The Chemistry of Behind the UV-Curable Nail Polishes
Abstract
1. Introduction
2. An Overview of the Polymerization Reactions
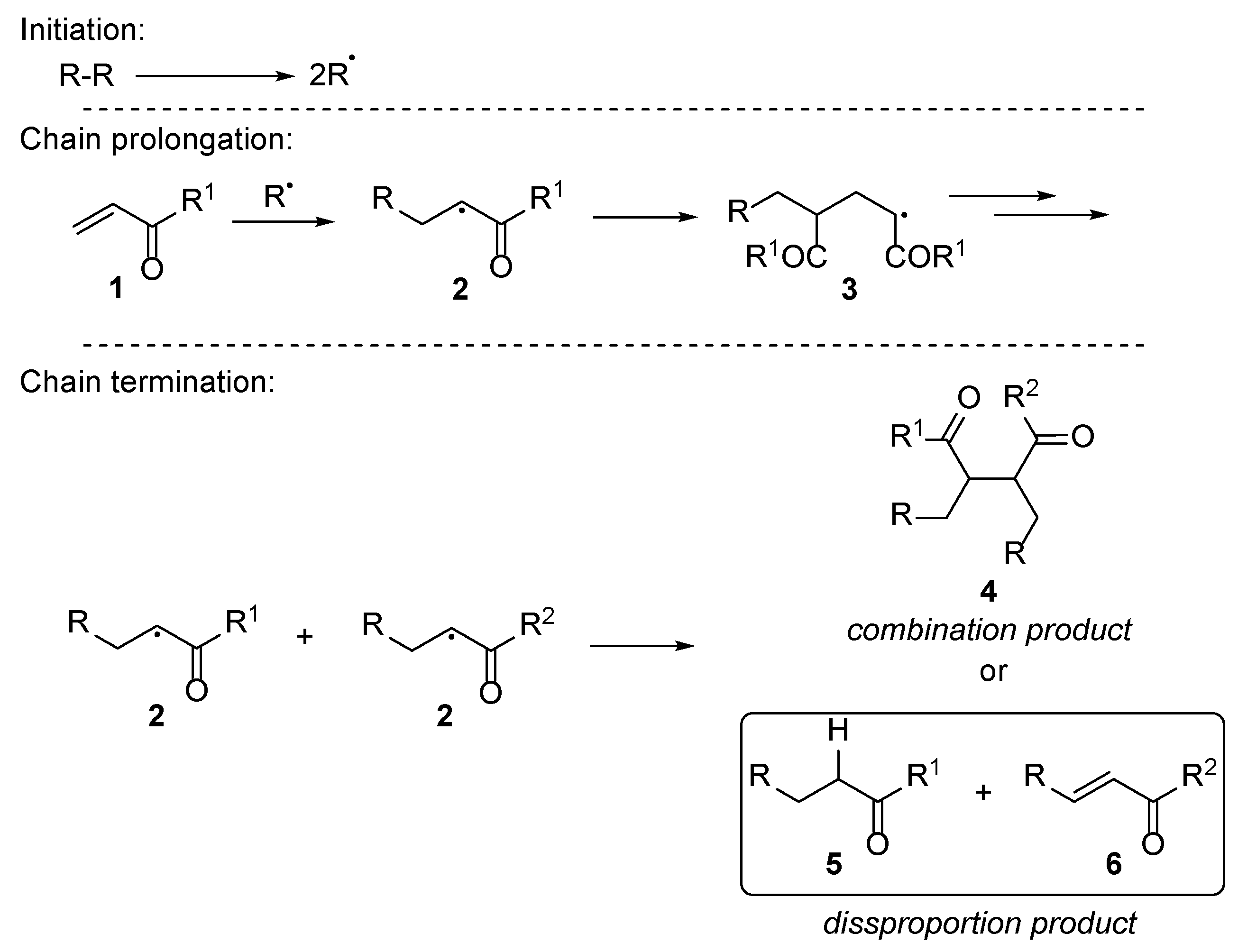
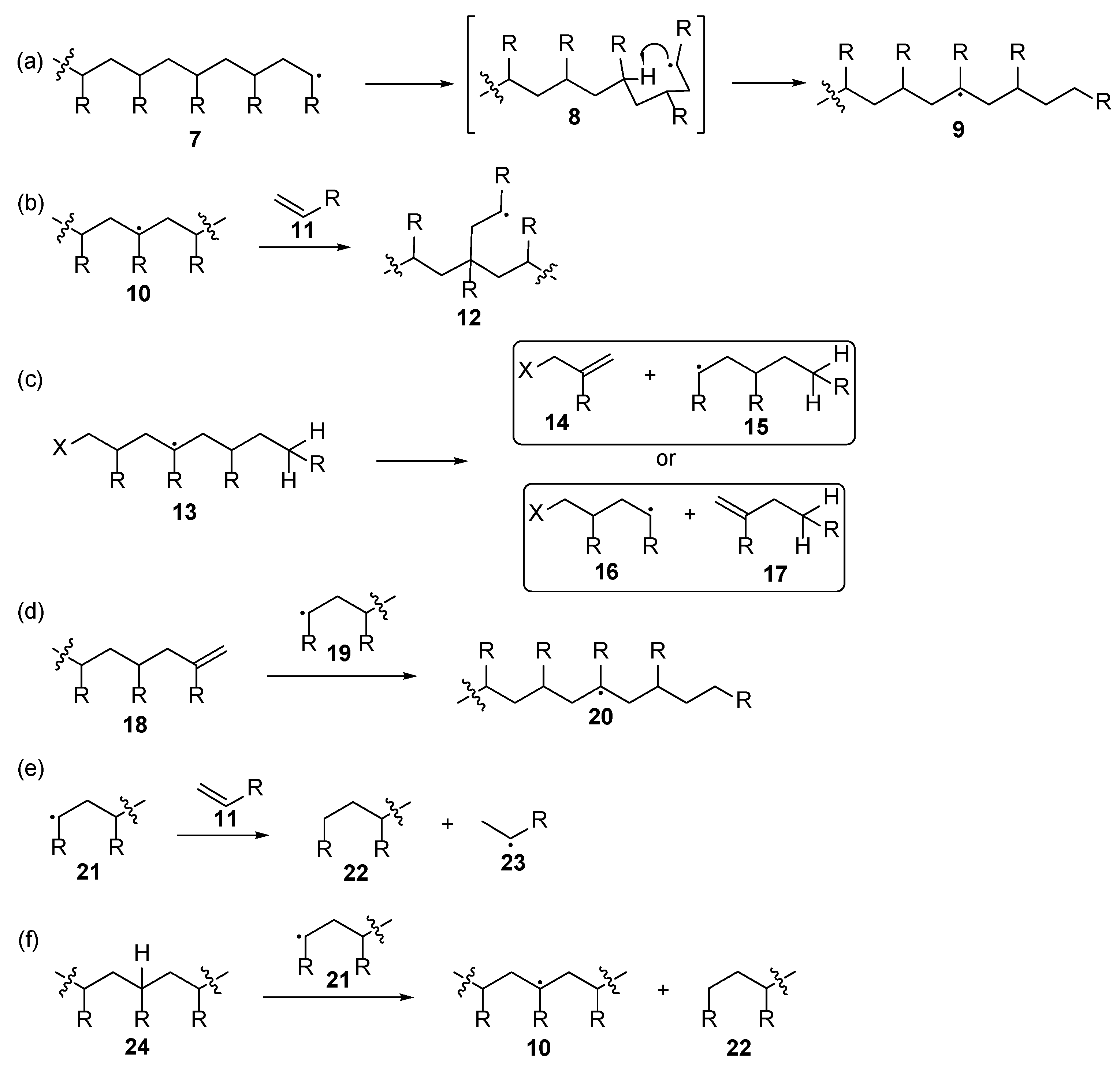
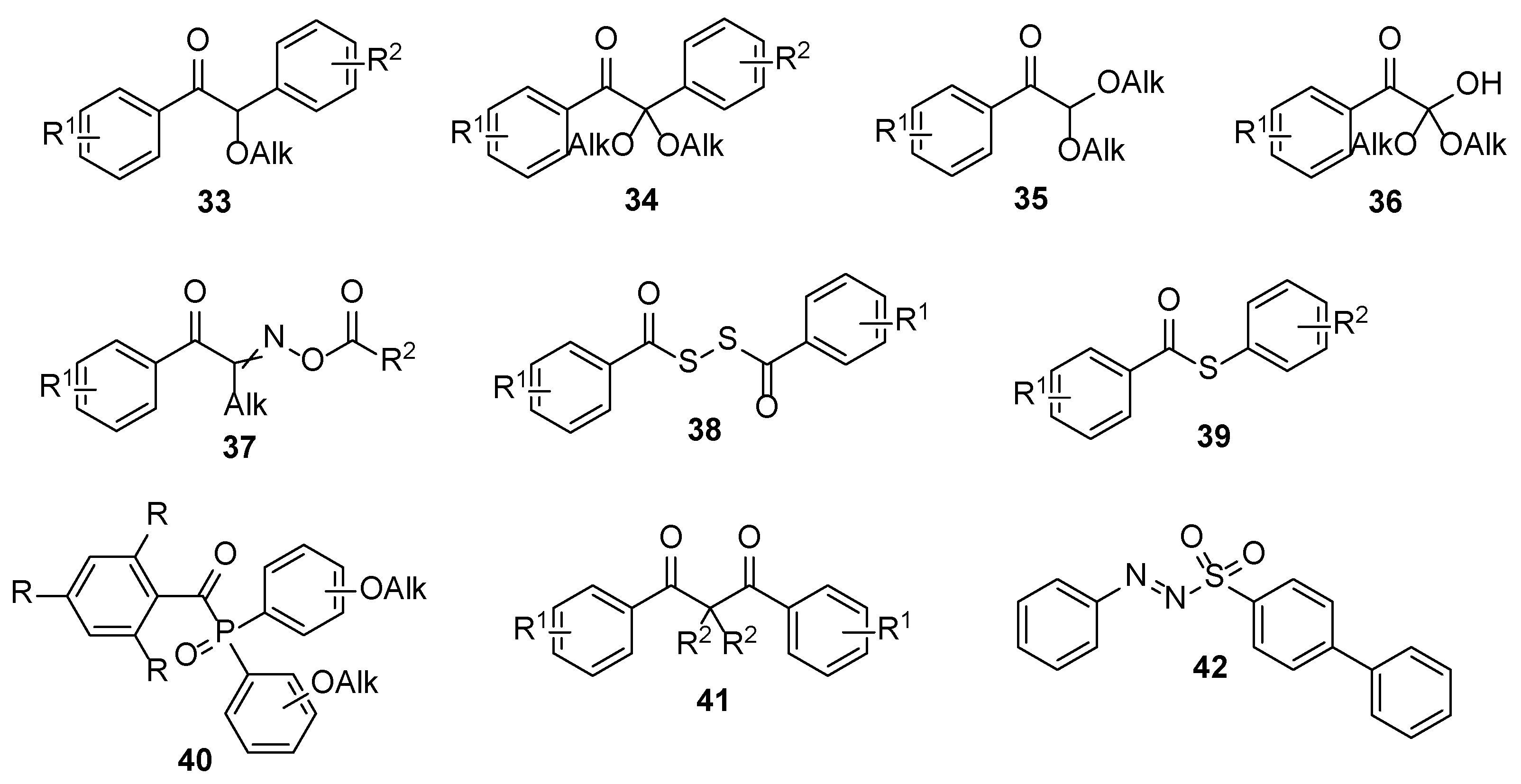
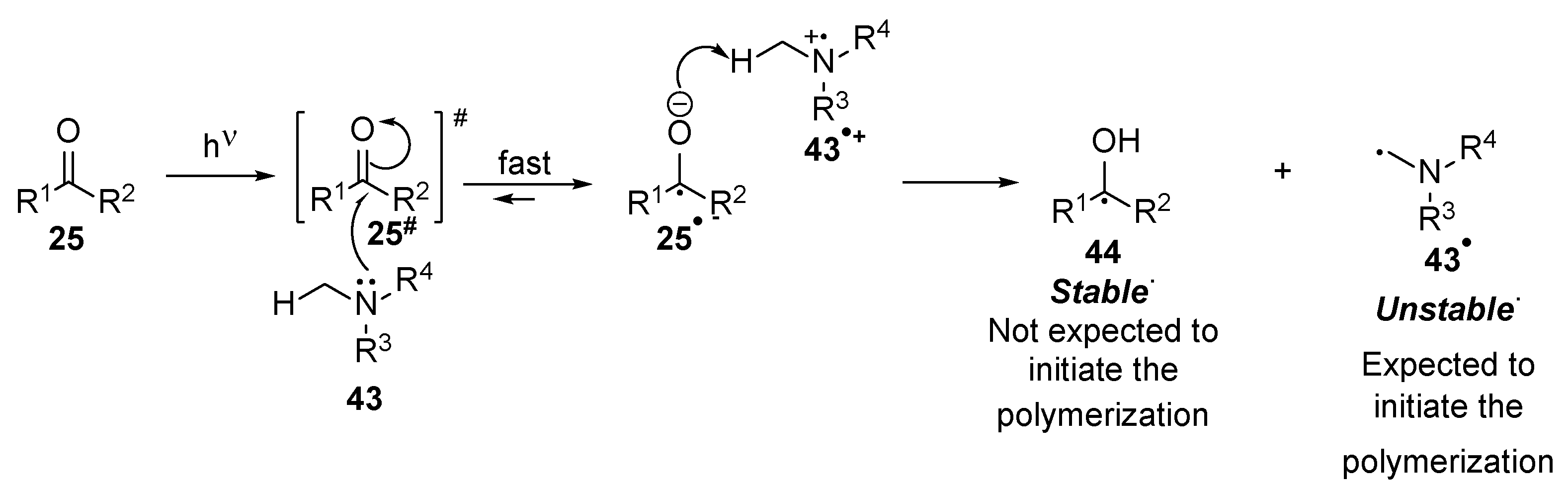
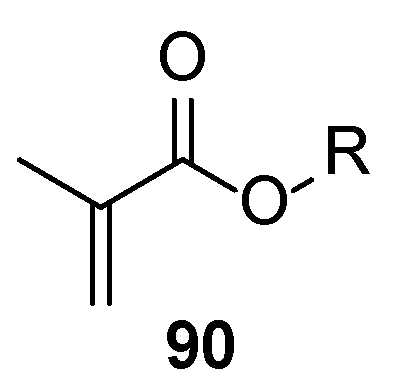
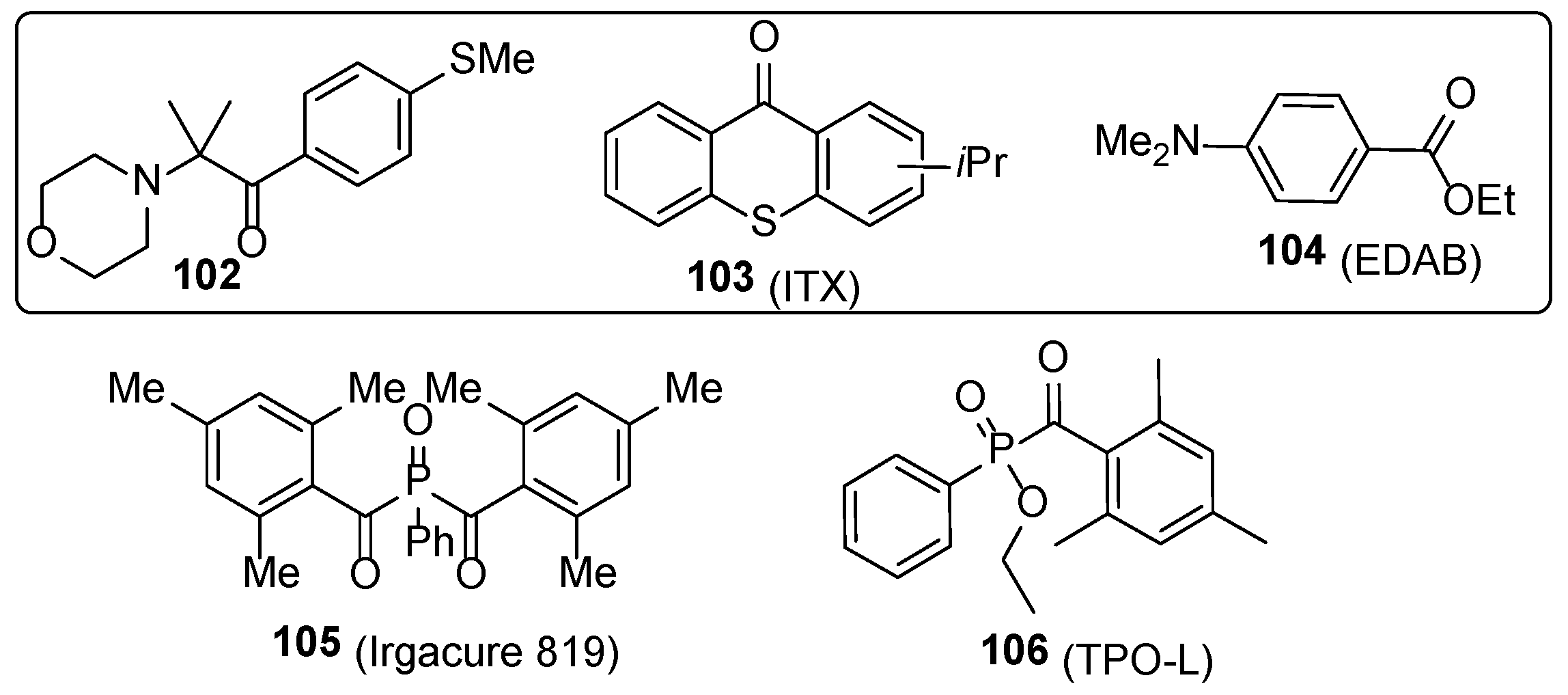
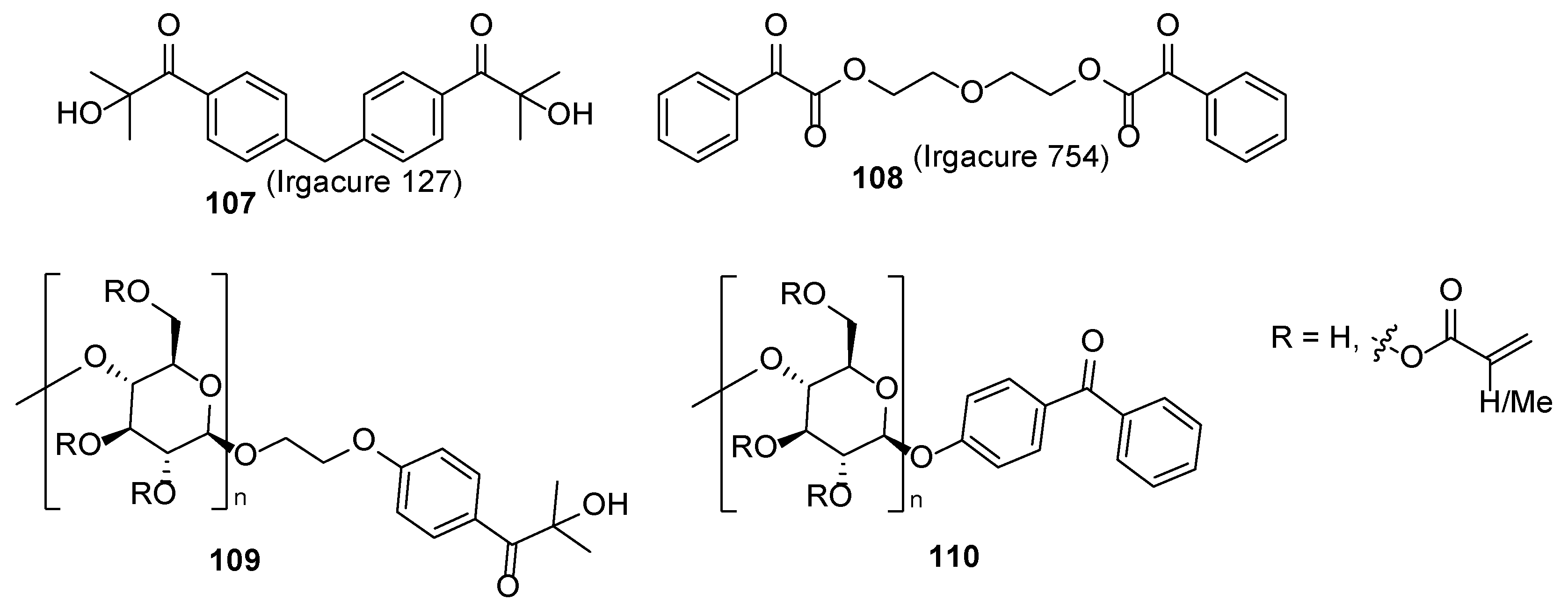
2.1. Acrylate Chemistry
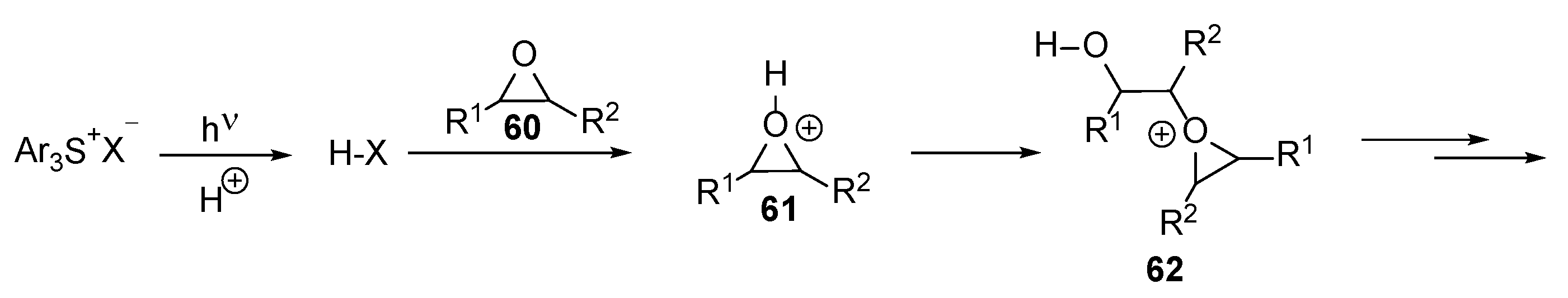
2.2. Epoxide Chemistry
2.3. Thiol-Ene Chemistry
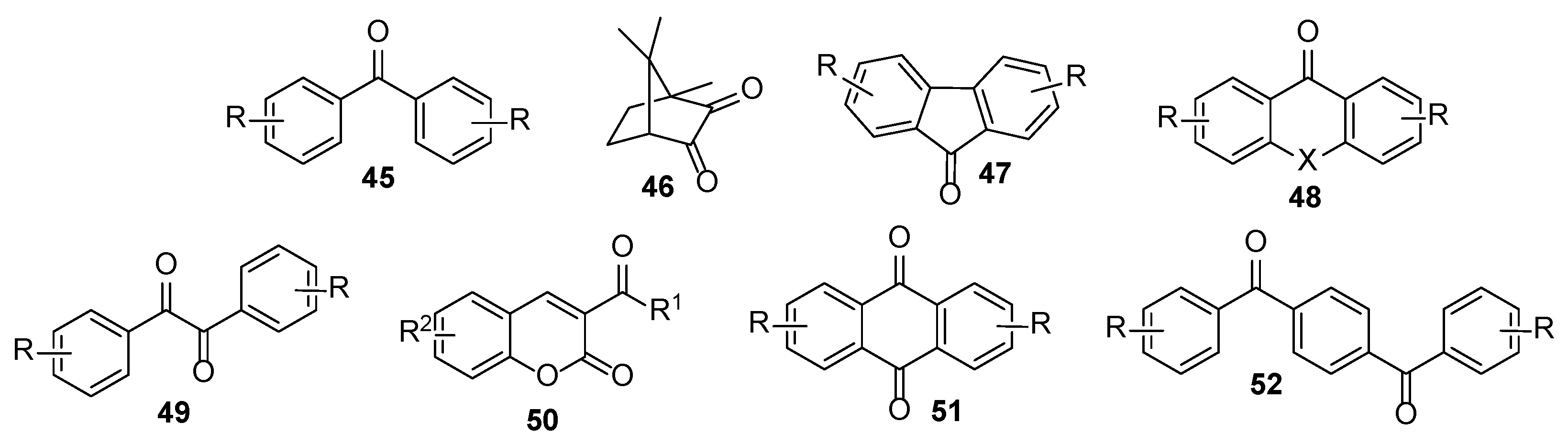
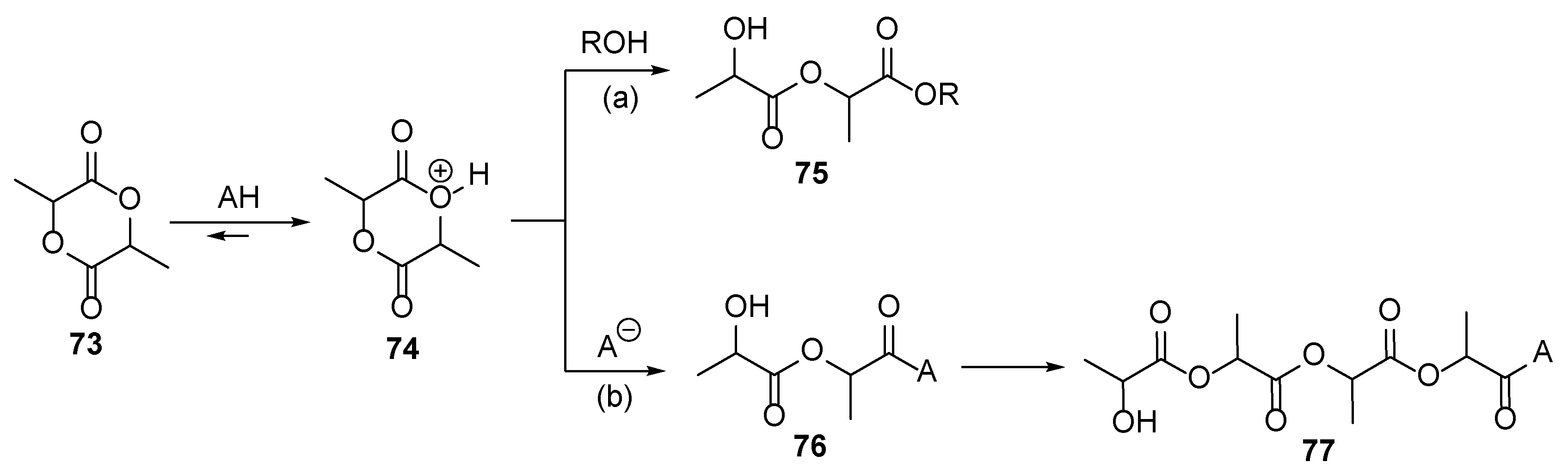
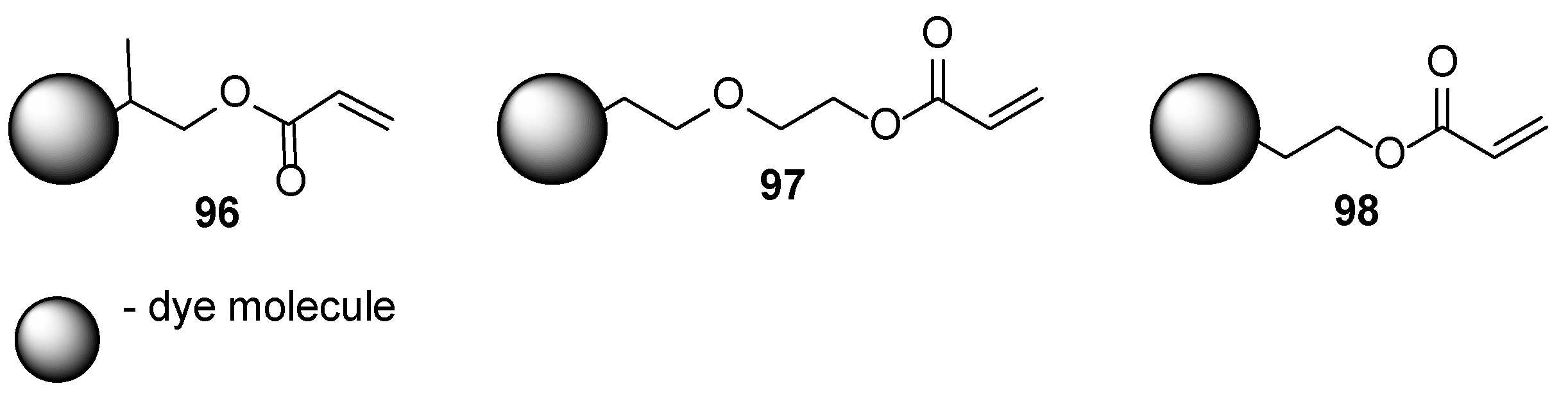
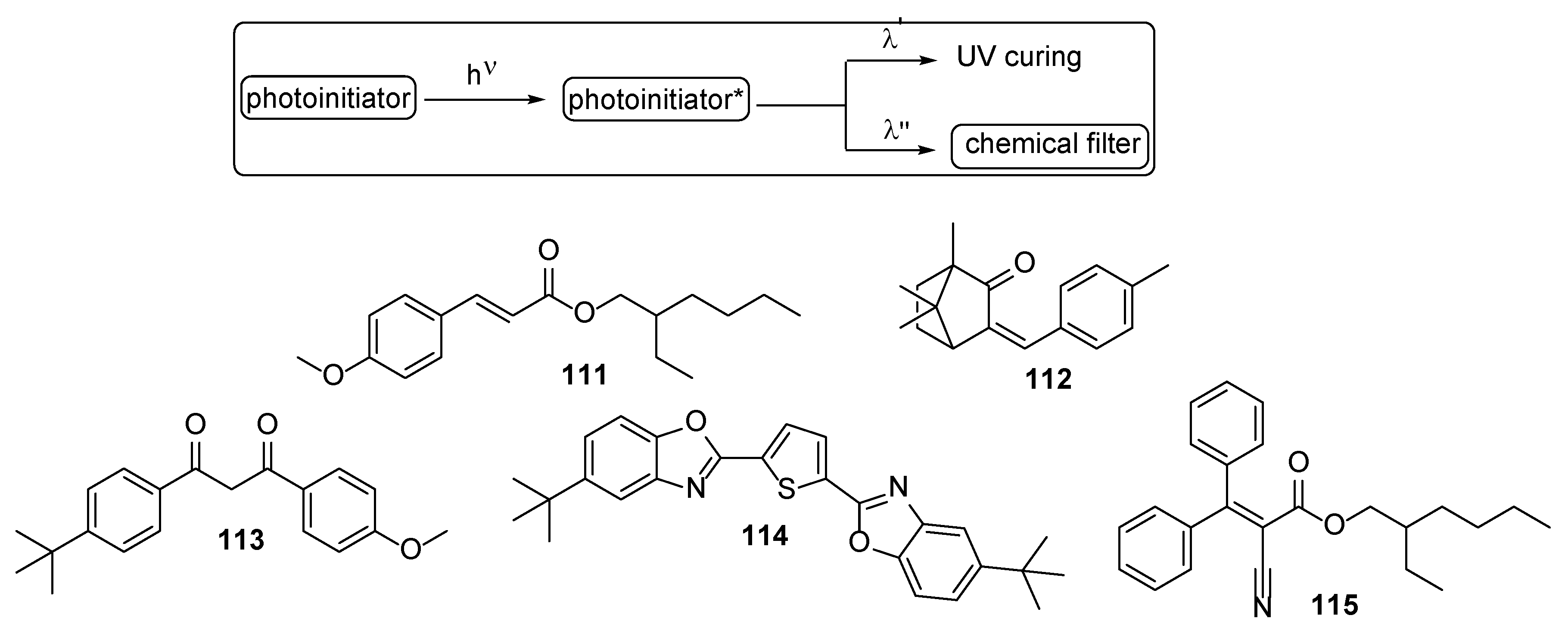
3. Acrylate-Based Compositions as the Film-Forming Agents
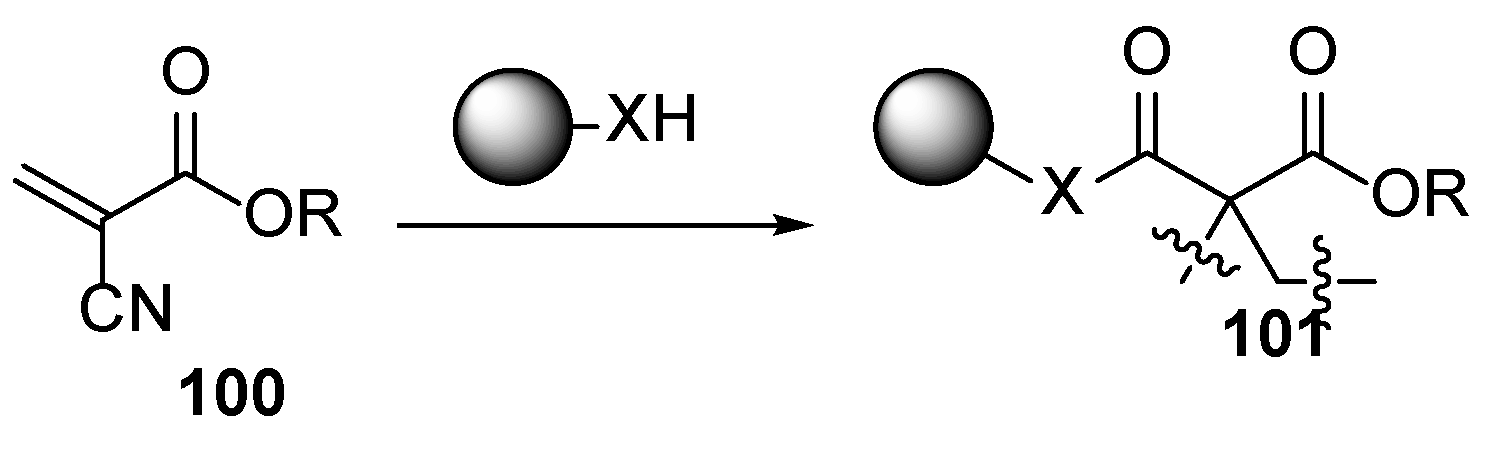
3.1. Petroleum-Based Acrylate Compositions
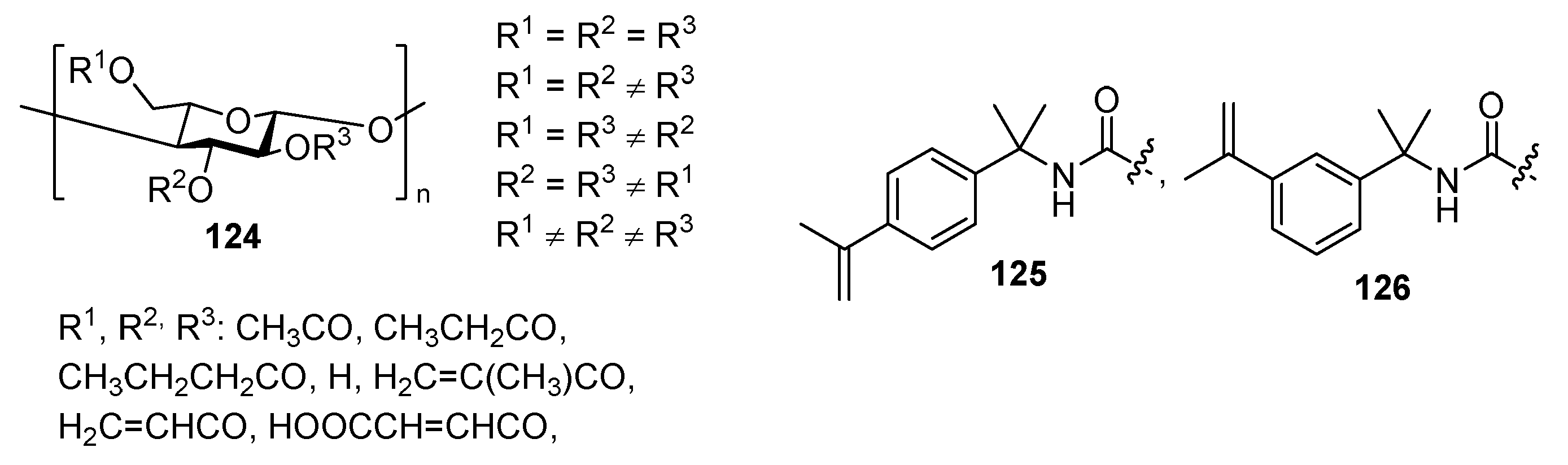
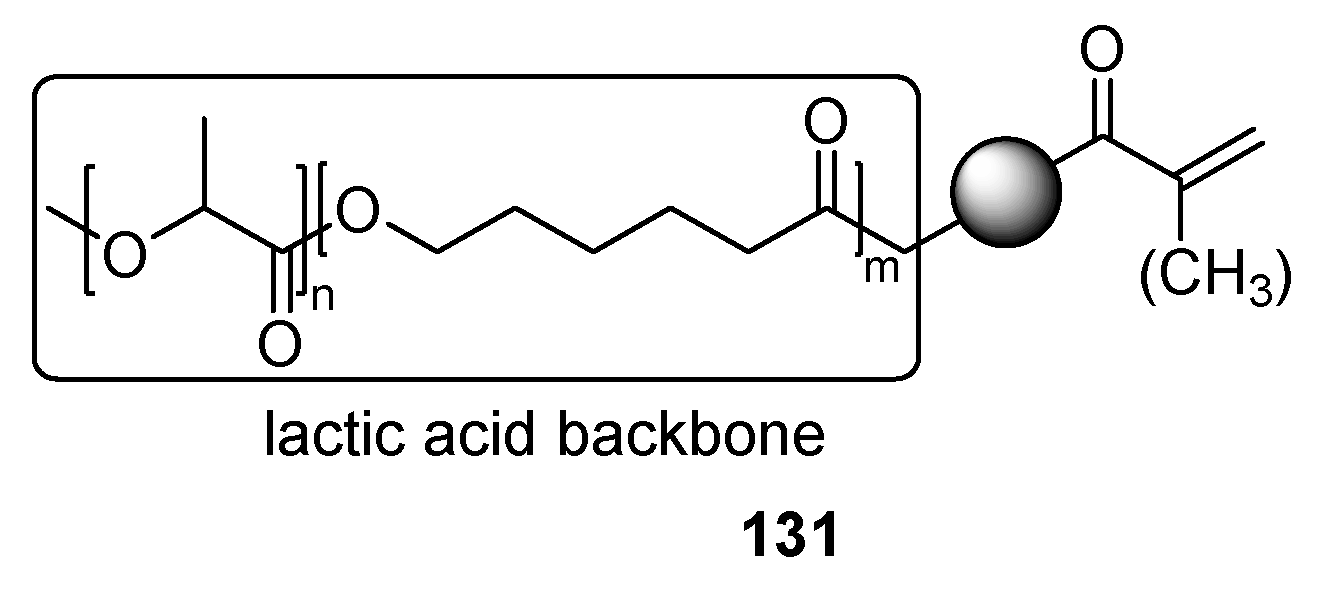
3.2. Bio-Based Acrylates
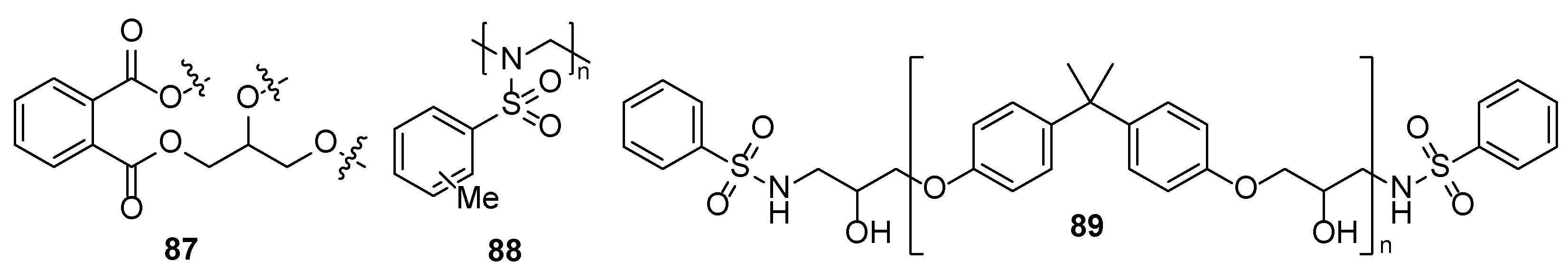
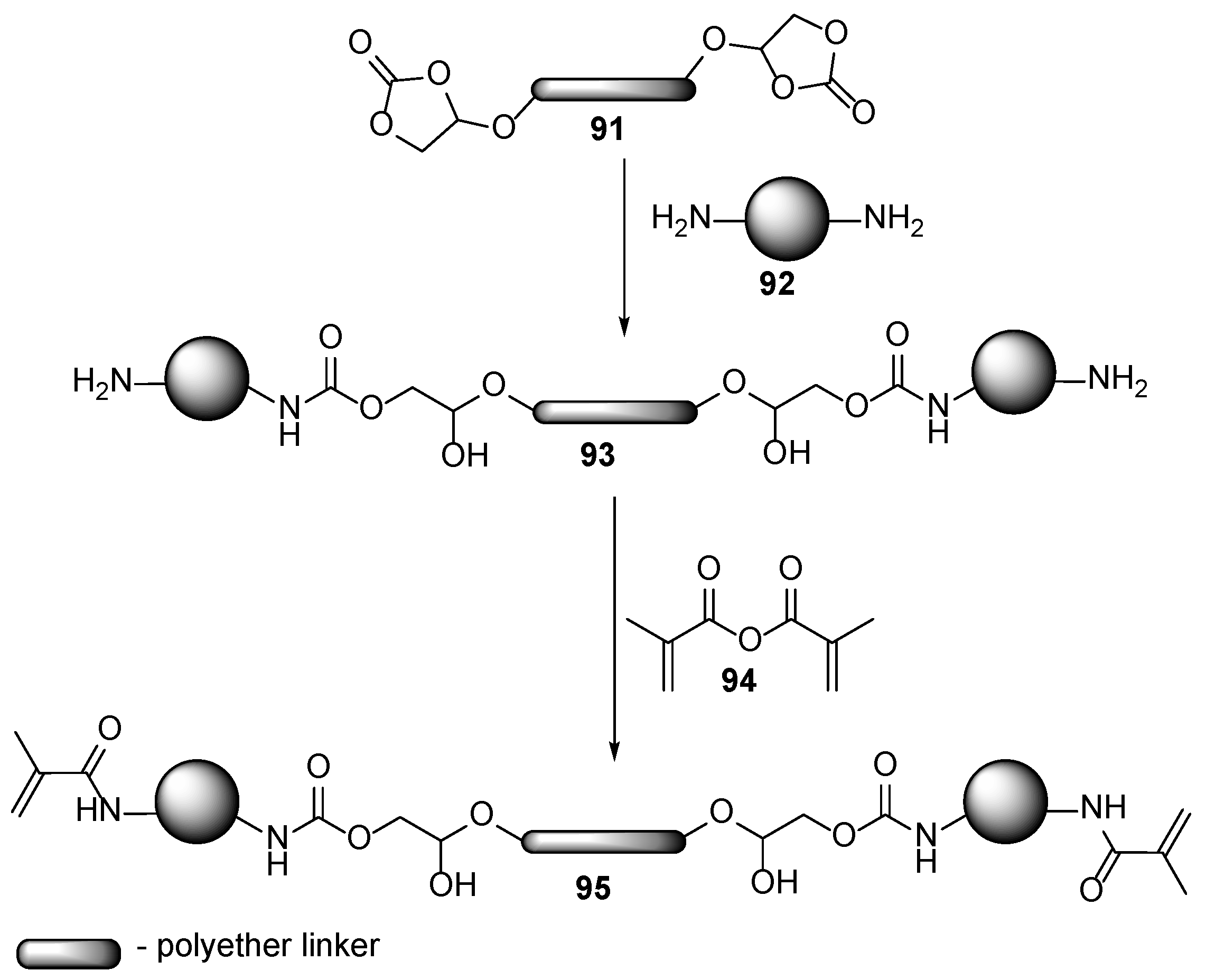
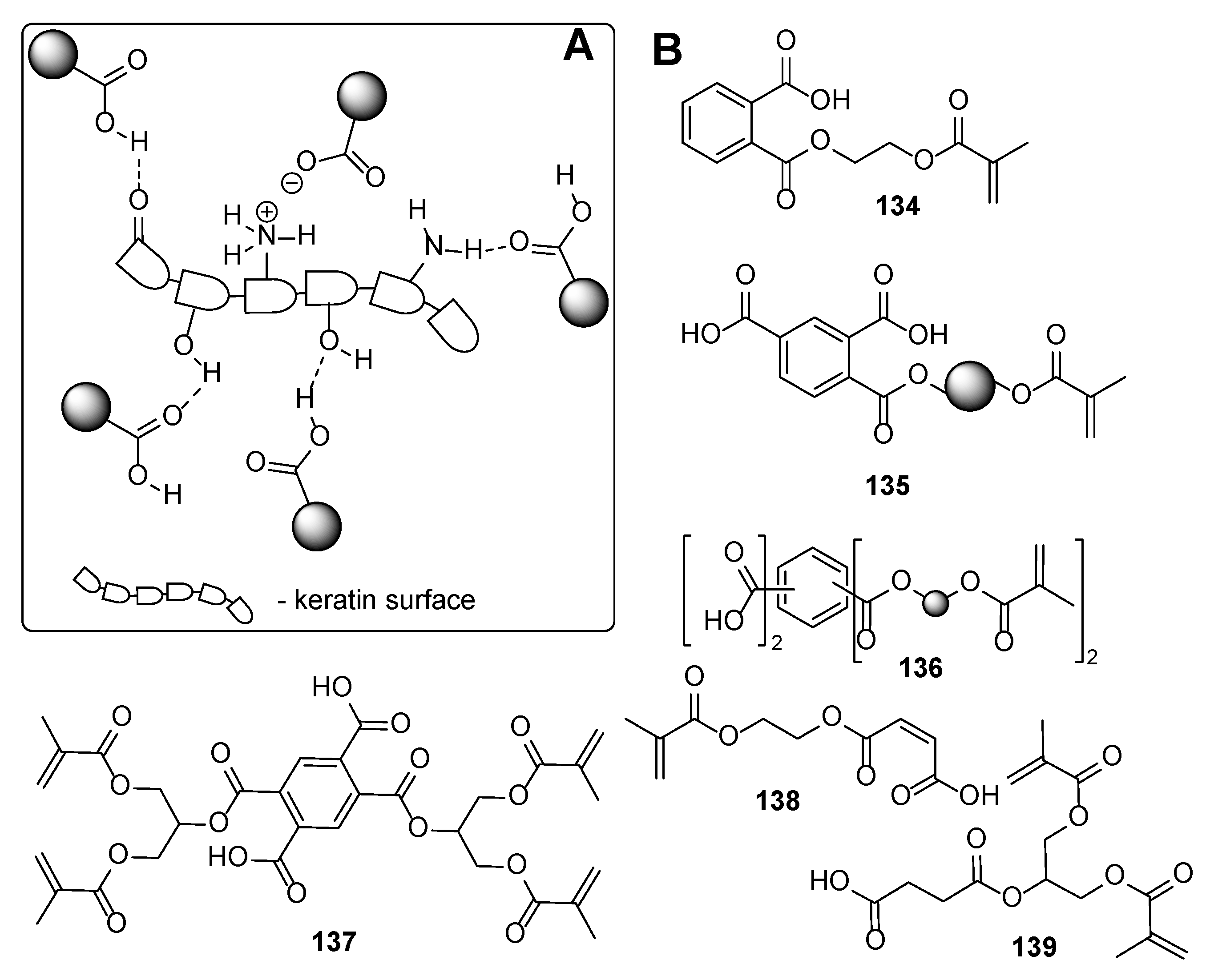
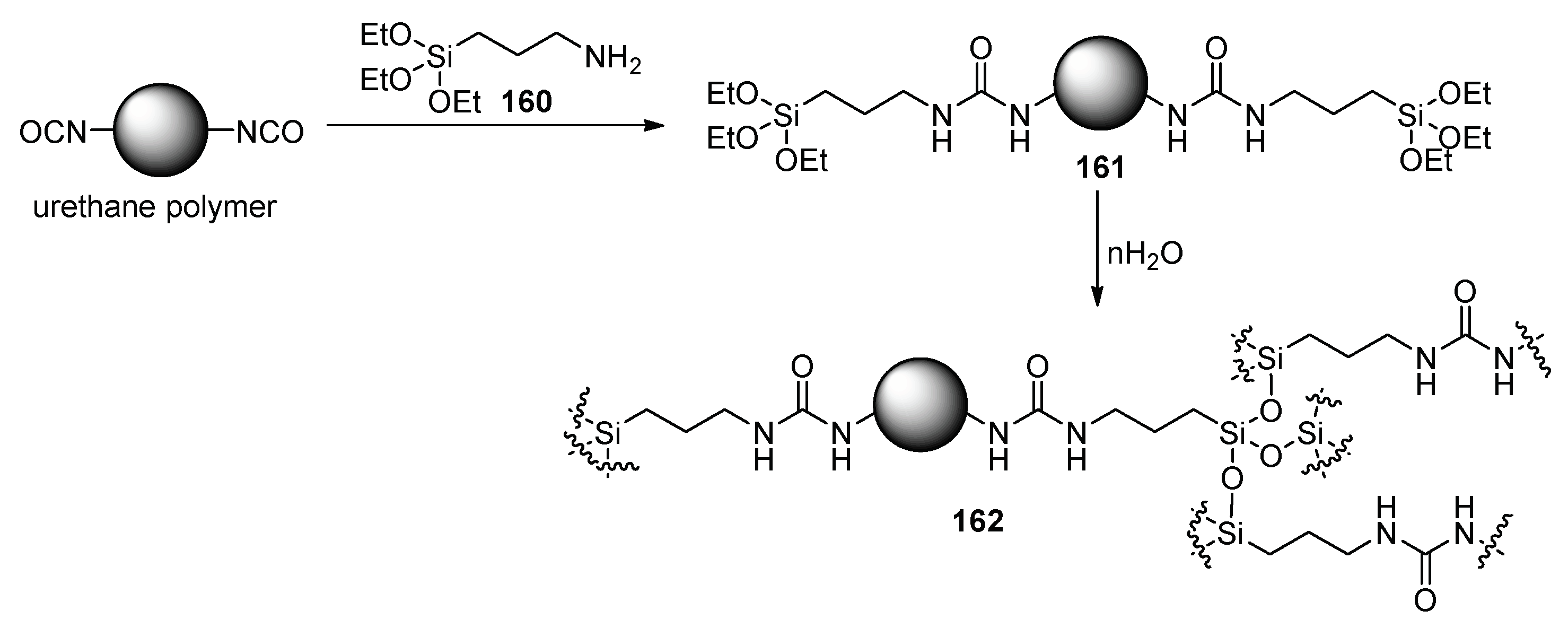
4. Additives for Improved Adhesion
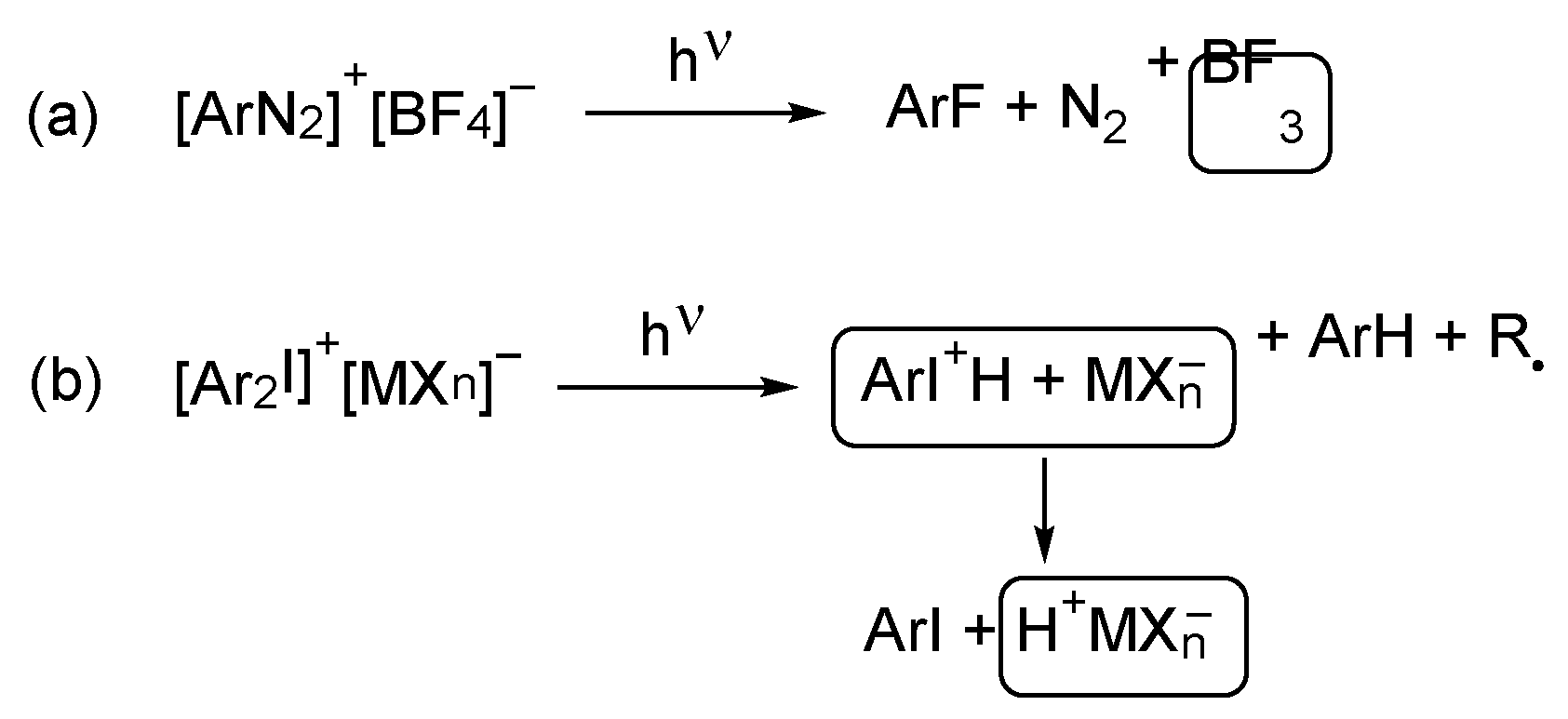
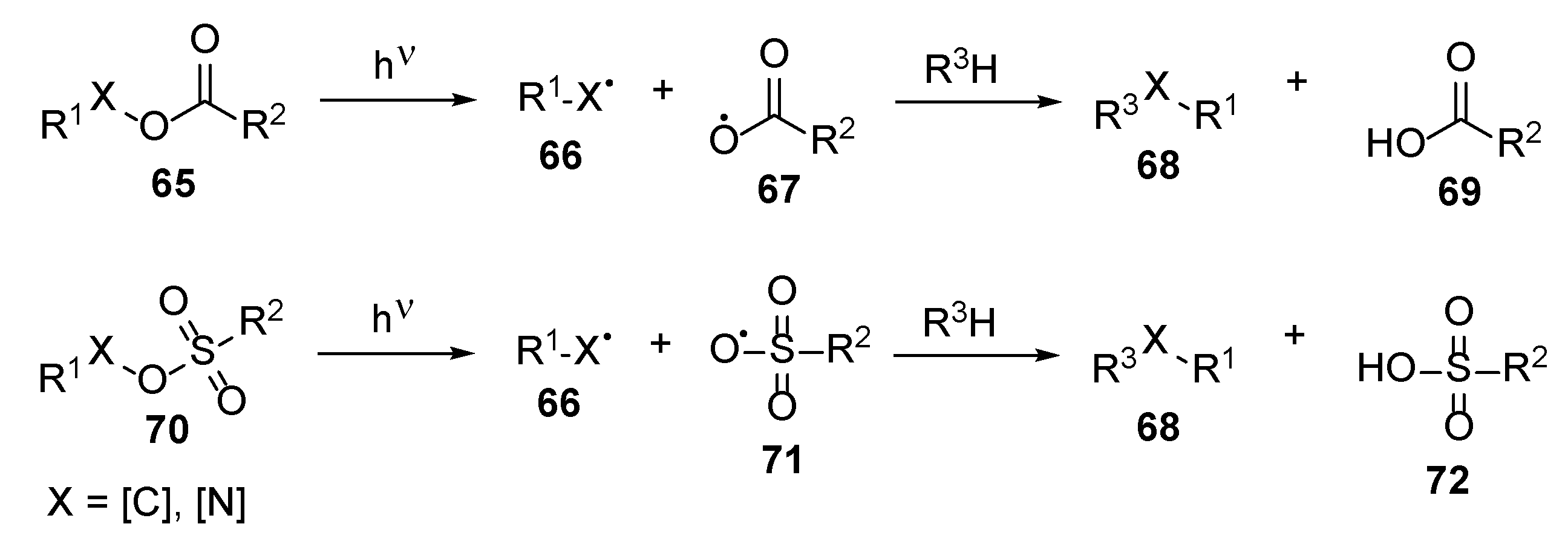
5. Photoinitiator-Free “On Nail” Curing Compositions
6. Other Nail Products
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EDAB | Ethyl 4-(dimethylamino)benzoate |
| DNA | Deoxyribonucleic acid |
| ITX | Isopropylthioxanthone |
| LED | Light-emitting diode |
| PDST | (4-Pheylthiophenyl)diphenylsulphonium triflate |
| UV | Ultraviolet |
| TPO-L | Ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate |
References
- Beauty & Personal Care—Worldwide. Available online: https://www.statista.com/outlook/cmo/beauty-personal-care/worldwide#revenue (accessed on 3 March 2025).
- The Beauty Market in 2023: A Special State of Fashion Report. Available online: https://www.mckinsey.com/industries/retail/our-insights/the-beauty-market-in-2023-a-special-state-of-fashion-report (accessed on 3 March 2025).
- UV Nail Gel—Global Market Activity, Trends, Opportunities and Growth Projections to 2030—Developed Regions Dominate, Developing Markets to Witness Fastest Growth. Available online: https://www.globenewswire.com/news-release/2024/07/24/2918112/0/en/UV-Nail-Gel-Global-Market-Activity-Trends-Opportunities-and-Growth-Projections-to-2030-Developed-Regions-Dominate-Developing-Markets-to-Witness-Fastest-Growth.html (accessed on 3 March 2025).
- Available online: https://www.nailsmag.com/encyclopedia/nsi-nail-systems-international (accessed on 12 April 2025).
- Baek, P.; Adler, B.L. Allergic contact dermatitis to isobornyl acrylate in a home nail glue. Contact Dermat. 2024, 91, 64–66. [Google Scholar] [CrossRef]
- Coe, J.; Robinson, R.; Wilkinson, S.M. Nail dystrophy mimicking psoriatic disease caused by contact allergy to nail varnish allergens including copolymers. Contact Dermat. 2021, 85, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Engelina, S.; Shim, T.N. Atypical cases of pseudo-psoriatic nails associated with acrylate contact allergy. Contact Dermat. 2021, 84, 342–344. [Google Scholar] [CrossRef]
- Walters, G.I.; Robertson, A.S.; Moore, V.C.; Burge, P.S. Occupational asthma caused by acrylic compounds from SHIELD surveillance (1989–2014). Occup. Med. 2017, 67, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Studholme, C. Novel investigational therapies for onychomycosis: An update. Expert Opin. Investig. Drugs 2016, 25, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Keskinkaya, Z.; Isik Mermutlu, S.; Ozge, K.; Cakir, H. Artificial nail modelling systems in healthcare workers: An emerging risk of contact sensitization to a well-known occupational allergen in an alternative way. Contact Dermat. 2024, 91, 38–44. [Google Scholar] [CrossRef]
- Gatica-Ortega, M.E.; Pastor-Nieto, M.A.; Gimenez-Arnau, A.M.; Mercader-Garcia, P.; Sanz-Sanchez, T.; Carrascosa-Carrillo, J.M.; Cordoba-Guijarro, S.; Sanchez-Perez, J.; Silvestre, J.F.; Frutos, F.J.O.; et al. 2-Hydroxyethyl methacrylate (2-HEMA) sensitization, a global epidemic at its peak in Spain? Contact Dermat. 2024, 90, 507–513. [Google Scholar] [CrossRef]
- Cordoba, S.; Rubio-Aguilera, R.F.; Baeza-Hernandez, G.; Borbujo, J. Allergic contact onycolysis due to acrylates in semipermanent nail-cosmetics. Piel 2023, 38, 477–4781. [Google Scholar]
- Lugović-Mihić, L.; Filija, E.; Varga, V.; Premuž, L.; Parać, E.; Tomašević, R.; Barac, E.; Špiljak, B. Unwanted Skin Reactions to Acrylates: An Update. Cosmetics 2024, 11, 127. [Google Scholar] [CrossRef]
- Beylin, D.; Kornhaber, R.; Le Lagadec, D.; Cleary, M. Assessing the Health Implications of UV/LED Nail Lamp Radiation Exposure During Manicure and Pedicure Procedures: A Scoping Review. Int. J. Dermatol. 2025, 64, 659–666. [Google Scholar] [CrossRef]
- Ardila Padilla, C.A.; Vignoni, M.; Serrano, M.P.; Dantola, M.L. Phototoxic Effects on Skin Biomolecules Induced by a Domestic Nail Polish Dryer Device. Chem. Res. Toxicol. 2025, 38, 182–192. [Google Scholar] [CrossRef]
- Stabicka-Jakubczyk, A.; Lewandowski, M.; Pastuszak, P.; Baranska-Rybak, W.; Gorska-Ponikowska, M. Influence of UV nail lamps radiation on human keratinocytes viability. Sci. Rep. 2023, 13, 22530. [Google Scholar]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Nakamura, Y.; Lee, R.; Coote, M.L.; Yamago, S. Termination mechanism of the radical polymerization of acrylates. Macromol. Rapid Commun. 2016, 37, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lugo, F.A.; Edeleva, M.; van Steenberge, P.H.M.; Sabbe, M.K. Secondary reactions during acrylate radical polymerization: Determining their rate coefficients. Polymer 2024, 300, 126938. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumuru, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevee, J. High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Qin, X.-H.; Ovsianikov, A.; Stampfl, J.; Liska, R. Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMat 2014, 15, 49–70. [Google Scholar] [CrossRef]
- Vazquez-Martel, C.; Mainik, P.; Blasco, E. Natural and naturally derived photoinitiating systems for light-based 3D printing. Org. Mater. 2022, 4, 281–291. [Google Scholar] [CrossRef]
- Muller, M.S.; Schlogl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent advances in type I photoinitiators for visible light induced photopolymerization. ChemPhotoChem 2022, 6, e202200091. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Allen, N.S. Photoinitiators for UV and visible curing coatings: Mechanisms and properties. J. Photochem. Photobiol. A 1996, 100, 101–107. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Bednarczyl, P.; Wroblewska, A.; Czech, Z.; Malko, M.W.; Rozwadowski, Z.; Markowska-Szczupak, A. Preparation, properties and potential applications of a photocurable varnish with pleasant limonene smell. Pol. J. Chem. Technol. 2016, 18, 13–19. [Google Scholar] [CrossRef]
- Taki, K.; Nakamura, T. Effects of curing conditions and formulations on residual monomer contents and temperature increase of a model UV gel nail formulation. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 111–118. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Mannari, V. Optimization the performance of sustainable nail gel compositions using a mixture experimental design methodology. Prog. Org. Coat. 2021, 153, 106168. [Google Scholar] [CrossRef]
- Malik, M.S.; Schlogl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Bomze, D.; Knaack, P.; Liska, R. Successful radical induced cationic frontal polymerization of epoxy-based monomers by C-C labile compounds. Polym. Chem. 2015, 6, 8161–8167. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Nie, J.; Zhu, X. Temperature controlled cationic photo-curing of a thick, dark composite. RSC Adv. 2017, 7, 4046–4053. [Google Scholar] [CrossRef]
- Basko, M.; Kubisa, P. Mechanism of propagation in the cationic polymerization of l,l-lactide. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7919–7923. [Google Scholar] [CrossRef]
- Singha, S.; Pan, S.; Talury, S.S.; Nguyen, G.; Tripathy, R.; De, P. Recent developments on cationic polymerization of vinyl ethers. ACS Polym. Au 2024, 4, 189–207. [Google Scholar] [CrossRef]
- Martin, C.J.; Rapenne, G.; Nakashima, T.; Kawai, T. Recent progress in development of photoacid generators. J. Photochem. Photobiol. C 2018, 34, 41–51. [Google Scholar] [CrossRef]
- Klikovits, N.; Knoock, P.; Bomze, D.; Krossing, I.; Liska, R. Novel photoacid generators for cationic photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Gershoni, G.; Dodiuk, H.; Tenne, R.; Kening, S. Cationic polymerized epoxy and radiation cured acrylate blend nanocomposites based on WS2 nanoparticles—Part A: Curing process and kinetics. J. Compos. Sci. 2023, 7, 41. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Sangermano, M. Advances in cationic photopolymerization. Pure Appl. Chem. 2012, 84, 2089–2133. [Google Scholar] [CrossRef]
- Feng, X.; Liu, R.; Liu, L.; Jin, Y.; Shi, Q.; Yan, P.; Wu, Y. Recent advances on visible light induced cationic polymerization. J. Polym. Sci. 2023, 61, 2411–2425. [Google Scholar] [CrossRef]
- Yang, Z.; Shan, J.; Huang, Y.; Dong, X.; Zheng, W.; Jin, Y.; Zhou, W. Preparation and mechanism of free-radical/cationic hybrid photosensitive resin with high tensile strength for three-dimensional printing applications. J. Appl. Polym. Sci. 2020, 138, e49881. [Google Scholar] [CrossRef]
- Ma, Y.; Dreiling, R.J.; Recker, E.A.; Kim, J.-W.; Shankel, S.L.; Hu, J.; Easley, A.D.; Page, Z.A.; Lambert, T.H.; Fors, B.P. Multimaterial thermoset synthesis: Switching polymerization mechanism with light dosage. ACS Cent. Sci. 2024, 10, 2125–2131. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, W.; Cui, W.; Fei, X.; Song, W. UV Cured Modified Thiol Resin Nail Polish and Preparation Method Thereof. CN Patent 111035575, 21 April 2020. [Google Scholar]
- Liang, W.; Cui, W.; Zhang, C.; Song, W.; Fei, X. UV-Curable Environment-Friendly Nail Polish and Preparation Method Thereof. CN Patent 110917068, 27 March 2020. [Google Scholar]
- Cramer, N.B.; Scott, J.P.; Bowman, N. Photopolymerizations of thiol-ene polymers without photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Clark, T.; Kwisnek, L.; Hoyle, C.E.; Nazarenko, S. Photopolymerization of thiol-ene systems based on oligomeric thiols. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 14–24. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Ahn, D.; Sathe, S.S.; Clarkson, B.H.; Scott, T.F. Heaarylbiimidazoles as visible light thiol-ene photoinitiators. Dent. Mater. 2015, 31, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, J.; Banaszak Holl, M.M.; Xiao, P. Thiol-ene photopolymerization under blue, green and red LED irradiation. ChemPhotoChem 2021, 5, 571–581. [Google Scholar] [CrossRef]
- Yue, J.; Nie, J.; Zhu, X. Thiol-divinylbenzene: A thiol-ene system with high storage stability. J. Photochem. Photobiol. A 2023, 436, 114417. [Google Scholar] [CrossRef]
- Uygun, M.; Tasdelen, M.A.; Yagci, Y. Influence of type of initiation on thiol-ene “click” chemistry. Macromol. Chem. Phys. 2010, 211, 103–110. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, X. LED-UV Phototherapy Glue Scrubbing-Free Sealing Layer Glue and Preparation Method Thereof. CN Patent 105878054, 24 August 2016. [Google Scholar]
- Xu, J. UV (Ultraviolet) Photocuring Red Nail Polish. CN Patent 104042452, 17 September 2014. [Google Scholar]
- Kergosien, G.; Riachi, C. Photocrosslinkable Nail Makeup Composition. U.S. Patent 2015/0297497, 22 October 2015. [Google Scholar]
- Kergosien, G.; Riachi, C.; Le Pape, M. Photo-Crosslinkable Varnish Compositions as Base Coating and Application Methods. U.S. Patent 2016/0303015, 20 October 2016. [Google Scholar]
- Ijdo, W.; Chen, Y.; Deshmukh, P. Acrylate Gel Nail Coating Compositions. U.S. Patent 2015/0359724, 17 December 2015. [Google Scholar]
- Kergosien, G.; Riachi, C.; Le Pape, M. Nail Makeup Method with Photocrosslinkable Vanrnish Compositions. U.S. Patent 2015/0313826, 5 November 2015. [Google Scholar]
- Zareanshahraki, F.; Asemani, H.R.; Skuza, J.; Mannari, V. Synthesis of non-isocyanate polyurethanes and their application in radiation-curable aerospace coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Wu, X.; Yang, W. UV-LED Nail Polish Gel and Preparation Method Thereof. CN Patent 109276467, 29 January 2019. [Google Scholar]
- Wu, T.; Li, Y.; Xiang, Y.; Cao, S.; Wu, X.; Yang, W.; Li, J. UV&LED Curing Aqueous Copolymerized Colored Nail Gel and Preparation Method Thereof. CN Patent 109758382, 17 May 2019. [Google Scholar]
- Wu, T.; Li, Y.; Xiang, Y.; Cao, S.; Wu, X.; Yang, W.; Li, J. UV&LED Solidified Aqueous Co-Polymerization Fluorescent Nail Polish Gel. CN Patent 109602637, 12 April 2019. [Google Scholar]
- Grigale-Sorocina, Z.; Kalnins, M.; Simanovska, J.; Vindedze, E.; Birks, I.; Brazdauska, E. Effect of additives on UV-activated urethane acrylate polymerization composite coatings. Mater. Sci. (Medžiagotyra) 2016, 22, 54–59. [Google Scholar] [CrossRef]
- Sirdesal, S.J. Composition Having a Reduced Exotherm in Actinic Curing of Urethane (Meth)acrylate Oligomers on Fingernails. Int. Patent Appl. 2014/134009, 4 September 2014. [Google Scholar]
- UV-Curable Gel Nail Polish Composition with Improved Acid Degradation Property and Uses Thereof. KR Patent 102350649, 12 January 2022.
- Tan, H.; Tan, Q.; Tan, D. Novel Nail Polish. CN Patent 110638689, 3 January 2020. [Google Scholar]
- Jin, J.; Dai, Y.; Ye, Z. Nail Polish System and Manicuring Method Thereof. CN Patent 110200848, 6 September 2019. [Google Scholar]
- Zhang, Y.; Wu, R.; Chang, W.; Li, Y.; Jin, C. Quick-Drying Nail Polish Gel and Preparation Method Thereof. CN Patent 105213221, 6 January 2016. [Google Scholar]
- Yu, M.; Ding, Z.; Xie, Y. Environment-Friendly UV Nail Polish Coating Composition and Preparing Method Thereof. CN Patent 105030567, 11 November 2015. [Google Scholar]
- Ding, J.; Chen, S.; Liao, Z.; Zhang, Z.; Xu, Q. Environment-Friendly UV Photocuring Nail Polish and Preparation Method Thereof. CN Patent 114191330, 18 March 2022. [Google Scholar]
- Wang, Y.; Li, G.; Ding, Q. Waterborne Photochromic Coating as Well as Nail Polish and Decorative Material. CN Patent 107325603, 7 November 2017. [Google Scholar]
- Bryson, I.; Thomson, D.; Li, J. UV Curable Nail Coating Compositions. Int. Patent Appl. 2015/049523, 9 April 2015. [Google Scholar]
- Mannari, V.M.; Zareanshahraki, F. Biobased, UV-Curable Nail Polish Compositions and Related Methods. U.S. Patent 2020/0078288, 12 March 2020. [Google Scholar]
- Ye, Q.; Yang, Z.; Huang, Y. Epoxidized Soybean Oil-Based Acrylate-Polyurethane UV (Ultraviolet) Light-Cured Resin and TPO (Thermoplastic Polyolefin) Photoinitiator-Free Plant Oil-Based Nail Polish Gel Prepared from Epoxy Soybean Oil-Based Acrylate-Polyurethane UV Light-Cured Resin. CN Patent 117003989, 7 November 2023. [Google Scholar]
- Zareanshahraki, F.; Mannari, V. “Green” UV-LED Gel Nail Polishes from Bio-Based Materials. Int. J. Cosmet. Sci. 2018, 40, 555–564. [Google Scholar] [CrossRef]
- Du, J.; Gu, H.; Dou, S. Preparation Method of Biobased Water-Borne UV (Ultraviolet) Curing Environment-Friendly Nail Polish. CN Patent 106821796, 13 June 2017. [Google Scholar]
- Cook, P.M. UV-Curable Nail Coating Nail Coating Formulations Containing Cellulose Esters with Ethylenically Unsaturated Pendant Groups. Int. Patent Appl. 98/48769, 5 November 1998. [Google Scholar]
- Kuang, M.; Lu, M.; Lu, Z.; Liu, M.; Lu, C.; Shang, Q. Preparation Method of High-Concentration Bio-Based UV Nail Polish Color Paste. CN Patent 115300416, 8 November 2022. [Google Scholar]
- Yamamoto, S.; Yamamoto, S.; Tanaka, H.; Matsuo, K. Photocurable Nail Coat Agent. JP Patent 2019094309, 20 June 2019. [Google Scholar]
- Lein, G. UV-Curable Nail Coating Formulations Based on Renewable Acids, Lactones Cyclic Ether, Lactams. U.S. Patent 2016/0184200, 30 June 2016. [Google Scholar]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Pawlikowska, M.; Czech, Z. Primers used in UV-curable nail varnishes. Int. J. Adhes. Adhes. 2017, 74, 177–180. [Google Scholar] [CrossRef]
- Mididoddi, P.K.; Prodduturi, S.; Repka, M.A. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev. Ind. Pharm. 2006, 32, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Understanding and Treating Various Skin Types: The Baumann Skin Type Indicator. Dermatol. Clin. 2008, 26, 359–373. [Google Scholar] [CrossRef]
- Hu, H.; Liang, L.; Liu, Y.; Hu, L.; Liang, J. Weak Ultraviolet Curing Composition. CN Patent 101164516, 23 April 2008. [Google Scholar]
- Jin, J.; Dai, Y.; Ye, Z. UV Glue for Primary Coating of Nail Painting. CN Patent 110585063, 20 December 2019. [Google Scholar]
- Pink, J.; Werner, D.H. Nail Gel Builder Composition. U.S. Patent 11000469, 11 May 2021. [Google Scholar]
- Lilley, P.H. Finishing Compounds for Radiation Curable Nail Coatings. U.S. Patent 6481444, 19 November 2002. [Google Scholar]
- Lilley, P.H. Radiation Curable Nail Coatings. U.S. Patent 2002/0010226, 24 January 2002. [Google Scholar]
- Sarbox® SB500E50. Available online: https://coatings.specialchem.com/product/m-sartomer-arkema-group-sarbox-sb500e50 (accessed on 3 March 2025).
- Vu, T.; Conger, C.; Larsen, D.M.; Valia, D.; Schoon, D.D. Compositions and Methods for UV-Curable Cosmetic Nail Coatings. Int. Patent Appl. 2012/121704, 13 September 2012. [Google Scholar]
- Flint, C. Nail Polish Formulation. U.S. Patent 10744349, 18 August 2020. [Google Scholar]
- Zhang, Y.; Hu, H.; Shi, H. UV-LED Fingernail Binding Agent and Preparation Method Thereof. CN Patent 107997990, 8 May 2018. [Google Scholar]
- Xie, G.; Guo, C. Polyurethane Modified Pure Acrylic Ester and Preparation Method Thereof, and Carving Rubber. CN Patent 116023581, 28 April 2023. [Google Scholar]
- Takemoto, K. Photocurable Composition to Be Used on Fingernails or Artificial Nails, Base Coat Agent Containing Same, Cured Article Thereof, Method for Producing Cured Article Thereof, Method for Detaching Cured Article Thereof, Method for Coating by Using Same, and Method for Using Same. U.S. Patent 2020/0214956, 9 July 2020. [Google Scholar]
- Komarova, E.Y. UV-UVV Curable, Wear-Resistant Nail Polish. U.S. Patent 20170312793, 2 November 2017. [Google Scholar]
- Grigale-Sorocina, Z.; Birks, I.; Novicka, E. Examining adhesion promoters in UV-cured nail coatings: A comprehensive investigation into composition, polymerization, and performance characteristics. Mater. Sci. (Medžiagotyra) 2024, 30, 467–472. [Google Scholar] [CrossRef]
- Brzozka, P.; Kolodziesjski, W. Sex-related differences in keratin from fingernail plates: A solid state carbon-13 NMR study. RSC Adv. 2017, 7, 28213–28223. [Google Scholar] [CrossRef]
- Greaves, M.S.; Moll, J.M.H. Amino Acid Composition of Human Nail, as measured by Gas-Liquid Chromatography. Clin. Chem. 1976, 22, 1608–1613. [Google Scholar] [CrossRef]
- Liu, X. Replaceable Volatile Solvent Based Phototherapy Nail Polish and Preparation Method Thereof. CN Patent 108434019, 24 August 2018. [Google Scholar]
- Zhou, X.Z.; Li, C.; Bui, H.S.; Simonnet, J.-T.; Riachi, C.; Kergosien, G. Nail Treatment System. U.S. Patent 10639255, 5 May 2020. [Google Scholar]
- Grigale-Sorocina, Z.; Kalnins, M.; Simanoska, J.; Vindedze, E.; Birks, I.; Brazdauska, E. Additives in UV-activated urethane acrylate polymerization composite coatings. Proc. Est. Acad. Sci. 2015, 64, 88–93. [Google Scholar] [CrossRef]
- Yuvavanich, S.; Pavlovic, E.; Bryson, P.H. Curable, Solvent Removable Gel for Nails. Int. Patent Appl. 2022/081628, 21 April 2022. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, Y. Quickly-Curing Strippable Nail Polish Gel and Preparation Method Thereof. CN Patent 105748317, 13 July 2016. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, Y.; Wu, Z. Strippable Nail Polish Glue and Synthetic Method. CN Patent 105640801, 8 June 2016. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, Y.; Xu, Y. Strippable Nail Polish Gel and Synthesis Method Thereof. CN Patent 105534741, 4 May 2016. [Google Scholar]
- Zhang, Y.; Yu, L.; Yang, Y.; Liang, H.; Li, Y. Peelable Nail Polish and Preparation Method Thereof. CN Patent 105596232, 25 May 2016. [Google Scholar]
- Zhang, Y.; Yu, L.; Yang, Y.; Chang, W.; Li, Y. Strippable Fluorescent Nail Lacquer Gel and Preparation Process Thereof. CN Patent 105496816, 20 April 2016. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, Y. Improved Peelable Soak-Off Gel and Preparation Method Thereof. CN Patent 105560082, 11 May 2016. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, B.; Wang, C.; Li, Y.; Wu, Z. Peelable Nail Polish Gel and Synthesis Process Thereof. CN Patent 105496817, 20 April 2016. [Google Scholar]
- Zhang, Y.; Chang, W.; Li, Y.; Xu, Y. Strippable Nail Polish Gel and Preparation Process Thereof. CN Patent 105534742, 4 May 2016. [Google Scholar]
- Zhou, X.; Simonnet, J.-T.; Bui, H.S.; Li, C.; Livingstone, E.C. Photo-Curable Resin for Cosmetic Application. Int. Patent Appl. 2014/105676, 3 July 2014. [Google Scholar]
- Bui, H.S.; Li, C.; Somonnet, J.-T.; Zhou, X.Z.; Lioutas, N.Z. Methods for Making Up a Keratinous Substrate. U.S. Patent 2013/0167859, 4 July 2013. [Google Scholar]
- Peng, X.; Liu, Y.; Xin, B.; Guo, H.; Yu, Y. Preparation and characterization of waterborne polyurethane nail enamel modified by silane coupling agent. J. Coat. Technol. Res. 2020, 17, 1377–1387. [Google Scholar] [CrossRef]
- Sin, S.M.; Jeon, Y.H.; Kuon, O.K. Gel Nail Sticker and Method for Manufacturing the Same. KR Patent 102208444, 28 January 2021. [Google Scholar]
- Kerai, L.V.; Hilton, S.; Murdan, S. UV-curable gel formulations: Potential drug carriers for the topical treatment of nail diseases. Int. J. Pharm. 2015, 492, 177–190. [Google Scholar] [CrossRef]
- Kerai, L.V.; Hilton, S.; Maugyeret, M.; Kazi, B.B.; Faull, J.; Bhakta, S.; Murdan, S. UV-curable gels as topical nail medicines: In vivo residence, anti-fungal efficacy and influence of gel components on their properties. Int. J. Pharm. 2016, 514, 244–254. [Google Scholar] [CrossRef]











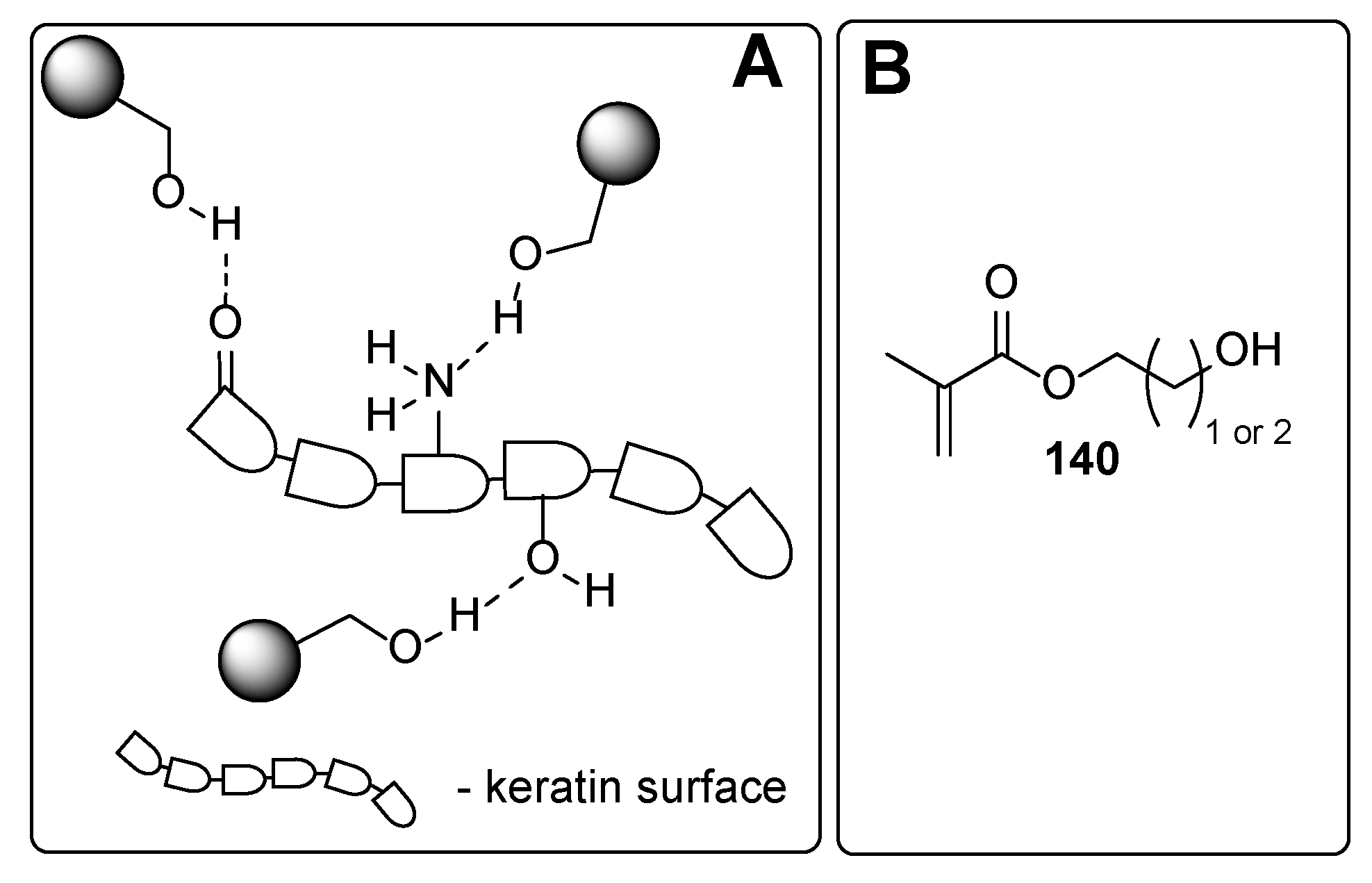
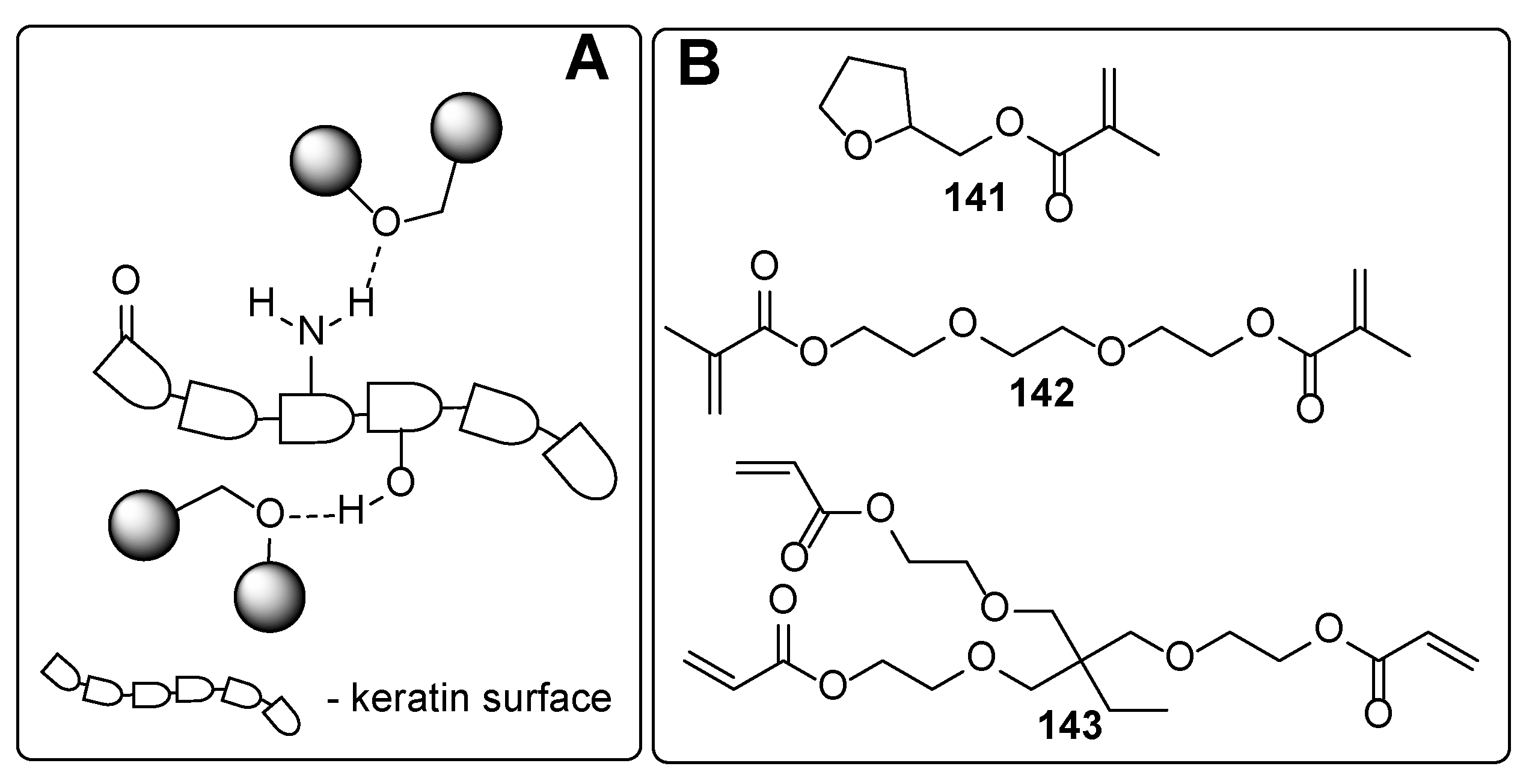

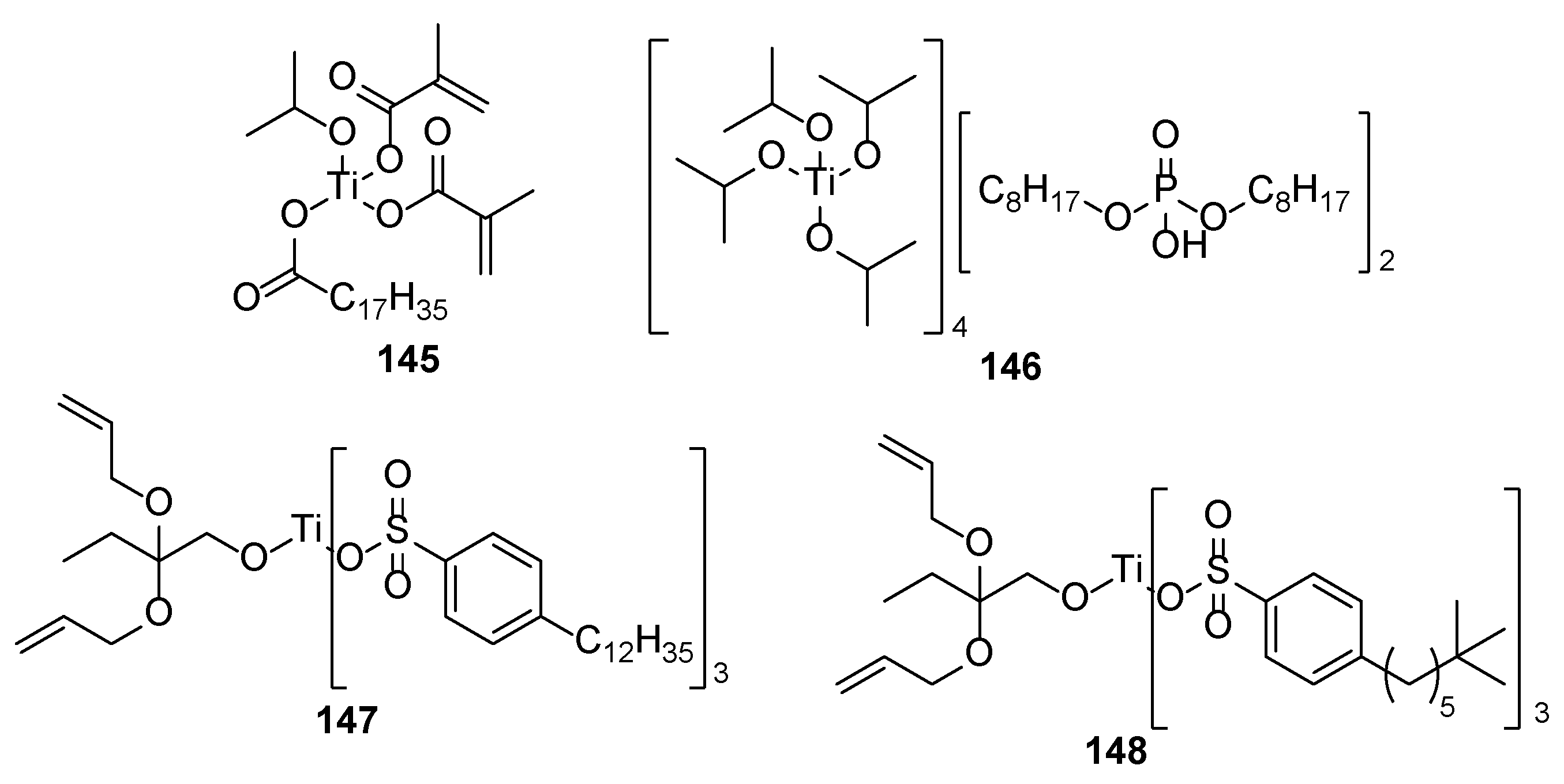


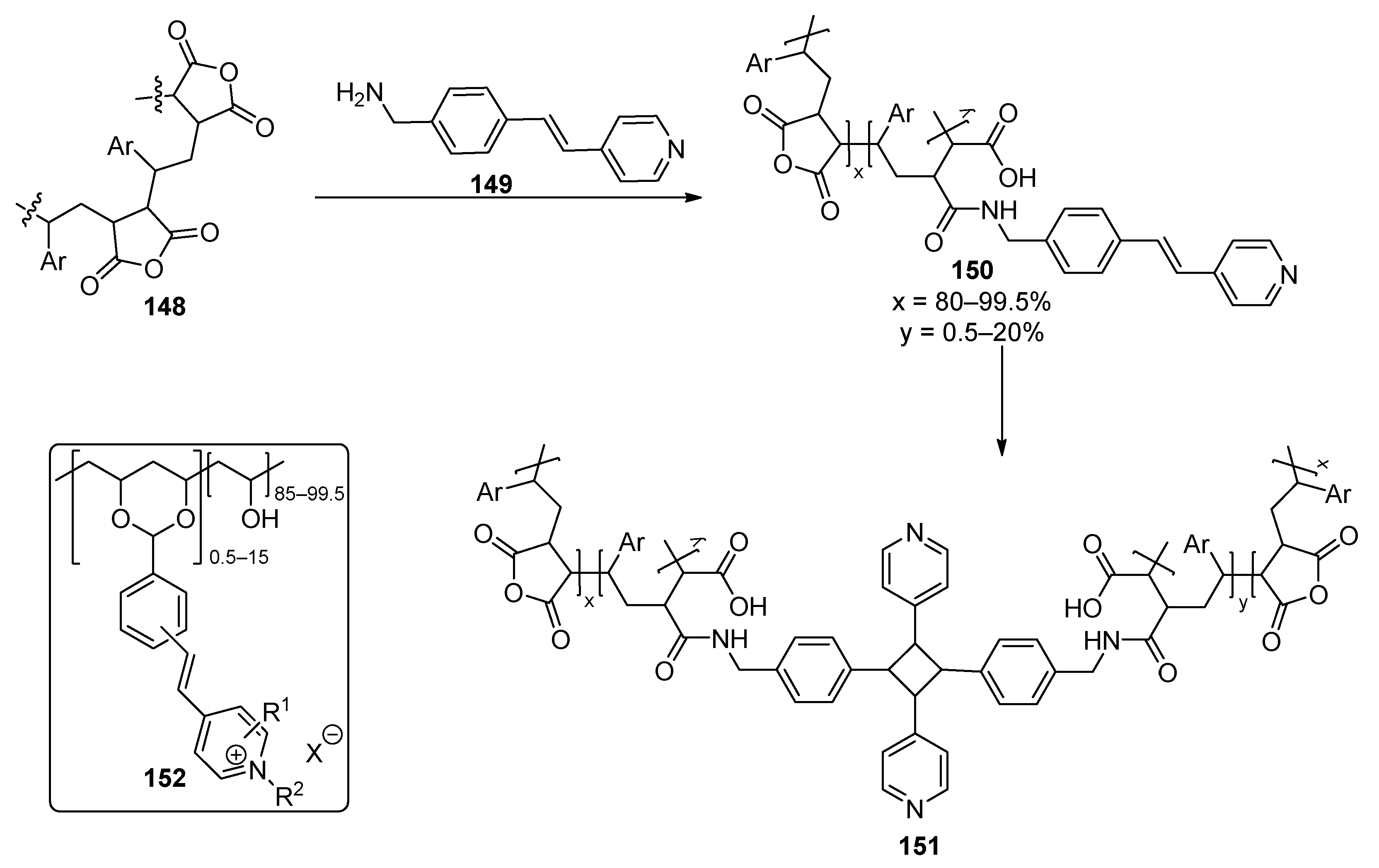
| Component | Advantages | Disadvantages |
|---|---|---|
| Acrylate-based compositions | ||
| (Meth)acrylate derived monomers and pre-polymers |
|
|
| Photoinitiators |
|
|
| Film-forming agents |
|
|
| Epoxide-based compositions | ||
| Epoxide moiety bearing monomers and pre-polymers |
|
|
| Photoinitiators |
|
|
| Thiole—ene compositions | ||
| Acrylates |
| |
| Thiols |
|
|
| Photoinitiators |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieriņa, I.; Grigale-Sorocina, Z.; Birks, I. The Chemistry of Behind the UV-Curable Nail Polishes. Polymers 2025, 17, 1166. https://doi.org/10.3390/polym17091166
Mieriņa I, Grigale-Sorocina Z, Birks I. The Chemistry of Behind the UV-Curable Nail Polishes. Polymers. 2025; 17(9):1166. https://doi.org/10.3390/polym17091166
Chicago/Turabian StyleMieriņa, Inese, Zane Grigale-Sorocina, and Ingmars Birks. 2025. "The Chemistry of Behind the UV-Curable Nail Polishes" Polymers 17, no. 9: 1166. https://doi.org/10.3390/polym17091166
APA StyleMieriņa, I., Grigale-Sorocina, Z., & Birks, I. (2025). The Chemistry of Behind the UV-Curable Nail Polishes. Polymers, 17(9), 1166. https://doi.org/10.3390/polym17091166






