Thermodynamic and Technological Compatibility of Polyvinyl Chloride, Thermoplastic Polyurethane, and Bio-Plasticizer Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Blends
2.2.2. Sample Preparation for TSD Test
2.2.3. Sample Preparation for Tensile Test
2.2.4. TSD Test for Polymers and Their Blends
2.2.5. TSD Test for the Bio-Plasticizer, Glycerol Diacetate Monolaurate
2.2.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.7. Dynamic Mechanical Analysis (DMA)
2.2.8. Scanning Electron Microscopy (SEM) Analysis
2.2.9. Tensile Testing
2.2.10. Hardness Measurement
2.2.11. Thermogravimetric Analysis (TGA)
3. Results and Discussion
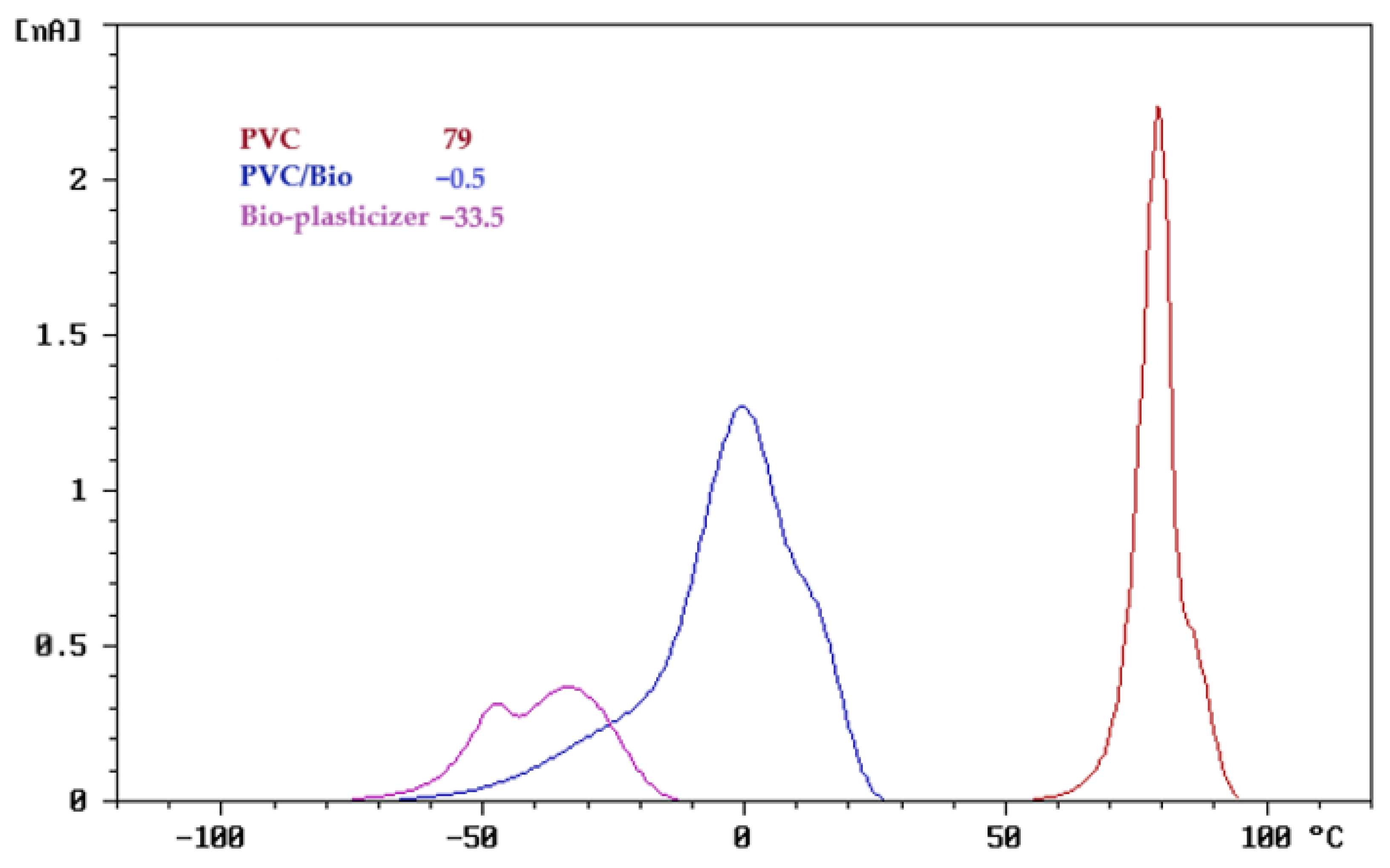
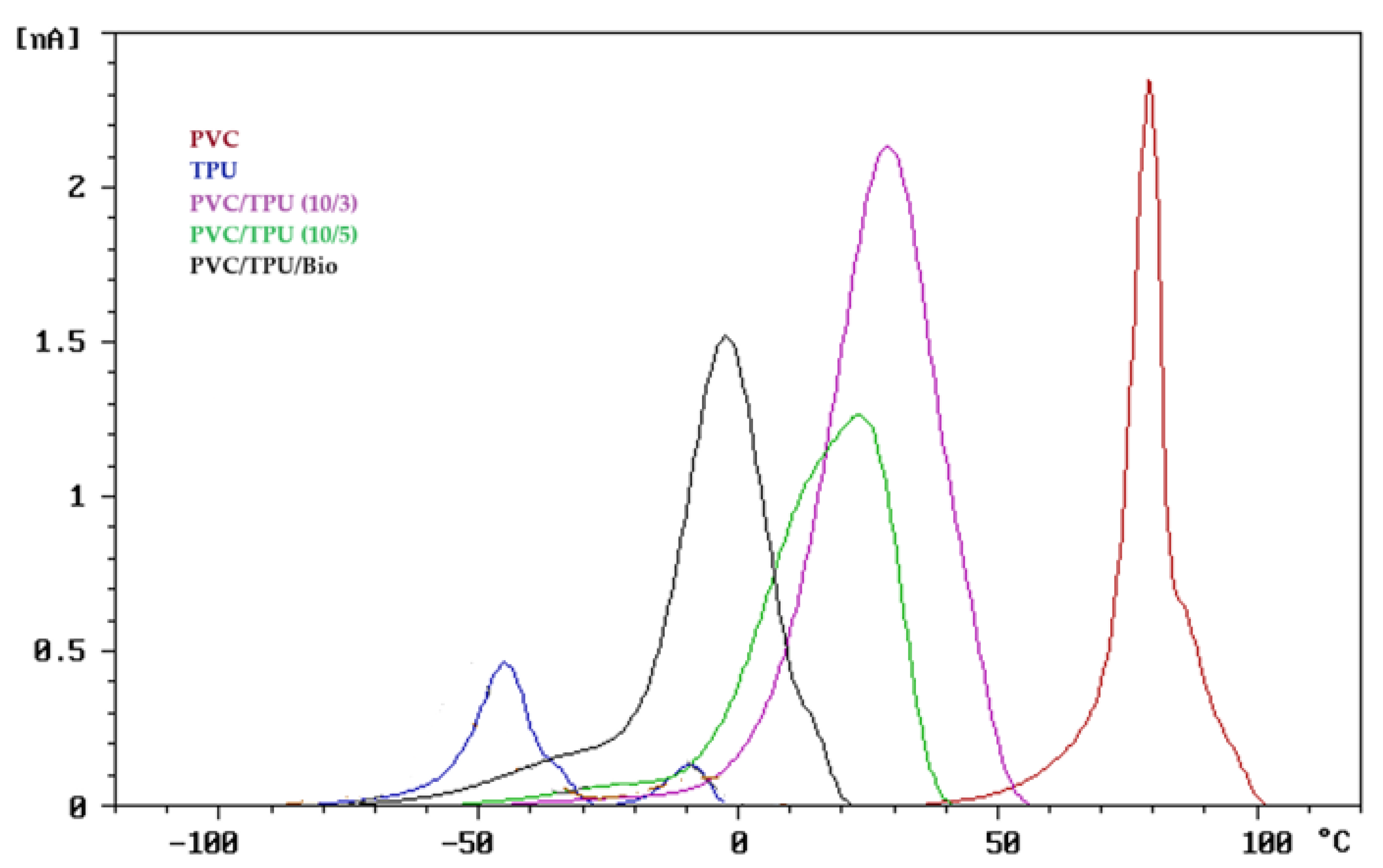
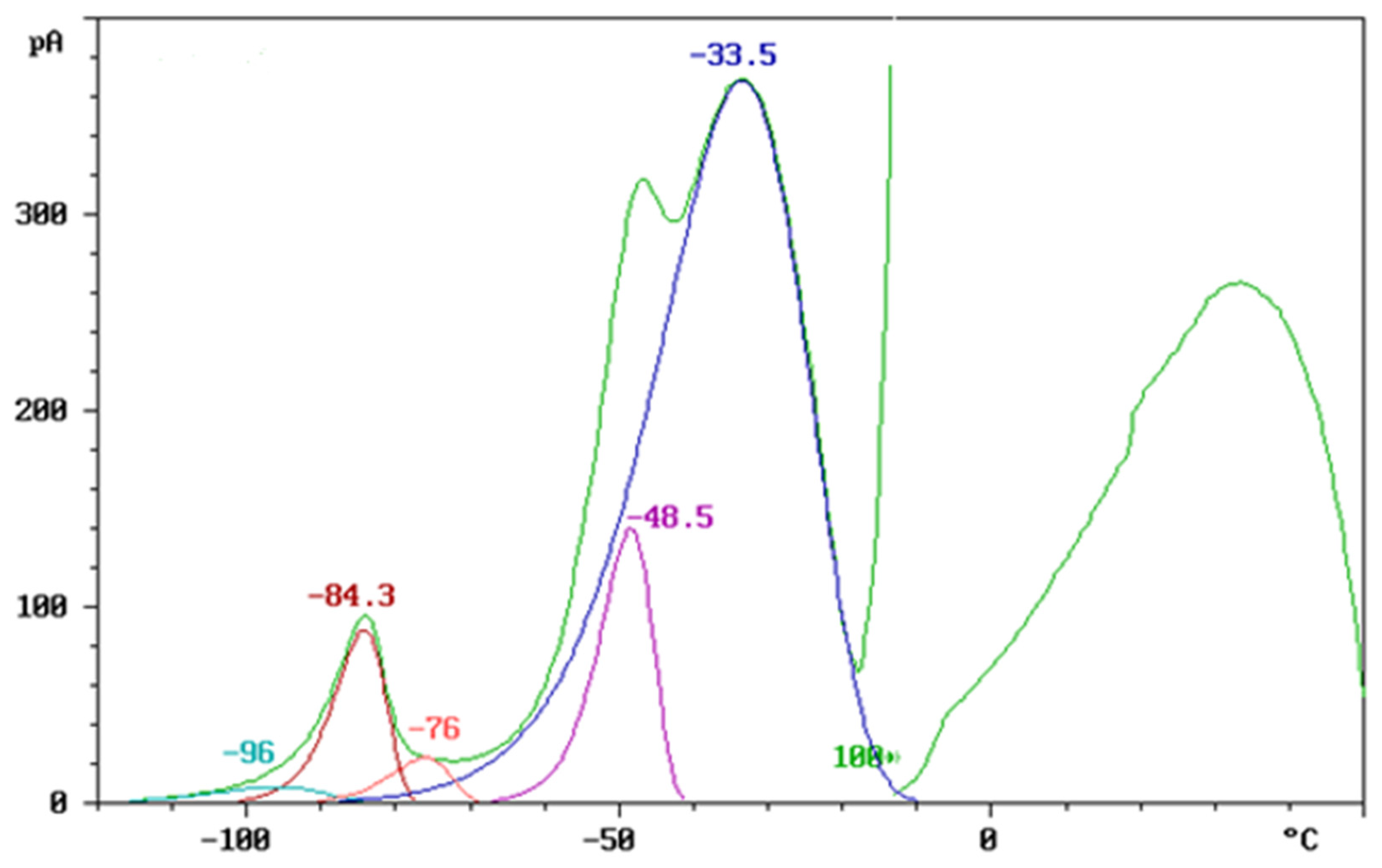
3.1. TSD Results of Polymer Blends
3.1.1. TSD Curve Analysis of Polymer Blends
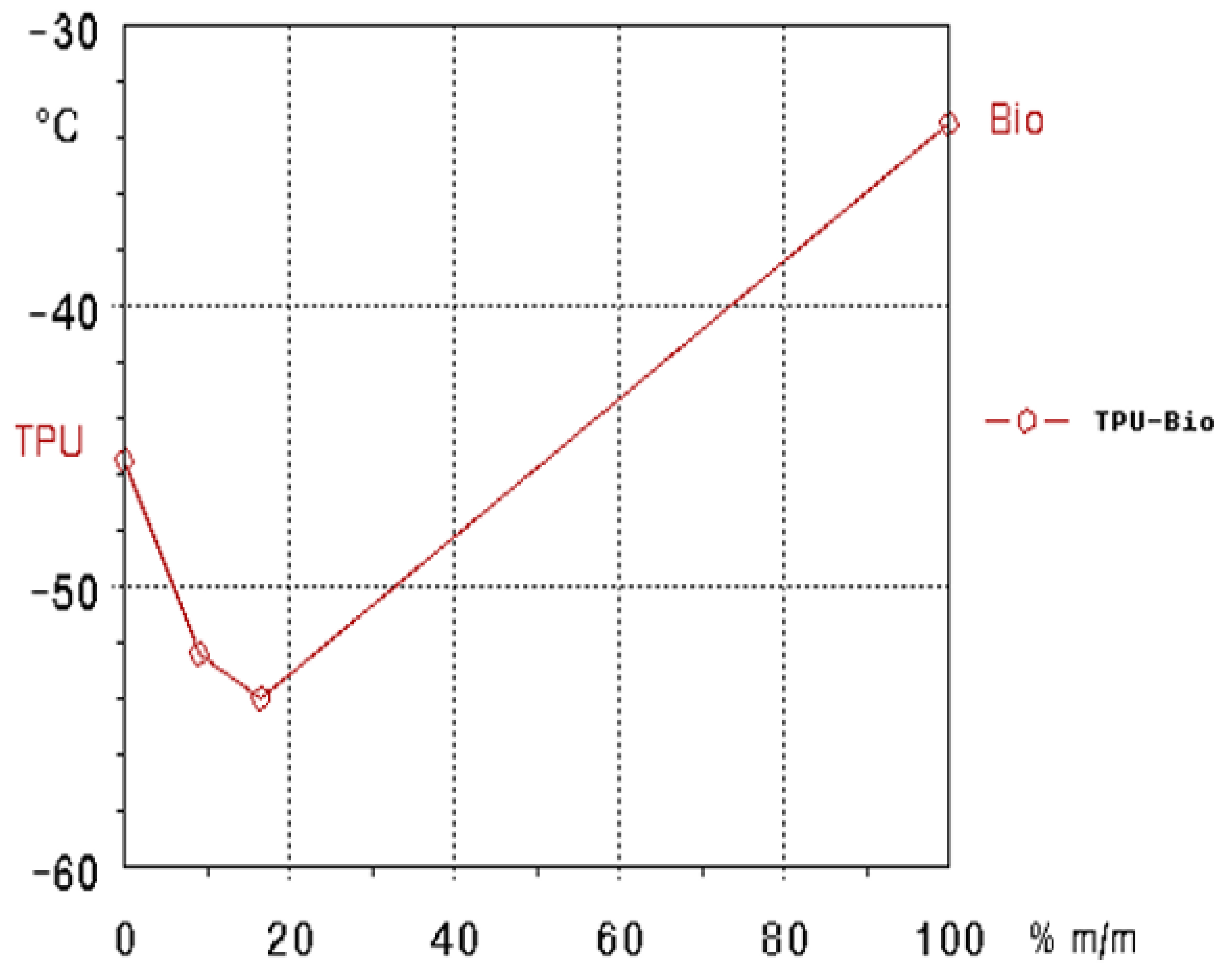
3.1.2. TSD Peak Maxima Trends
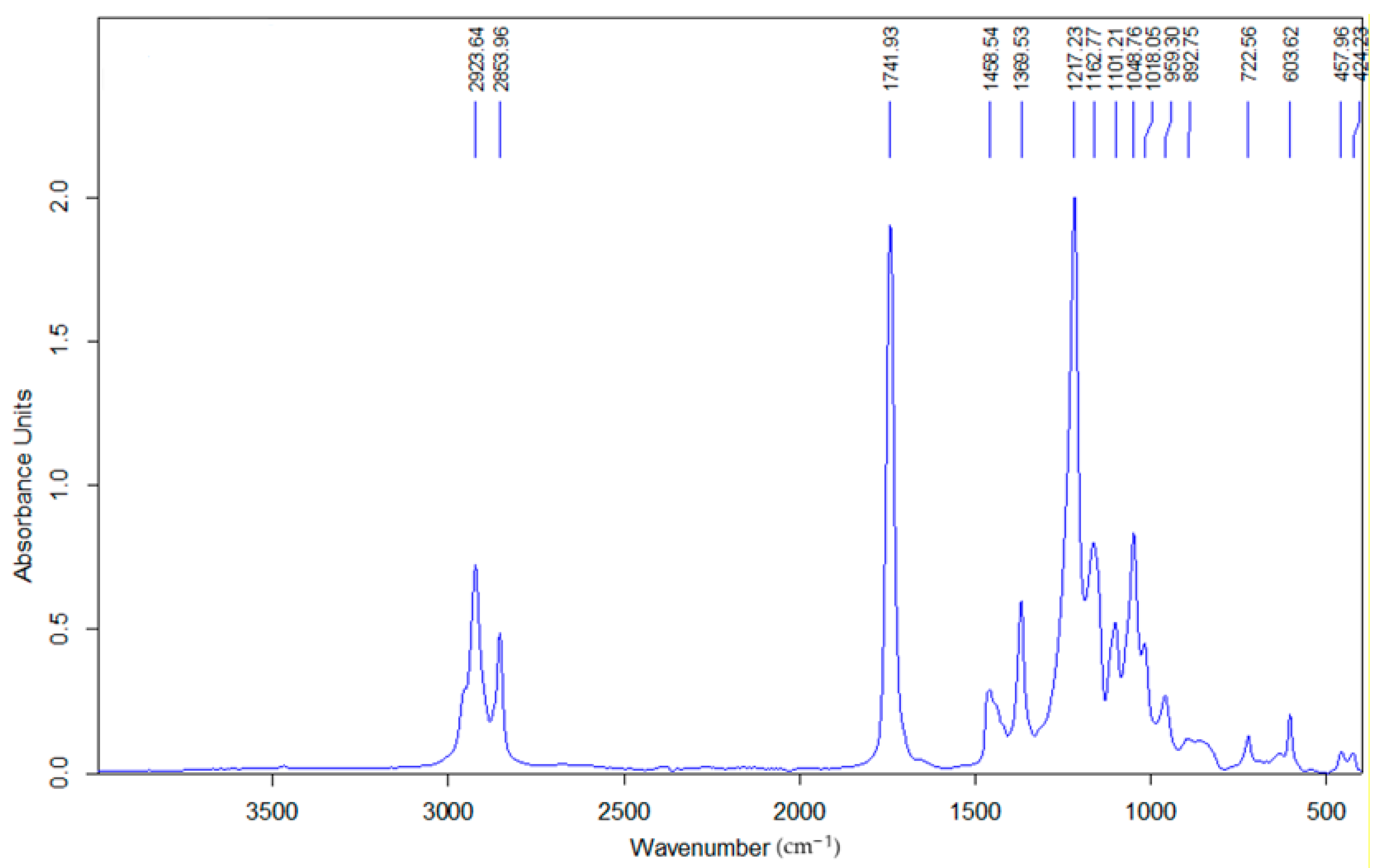
3.2. FTIR Analysis of Glycerol Diacetate Monolaurate
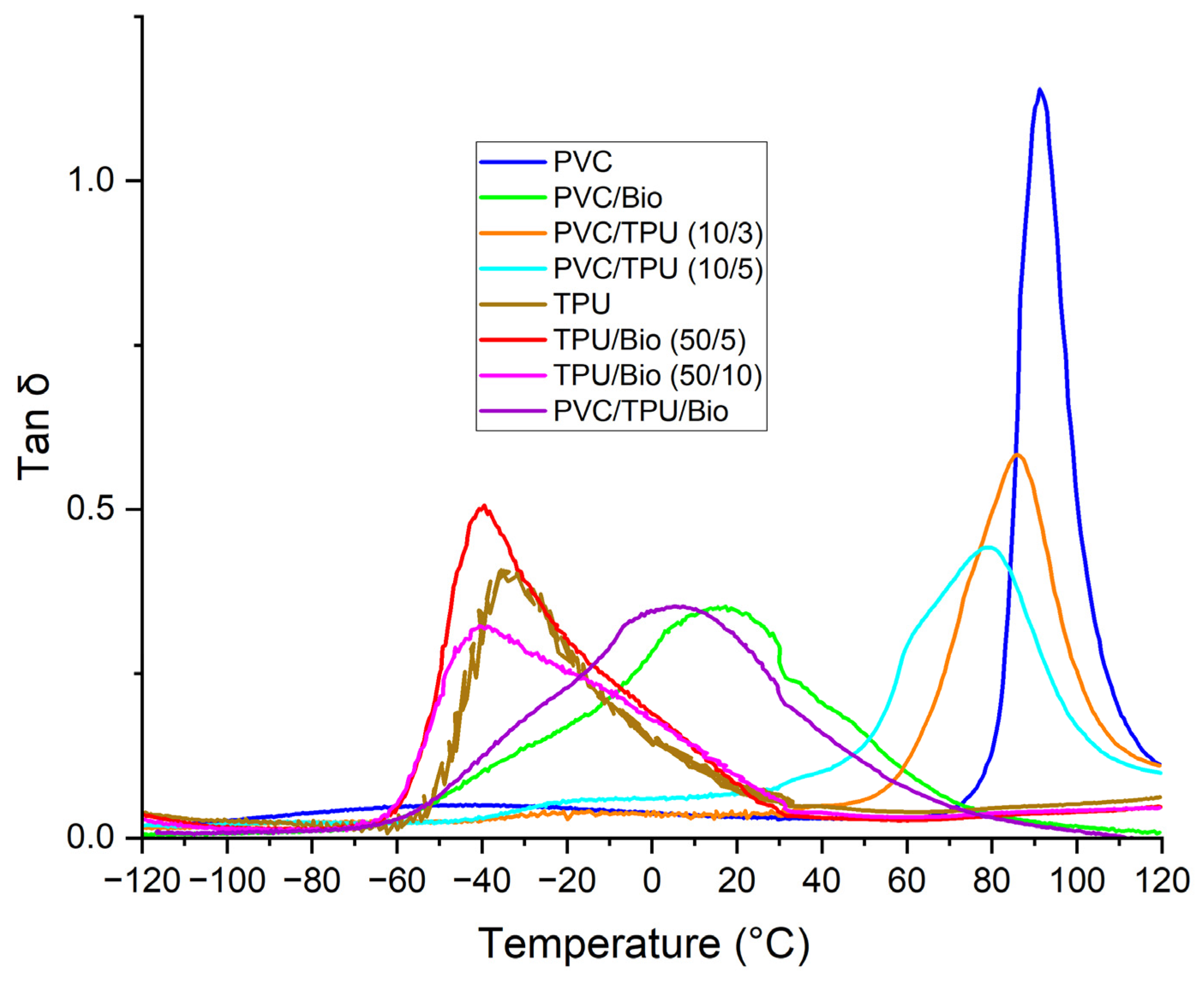
3.3. Dynamic Mechanical Analysis (DMA) of Polymer Blends
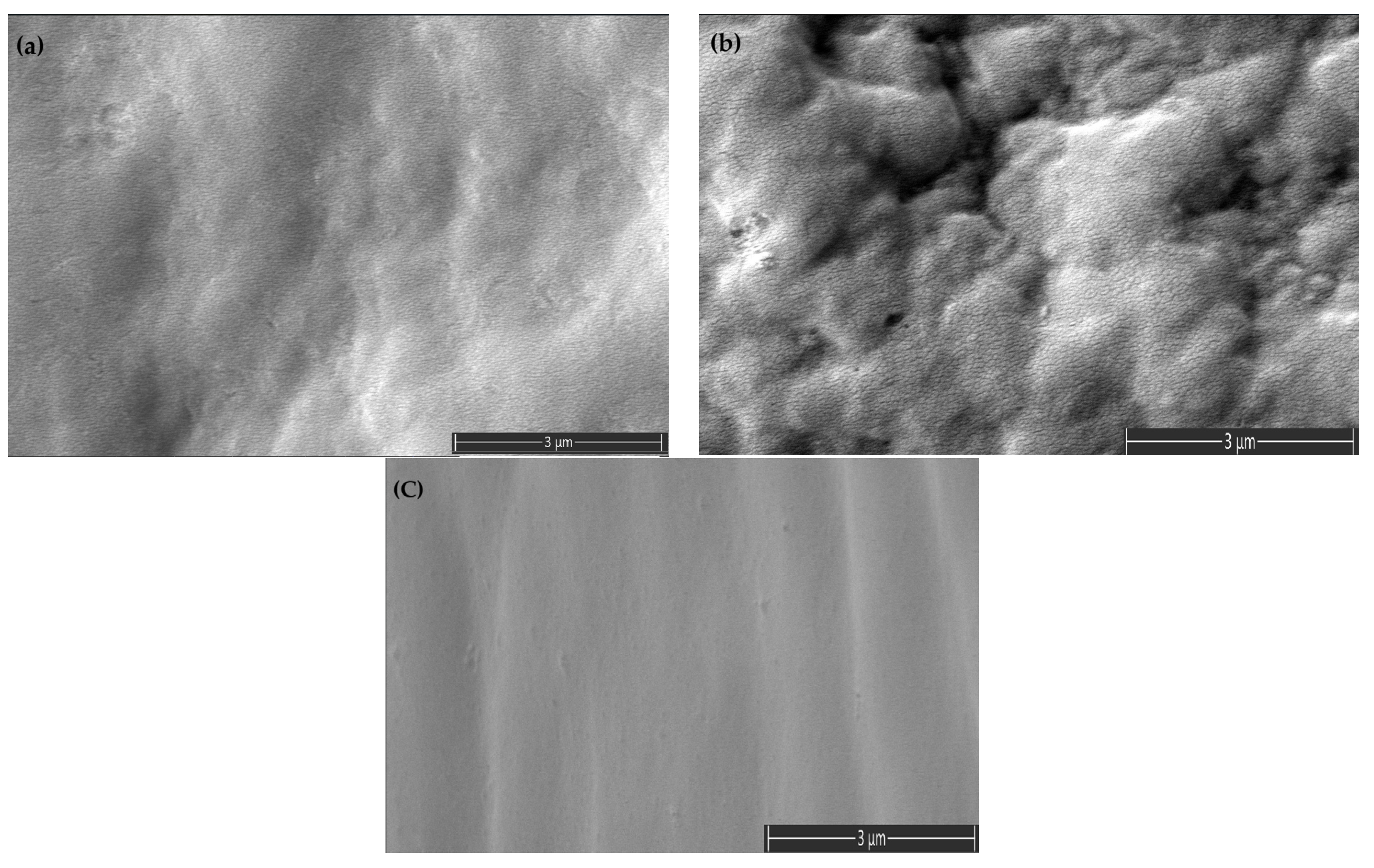
3.4. Scanning Electron Microscopy (SEM) Analysis of Polymer Blends
3.5. Results of Mechanical Measurements
3.5.1. Tensile Properties
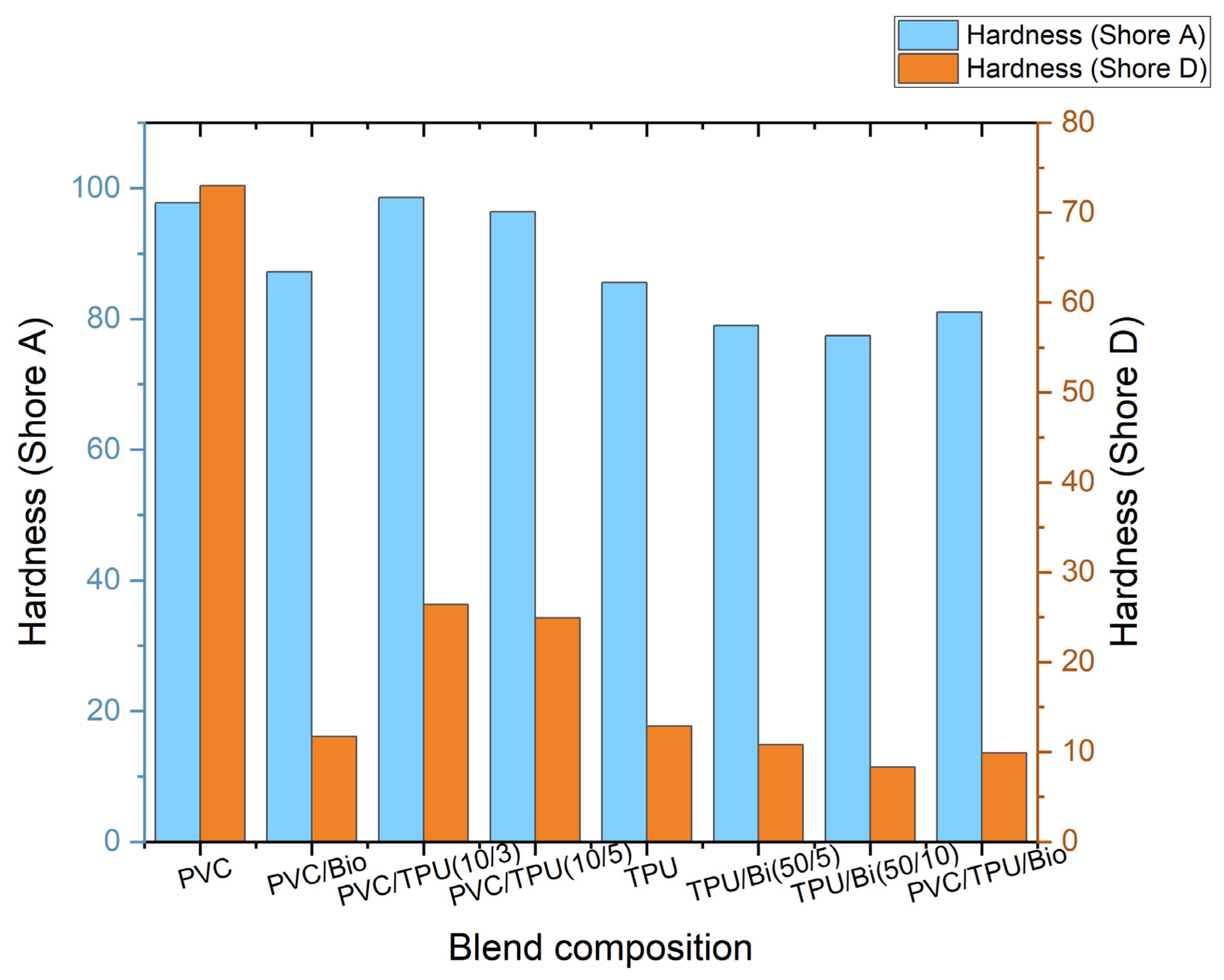
3.5.2. Results of Hardness Testing
3.6. Thermal Degradation Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robeson, L. Historical perspective of advances in the science and technology of polymer blends. Polymers 2014, 6, 1251–1265. [Google Scholar] [CrossRef]
- Sangkhawasi, M.; Remsungnen, T.; Vangnai, A.S.; Maitarad, P.; Rungrotmongkol, T. Prediction of the glass transition temperature in polyethylene terephthalate/polyethylene vanillate (PET/PEV) blends: A molecular dynamics study. Polymers 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Palabiyik, M.; Bahadur, S. Mechanical and tribological properties of polyamide 6 and high density polyethylene polyblends with and without compatibilizer. Wear 2000, 246, 149–158. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; Ramos-Olmos, M.; Pájaro-Gómez, N. Bibliometric and Co-Occurrence Study of Process System Engineering (PSE) Applied to the Polyvinyl Chloride (PVC) Production. Materials 2023, 16, 6932. [Google Scholar] [CrossRef]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Misidentification of PVC microplastics in marine environmental samples. TrAC Trends Anal. Chem. 2022, 153, 116649. [Google Scholar] [CrossRef]
- Tian, Y.; Han, M.; Gu, D.; Bi, Z.; Gu, N.; Hu, T.; Li, G.; Zhang, N.; Lu, J. PVC Dechlorination for Facilitating Plastic Chemical Recycling: A Systematic Literature Review of Technical Advances, Modeling and Assessment. Sustainability 2024, 16, 8331. [Google Scholar] [CrossRef]
- Kobetičová, K.; Černý, R. Ecotoxicity assessment of short-and medium-chain chlorinated paraffins used in polyvinyl-chloride products for construction industry. Sci. Total Environ. 2018, 640, 523–528. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and chemical modifications of poly (vinyl chloride) materials to prevent plasticizer migration-Still on the run. React. Funct. Polym. 2020, 147, 104458. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K. A brief review of poly (vinyl chloride)(PVC) recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Mohan, C.; Negi, A. An investigative study on the structural, thermal and mechanical properties of clay-based PVC polymer composite films. Polymers 2023, 15, 1922. [Google Scholar] [CrossRef] [PubMed]
- Somdee, P.; Lassú-Kuknyó, T.; Kónya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of bio-based polyurethanes: Perspective on applications and bio-degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Backes, E.H.; Harb, S.V.; Pinto, L.A.; de Moura, N.K.; de Melo Morgado, G.F.; Marini, J.; Passador, F.R.; Pessan, L.A. Thermoplastic polyurethanes: Synthesis, fabrication techniques, blends, composites, and applications. J. Mater. Sci. 2024, 59, 1123–1152. [Google Scholar] [CrossRef]
- Oh, J.; Kim, Y.K.; Hwang, S.-H.; Kim, H.-C.; Jung, J.-H.; Jeon, C.-H.; Kim, J.; Lim, S.K. Synthesis of thermoplastic polyurethanes containing bio-based polyester polyol and their fiber property. Polymers 2022, 14, 2033. [Google Scholar] [CrossRef]
- da Silva, C.A.; dos Santos, R.N.; Oliveira, G.G.; de Souza Ferreira, T.P.; de Souza, N.L.G.D.; Soares, A.S.; de Melo, J.F.; Colares, C.J.G.; de Souza, U.J.B.; de Araújo-Filho, R.N. Biodiesel and bioplastic production from waste-cooking-oil transesterification: An environmentally friendly approach. Energies 2022, 15, 1073. [Google Scholar] [CrossRef]
- Schwartz, G.A.; Paluch, M.; Alegría, Á.; Colmenero, J. High pressure dynamics of polymer/plasticizer mixtures. J. Chem. Phys. 2009, 131, 044906. [Google Scholar] [CrossRef]
- White, C.; Pejcic, B.; Myers, M.; Qi, X. Development of a plasticizer-poly (methyl methacrylate) membrane for sensing petroleum hydrocarbons in water. Sens. Actuators B Chem. 2014, 193, 70–77. [Google Scholar] [CrossRef]
- Czogała, J.; Pankalla, E.; Turczyn, R. Recent attempts in the design of efficient PVC plasticizers with reduced migration. Materials 2021, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Román, K.; Zsoldos, G. PVC/LDPE Blends: Relationship Between Thermal/Mechanical Properties, Structure and Blend Behaviour. Int. J. Eng. Manag. Sci. 2019, 4, 162–165. [Google Scholar] [CrossRef]
- Arrighi, V.; Cowie, J.M.; Fuhrmann, S.; Youssef, A. Miscibility criterion in polymer blends and its determination. In Encyclopedia of Polymer Blends; Wiley: Hoboken, NJ, USA, 2010; pp. 153–198. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Marossy, K.; Tóth, J. Anomalous behaviour of PVC–CPVC–CPE blends. Plast. Rubber Compos. 2005, 34, 438–442. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: A literature review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef]
- Feng, G.; Hu, L.; Ma, Y.; Jia, P.; Hu, Y.; Zhang, M.; Liu, C.; Zhou, Y. An efficient bio-based plasticizer for poly (vinyl chloride) from waste cooking oil and citric acid: Synthesis and evaluation in PVC films. J. Clean. Prod. 2018, 189, 334–343. [Google Scholar] [CrossRef]
- Kollár, M.; Zsoldos, G. Investigating poly-(vinyl-chloride)-polyethylene blends by thermal methods. J. Therm. Anal. Calorim. 2012, 107, 645–650. [Google Scholar] [CrossRef]
- Radhakrishnan Nair, M.; Gopinathan Nair, M. Compatibility studies and characterisation of a PVC/NR blend system using NR/PU block copolymer. Polym. Bull. 2006, 56, 619–631. [Google Scholar] [CrossRef]
- De Feo, G.; Ferrara, C.; Giordano, L.; Ossèo, L.S. Assessment of Three Recycling Pathways for Waste Cooking Oil as Feedstock in the Production of Biodiesel, Biolubricant, and Biosurfactant: A Multi-Criteria Decision Analysis Approach. Recycling 2023, 8, 64. [Google Scholar] [CrossRef]
- Marossy, K. Practical approach to thermally stimulated discharge (TSD) method on polymers. J. Therm. Anal. Calorim. 2017, 129, 161–170. [Google Scholar] [CrossRef]
- Ihemaguba, C.L.; Marossy, K. Combined thermal analysis of fluid plasticizers. J. Therm. Anal. Calorim. 2020, 147, 195–201. [Google Scholar] [CrossRef]
- Tamási, K.; Marossy, K. Combined thermal analysis of plant oils. J. Therm. Anal. Calorim. 2021, 147, 2047–2054. [Google Scholar] [CrossRef]
- Marossy, K. Thermally stimulated discharge (TSD) study of low-temperature transitions in chlorine-containing polymers. J. Therm. Anal. Calorim. 2023, 148, 1899–1903. [Google Scholar] [CrossRef]






| PVC | PVC/Bio | PVC/TPU (10/3) | PVC/TPU (10/5) | TPU | TPU/Bio(50/5) | TPU/Bio(50/10) | PVC/TPU/Bio | |
|---|---|---|---|---|---|---|---|---|
| PVC base mixture | 100 | 100 | 100 | 100 | - | - | - | 100 |
| TPU | - | - | 30 | 50 | 100 | 100 | 100 | 20 |
| Bio plasticizer | - | 30 | - | - | - | 10 | 20 | 50 |
| CaZn stab. | 1.2 | 1.2 | 1.2 | 1.2 | - | - | - | 1.2 |
| E-wax | 0.3 | 0.3 | 0.3 | 0.3 | - | - | - | 0.3 |
| PVC | PVC/Bio | PVC/TPU (10/3) | PVC/TPU (10/5) | TPU | TPU/Bio(50/5) | TPU/Bio(50/10) | PVC/TPU/Bio | |
|---|---|---|---|---|---|---|---|---|
| Tensile strength (MPa) | 52.2 | 20.8 | 43.3 | 27.8 | 52.9 | 49.9 | 34.2 | 27 |
| Young’s modulus (MPa) | 2768 | 5.9 | 987.2 | 457.5 | 8.2 | 6.9 | 3.5 | 4.9 |
| Elongation at break (%) | 86 | 310 | 225 | 194 | 610 | 615 | 620 | 360 |
| Hardness (Shore A) | 97.78 | 87.2 | 98.6 | 99.4 | 85.6 | 79 | 77.4 | 81 |
| Hardness (Shore D) | 83 | 11.7 | 26.4 | 24.9 | 12.9 | 10.8 | 8.3 | 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minale, Y.F.; Gajdoš, I.; Štefčák, P.; Dulebová, Ľ.; Jachowicz, T.; Szabó, T.; Ádámné Major, A.; Marossy, K. Thermodynamic and Technological Compatibility of Polyvinyl Chloride, Thermoplastic Polyurethane, and Bio-Plasticizer Blends. Polymers 2025, 17, 1149. https://doi.org/10.3390/polym17091149
Minale YF, Gajdoš I, Štefčák P, Dulebová Ľ, Jachowicz T, Szabó T, Ádámné Major A, Marossy K. Thermodynamic and Technological Compatibility of Polyvinyl Chloride, Thermoplastic Polyurethane, and Bio-Plasticizer Blends. Polymers. 2025; 17(9):1149. https://doi.org/10.3390/polym17091149
Chicago/Turabian StyleMinale, Yitbarek Firew, Ivan Gajdoš, Pavol Štefčák, Ľudmila Dulebová, Tomasz Jachowicz, Tamás Szabó, Andrea Ádámné Major, and Kálmán Marossy. 2025. "Thermodynamic and Technological Compatibility of Polyvinyl Chloride, Thermoplastic Polyurethane, and Bio-Plasticizer Blends" Polymers 17, no. 9: 1149. https://doi.org/10.3390/polym17091149
APA StyleMinale, Y. F., Gajdoš, I., Štefčák, P., Dulebová, Ľ., Jachowicz, T., Szabó, T., Ádámné Major, A., & Marossy, K. (2025). Thermodynamic and Technological Compatibility of Polyvinyl Chloride, Thermoplastic Polyurethane, and Bio-Plasticizer Blends. Polymers, 17(9), 1149. https://doi.org/10.3390/polym17091149







