Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Preparation of Polymeric Solutions
2.3. Leachability
2.4. Dimensional Stability
2.5. FTIR Analysis
2.6. Thermal Stability
2.7. Surface Properties
2.8. Wood Durability
2.9. Statistical Analysis
3. Results
3.1. WPG and Leachability
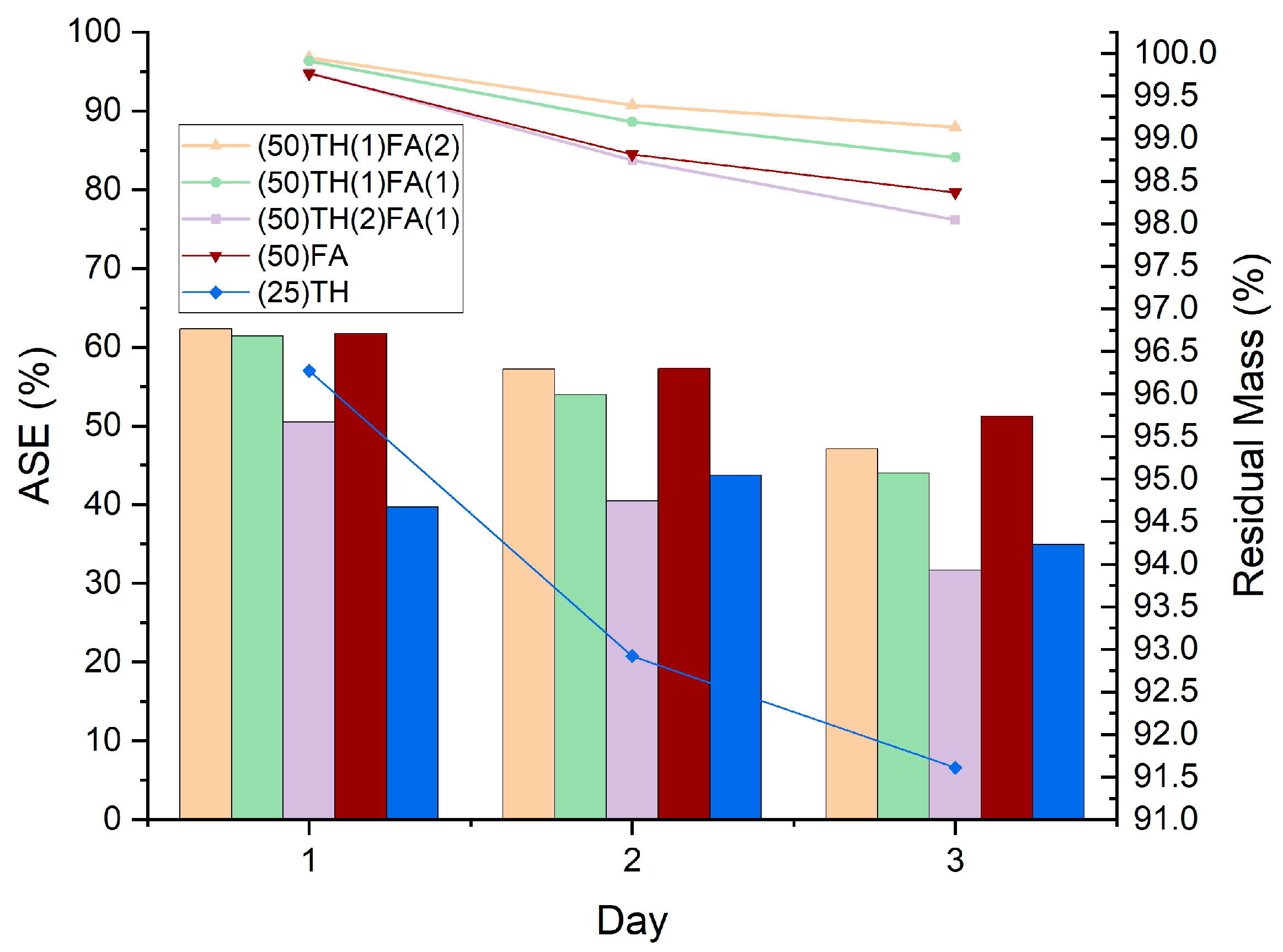
3.2. Dimensional Stability
3.3. FTIR Analysis
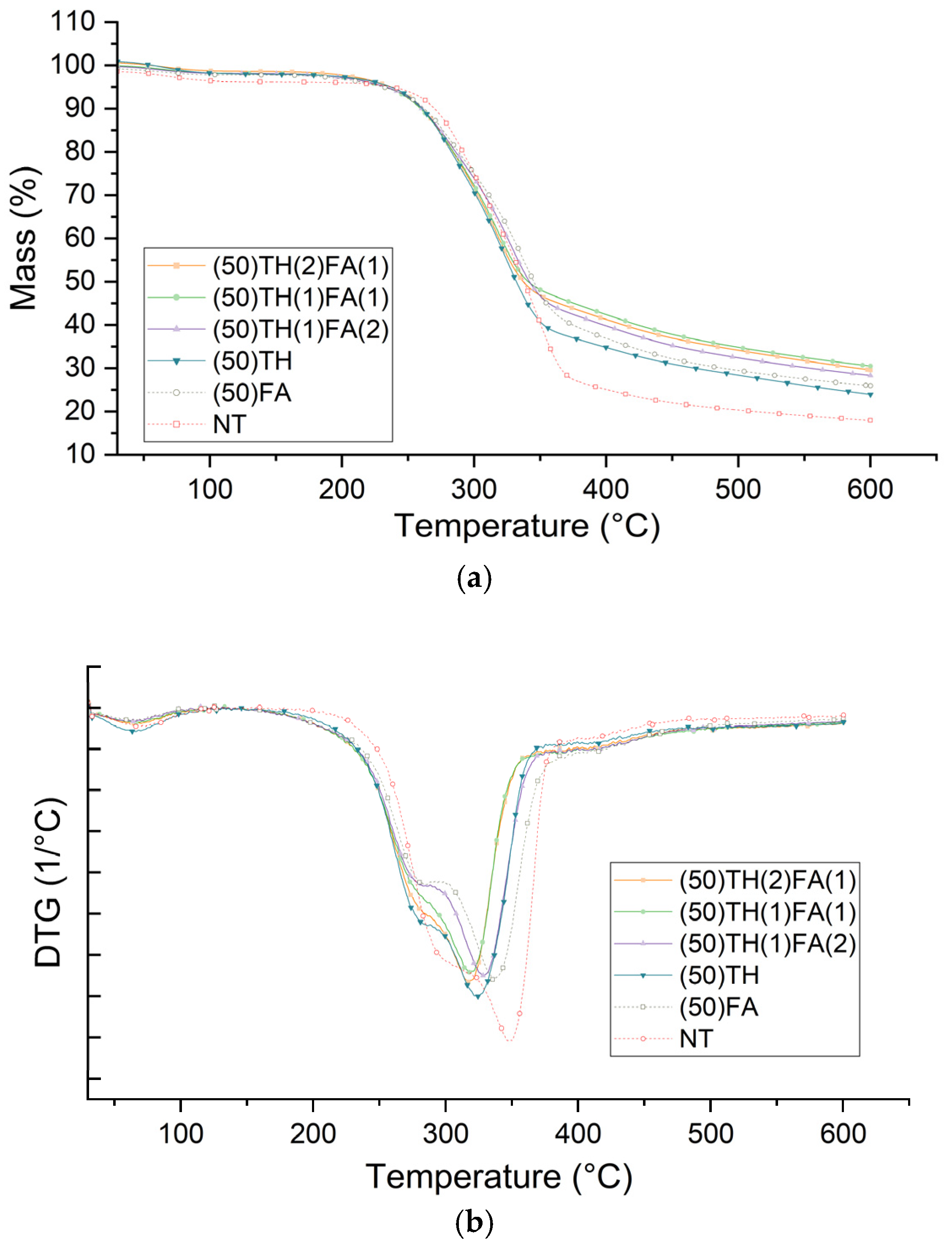
3.4. Thermal Stability
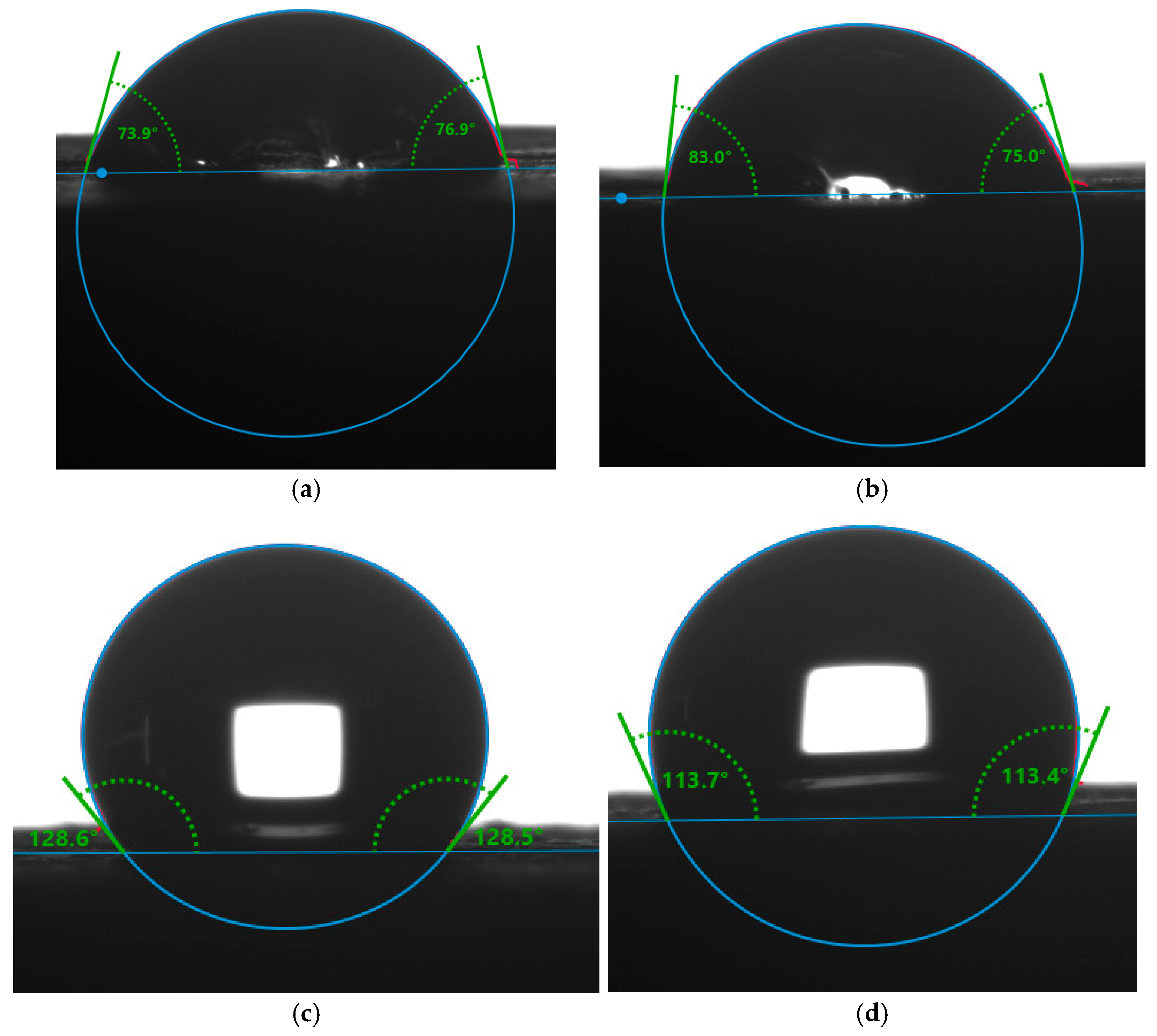
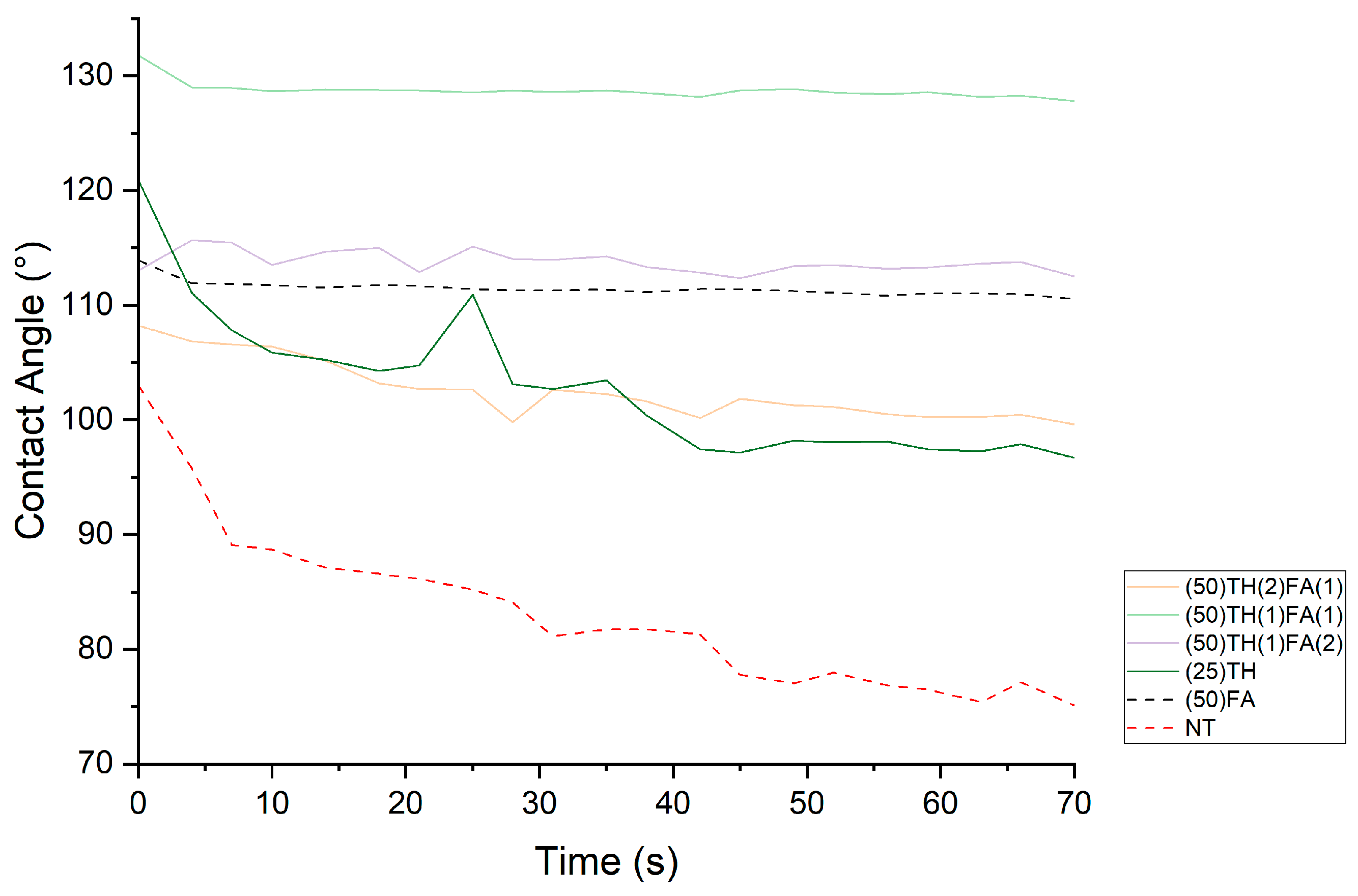
3.5. Surface Properties
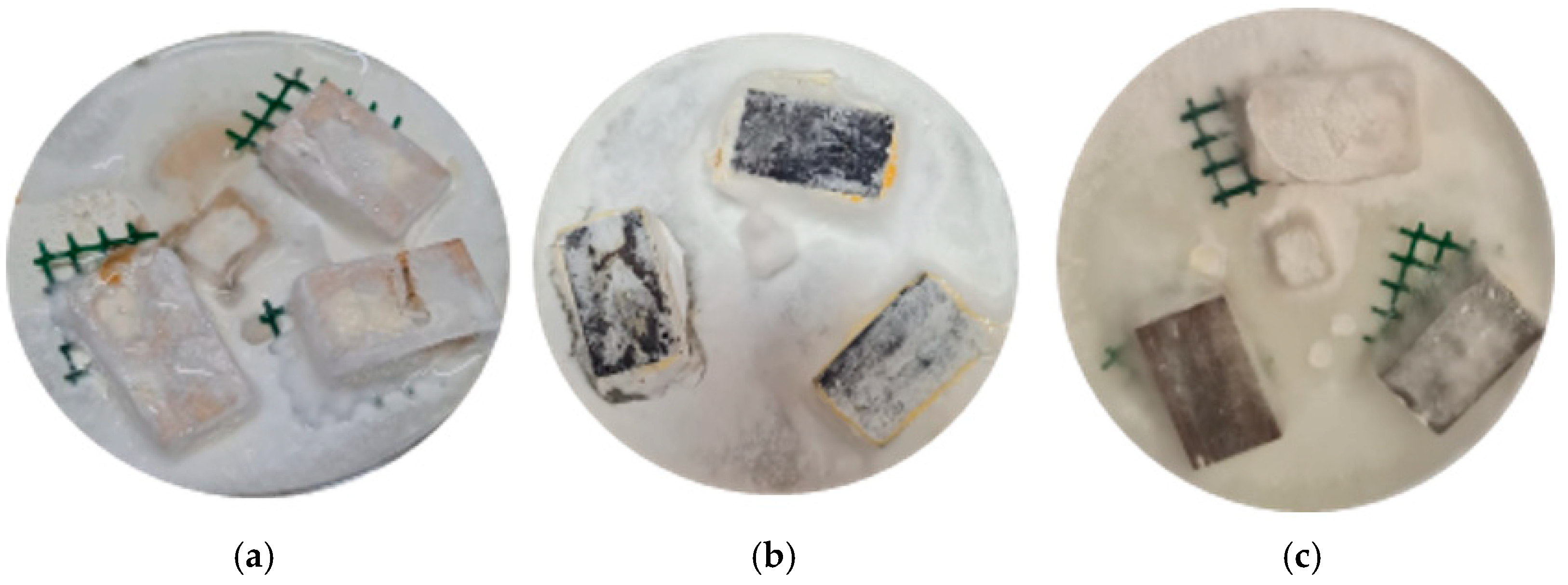
3.6. Wood Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowell, R.M.; Dickerson, J.P. Acetylation of Wood; ACS Publications: Washington, DC, USA, 2014; pp. 301–327. [Google Scholar]
- Calovi, M.; Zanardi, A.; Rossi, S. Recent Advances in Bio-Based Wood Protective Systems: A Comprehensive Review. Appl. Sci. 2024, 14, 736. [Google Scholar] [CrossRef]
- Militz, H.; Lande, S. Challenges in Wood Modification Technology on the Way to Practical Applications. Wood Mater. Sci. Eng. 2009, 4, 23–29. [Google Scholar] [CrossRef]
- Mantanis, G.I. Chemical Modification of Wood by Acetylation or Furfurylation: A Review of the Present Scaled-up Technologies. Bioresources 2017, 12, 4478–4489. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Altgen, M.; Emmerich, L.; Guigo, N.; Keplinger, T.; Kymäläinen, M.; Thybring, E.E.; Thygesen, L.G. Review of Wood Modification and Wood Functionalization Technologies. Forests 2022, 13, 1004. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. South Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Tascioglu, C.; Yalcin, M.; Sen, S.; Akcay, C. Antifungal Properties of Some Plant Extracts Used as Wood Preservatives. Int. Biodeterior. Biodegrad. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević Kovač, M.; Pavić, V. A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents. Molecules 2024, 29, 2615. [Google Scholar] [CrossRef]
- Bello, A.; Bergmann, U.; Vepsäläinen, J.; Leiviskä, T. Effects of Tree Harvesting Time and Tannin Cold/Hot-Water Extraction Procedures on the Performance of Spruce Tannin Biocoagulant for Water Treatment. Chem. Eng. J. 2022, 449, 137809. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why Is It Important to Understand the Nature and Chemistry of Tannins to Exploit Their Potential as Nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- Dhawale, P.V.; Vineeth, S.K.; Gadhave, R.V.; Fatima, M.J.J.; Supekar, M.V.; Thakur, V.K.; Raghavan, P. Tannin as a Renewable Raw Material for Adhesive Applications: A Review. Mater. Adv. 2022, 3, 3365–3388. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Biofoams-A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Vera, M.; Urbano, B.F. Tannin Polymerization: An Overview. Polym. Chem. 2021, 12, 4272–4290. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins Medical / Pharmacological and Related Applications: A Critical Review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Ojo, M.A. Tannins in Foods: Nutritional Implications and Processing Effects of Hydrothermal Techniques on Underutilized Hard-to-Cook Legume Seeds—A Review. Prev. Nutr. Food Sci. 2022, 27, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a Sustainable Raw Material for Green Chemistry: A Review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Kavitha, V.U.; Kandasubramanian, B. Tannins for Wastewater Treatment. SN Appl. Sci. 2020, 2, 1081. [Google Scholar] [CrossRef]
- Mubarok, M.; Gérardin-Charbonnier, C.; Azadeh, E.; Akong, F.O.; Dumarçay, S.; Pizzi, A.; Gérardin, P. Modification of Wood by Tannin-Furfuryl Alcohol Resins–Effect on Dimensional Stability, Mechanical Properties and Decay Durability. J. Renew. Mater. 2023, 11, 505–521. [Google Scholar] [CrossRef]
- López-Gómez, Y.M.; Barbero-López, A.; Suvanto, S.; Venäläinen, M.; Haapala, A. Effects of Tannin-Geopolymer Impregnation on Wood: Leachability, Biodegradation Resistance and Mechanical Properties. Eur. J. Wood Wood Prod. 2025, 83, 17. [Google Scholar] [CrossRef]
- Sari, R.A.L.; Lubis, M.A.R.; Sari, R.K.; Kristak, L.; Iswanto, A.H.; Mardawati, E.; Fatriasari, W.; Lee, S.H.; Reh, R.; Sedliacik, J.; et al. Properties of Plywood Bonded with Formaldehyde-Free Adhesive Based on Poly(Vinyl Alcohol)–Tannin–Hexamine at Different Formulations and Cold-Pressing Times. J. Compos. Sci. 2023, 7, 113. [Google Scholar] [CrossRef]
- Pizzi, A.; Baecker, A. A New Boron Fixation Mechanism for Environment Friendly Wood Preservatives. HFSG 1996, 50, 507–510. [Google Scholar] [CrossRef]
- Sommerauer, L.; Thevenon, M.-F.; Petutschnigg, A.; Tondi, G. Effect of Hardening Parameters of Wood Preservatives Based on Tannin Copolymers. Holzforschung 2019, 73, 457–467. [Google Scholar] [CrossRef]
- Grosse, C.; Noël, M.; Thévenon, M.-F.; Gérardin, P. Improvement of Modified Wood Properties with Addition of Chestnut Tannins in Lactic Acid-Based Treatments. J. Wood Chem. Technol. 2019, 39, 124–135. [Google Scholar] [CrossRef]
- Baysal, E.; Ozaki, S.K.; Yalinkilic, M. Dimensional Stabilization of Wood Treated with Furfuryl Alcohol Catalysed by Borates. Wood Sci. Technol. 2004, 38, 405–415. [Google Scholar] [CrossRef]
- NF EN 113-1; Durabilité du Bois et des Matériaux Dérivés du Bois—Méthode d’essai Vis-à-Vis des Champignons Basidiomycètes—Partie 1: Détermination de L’efficacité Protectrice de Produits de Préservation. AFNOR: Paris, France, 2020.
- NF X 41-568; Produits de Préservation du Bois—Méthode de Laboratoire Pour Obtenir des Échantillons Pour Analyse Pour Mesurer Les Pertes Après Délavage à l’eau Ou à l’eau de Mer Synthétique. AFNOR: Paris, France, 2014.
- Rowell, R.M. Chemical Modification of Wood. In Handbook of Engineering Biopolymers; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2007; pp. 673–691. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- EN 350-1; Durabilité du Bois et des Matériaux Dérivés du Bois—Méthodes d’essai et de Classification de La Durabilité Vis-à-Vis des Agents Biologiques du Bois et des Matériaux Dérivés du Bois. AFNOR: Paris, France, 2016.
- Sejati, P.S.; Imbert, A.; Gérardin-Charbonnier, C.; Dumarçay, S.; Fredon, E.; Masson, E.; Nandika, D.; Priadi, T.; Gérardin, P. Tartaric Acid Catalyzed Furfurylation of Beech Wood. Wood Sci. Technol. 2017, 51, 379–394. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Jakab, E.; Badea, E.; Barta-Rajnai, E.; Şendrea, C.; Czégény, Z. Thermal Degradation Study of Vegetable Tannins and Vegetable Tanned Leathers. J. Anal. Appl. Pyrolysis 2019, 138, 178–187. [Google Scholar] [CrossRef]
- Pranger, L.A.; Nunnery, G.A.; Tannenbaum, R. Mechanism of the Nanoparticle-Catalyzed Polymerization of Furfuryl Alcohol and the Thermal and Mechanical Properties of the Resulting Nanocomposites. Compos. B Eng. 2012, 43, 1139–1146. [Google Scholar] [CrossRef]
- Falavinha, J.V.D.; De Cademartori, P.H.G.; Gérardin, P.; Pizzi, A.; Gérardin-Charbonnier, C. New Rigid Furan Biofoams Based on Hydrolysable Chesnut (Castanea sativa) Tannin by Chemical Expansion. J. Renew. Mater. 2025, 13, 4. [Google Scholar] [CrossRef]
- Mubarok, M.; Azadeh, E.; Obounou Akong, F.; Dumarçay, S.; Gérardin, P.; Gérardin-Charbonnier, C. Effect of Tannins Addition on Thermal Stability of Furfurylated Wood. Polymers 2023, 15, 2044. [Google Scholar] [CrossRef]
- Martha, R.; Mubarok, M.; Batubara, I.; Rahayu, I.S.; Setiono, L.; Darmawan, W.; Akong, F.O.; George, B.; Gérardin, C.; Gérardin, P. Effect of Furfurylation Treatment on Technological Properties of Short Rotation Teak Wood. J. Mater. Res. Technol. 2021, 12, 1689–1699. [Google Scholar] [CrossRef]
- Beall, F.C.; Eickner, H.W. Thermal Degradation of Wood Components: A Review of the Literature; United States Department of Agriculture: Washington, DC, USA, 1970.
- Acosta, A.P.; Beltrame, R.; Missio, A.L.; de Avila Delucis, R.; Gatto, D.A. Juvenile and Mature Woods from Pine Subjected to in Situ Polymerization with Furfuryl Alcohol. Wood Mater. Sci. Eng. 2022, 17, 151–156. [Google Scholar] [CrossRef]
- Danish, M. Contact Angle Studies of Hydrophobic and Hydrophilic Surfaces. In Handbook of Magnetic Hybrid Nanoalloys and Their Nanocomposites; Springer International Publishing: Cham, Switzerland, 2022; pp. 761–782. [Google Scholar]

| Treatment ID | Tannin Ratio | Furfuryl Alcohol Ratio |
|---|---|---|
| * (n)TH(2)FA(1) | 2 | 1 |
| * (n)TH(1)FA(1) | 1 | 1 |
| * (n)TH(1)FA(2) | 1 | 2 |
| * (n)FA | 0 | 1 |
| * (n)TH | 1 | 0 |
| ID | WPG (%) * | WPL (%) * | FWPG (%) * |
|---|---|---|---|
| (50)TH(2)FA(1) | 55.4 ± 3.2 A ** | 4.9 ± 0.2 C | 48.3 ± 3.5 A |
| (50)TH(1)FA(1) | 50.5 ± 2.6 B | 4.0 ± 0.4 B | 45.2 ± 2.9 B |
| (50)TH(1)FA(2) | 49.1 ± 2.7 B | 3.8 ± 0.3 B | 42.9 ± 1.9 B |
| (50)FA | 25.2 ± 2.0 CD | 1.5 ± 0.2 A | 23.8 ± 2.2 C |
| (25)TH(2)FA(1) | 26.6 ± 1.2 C | 8.2 ± 0.3 F | 16.6 ± 0.9 D |
| (25)T(1)FA(1) | 25.5 ± 1.2 CD | 5.5 ± 0.6 D | 18.9 ± 1.7 D |
| (25)TH(1)FA(2) | 19.7 ± 1.1 E | 6.3 ± 0.6 E | 12.3 ± 1.5 E |
| (25)TH | 24.0 ± 1.6 D | 14.4 ± 0.9 G | 6.0 ± 1.7 G |
| (25)FA | 10.3 ± 0.9 F | 1.6 ± 0.2 A | 8.6 ± 1.2 F |
| ID | ASE (%) | Total Mass Loss (%) * | |
|---|---|---|---|
| First Cycle * | Last Cycle * | ||
| (50)TH(2)FA(1) | 50.5 ± 4.7 B ** | 31.7 ± 2.6 C | 2.0 ± 0.1 |
| (50)TH(1)FA(1) | 61.4 ± 1.0 A | 44.0 ± 1.1 B | 1.2 ± 0.1 |
| (50)TH(1)FA(2) | 62.3 ± 2.5 A | 47.1 ± 2.5 AB | 0.9 ± 0.01 |
| (50)FA | 61.7 ± 4.0 A | 51.2 ± 1.7 A | 1.6 ± 0.2 |
| (25)TH | 39.7 ± 5.1 C | 34.9 ± 5.3 C | 8.4 ± 1.1 |
| ID | Contact Angle (°) | Surface Free Energy (mN/m) | |
|---|---|---|---|
| Water * | Diiodomethane * | ||
| (50)TH(2)FA(1) | 107.4 ± 4.7 | 50.5 ± 1.2 | 34.04 ± 4.4 |
| (50)TH(1)FA(1) | 123.9 ± 4.8 | 49.1 ± 3.8 | 37.3 ± 0.9 |
| (50)TH(1)FA(2) | 114.9 ± 5.9 | 49.6 ± 1.4 | 35.7 ± 0.04 |
| (50)FA | 111.8 ± 0.8 | 47.26 ± 2.6 | 36.3 ± 1.3 |
| (25)TH | 99.5 ± 1.3 | 48.9 ± 4.0 | 35.0 ± 2.3 |
| NT | 88.4 ± 9.1 | 51.4 ± 1.8 | 35.6 ± 3.4 |
| ID | Trametes versicolor | Coniophora puteanea | ||||
|---|---|---|---|---|---|---|
| WLDNL (%) * | WLDL (%) * | DC * | WLDNL (%) * | WLDL (%) * | DC * | |
| (50)TH(2)FA(1) | 6.3 ± 0.5 (BC) ** | 2.7 ± 0.3 (A) | D1 | 5.6 ± 0.7 (CD) | 2.4 ± 0.3 AB | D1 |
| (50)TH(1)FA(1) | 4.7 ± 0.9 (AB) | 3.3 ± 1.2 (A) | D1 | 4.1 ± 0.2 (C) | 2.0 ± 0.5 (AB) | D1 |
| (50)TH(1)FA(2) | 4.1 ± 0.7 (AB) | 1.2 ± 0.3 (A) | D1 | 3.6 ± 0.3 (BC) | 1.3 ± 0.3 (AB) | D1 |
| (50)FA | 2.8 ± 1.6 (A) | 9.3 ± 4.0 (A) | D2 | 1.1 ± 0.4 (AB) | 0.6 ± 0.3 (A) | D1 |
| (25)TH(2)FA(1) | 5.0 ± 0.5 (AB) | 25.9 ± 2.5 (B) | D4 | 3.3 ± 0.3 (BC) | 6.7 ± 3.7 (B) | D2 |
| (25)TH(1)FA(1) | 4.2 ± 0.3 (AB) | 26.1 ± 2.0 (BC) | D4 | 3.4 ± 0.3 (BC) | 2.5 ± 2.5 (AB) | D1 |
| (25)TH(1)FA(2) | 8.1 ± 3.1 (CD) | 20.5 ± 7.2 (B) | D4 | 3.6 ± 0.4 (BC) | 0.6 ± 0.8 (A) | D1 |
| (25)TH | 10.6 ± 1.2 (D) | 34.8 ± 5.0 (DC) | D5 | 7.4 ± 2.0 (D) | 15.1 ± 9.9 (C) | D4 |
| (25)FA | 23.2 ± 1.2 (E) | 26.0 ± 9.3 (B) | D4 | 0.6 ± 0.1 (A) | 4.4 ± 5.1 (A) | D1 |
| NT | 38.6 ± 3.6 (F) | 38.6 ± 3.6 (D) | - | 27.6 ± 5.3 (E) | 27.6 ± 5.3 (D) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorini Falavinha, J.V.; Gérardin, P.; Gonzales De Cademartori, P.H.; Gérardin-Charbonnier, C. Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification. Polymers 2025, 17, 1159. https://doi.org/10.3390/polym17091159
Dorini Falavinha JV, Gérardin P, Gonzales De Cademartori PH, Gérardin-Charbonnier C. Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification. Polymers. 2025; 17(9):1159. https://doi.org/10.3390/polym17091159
Chicago/Turabian StyleDorini Falavinha, João Vitor, Philippe Gérardin, Pedro Henrique Gonzales De Cademartori, and Christine Gérardin-Charbonnier. 2025. "Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification" Polymers 17, no. 9: 1159. https://doi.org/10.3390/polym17091159
APA StyleDorini Falavinha, J. V., Gérardin, P., Gonzales De Cademartori, P. H., & Gérardin-Charbonnier, C. (2025). Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification. Polymers, 17(9), 1159. https://doi.org/10.3390/polym17091159







