Dual Flame-Retardant and Curing-Agent Effects of Phytic Acid–Guanazole as an Additive in Fire-Protective Coatings for Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of PG
2.2.2. Synthesis of KH550-Modified UF Resin
2.2.3. Fabrication of Flame-Retardant Wood Samples
2.3. Sample Characterization
2.3.1. Structural Characterization and Thermal Analysis
2.3.2. Flammability Tests
2.3.3. Post-Combustion Char Assessment
3. Results and Discussion
3.1. Synthesis and Characterization for PG and Flame-Retardant Coating Samples with Different Formulations
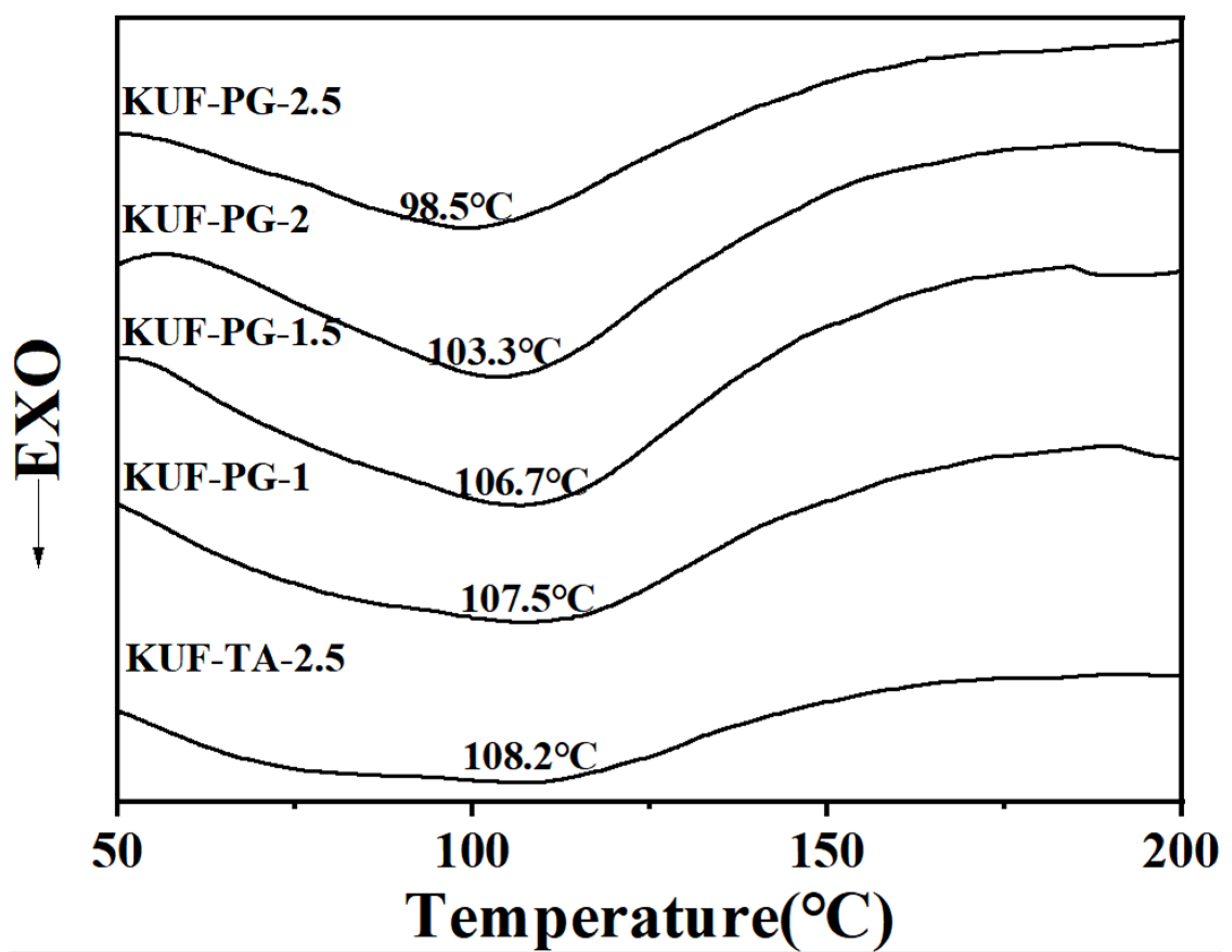
3.2. Thermal Performance of PGKUFs
3.3. Flame-Retardant Performance of PGKUFWs
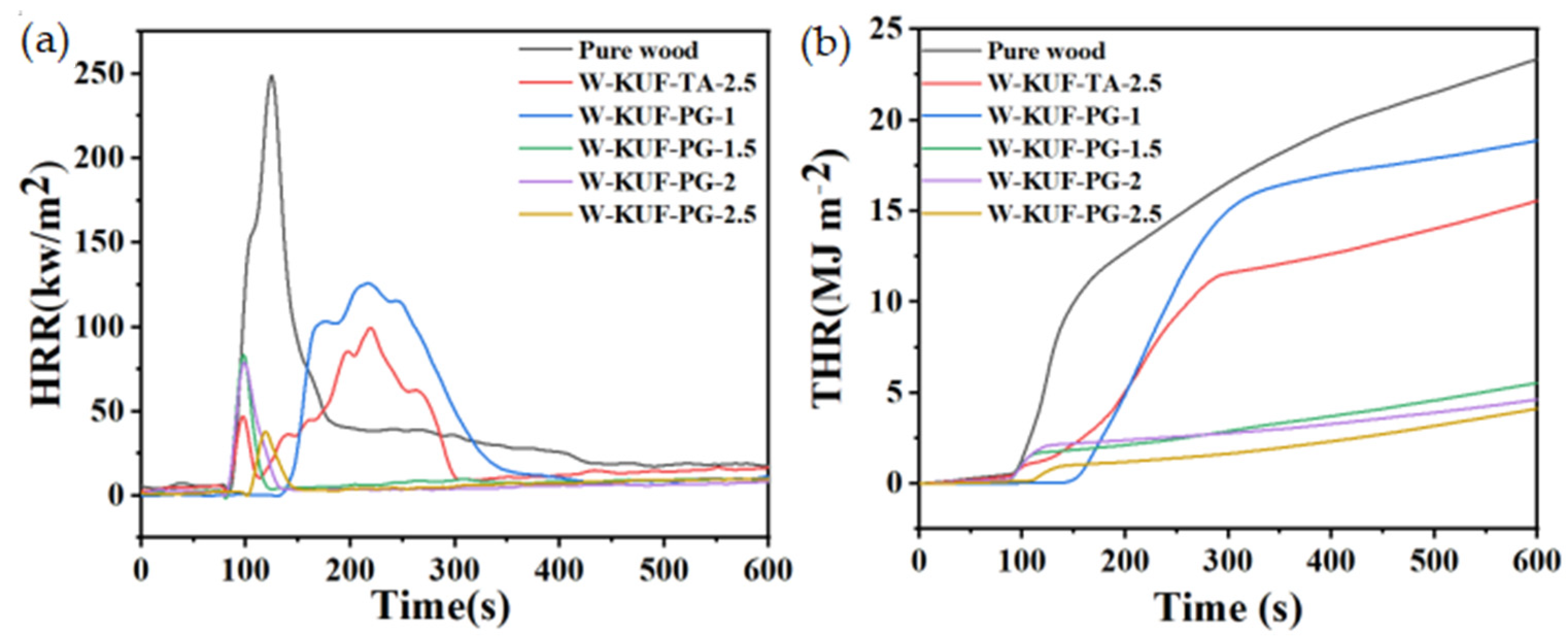
3.3.1. Heat Release Rate and Total Heat Release
3.3.2. Vertical Flammability and LOI Results
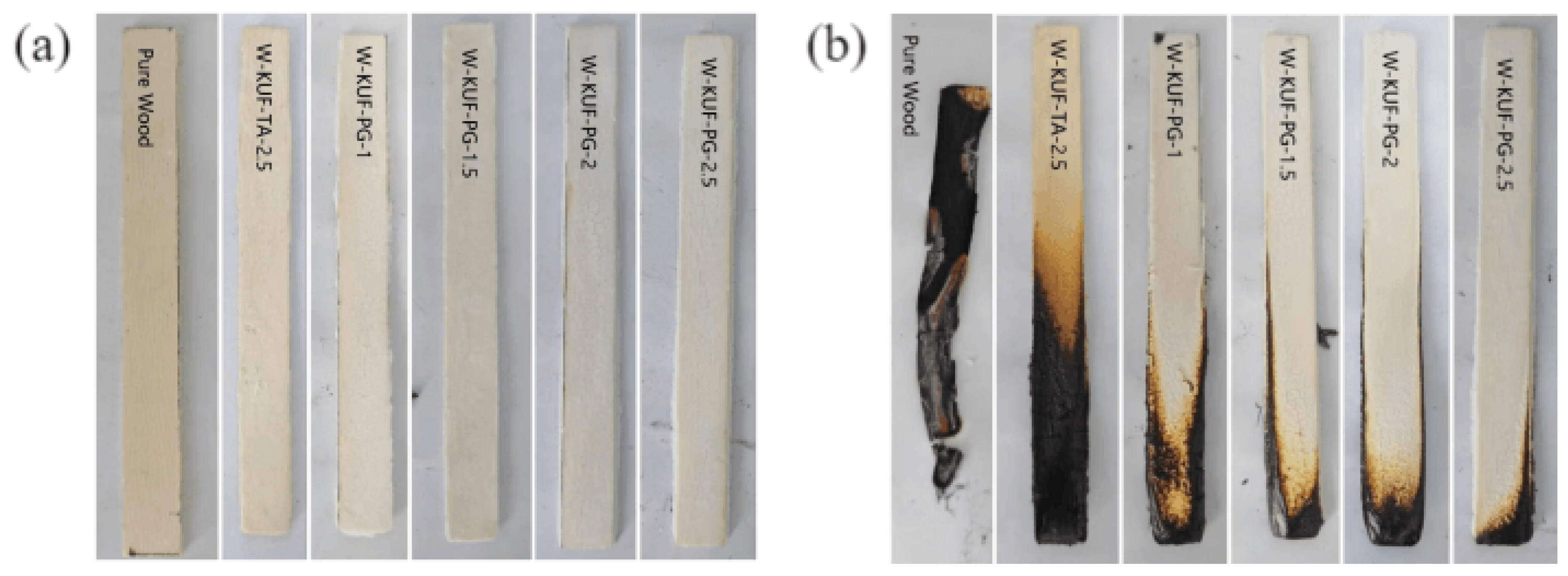
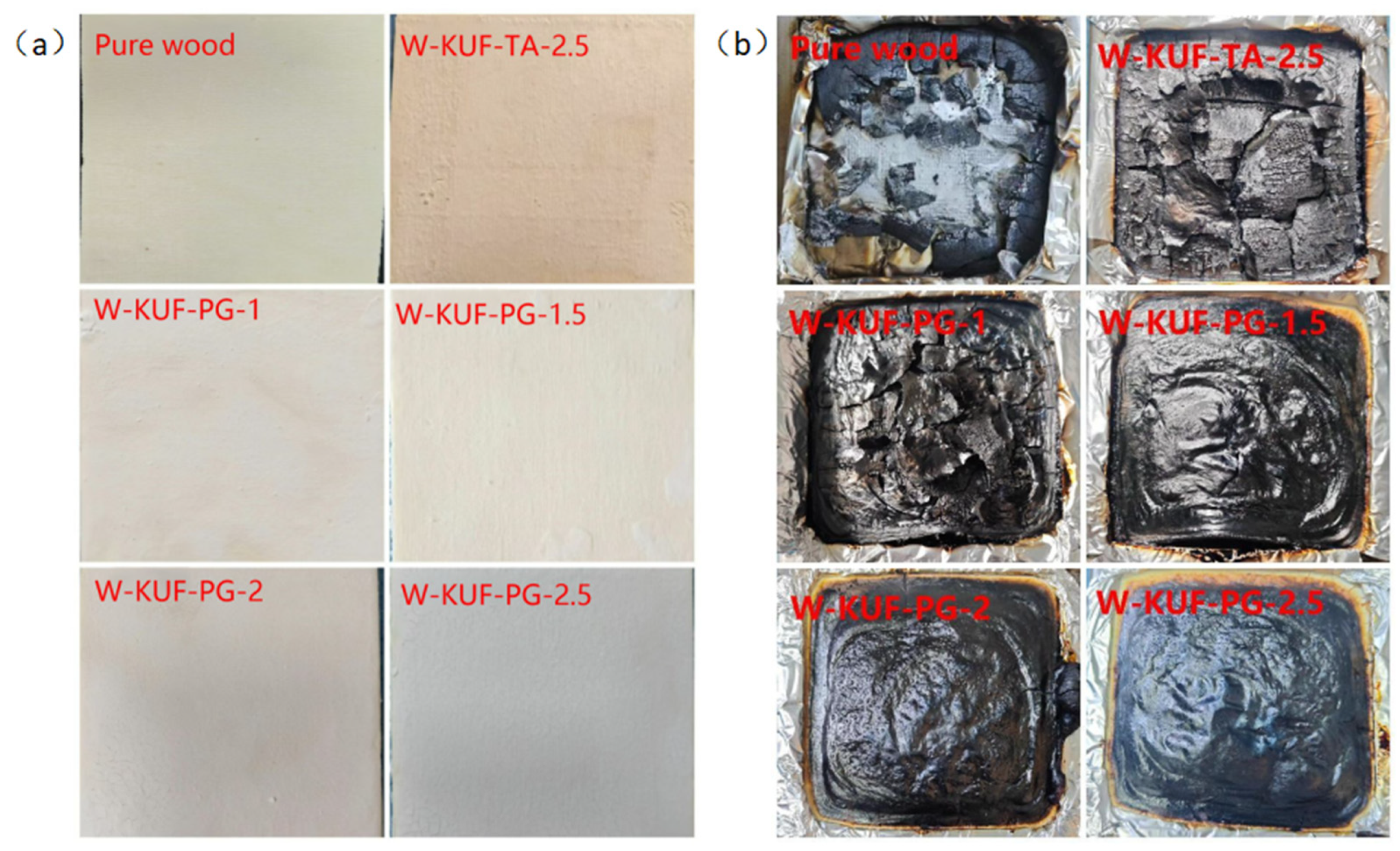
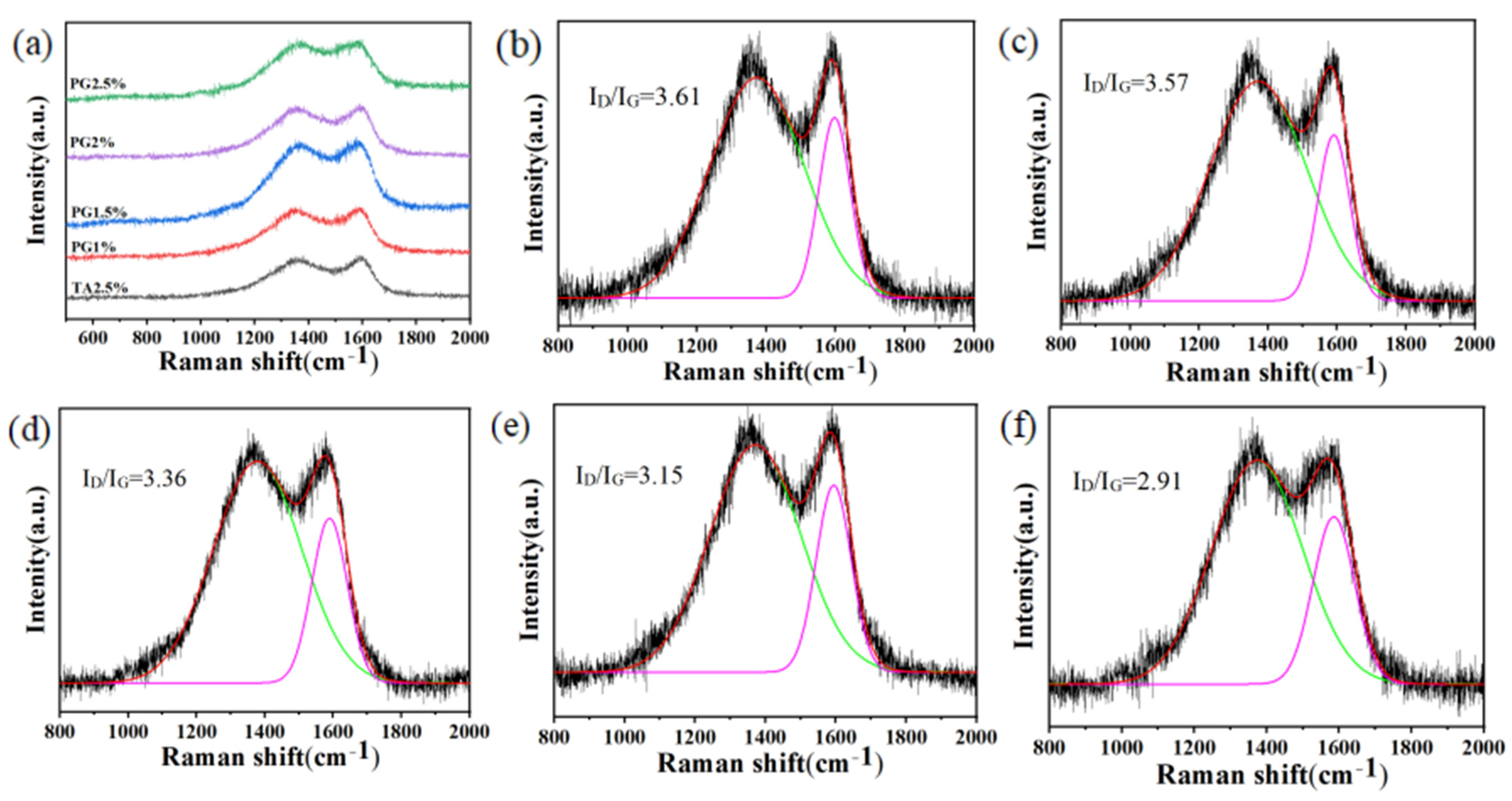
3.4. Analysis of Char Residue After Cone Calorimetry Combustion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, C.; Gao, Y.; Li, Y.; Yan, L.; Zhu, D.; Guo, S.; Ou, C.; Wang, Z. A flame-retardant densified wood as robust and fire-safe structural material. Wood Sci. Technol. 2022, 57, 111–134. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Chen, P. Epoxy-modified silicone resin based N/P/Si synergistic flame-retardant coating for wood surface. Prog. Org. Coat. 2022, 170, 106953. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Tang, Z.-C.; Lin, X.-Z.; Jin, J.; Li, Z.-Y.; Deng, C. Phosphorus/sulfur-containing aliphatic polyamide curing agent endowing epoxy resin with well-balanced flame safety, transparency and refractive index. Mater. Des. 2020, 187, 108417. [Google Scholar] [CrossRef]
- Gao, S.; Cheng, Z.; Zhou, X.; Liu, Y.; Chen, R.; Wang, J.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Unexpected role of amphiphilic lignosulfonate to improve the storage stability of urea formaldehyde resin and its application as adhesives. Int. J. Biol. Macromol. 2020, 161, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupe, A.; Molinari, F.; Eisenberg, P.; Escobar, M.M. Adhesive properties of urea-formaldehyde resins blended with soy protein concentrate. Adv. Compos. Hybrid Mater. 2020, 3, 213–221. [Google Scholar] [CrossRef]
- Cao, L.; Pizzi, A.; Zhang, Q.; Tian, H.; Lei, H.; Xi, X.; Du, G. Preparation and characterization of a novel environment-friendly urea-glyoxal resin of improved bonding performance. Eur. Polym. J. 2022, 162, 110915. [Google Scholar] [CrossRef]
- Song, F.; Liu, T.; Fan, Q.; Li, D.; Ou, R.; Liu, Z.; Wang, Q. Sustainable, high-performance, flame-retardant waterborne wood coatings via phytic acid based green curing agent for melamine-urea-formaldehyde resin. Prog. Org. Coat. 2022, 162, 106597. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, H.; Gao, Z.; Huang, Y.; Liang, J.; Zhou, H.; Sun, J. Preparation of waterborne intumescent flame-retardant coatings using adenosine-based phosphonates for wood surfaces. Prog. Org. Coat. 2024, 187, 108061. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Li, D.; He, S.; Zhang, F.; Zhang, G. A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr. Polym. 2017, 175, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, G. Biomacromolecules and Bio-Sourced Products for the Design of Flame Retarded Fabrics: Current State of the Art and Future Perspectives. Molecules 2019, 24, 3774. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Zhang, Q.-Y.; Cheng, B.-W.; Ren, Y.-L.; Zhang, Y.-G.; Ding, C. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 2018, 25, 799–811. [Google Scholar] [CrossRef]
- Gao, M.; Ling, B.; Yang, S.; Zhao, M. Flame retardance of wood treated with guanidine compounds characterized by thermal degradation behavior. J. Anal. Appl. Pyrolysis 2005, 73, 151–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, Y. Green synthesis of chitosan–phytic acid polymers and nanoparticles. Ind. Crops Prod. 2023, 199, 116747. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, J.; Zhong, Z.; Zhang, Y.; Zhang, F.; Wang, M. Synthesis of phytic acid-modified chitosan and the research of the corrosion inhibition and antibacterial properties. Int. J. Biol. Macromol. 2023, 253, 126905. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Ou, M.; Wang, S.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Preparation of a Highly Flame-Retardant Urea–Formaldehyde Resin and Flame Retardance Mechanism. Polymers 2024, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ji, S.; Zhang, J.; Liu, Q.; He, F.; Peng, S.; Li, Y. Tannic acid encountering ovalbumin: A green and mild strategy for superhydrophilic and underwater superoleophobic modification of various hydrophobic membranes for oil/water separation. J. Mater. Chem. A 2018, 6, 13959–13967. [Google Scholar] [CrossRef]

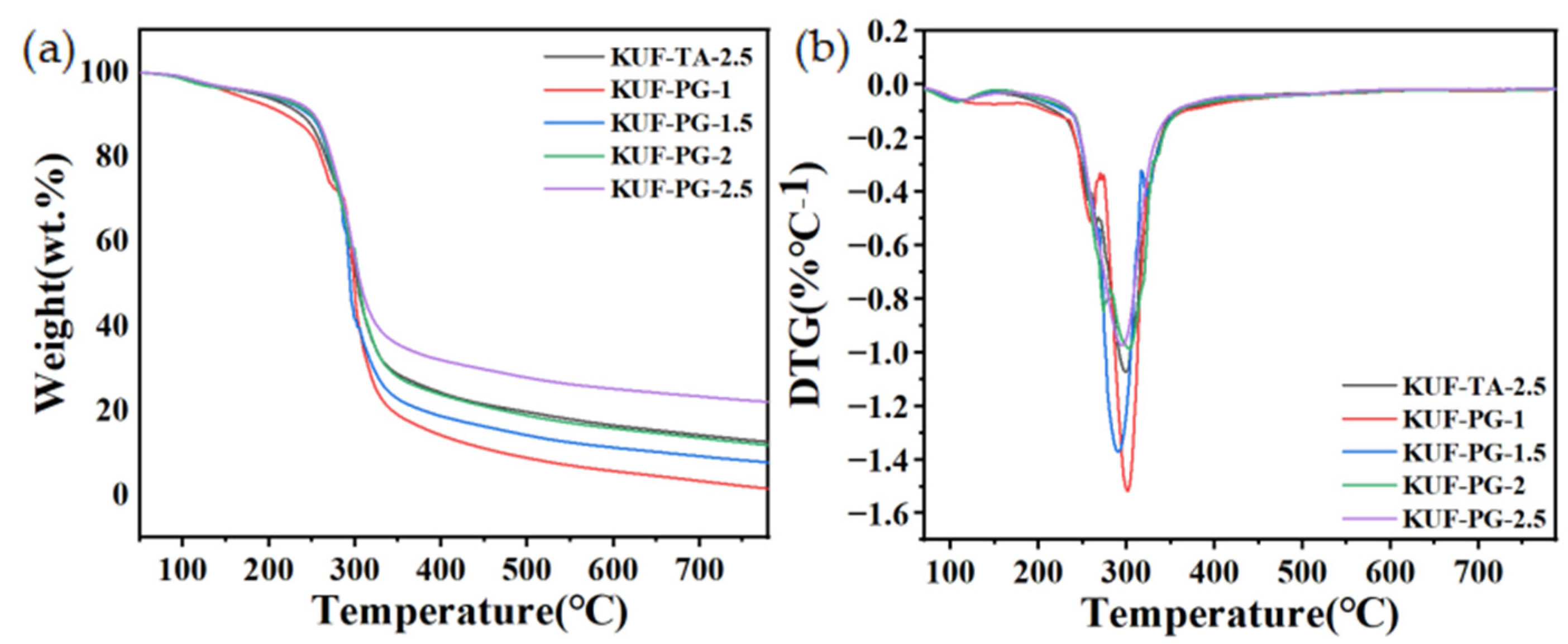

| Sample | Td5% (°C) | Tmax (°C) | R800 |
|---|---|---|---|
| KUF-TA-2.5 | 181.3 | 298.7 | 12.5 |
| KUF-PG-1 | 157.6 | 300.3 | 1.3 |
| KUF-PG-1.5 | 188.5 | 289.4 | 7.6 |
| KUF-PG-2 | 190.1 | 302.7 | 11.7 |
| KUF-PG-2.5 | 195.3 | 294.8 | 21.9 |
| Sample | LOI (%) | t1: After-Flame Time (1st Ignition) (s) | t2: Total After-Flame + Afterglow Time (2nd Ignition) (s) | Dripping Occurrence | Cotton Ignition | UL-94 Rating |
|---|---|---|---|---|---|---|
| Pure Wood | 20.5 | >60 | - | Yes | Yes | No |
| KUF-TA-2.5 | 31.5 | 0.9 | 34.7 | No | No | V-1 |
| KUF-PG-1 | 28.8 | 2.2 | 24.5 | No | No | V-1 |
| KUF-PG-1.5 | 29.9 | 1.4 | 12.3 | No | No | V-0 |
| KUF-PG-2 | 32.1 | 1.2 | 3 | No | No | V-0 |
| KUF-PG-2.5 | 32.7 | 1.2 | 1.3 | No | No | V-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zou, Y.; Xiang, C.; Wei, A.; Wei, Y.; Sun, L. Dual Flame-Retardant and Curing-Agent Effects of Phytic Acid–Guanazole as an Additive in Fire-Protective Coatings for Wood. Polymers 2025, 17, 1169. https://doi.org/10.3390/polym17091169
Zheng X, Zou Y, Xiang C, Wei A, Wei Y, Sun L. Dual Flame-Retardant and Curing-Agent Effects of Phytic Acid–Guanazole as an Additive in Fire-Protective Coatings for Wood. Polymers. 2025; 17(9):1169. https://doi.org/10.3390/polym17091169
Chicago/Turabian StyleZheng, Xue, Yongjin Zou, Cuili Xiang, An Wei, Yuhong Wei, and Lixian Sun. 2025. "Dual Flame-Retardant and Curing-Agent Effects of Phytic Acid–Guanazole as an Additive in Fire-Protective Coatings for Wood" Polymers 17, no. 9: 1169. https://doi.org/10.3390/polym17091169
APA StyleZheng, X., Zou, Y., Xiang, C., Wei, A., Wei, Y., & Sun, L. (2025). Dual Flame-Retardant and Curing-Agent Effects of Phytic Acid–Guanazole as an Additive in Fire-Protective Coatings for Wood. Polymers, 17(9), 1169. https://doi.org/10.3390/polym17091169





