Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Polysaccharides
2.3. Characterization of Polysaccharides from DPs
2.3.1. Molecular Weight (Mw) Distribution Analysis
2.3.2. Monosaccharide Compositional Analysis
2.3.3. Methylation Analysis
2.4. Effects of Polysaccharides on Macrophage Functions
2.4.1. Cell Culture
2.4.2. Cell Viability Assay
2.4.3. NO Assay
2.4.4. Measurements of Apoptosis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight (Mw) Distribution Analysis of DPs
3.2. Monosaccharide Compositions of DPs
3.3. Glycosidic Linkages of DPs
3.4. Effects of DPs on Macrophage Functions
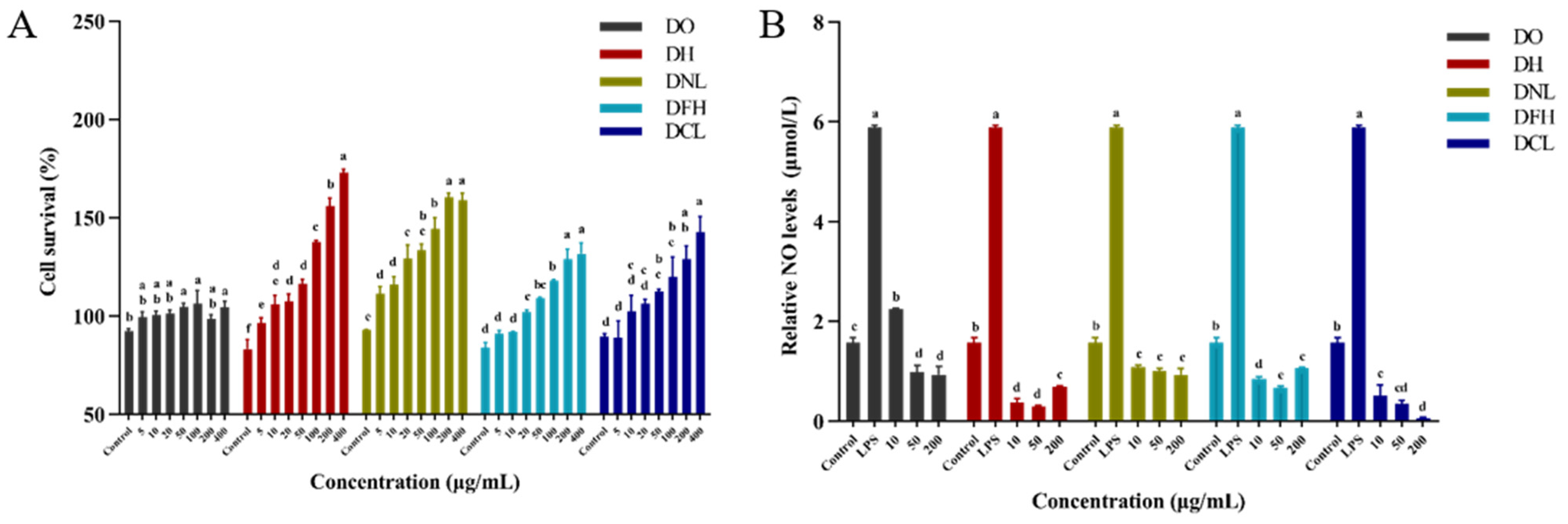
3.4.1. Effects of DPs on Cell Survival
3.4.2. Effects of DPs on NO Production
3.4.3. Effects of DPs on Apoptosis
3.5. Correlation Analysis of Structure and Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional Uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021, 12, 726528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: A review. J. Ethnopharmacol. 2021, 270, 113851. [Google Scholar] [CrossRef]
- Fan, C.; Sun, X.; Wang, X.; Yu, H. Therapeutic potential of the chemical composition of Dendrobium nobile Lindl. Front. Pharmacol. 2023, 14, 1163830. [Google Scholar] [CrossRef]
- Fu, X.; Chen, S.; Xian, S.; Wu, Q.; Shi, J.; Zhou, S. Dendrobium and its active ingredients: Emerging role in liver protection. Biomed. Pharmacother. 2023, 157, 114043. [Google Scholar] [CrossRef]
- Gao, L.; Wang, F.; Hou, T.; Geng, C.; Xu, T.; Han, B.; Liu, D. Dendrobium huoshanense C.Z.Tang et S.J.Cheng: A review of its traditional uses, phytochemistry, and pharmacology. Front. Pharmacol. 2022, 13, 920823. [Google Scholar] [CrossRef]
- Shi, D.C.; Wang, P.Y.; Xu, L.; Zhu, H.; Zhang, W.Y.; Wu, Q.Y.; Bu, T.T.; Tian, B.M.; Sun, P.L.; Cai, M. Potential of Dendrobium officinale oligosaccharides to alleviate chronic colitis by modulating inflammation and gut microbiota. Food Med. Homol. 2024, 2, 9420077. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, L.X.; Han, B.X.; Wu, D.T.; Cheong, K.L.; Chen, N.F.; Zhao, J.; Li, S.P. Qualitative and quantitative analysis of specific polysaccharides in Dendrobium huoshanense by using saccharide mapping and chromatographic methods. J. Pharm. Biomed. Anal. 2016, 129, 163–171. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, B.; Zhang, X.; Zhao, J.; Li, S.; Zhao, J. Characterization and immunological activity of polysaccharides from two types of Dendrobium devonianum with different appearance. J. Pharm. Biomed. Anal. 2023, 223, 115146. [Google Scholar] [CrossRef]
- Ma, K.; Yi, X.; Yang, S.T.; Zhu, H.; Liu, T.Y.; Jia, S.S.; Fan, J.H.; Hu, D.J.; Lv, G.P.; Huang, H. Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo. Int. J. Biol. Macromol. 2024, 265, 130988. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, B.; Chen, Z.; Cao, W.; Wang, J.; Li, S.; Zhao, J. Effects of steam on polysaccharides from Polygonatum cyrtonema based on saccharide mapping analysis and pharmacological activity assays. Chin. Med. 2022, 17, 97. [Google Scholar] [CrossRef]
- Cheong, K.L.; Wu, D.T.; Zhao, J.; Li, S.P. A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J. Chromatogr. A 2015, 1400, 98–106. [Google Scholar] [CrossRef]
- Cheong, K.L.; Wu, D.T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.J.; Li, S.P. Qualitation and quantification of specific polysaccharides from Panax species using GC–MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, Y.; Liu, X.; Ren, X.; Dong, Y.; Ma, J.; Song, R.; Wei, J.; Yu, A.; et al. A systematic review on polysaccharides from Dendrobium genus: Recent advances in the preparation, structural characterization, bioactive molecular mechanisms, and applications. Am. J. Chin. Med. 2022, 50, 471–509. [Google Scholar] [CrossRef]
- Lin, S.; Li, H.Y.; Yuan, Q.; Nie, X.R.; Zhou, J.; Wei, S.Y.; Du, G.; Zhao, L.; Wang, S.P.; Zhang, Q.; et al. Structural characterization, antioxidant activity, and immunomodulatory activity of non-starch polysaccharides from Chuanminshen violaceum collected from different regions. Int. J. Biol. Macromol. 2020, 143, 902–912. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Chen, L.X.; Chen, X.Q.; Lu, J.H.; Zhao, J.; Li, S.P. Chemical characterization and immunomodulatory activity of acetylated polysaccharides from Dendrobium devonianum. Carbohyd. Polym. 2018, 180, 238–245. [Google Scholar] [CrossRef]
- Wu, Y.G.; Wang, K.W.; Zhao, Z.R.; Zhang, P.; Liu, H.; Zhou, G.J.; Cheng, Y.; Wu, W.J.; Cai, Y.H.; Wu, B.L.; et al. A novel polysaccharide from Dendrobium devonianum serves as a TLR4 agonist for activating macrophages. Int. J. Biol. Macromol. 2019, 133, 564–574. [Google Scholar] [CrossRef]
- Scarano, S.; Pascale, E.; Minunni, M. The early nucleation stage of gold nanoparticles formation in solution as powerful tool for the colorimetric determination of reducing agents: The case of xylitol and total polyols in oral fluid. Anal. Chim. Acta 2017, 993, 71–78. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, R.; Wu, J.; Cai, X.; Deng, G.; Lv, J.; Ma, M.; Yu, N.; Yao, L.; Peng, D. Structural characterization and improves cognitive disorder in ageing mice of a glucomannan from Dendrobium huoshanense. Int. J. Biol. Macromol. 2024, 269, 131995. [Google Scholar] [CrossRef]
- Wu, D.T.; Meng, L.Z.; Wang, L.Y.; Lv, G.P.; Cheong, K.L.; Hu, D.J.; Guan, J.; Zhao, J.; Li, S.P. Chain conformation and immunomodulatory activity of a hyperbranched polysaccharide from Cordyceps sinensis. Carbohyd. Polym. 2014, 110, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yi, X.; Jia, S.S.; Liu, C.Y.; Han, Z.W.; Han, B.X.; Jiang, G.C.; Ding, Z.F.; Wang, R.L.; Lv, G.P. Optimization of three extraction methods and their effect on the structure and antioxidant activity of polysaccharides in Dendrobium huoshanense. Molecules 2023, 28, 8019. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, S.; Yu, L.; Li, Y.; Liu, Y.; Ye, X.; Zheng, X. Apios americana medikus tuber polysaccharide exerts anti-inflammatory effects by activating autophagy. Int. J. Biol. Macromol. 2019, 130, 892–902. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, J.; Sun, J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol. Lett. 2012, 214, 91–98. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, Y.; Liang, L.; Qin, X.; Dong, X.; Guo, Y.; Yu, Q.; Zhang, G.; Hu, X. Effects of the Lactobacillus fermentation on Dendrobium officinale polysaccharide: Structural, physicochemical properties and in vitro bioactivities. Food Biosci. 2024, 61, 105003. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.; Zhang, L.; Yao, L.; Cen, L.; Li, L.; Lv, Y.; Wei, C. Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: Optimization, physicochemical properties and anti-inflammatory activity. Foods 2022, 11, 2957. [Google Scholar] [CrossRef]
- Khan, S.G.; Melikian, N.; Shabeeh, H.; Cabaco, A.R.; Martin, K.; Khan, F.; O’Gallagher, K.; Chowienczyk, P.J.; Shah, A.M. The human coronary vasodilatory response to acute mental stress is mediated by neuronal nitric oxide synthase. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Huang, J.; Wang, Y.; Xie, M. Polysaccharide from Ganoderma atrum evokes antitumor activity via Toll-like receptor 4-mediated NF-kappaB and mitogen-activated protein kinase signaling pathways. J. Agric. Food Chem. 2013, 61, 3676–3682. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Zhong, K.; Wu, Z.; Wang, C.; Ding, Z.; Chen, S.; Zhang, J. Salecan ameliorates LPS-induced acute lung injury through regulating Keap1-Nrf2/HO-1 pathway in mice. Int. Immunopharmacol. 2024, 128, 111512. [Google Scholar] [CrossRef]
- Wang, C.X.; Zheng, L.Y.; Liu, S.N.; Guo, X.X.; Yang, Y.Q.; Gao, M.J.; Cui, X.M.; Yang, Y. A novel acidic polysaccharide from the residue of Panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020, 149, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, Y.; Lv, Y.; Li, X.; Chen, L.; Huang, Z.; Zhou, J.; Wang, Y.; Wang, X.; Wang, X.; et al. Dendrobium officinale polysaccharides attenuate uropathogenic Escherichia coli (UPEC)-induced pyroptosis in macrophage cells. Biomed. Pharmacother. 2022, 151, 113098. [Google Scholar] [CrossRef]
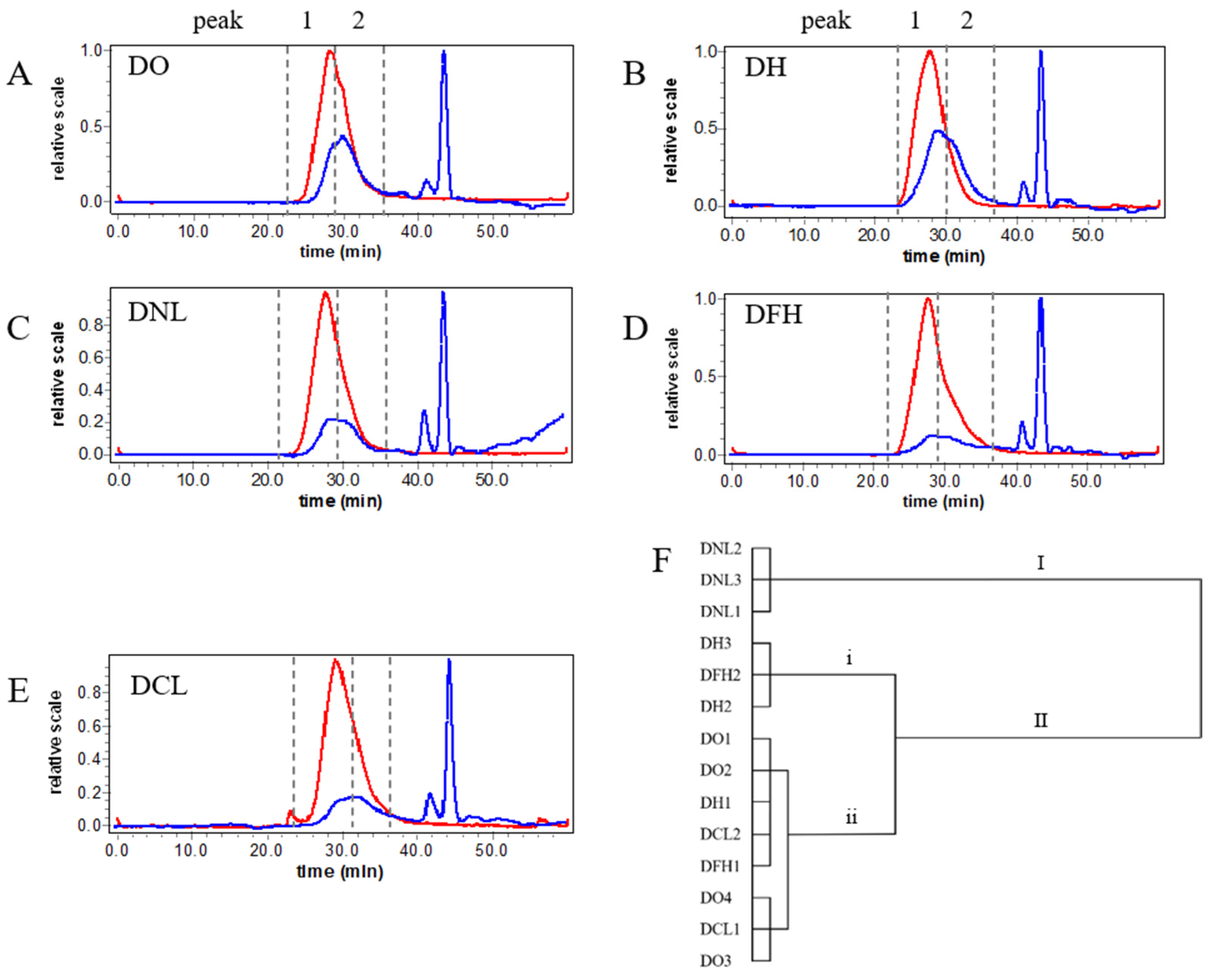



| No. | Species | Codes | Origins |
|---|---|---|---|
| 1 | Dendrobium officinale Kimura et Migo | DO1 | Anhui Province |
| 2 | DO2 | Anhui Province | |
| 3 | DO3 | Yunnan Province | |
| 4 | DO4 | Yunnan Province | |
| 5 | Dendrobium huoshanense C. Z. Tang & S. J. Cheng | DH1 | Anhui Province |
| 6 | DH2 | Anhui Province | |
| 7 | DH3 | Anhui Province | |
| 8 | Dendrobium nobile Lindl. | DNL1 | Yunnan Province |
| 9 | DNL2 | Guizhou Province | |
| 10 | DNL3 | Yunnan Province | |
| 11 | Dendrobium fimbriatum Hook. | DFH1 | Yunnan Province |
| 12 | DFH2 | Yunnan Province | |
| 13 | Dendrobium chrysanthum Lindl. | DCL1 | Yunnan Province |
| 14 | DCL2 | Yunnan Province |
| Peak 1 | Peak 2 | |||
|---|---|---|---|---|
| Mw (×105 Da) | PDI | Mw (×105 Da) | PDI | |
| DO1 | 8.905 ± 0.039 b | 1.194 ± 0.057 | 2.119 ± 0.026 b | 1.047 ± 0.042 |
| DO2 | 12.330 ± 0.043 a | 1.142 ± 0.049 | 2.829 ± 0.033 a | 1.068 ± 0.029 |
| DO3 | 5.698 ± 0.046 c | 1.208 ± 0.053 | 1.539 ± 0.049 c | 1.049 ± 0.054 |
| DO4 | 5.016 ± 0.026 d | 1.271 ± 0.024 | 1.236 ± 0.033 d | 1.133 ± 0.020 |
| Average | 7.987 ± 3.035 AB | 1.204 ± 0.063 | 1.204 ± 0.063 AB | 1.074 ± 0.049 |
| DH1 | 7.978 ± 0.044 a | 1.197 ± 0.061 | 1.727 ± 0.051 a | 1.078 ± 0.057 |
| DH2 | 4.546 ± 0.055 c | 1.121 ± 0.063 | 1.553 ± 0.058 b | 1.071 ± 0.070 |
| DH3 | 4.727 ± 0.024 b | 1.156 ± 0.033 | 1.699 ± 0.032 a | 1.094 ± 0.041 |
| Average | 5.750 ± 1.673 AB | 1.158 ± 0.057 | 1.660 ± 0.091 AB | 1.081 ± 0.051 |
| DNL1 | 5.594 ± 0.027 b | 1.287 ± 0.058 | 1.579 ± 0.030 a | 1.053 ± 0.048 |
| DNL2 | 4.810 ± 0.043 c | 1.226 ± 0.065 | 1.274 ± 0.027 b | 1.120 ± 0.081 |
| DNL3 | 5.760 ± 0.053 a | 1.172 ± 0.029 | 1.584 ± 0.025 a | 1.166 ± 0.037 |
| Average | 5.388 ± 0.441 AB | 1.228 ± 0.068 | 1.479 ± 0.156 AB | 1.113 ± 0.071 |
| DFH1 | 6.514 ± 0.047 b | 1.286 ± 0.039 | 1.640 ± 0.041 b | 1.031 ± 0.038 |
| DFH2 | 13.540 ± 0.033 a | 1.085 ± 0.046 | 4.960 ± 0.021 a | 1.642 ± 0.030 |
| Average | 10.027 ± 3.848 A | 1.186 ± 0.117 | 3.300 ± 1.819 A | 1.337 ± 0.336 |
| DCL1 | 3.364 ± 0.058 a | 1.174 ± 0.037 | 0.855 ± 0.047 a | 1.123 ± 0.087 |
| DCL2 | 3.343 ± 0.061 a | 1.622 ± 0.039 | 0.563 ± 0.049 b | 1.117 ± 0.072 |
| Average | 3.354 ± 0.054 B | 1.398 ± 0.248 | 0.709 ± 0.166 B | 1.120 ± 0.071 |
| Ara | Xyl | Man | Glc | Gal | Am/Ag | Am/Ag Range | |
|---|---|---|---|---|---|---|---|
| DO1 | 0.41 | 0.36 | 122.42 | 31.84 | 1.00 a | 3.84 | 2.23–4.22 |
| DO2 | 0.35 | 0.44 | 121.13 | 28.72 | 1.00 | 4.22 | |
| DO3 | 0.47 | 0.48 | 333.50 | 127.77 | 1.00 | 2.61 | |
| DO4 | 0.83 | 0.89 | 339.90 | 152.69 | 1.00 | 2.23 | |
| Average | 0.52 ± 0.22 | 0.54 ± 0.24 | 229.24 ± 124.12 | 85.26 ± 64.30 | 1.00 | 3.23 ± 0.96 | |
| DH1 | 0.58 | 0.00 | 348.47 | 102.61 | 1.00 | 3.40 | 3.40–4.49 |
| DH2 | 0.69 | 0.00 | 485.41 | 137.17 | 1.00 | 3.54 | |
| DH3 | 0.73 | 0.70 | 402.77 | 89.64 | 1.00 | 4.49 | |
| Average | 0.67 ± 0.08 | 0.23 ± 0.40 | 412.22 ± 68.96 | 109.81 ± 24.57 | 1.00 | 3.81 ± 0.59 | |
| DNL1 | 0.40 | 0.00 | 289.32 | 105.23 | 1.00 | 2.75 | 2.75–4.76 |
| DNL2 | 0.63 | 0.78 | 294.01 | 71.21 | 1.00 | 4.13 | |
| DNL3 | 0.71 | 0.87 | 274.61 | 57.74 | 1.00 | 4.76 | |
| Average | 0.58 ± 0.16 | 0.55 ± 0.48 | 285.98 ± 10.12 | 78.06 ± 24.47 | 1.00 | 3.88 ± 1.03 | |
| DFH1 | 0.81 | 0.61 | 97.35 | 232.99 | 1.00 | 0.42 | 0.42–0.48 |
| DFH2 | 0.84 | 0.81 | 111.79 | 232.23 | 1.00 | 0.48 | |
| Average | 0.83 ± 0.02 | 0.71 ± 0.14 | 104.57 ± 10.21 | 232.61 ± 0.54 | 1.00 | 0.45 ± 0.04 | |
| DCL1 | 0.70 | 0.77 | 44.89 | 52.15 | 1.00 | 0.86 | 0.76–0.86 |
| DCL2 | 0.49 | 0.42 | 30.38 | 40.03 | 1.00 | 0.76 | |
| Average | 0.60 ± 0.15 | 0.60 ± 0.25 | 37.64 ± 10.26 | 46.09 ± 8.57 | 1.00 | 0.81 ± 0.07 |
| Peak | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Glycosidic Bonds | Araf (1→ | →5) Araf (1→ | Glcp (1→ | →3,5) Araf (1→ | →4) Manp (1→ | →4) Glcp (1→ | →4,6) Manp (1→ | →4,6) Glcp (1→ | →4)Manp(1→/→4)Glcp(1→ | →4)Manp (1→/→4) Glcp(1→ Average Value |
| DO1 | 0.03 | 0.79 | 0.99 | 1.00 a | 10.09 | 3.63 | 0.05 | 0.03 | 2.78 | 2.85 |
| DO2 | / b | 1.41 | 0.78 | 1.00 | 10.75 | 4.11 | 0.04 | 0.04 | 2.62 | |
| DO3 | 0.03 | 0.66 | 0.65 | 1.00 | 11.18 | 3.43 | 0.85 | 0.27 | 3.26 | |
| DO4 | 0.03 | 3.08 | 0.56 | 1.00 | 10.26 | 3.73 | 0.80 | 0.23 | 2.75 | |
| DH1 | 0.06 | 0.12 | 0.58 | 1.00 | 20.72 | 7.23 | 0.10 | 0.09 | 2.87 | 2.92 |
| DH2 | 0.14 | 0.01 | 0.66 | 1.00 | 28.61 | 9.89 | 0.20 | 0.16 | 2.89 | |
| DH3 | 0.41 | 11.05 | 2.85 | 1.00 | 46.11 | 15.31 | 0.87 | 0.28 | 3.01 | |
| DNL1 | 0.03 | 1.35 | 0.94 | 1.00 | 8.61 | 5.94 | 0.24 | 0.20 | 1.45 | 1.50 |
| DNL2 | 0.02 | 0.48 | 0.80 | 1.00 | 9.75 | 7.05 | 0.19 | 0.19 | 1.38 | |
| DNL3 | 0.02 | 1.93 | 0.99 | 1.00 | 8.62 | 5.16 | 0.06 | 0.09 | 1.67 | |
| DFH1 | 0.03 | 1.78 | 0.91 | 1.00 | 7.95 | 4.66 | 0.05 | 0.08 | 1.71 | 1.45 |
| DFH2 | / | 0.20 | 1.11 | 1.00 | 7.64 | 6.43 | 0.03 | 0.06 | 1.19 | |
| DCL1 | 0.48 | 0.75 | 1.26 | 1.00 | 9.58 | 9.43 | 0.01 | 0.03 | 1.02 | 1.05 |
| DCL2 | 0.01 | 0.69 | 1.55 | 1.00 | 11.43 | 10.49 | 0.03 | 0.07 | 1.09 |
| NO Production | |||||
|---|---|---|---|---|---|
| Molecular Weight Distribution | Monosaccharide Composition | Glycosidic Linkage Types | |||
| Peaks | Correlation Coefficient | Monosaccharide | Correlation Coefficient | Glycosidic Bond | Correlation Coefficient |
| Peak 1 | 0.748 | Xyl | 0.746 | →4)Manp(1→/→4)Glcp(1→ | 0.765 |
| Peak 2 | 0.682 | Gal | 0.731 | →4,6)Glcp(1→ | 0.740 |
| Am/Ag | 0.724 | →3,5)Araf(1→ | 0.731 | ||
| Man | 0.671 | Glcp(1→ | 0.638 | ||
| Ara | 0.667 | →5)Araf(1→ | 0.637 | ||
| Glc | 0.632 | →4)Manp(1→ | 0.593 | ||
| →4)Glcp(1→ | 0.585 | ||||
| →4,6)Manp(1→ | 0.568 | ||||
| Araf(1→ | 0.427 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Zhang, H.-W.; Fan, J.-H.; Jia, S.-S.; Yi, X.; Han, Z.-W.; Wang, R.-L.; Qiu, H.-W.; Lv, G.-P. Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium. Polymers 2025, 17, 1164. https://doi.org/10.3390/polym17091164
Zhu H, Zhang H-W, Fan J-H, Jia S-S, Yi X, Han Z-W, Wang R-L, Qiu H-W, Lv G-P. Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium. Polymers. 2025; 17(9):1164. https://doi.org/10.3390/polym17091164
Chicago/Turabian StyleZhu, Hua, Hui-Wen Zhang, Jia-Hao Fan, Si-Si Jia, Xin Yi, Zi-Wei Han, Ren-Lei Wang, Hong-Wei Qiu, and Guang-Ping Lv. 2025. "Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium" Polymers 17, no. 9: 1164. https://doi.org/10.3390/polym17091164
APA StyleZhu, H., Zhang, H.-W., Fan, J.-H., Jia, S.-S., Yi, X., Han, Z.-W., Wang, R.-L., Qiu, H.-W., & Lv, G.-P. (2025). Study on Differences in Structure and Anti-Inflammatory Activity of Polysaccharides in Five Species of Dendrobium. Polymers, 17(9), 1164. https://doi.org/10.3390/polym17091164






