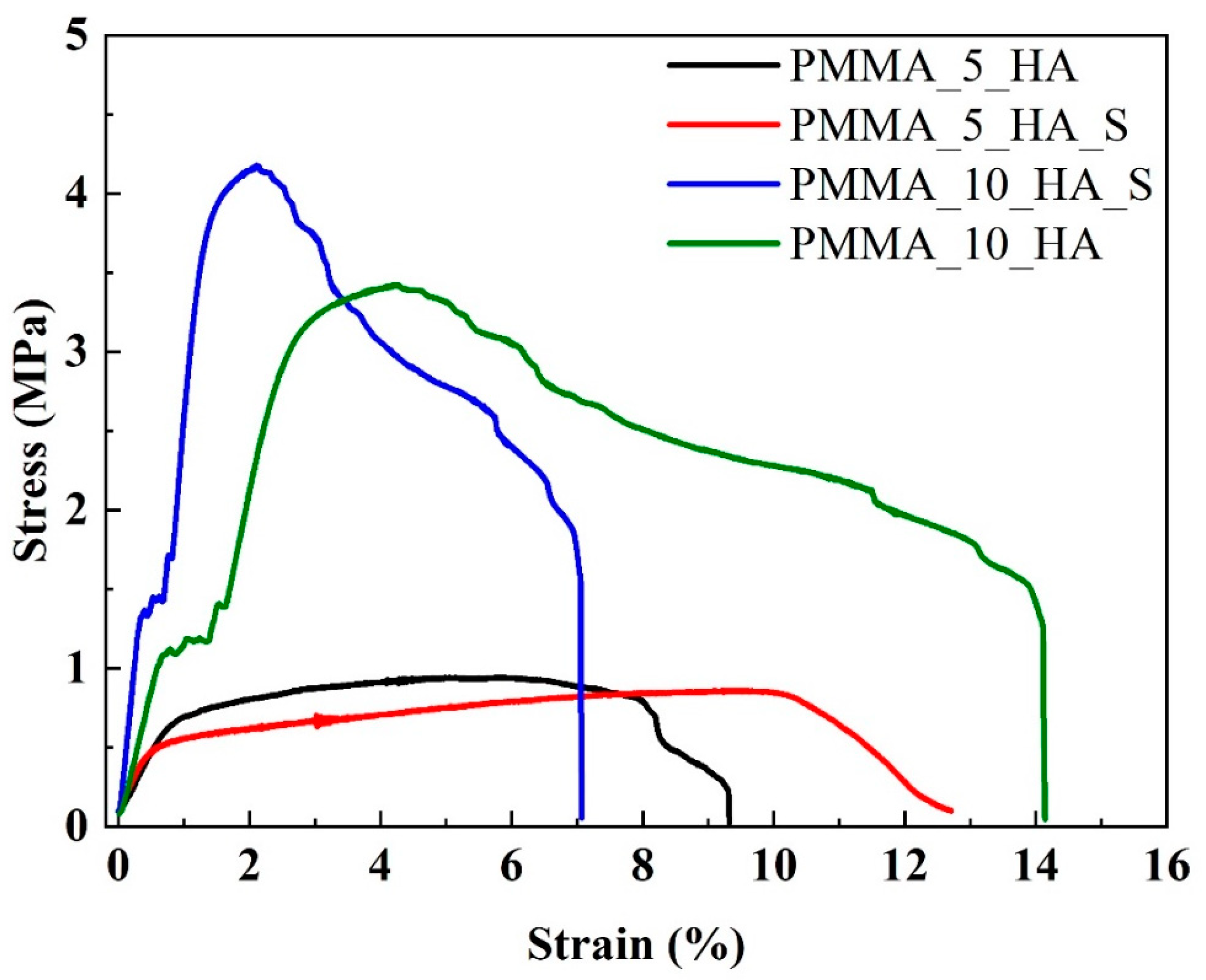
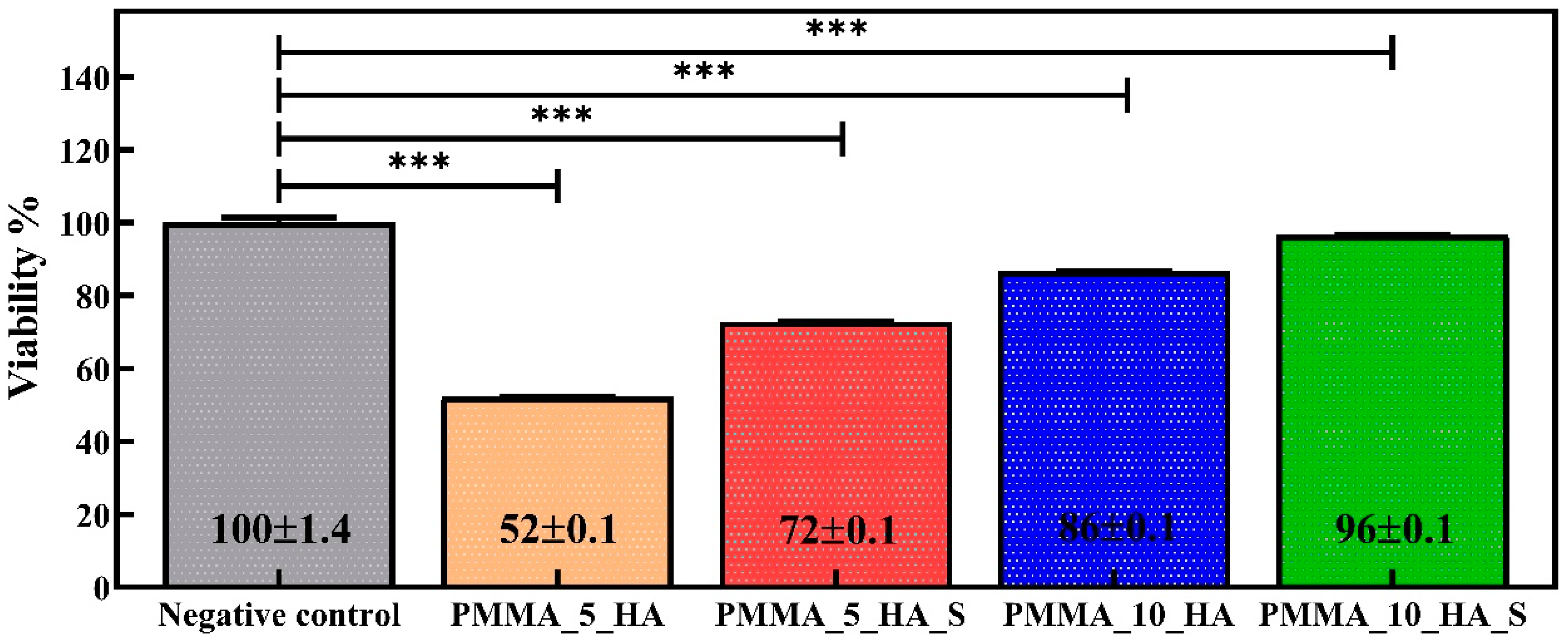
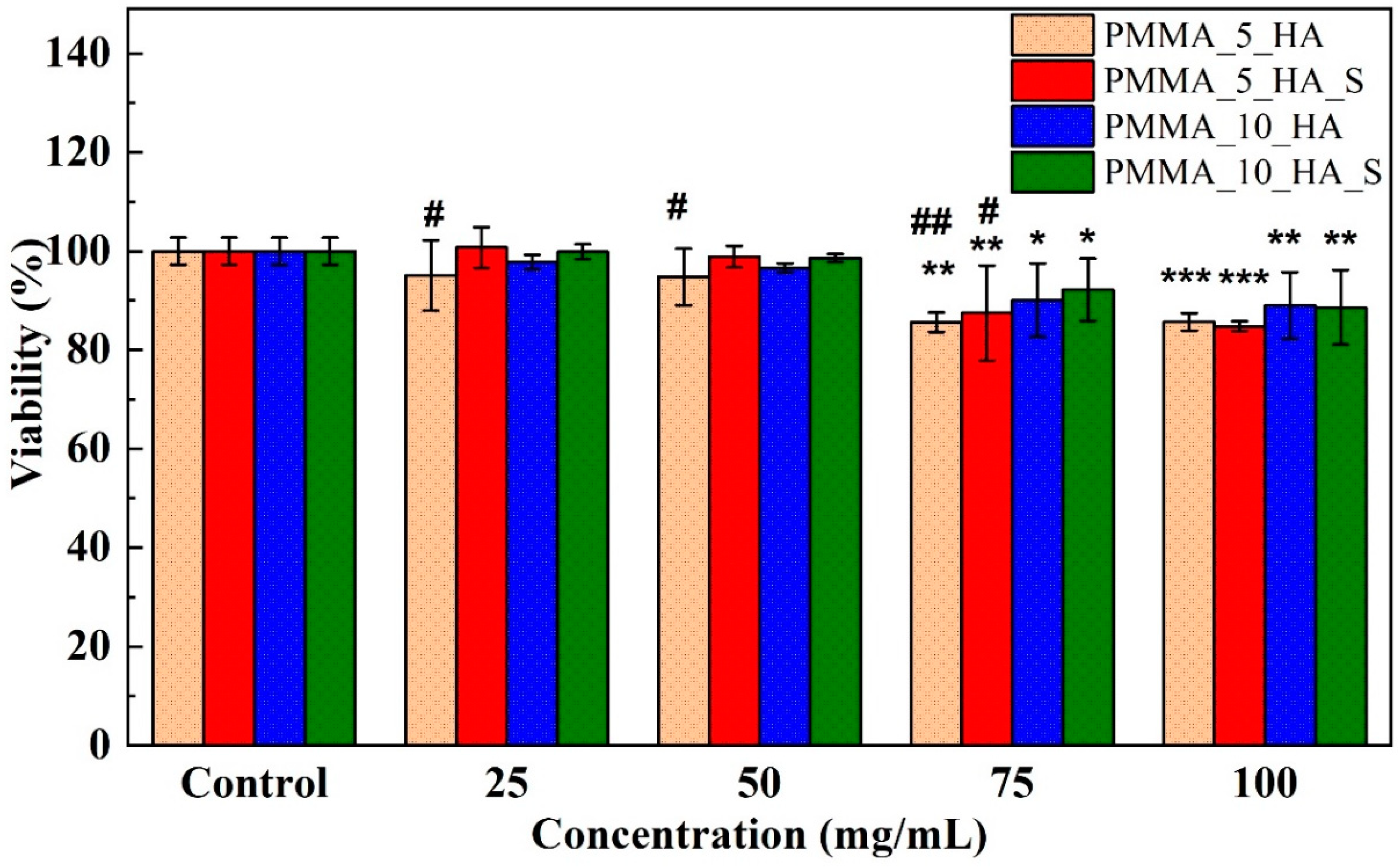
| Sample Code | Stress (MPa) | Elongation (%) |
|---|---|---|
| PMMA_5_HA | 0.95 ± 1.45 * | 9.3 ± 2.24 * |
| PMMA_5_HA_S | 0.85 ± 1.20 * | 12.7 ± 2.73 * |
| PMMA_10_HA | 3.42 ± 1.56 * | 14.2 ± 2.87 * |
| PMMA_10_HA_S | 4.16 ± 2.13 * | 7.1 ± 1.95 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oruc, M.E.; Evcimen Duygulu, N.; Onder, B.; Yelkenci, A.; Ustündag, C.B.; Ciftci, F. Nano-Hydroxyapatite/Poly(methyl methacrylate) Composite Bone Scaffold: Surfactant Surface Effects. Polymers 2025, 17, 1148. https://doi.org/10.3390/polym17091148
Oruc ME, Evcimen Duygulu N, Onder B, Yelkenci A, Ustündag CB, Ciftci F. Nano-Hydroxyapatite/Poly(methyl methacrylate) Composite Bone Scaffold: Surfactant Surface Effects. Polymers. 2025; 17(9):1148. https://doi.org/10.3390/polym17091148
Chicago/Turabian StyleOruc, Muhammed Enes, Nilüfer Evcimen Duygulu, Betul Onder, Aslihan Yelkenci, Cem Bülent Ustündag, and Fatih Ciftci. 2025. "Nano-Hydroxyapatite/Poly(methyl methacrylate) Composite Bone Scaffold: Surfactant Surface Effects" Polymers 17, no. 9: 1148. https://doi.org/10.3390/polym17091148
APA StyleOruc, M. E., Evcimen Duygulu, N., Onder, B., Yelkenci, A., Ustündag, C. B., & Ciftci, F. (2025). Nano-Hydroxyapatite/Poly(methyl methacrylate) Composite Bone Scaffold: Surfactant Surface Effects. Polymers, 17(9), 1148. https://doi.org/10.3390/polym17091148







