Fabrication of Poly(s-triazine-co-o-aminophenol) Conducting Polymer via Electropolymerization and Its Application in Aqueous Charge Storage
Abstract
1. Introduction
2. Experimental
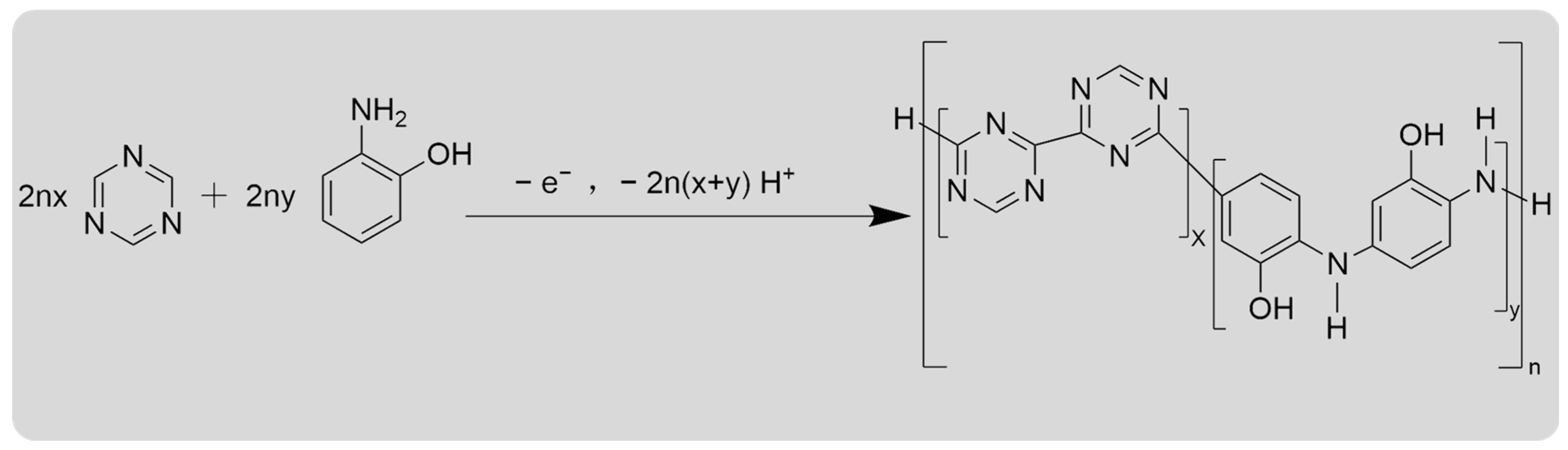
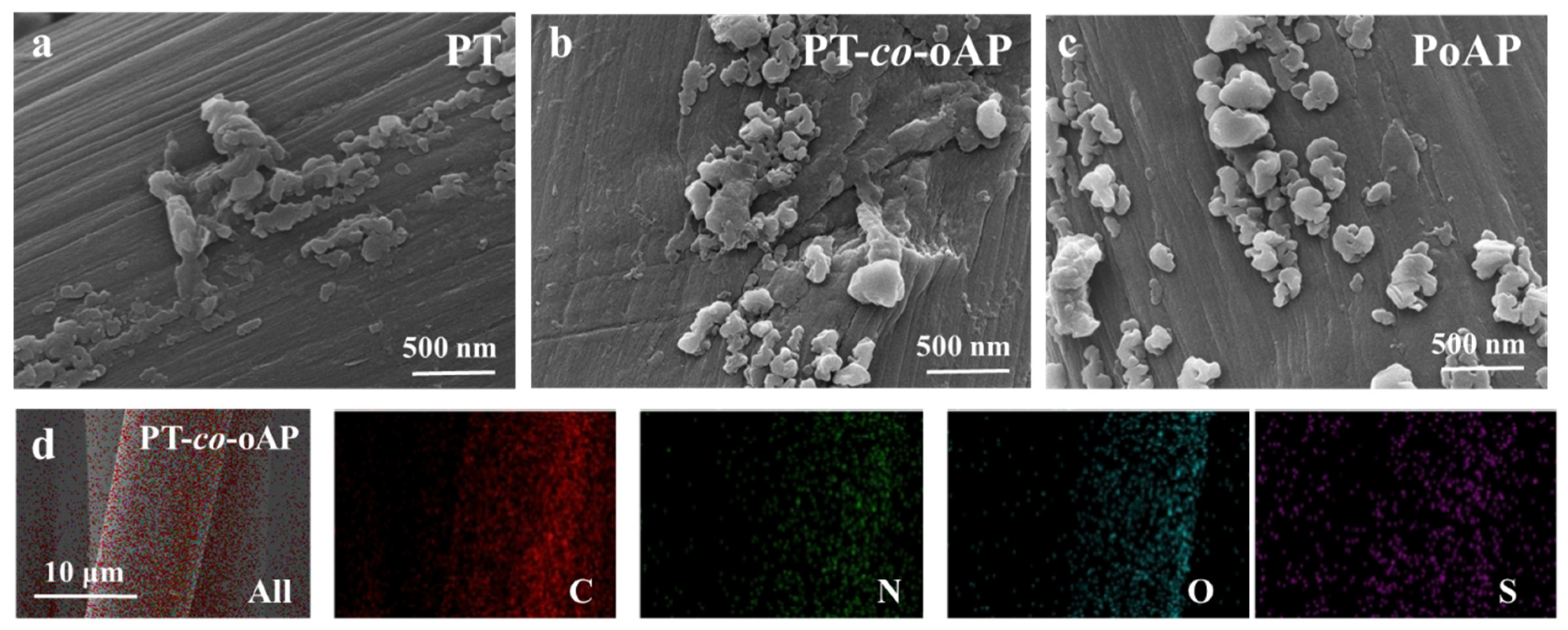
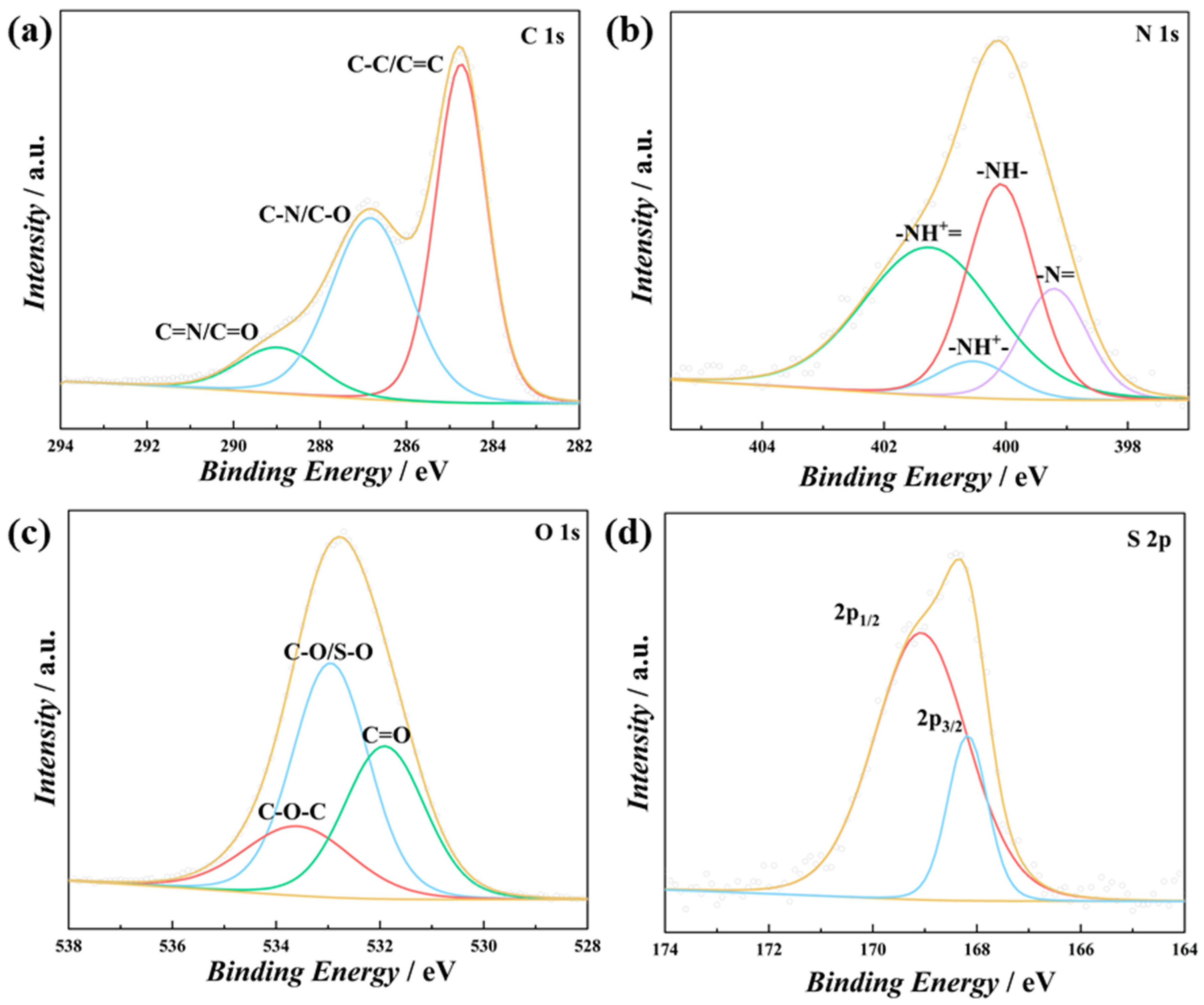
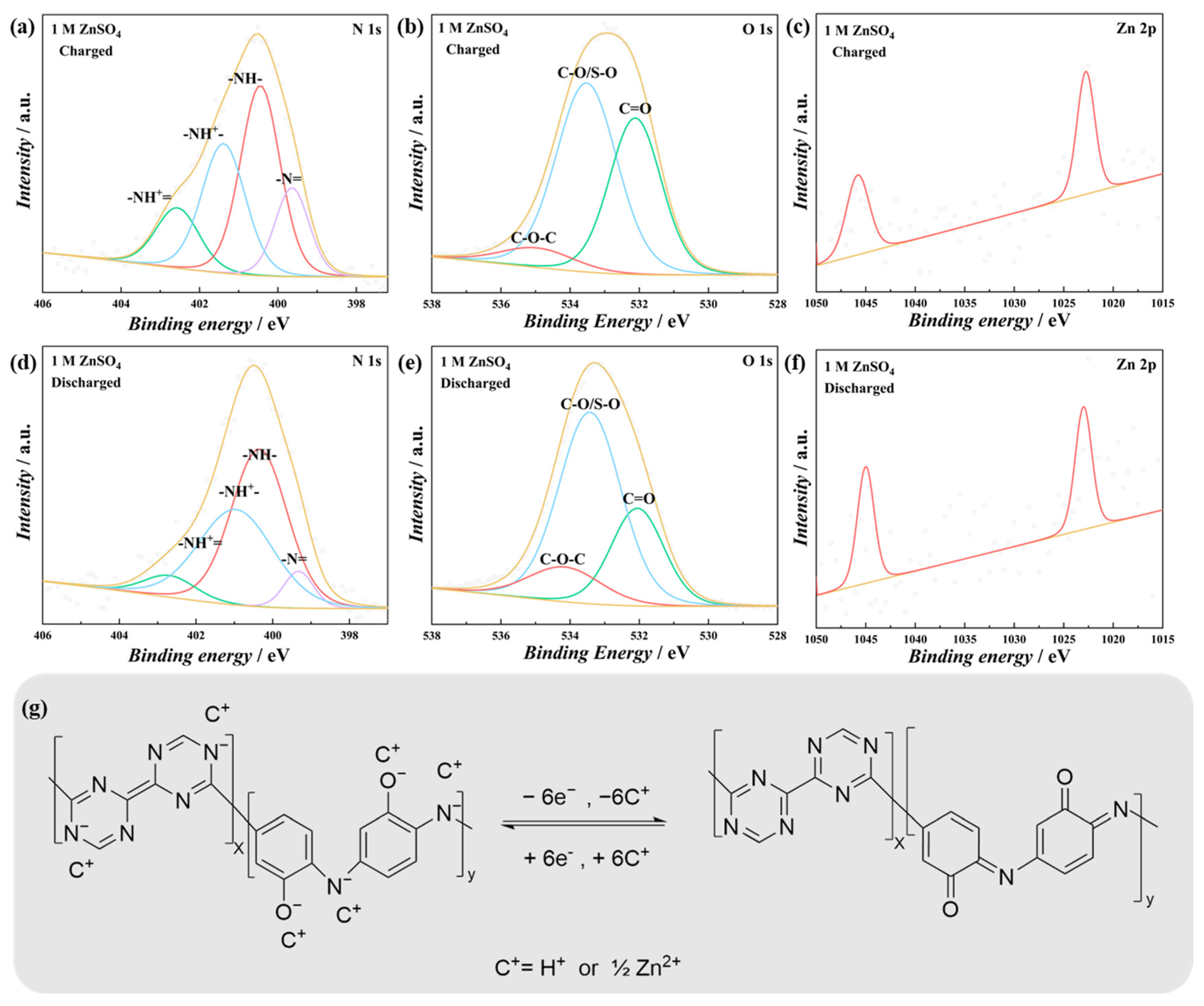
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Jadoon, S.; Iqbal, M.Z.; Hegazy, H.H.; Ahmad, Z.; Yahia, I.S. Recent progress in polypyrrole and its composites with carbon, metal oxides, sulfides and other conducting polymers as an emerging electrode material for asymmetric supercapacitors. J. Energy Storage 2024, 85, 110955. [Google Scholar] [CrossRef]
- Bashir, S.; Hasan, K.; Hina, M.; Ali Soomro, R.; Mujtaba, M.A.; Ramesh, S.; Ramesh, K.; Duraisamy, N.; Manikam, R. Conducting polymer/graphene hydrogel electrodes based aqueous smart Supercapacitors: A review and future prospects. J. Electroanal. Chem. 2021, 898, 115626. [Google Scholar] [CrossRef]
- del Valle, M.A.; Gacitúa, M.A.; Hernández, F.; Luengo, M.; Hernández, L.A. Nanostructured Conducting Polymers and Their Applications in Energy Storage Devices. Polymers 2023, 15, 1450. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, S.; Wang, R.; Zhang, L.; Ma, Q.; Ren, X.; Gao, Y.; Wang, Z.; Guo, Z.; Zhang, C. Covalent triazine frameworks for advanced energy storage: Challenges and new opportunities. Energy Environ. Sci. 2023, 16, 3181–3213. [Google Scholar] [CrossRef]
- Pei, S.; Lan, B.; Bai, X.; Liu, Y.; Li, X.; Wang, C. Electropolymerization of s-Triazines and Their Charge Storage Performance in Aqueous Acidic Electrolytes. Polymers 2024, 16, 3266. [Google Scholar] [CrossRef]
- Tucceri, R.; Arnal, P.M.; Scian, A.N. Poly(o-aminophenol) film electrodes: Synthesis and characterization and formation mechanisms—A review article. Can. J. Chem. 2013, 91, 91–112. [Google Scholar] [CrossRef]
- Heli, H.; Yadegari, H.; Jabbari, A. Graphene nanosheets-poly(o-aminophenol) nanocomposite for supercapacitor applications. Mater. Chem. Phys. 2012, 134, 21–25. [Google Scholar] [CrossRef]
- Ehsani, A.; Mohammad, S.H.; Kowsari, E.; Safari, R.; Torabian, J.; Kazemi, S. Nanocomposite of p-type conductive polymer/functionalized graphene oxide nanosheets as novel and hybrid electrodes for highly capacitive pseudocapacitors. J. Colloid Interface Sci. 2016, 478, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Fotouhi, L.; Ehsani, A.; Shiri, H.M. Novel electroactive nanocomposite of POAP for highly efficient energy storage and electrocatalyst: Electrosynthesis and electrochemical performance. J. Colloid Interface Sci. 2016, 484, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Fu, H.; Zhang, L.; Dong, Y.; Li, W.; Ouyang, M.; Zhang, C. Conjugated polymer multilayer by in situ electrochemical polymerization for black-to-transmissive eletrochromism. Chem. Eng. J. 2021, 406, 126819. [Google Scholar] [CrossRef]
- Jadoun, S.; Riaz, U. A review on the chemical and electrochemical copolymerization of conducting monomers: Recent advancements and future prospects. Polym. Plast. Technol. Mater. 2020, 59, 484–504. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Zhou, Z.; Li, Y.; Li, Y.; Hou, W.; Liu, S.; Tian, Y. Electrodeposited Poly(5-Amino-2-Naphthalenesulfonic Acid-co-o-Aminophenol) as the Electrode Material for Flexible Supercapacitor. Small 2024, 20, 2305994. [Google Scholar] [CrossRef]
- Mansha, M.; Ahmad, T.; Ullah, N.; Akram Khan, S.; Ashraf, M.; Ali, S.; Tan, B.; Khan, I. Photocatalytic Water-Splitting by Organic Conjugated Polymers: Opportunities and Challenges. Chem. Rec. 2022, 22, e202100336. [Google Scholar] [CrossRef]
- An, N.; Guo, C.; Li, W.; Wei, M.; Liu, L.; Meng, C.; Sun, D.; Lei, Y.; Hu, Z.; Zhao, L. Electropolymerization nanoarchitectonics of polyaminoanthraquinone/carbon cloth flexible electrode with nano-spines array structure for high-performance supercapacitor. J. Energy Storage 2024, 75, 109558. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Liu, P. Boosting electrochemical property of carbon cloth for supercapacitors with electrodeposited aniline-based copolymers. Electrochim. Acta 2023, 462, 142706. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting polymers: Past, present, future. J. Electrochem. Sci. Eng. 2018, 8, 3–37. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Yan, H.; Mu, X.; Song, Y.; Qin, Z.; Guo, D.; Sun, X.; Liu, X.-X. Protonating imine sites of polyaniline for aqueous zinc batteries. Chem. Commun. 2022, 58, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, S.J.; Kim, H.G.; Jee, Y.; Huh, S.H. Two-Dimensional Hydration and Triple-Interlayer Lattice Structures in Sulfate-Intercalated Graphene Oxide Nanosheets. Minerals 2024, 14, 1030. [Google Scholar] [CrossRef]
- Chun, S.-E.; Evanko, B.; Wang, X.; Vonlanthen, D.; Ji, X.; Stucky, G.D.; Boettcher, S.W. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 2015, 6, 7818. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Chen, J.; Yi, L.; Zhan, H.; Wen, Z. Fast Redox Kinetics in Bi-Heteroatom Doped 3D Porous Carbon Nanosheets for High-Performance Hybrid Potassium-Ion Battery Capacitors. Adv. Energy Mater. 2019, 9, 1901533. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Mittal, U.; Kundu, D. Electrochemical Stability of Prospective Current Collectors in the Sulfate Electrolyte for Aqueous Zn-Ion Battery Application. J. Electrochem. Soc. 2021, 168, 090560. [Google Scholar] [CrossRef]
- Ye, S.; Sheng, S.; Yao, H.; Chen, Q.; Meng, L.; Yang, Y. One-stone-for-two-birds strategy to enhance the zinc ion storage performance of PANI in low-cost ZnSO4 electrolyte. J. Energy Storage 2024, 100, 113674. [Google Scholar] [CrossRef]
- Yue, J.; Chen, S.; Yang, J.; Li, S.; Tan, G.; Zhao, R.; Wu, C.; Bai, Y. Multi-Ion Engineering Strategies toward High Performance Aqueous Zinc-Based Batteries. Adv. Mater. 2024, 36, 2304040. [Google Scholar] [CrossRef]
- Chen, X.; Xie, X.; Ruan, P.; Liang, S.; Wong, W.-Y.; Fang, G. Thermodynamics and Kinetics of Conversion Reaction in Zinc Batteries. ACS Energy Lett. 2024, 9, 2037–2056. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Patil, S.S.; Patil, P.S. 3D Bode analysis of nickel pyrophosphate electrode: A key to understanding the charge storage dynamics. Electrochim. Acta 2023, 451, 142278. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, H.; Ran, F. Fast-charging cathode materials for lithium & sodium ion batteries. Mater. Today 2023, 63, 360–379. [Google Scholar] [CrossRef]
- Cha, H.L.; Park, J.W.; Yun, J.-I. Determination of the Diffusion Coefficient in Electrodeposition Reactions by Electrochemical Impedance Spectroscopy: A Case Study of Cobalt in Molten LiCl-KCl Salt. J. Electrochem. Soc. 2024, 171, 036503. [Google Scholar] [CrossRef]
- Perdana, M.Y.; Johan, B.A.; Abdallah, M.; Hossain, M.E.; Aziz, M.A.; Baroud, T.N.; Drmosh, Q.A. Understanding the Behavior of Supercapacitor Materials via Electrochemical Impedance Spectroscopy: A Review. Chem. Rec. 2024, 24, e202400007. [Google Scholar] [CrossRef]
- Patra, A.; Namsheer, K.; Jose, J.R.; Sahoo, S.; Chakraborty, B.; Rout, C.S. Understanding the charge storage mechanism of supercapacitors: In situ/operando spectroscopic approaches and theoretical investigations. J. Mater. Chem. A 2021, 9, 25852–25891. [Google Scholar] [CrossRef]
- Shi, H.-Y.; Ye, Y.-J.; Liu, K.; Song, Y.; Sun, X. A Long-Cycle-Life Self-Doped Polyaniline Cathode for Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2018, 57, 16359–16363. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and confusions in supercapacitor and battery: Theory and solutions. J. Power Sources 2018, 401, 213–223. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Kanjanaboos, P.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Shaikh, J.S. Engineering of Battery Type Electrodes for High Performance Lithium Ion Hybrid Supercapacitors. ChemElectroChem 2021, 8, 4686–4724. [Google Scholar] [CrossRef]
- Beyers, I.; Bensmann, A.; Hanke-Rauschenbach, R. Ragone plots revisited: A review of methodology and application across energy storage technologies. J. Energy Storage 2023, 73, 109097. [Google Scholar] [CrossRef]
- Khanra, P.; Kuila, T.; Bae, S.H.; Kim, N.H.; Lee, J.H. Electrochemically exfoliated graphene using 9-anthracene carboxylic acid for supercapacitor application. J. Mater. Chem. 2012, 22, 24403–24410. [Google Scholar] [CrossRef]
- Ganesh, V.; Lakshminarayanan, V.; Pitchumani, S. Assessment of Liquid Crystal Template Deposited Porous Nickel as a Supercapacitor Electrode Material. Electrochem. Solid-State Lett. 2005, 8, A308. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Zhou, Z.; Tian, Q.; Cao, X.; Wu, Y.; Liu, S.; Wang, J. Electropolymerized 1, 10-phenanthroline as the electrode material for aqueous supercapacitors. Chem. Eng. J. 2022, 433, 134483. [Google Scholar] [CrossRef]
- Khan, M.Z.; Gul, I.H.; Baig, M.M.; Akram, M.A. Facile synthesis of a multifunctional ternary SnO2/MWCNTs/PANI nanocomposite: Detailed analysis of dielectric, electrochemical, and water splitting applications. Electrochim. Acta 2023, 441, 141816. [Google Scholar] [CrossRef]
- Lv, T.R.; Zhang, W.H.; Yang, Y.Q.; Zhang, J.C.; Yin, M.J.; Yin, Z.; Yong, K.T.; An, Q.F. Micro/Nano-Fabrication of Flexible Poly (3, 4-Ethylenedioxythiophene)-Based Conductive Films for High-Performance Microdevices. Small 2023, 19, 2301071. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, Y.; Wu, Y.; Zhou, Z.; Li, Y.; Wang, J.; Liu, S.; Wang, C. Electropolymerization of 5-amino-2-naphthalenesulfonic acid and their application as the electrode material for supercapacitors. J. Energy Storage 2023, 72, 108308. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Zhong, L. Cellulose carbon aerogel/PPy composites for high-performance supercapacitor. Carbohydr. Polym. 2019, 215, 322–329. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Lan, B.; Li, X.; Yi, X.; Pei, S.; Wang, C. Fabrication of Poly(s-triazine-co-o-aminophenol) Conducting Polymer via Electropolymerization and Its Application in Aqueous Charge Storage. Polymers 2025, 17, 1160. https://doi.org/10.3390/polym17091160
Bai X, Lan B, Li X, Yi X, Pei S, Wang C. Fabrication of Poly(s-triazine-co-o-aminophenol) Conducting Polymer via Electropolymerization and Its Application in Aqueous Charge Storage. Polymers. 2025; 17(9):1160. https://doi.org/10.3390/polym17091160
Chicago/Turabian StyleBai, Xueting, Bo Lan, Xinyang Li, Xinlan Yi, Shaotong Pei, and Chao Wang. 2025. "Fabrication of Poly(s-triazine-co-o-aminophenol) Conducting Polymer via Electropolymerization and Its Application in Aqueous Charge Storage" Polymers 17, no. 9: 1160. https://doi.org/10.3390/polym17091160
APA StyleBai, X., Lan, B., Li, X., Yi, X., Pei, S., & Wang, C. (2025). Fabrication of Poly(s-triazine-co-o-aminophenol) Conducting Polymer via Electropolymerization and Its Application in Aqueous Charge Storage. Polymers, 17(9), 1160. https://doi.org/10.3390/polym17091160





