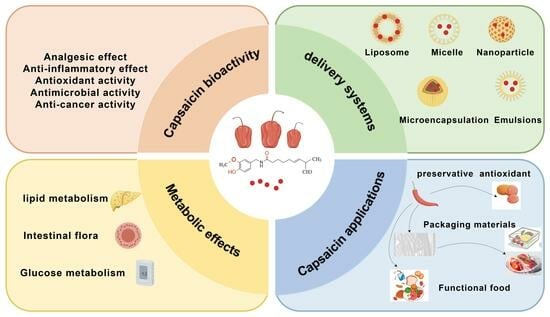
Recent Advances in the Applications and Studies of Polysaccharide-, Protein-, and Lipid-Based Delivery Systems in Enhancing the Bioavailability of Capsaicin—A Review
Abstract
1. Introduction
2. Bioactivities of CAP
2.1. Analgesic Effect
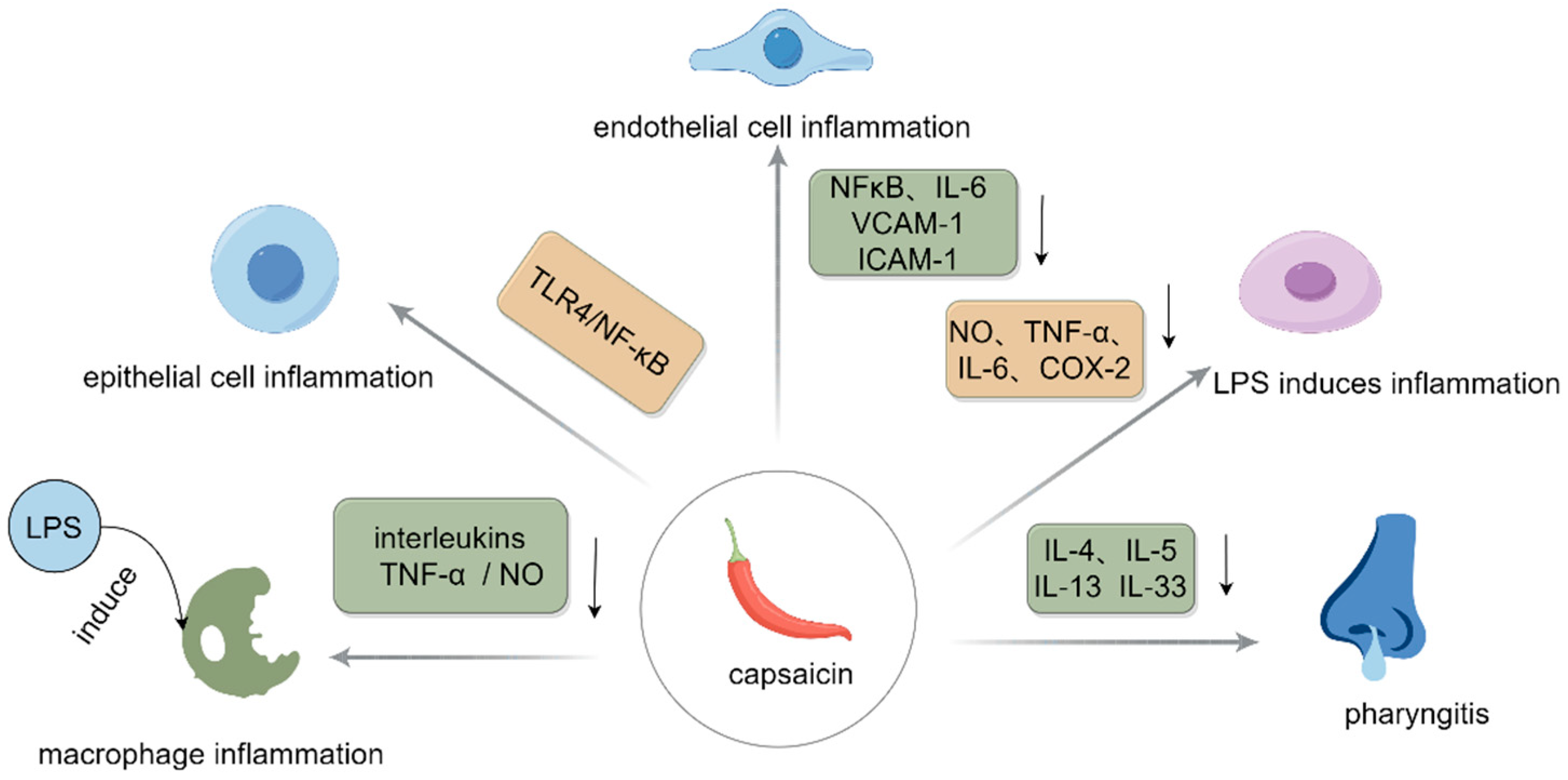
2.2. Anti-Inflammatory Effect
2.3. Antioxidant Activity
2.4. Antimicrobial Activity
2.5. Anticancer Activity
3. Impacts of CAP on In Vivo Metabolism
3.1. Impacts of CAP on Lipid Metabolic Pathways
3.2. Effects of CAP on the Mechanisms of Glucose Metabolism Pathways
3.3. Effects on Metabolic Pathways of Intestinal Flora
4. Novel Delivery Systems for CAP
4.1. Nanoliposomes
4.2. Nanoparticles
4.3. Emulsions
4.4. Micelles and Microcapsules
4.5. Other Delivery Systems
5. Various Applications for CAP
5.1. Application of CAP for Food Preservation
5.1.1. CAP Is Applied as a Preservative and Antioxidant for Food Preservation
5.1.2. CAP as a Plant Active Ingredient Added to Food Packaging Materials for Food Preservation
5.1.3. Preparation of Functional Foods
5.2. Applications of CAP in Combating Challenging Diseases
6. The Challenges Faced by CAP
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X-protein |
| BSH | bile salt hydrolase |
| COX-2 | cyclooxygenase-2 |
| CAT | catalase |
| CDK8 | cyclin-dependent kinase 8 |
| Cyt c | cytochrome C |
| CYP7A1 | protein cholesterol 7α-hydroxylase |
| CDCA | chenodeoxycholic acid |
| CAMKK2 | calmodulin-dependent protein kinase kinase 2 |
| cAMP | cyclic adenosine monophosphate |
| DCA | deoxycholic acid |
| FBI-1 | human immunodeficiency virus-1 |
| Fgf15 | fibroblast growth factor 15 |
| GSH | glutathione |
| GLUT1/2/4 | glucose transporter 1/2/4 |
| GLP-1 | glucagon-like peptide-1 |
| IL-4/5/6/13/13 | interleukin-4/5/6/13/33 |
| IL-1β | interleukin-1β |
| IRS1/2 | insulin receptor substrate 1/2 |
| ICAM-1 | intercellular cell adhesion molecule-1 |
| Junb | Jun B proto-oncogene |
| Ki-67 | proliferation-related protein |
| LPS | lipopolysaccharide |
| mTOR | mammalian target of rapamycin |
| mPTP | mitochondrial permeability transition pore |
| NF-kB | nuclear factor-kappa B |
| Nrf2 | NF-E2-related factor 2 |
| NOX4 | NADPH oxidase 4 |
| Nr4a3 | Nuclear Receptor Subfamily 4 Group A Member 3 |
| PI3K | phosphate inosine 3 kinase |
| PDX-1 | pancreatic duodenal homeobox 1 |
| PPAR-a/γ | peroxisome proliferator-activated receptor a/γ |
| PGC-1a | peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SIRT1 | silent information regulator 1 |
| SREBP-1c | sterol regulatory element binding protein-1c |
| SCFAs | short-chain fatty acids |
| TNF-a | tumor necrosis factor-alpha |
| TLR4 | toll-like receptor 4 |
| TRPV1 | transient receptor potential cation channel subfamily V member 1 |
| TC | total cholesterol |
| TG | triglyceride |
| T-β-MCA | tauro-β-muricholic acid |
| UCP2 | uncoupling protein 2 |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Kalaiyarasi, D.; Manobharathi, V.; Mirunalini, S. Pharmacological Properties and Health Benefits of Capsicum Species: A Comprehensive Review. In Capsicum; Orlex Baylen, Y., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. 3. [Google Scholar] [CrossRef]
- Jaiswal, V.; Gahlaut, V.; Kumar, N.; Ramchiary, N. Genetics, Genomics and Breeding of Chili Pepper Capsicum frutescens L. and Other Capsicum Species. In Advances in Plant Breeding Strategies: Vegetable Crops: Volume 9: Fruits and Young Shoots; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 59–86. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, D.; Zhang, L.; Zhao, C.; Shu, H.; Cheng, S.; Wang, Z.; Zhu, J.; Liu, P. Identification and expression analysis of capsaicin biosynthesis pathway genes at genome level in Capsicum chinense. Biotechnol. Biotechnol. Equip. 2022, 36, 232–244. [Google Scholar] [CrossRef]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Núñez-Mexía, S.A.; Cortez-Rocha, M.O.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Rosas-Durazo, A.d.J.; Parra-Vergara, N.V.; Plascencia-Jatomea, M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers 2022, 14, 2774. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fang, S.; Xie, Y.; Mei, J.; Xie, J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings 2024, 14, 338. [Google Scholar] [CrossRef]
- Shi, P.; Mei, J.; Xie, J. Impact of pretreatment sterilization techniques and ginger (Zingiber officinale roscoe) essential oil-based active packaging on the quality of crucian carp (Carassius auratus) during cold storage. J. Stored Prod. Res. 2025, 112, 102598. [Google Scholar] [CrossRef]
- Gálvez, R.; Navez, M.-L.; Moyle, G.; Maihöfner, C.; Stoker, M.; Ernault, E.; Nurmikko, T.J.; Attal, N. Capsaicin 8% Patch Repeat Treatment in Nondiabetic Peripheral Neuropathic Pain: A 52-Week, Open-Label, Single-Arm, Safety Study. Clin. J. Pain. 2017, 33, 921–931. [Google Scholar] [CrossRef]
- Goncalves, D.; Rebelo, V.; Barbosa, P.; Gomes, A. 8% capsaicin patch in treatment of peripheral neuropathic pain. Pain. Physician 2020, 23, E541. [Google Scholar] [CrossRef]
- Wang, S.; Bian, C.; Yang, J.; Arora, V.; Gao, Y.; Wei, F.; Chung, M.-K. Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. Eneuro 2020, 7, 2–12. [Google Scholar] [CrossRef]
- Tshering, G.; Posadzki, P.; Kongkaew, C. Efficacy and safety of topical capsaicin in the treatment of osteoarthritis pain: A systematic review and meta-analysis. Phytother. Res. 2024, 38, 3695–3705. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.-K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef]
- Olusanya, A.; Yearsley, A.; Brown, N.; Braun, S.; Hayes, C.; Rose, E.; Connolly, B.; Dicks, M.; Beal, C.; Helmonds, B. Capsaicin 8% patch for spinal cord injury focal neuropathic pain, a randomized controlled trial. Pain. Med. 2023, 24, 71–78. [Google Scholar] [CrossRef]
- Nathan, C. Nonresolving inflammation redux. Immunity 2022, 55, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Zhang, L.; An, N.; Ni, W.; Gao, Q.; Yu, Y. Capsaicin affects macrophage anti-inflammatory activity via the MAPK and NF-κB signaling pathways. Int. J. Vitam. Nutr. Res. 2023, 93, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, B.; Friesen, M.; Liu, S.; Zhu, C.; Yang, C. Capsaicin attenuates lipopolysaccharide-induced inflammation and barrier dysfunction in intestinal porcine epithelial cell line-J2. Front. Physiol. 2021, 12, 715469. [Google Scholar] [CrossRef] [PubMed]
- Thongin, S.; Den-Udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Wu, X.; Zhang, X.; Hu, C.; Kang, Y.; Lin, J.; Li, J.; Huang, Y.; Zhang, X. Enhanced anti-inflammatory effects of silibinin and capsaicin combination in lipopolysaccharide-induced RAW264. 7 cells by inhibiting NF-κB and MAPK activation. Front. Chem. 2022, 10, 934541. [Google Scholar] [CrossRef]
- Oner, F.; Kozan, G.; Kara, A. The Therapeutic Effect of Capsaicin and/or Steroids on Inflammation in an Experimental Allergic Rhinitis Model. Asthma Allergy Immunol. 2023, 21, 192–198. [Google Scholar] [CrossRef]
- Sattler, K.; El-Battrawy, I.; Cyganek, L.; Lang, S.; Lan, H.; Li, X.; Zhao, Z.; Utikal, J.; Wieland, T.; Borggrefe, M. TRPV1 activation and internalization is part of the LPS-induced inflammation in human iPSC-derived cardiomyocytes. Sci. Rep. 2021, 11, 14689. [Google Scholar] [CrossRef]
- Kilinc, Y.B.; Dilek, M.; Kilinc, E.; Torun, I.E.; Saylan, A.; Duzcu, S.E. Capsaicin attenuates excitotoxic-induced neonatal brain injury and brain mast cell-mediated neuroinflammation in newborn rats. Chem. Biol. Interact. 2023, 376, 110450. [Google Scholar] [CrossRef]
- Gangabhagirathi, R.; Joshi, R. Antioxidant activity of capsaicin on radiation-induced oxidation of murine hepatic mitochondrial membrane preparation. Res. Rep. Biochem. 2015, 5, 163–171. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Cheng, K.; Zhang, L.; Wang, T. Capsaicin alleviates the intestinal oxidative stress via activation of TRPV1/PKA/UCP2 and Keap1/Nrf2 pathways in heat-stressed mice. J. Funct. Foods 2023, 108, 105749. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gour, J.K.; Rizvi, S.I. Capsaicin has potent anti-oxidative effects in vivo through a mechanism which is non-receptor mediated. Arch. Physiol. Biochem. 2022, 128, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Zuñiga-Ramos, L.M.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin and sulforaphane prevent experimental liver fibrosis via upregulation of peroxisome proliferator-activated receptor gamma and nuclear factor (erythroid-derived 2)-like 2. J. Funct. Foods 2019, 52, 382–388. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Abdel-Rahman, R.F.; Sleem, A.A.; Farrag, A.R. Modulation of lipopolysaccharide-induced oxidative stress by capsaicin. Inflammopharmacology 2012, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wei, X.; Wu, Y.; Nan, S.; Feng, J.; Wang, F.; Yao, M.; Nie, C. Capsaicin Modulates Hepatic and Intestinal Inflammation and Oxidative Stress by Regulating the Colon Microbiota. Antioxidants 2024, 13, 942. [Google Scholar] [CrossRef]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial properties of capsaicin: Available data and future research perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Colina, J.; Guzmán-Rodríguez, L.; Sierra-Carmona, C.G.; Farías-Campomanes, Á.M.; García-Pinilla, S.; González-Tijera, M.M.; Malagón-Alvira, K.O.; Peredo-Lovillo, A. C apsicum fruits as functional ingredients with antimicrobial activity: An emphasis on mechanisms of action. J. Food Sci. Technol. 2023, 60, 2725–2735. [Google Scholar] [CrossRef]
- Jones, N.L.; Shabib, S.; Sherman, P.M. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol. Lett. 1997, 146, 223–227. [Google Scholar] [CrossRef]
- Zhai, X.; Ju, P.; Guan, F.; Ren, Y.; Liu, X.; Wang, N.; Zhang, Y.; Duan, J.; Wang, C.; Hou, B. Electrodeposition of capsaicin-induced ZnO/Zn nanopillar films for marine antifouling and antimicrobial corrosion. Surf. Coat. Technol. 2020, 397, 125959. [Google Scholar] [CrossRef]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Anticandidal activity of capsaicin and its effect on ergosterol biosynthesis and membrane integrity of Candida albicans. Int. J. Mol. Sci. 2023, 24, 1046. [Google Scholar] [CrossRef]
- Ardi Putra, F.R.; Irmawati, A. A review: A role of capsaicin to regulating T2R and TRPV1 and its association in cancer development. J. Biosains Pascasarj. 2023, 25, 90–91. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Li, H.X.; Hou, X.J.; Huang, M.Y.; Zhu, Z.M.; Wei, L.X.; Tang, C.X. Capsaicin suppresses hepatocarcinogenesis by inhibiting the stemness of hepatic progenitor cells via SIRT1/SOX2 signaling pathway. Cancer Med. 2022, 11, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, C. Capsaicin inhibits cell proliferation by enhancing oxidative stress and apoptosis through SIRT1/NOX4 signaling pathways in HepG2 and HL-7702 cells. J. Biochem. Mol. Toxicol. 2022, 36, e22974. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jia, H.; Zhang, Z.; Li, S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020, 22, 4868–4876. [Google Scholar] [CrossRef]
- Han, T.-H.; Park, M.K.; Nakamura, H.; Ban, H.S. Capsaicin inhibits HIF-1α accumulation through suppression of mitochondrial respiration in lung cancer cells. Biomed. Pharmacother. 2022, 146, 112500. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin induces mitochondrial dysfunction and apoptosis in anaplastic thyroid carcinoma cells via TRPV1-mediated mitochondrial calcium overload. Cell. Signal. 2020, 75, 109733. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, C.; Jiang, W.; Yang, W.; Qin, Q.; Tan, Q.; Lian, B.; Liang, Z.; Wei, C. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-κB pathway. Drug Des. Dev. Ther. 2021, 15, 125–140. [Google Scholar] [CrossRef]
- Takkem, A.; Zakaraia, S.; Silan, A.; Alghazawi, M.; Sahyouni, W.; Al-Manadili, A. The Apoptotic and Antiproliferative Effects of Capsaicin in the Developmental Stages of Oral Squamous Cell Carcinoma Induced in Hamsters. Cureus 2022, 14, e26073. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, W.; Zhang, X.; Mao, J.; Zhang, Q.; Zhao, W.; Zhang, S.; Xie, J. Recent advances in analysis of capsaicin and its effects on metabolic pathways by mass spectrometry. Front. Nutr. 2023, 10, 1227517. [Google Scholar] [CrossRef]
- Gong, T.; Wang, H.; Liu, S.; Zhang, M.; Xie, Y.; Liu, X. Capsaicin regulates lipid metabolism through modulation of bile acid/gut microbiota metabolism in high-fat-fed SD rats. Food Nutr. Res. 2022, 66, 8289. [Google Scholar] [CrossRef]
- Li, R.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.-T.; Lu, M. Capsaicin attenuates oleic acid-induced lipid accumulation via the regulation of circadian clock genes in HepG2 cells. J. Agric. Food Chem. 2021, 70, 794–803. [Google Scholar] [CrossRef]
- Bort, A.; Sánchez, B.G.; Mateos-Gómez, P.A.; Díaz-Laviada, I.; Rodríguez-Henche, N. Capsaicin targets lipogenesis in HepG2 cells through AMPK activation, AKT inhibition and PPARs regulation. Int. J. Mol. Sci. 2019, 20, 1660. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.-W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Dai, P. Capsaicin directly promotes adipocyte browning in the chemical compound-induced brown adipocytes converted from human dermal fibroblasts. Sci. Rep. 2022, 12, 6612. [Google Scholar] [CrossRef]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, L.; Xu, F.; Hui, Y.; Lu, H.; Liu, X. TRPV1 receptor-mediated hypoglycemic mechanism of capsaicin in streptozotocin-induced diabetic rats. Front. Nutr. 2021, 8, 750355. [Google Scholar] [CrossRef]
- Hui, S.; Liu, Y.; Chen, M.; Wang, X.; Lang, H.; Zhou, M.; Yi, L.; Mi, M. Capsaicin improves glucose tolerance and insulin sensitivity through modulation of the gut microbiota-bile acid-FXR axis in type 2 diabetic db/db mice. Mol. Nutr. Food Res. 2019, 63, 1900608. [Google Scholar] [CrossRef]
- Gong, T.; Wang, H.; Lei, J.; Jiang, L.; Yuan, M. Capsaicin Regulates Glucose Metabolism in Rats Fed with Dietary Fiber by Regulating Microbiota-Short Chain Fatty Acids. Indian. J. Pharm. Sci. 2023, 85, 1099–1109. [Google Scholar] [CrossRef]
- Hui, S.; Huang, L.; Wang, X.; Zhu, X.; Zhou, M.; Chen, M.; Yi, L.; Mi, M. Capsaicin improves glucose homeostasis by enhancing glucagon-like peptide-1 secretion through the regulation of bile acid metabolism via the remodeling of the gut microbiota in male mice. FASEB J. 2020, 34, 8558–8573. [Google Scholar] [CrossRef]
- Ferdowsi, P.V.; Ahuja, K.D.; Beckett, J.M.; Myers, S. Capsaicin and zinc promote glucose uptake in C2C12 skeletal muscle cells through a common calcium signalling pathway. Int. J. Mol. Sci. 2022, 23, 2207. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Kang, Z.-Q.; Hu, J.-L.; Chen, M.-Y.; Mao, Y.; Xie, L.-F.; Yang, N.; Liu, T.; Zhang, W.; Huang, W.-H. Effects of capsaicin on the hypoglycemic regulation of metformin and gut microbiota profiles in type 2 diabetic rats. Am. J. Chin. Med. 2022, 50, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, X.; Chen, Y.; Zhang, D.; Chen, D.; Chen, L.; Lin, J. Study on the effect of capsaicin on the intestinal flora through high-throughput sequencing. ACS Omega 2020, 5, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio 2017, 8, 10. [Google Scholar] [CrossRef]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef]
- Gong, T.; Zhou, Y.; Shi, Q.; Li, Y.; Wang, H.; Zhang, M.; Liao, L. Capsaicin modulates Akkermansia muciniphila abundance by enhancing MUCIN2 levels in mice fed with high-fat diets. Food Nutr. Res. 2023, 67, 9990. [Google Scholar] [CrossRef]
- Santos, E.A.; Silva, J.L.; Leocádio, P.C.; Andrade, M.E.R.; Queiroz-Junior, C.M.; Oliveira, N.S.; Alves, J.L.; Oliveira, J.S.; Aguilar, E.C.; Boujour, K. Cutaneous Application of Capsaicin Cream Reduces Clinical Signs of Experimental Colitis and Repairs Intestinal Barrier Integrity by Modulating the Gut Microbiota and Tight Junction Proteins. ACS Pharmacol. Transl. Sci. 2024, 12, 2143–2153. [Google Scholar] [CrossRef]
- Elmas, C.; Gezer, C. Anti-obesity Effects of Capsaicin via Gut Microbiota. In Capsaicinoids: From Natural Sources to Biosynthesis and Their Clinical Applications; Swamy, M.K., Kumar, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 409–426. [Google Scholar] [CrossRef]
- Waheed, A.; Arshad, L.; Tabassum, S.; Zahid, I.; Ahmed, H.; Akram, S.; Mushtaq, M. Chapter 29—Capsaicin. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 659–680. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Sahin, K.; Kucuk, O.; Orhan, C.; Sahin, E.; Fowler, K.; White, T.; Durkee, S.; Bellamine, A. Bioavailability of a Capsaicin Lipid Multi-Particulate Formulation in Rats. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 645–650. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial lipid-based carrier for non-invasive capsaicin delivery; manufacturing scale-up and human irritation assessment. Molecules 2020, 25, 5575. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. Eur. J. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, J.; Mu, Y.; Foda, M.F.; Han, H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials 2022, 284, 121520. [Google Scholar] [CrossRef] [PubMed]
- Abulencia, A.B.; Vidallon, M.L.P.; Almeda, R.A.; Salamanez, K.C.; Rodriguez, E.B. Rice bran phospholipid-based nanovesicles for enhanced oral and topical delivery of capsaicinoids. J. Drug Deliv. Sci. Technol. 2020, 60, 102005. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Adu-Frimpong, M.; Zou, Z.; Jin, Z.; Zhang, P.; Xue, Y.; Li, S.; Xu, Y.; Yu, J.; et al. HA/PLA Composite Nanoparticles for Enhanced Oral Bioavailability of Capsaicin: Fabrication, Characterization and in vitro-in vivo Evaluation. ChemistrySelect 2024, 9, e202303080. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Sui, Z.; Lu, W.; Corke, H. Soybean lecithin-stabilized oil-in-water (O/W) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. 2021, 338, 128071. [Google Scholar] [CrossRef]
- Oprea, I.; Fărcaș, A.C.; Leopold, L.F.; Diaconeasa, Z.; Coman, C.; Socaci, S.A. Nano-Encapsulation of Citrus Essential Oils: Methods and Applications of Interest for the Food Sector. Polymers 2022, 14, 4505. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Cheng, C.; McClements, D.J.; Chen, X.; Zou, L.; Liu, W. Encapsulation of hydrophobic capsaicin within the aqueous phase of water-in-oil high internal phase emulsions: Controlled release, reduced irritation, and enhanced bioaccessibility. Food Hydrocoll. 2022, 123, 107184. [Google Scholar] [CrossRef]
- Luo, N.; Ye, A.; Wolber, F.M.; Singh, H. Digestion behaviour of capsaicinoid-loaded emulsion gels and bioaccessibility of capsaicinoids: Effect of emulsifier type. Curr. Res. Food Sci. 2023, 6, 100473. [Google Scholar] [CrossRef]
- Xu, J. Development of Capsaicin-based Nanoemulsions: Structural Characterisation and In Vitro Evaluation. Master Thesis, University of Leeds, Leeds, UK, 2021. [Google Scholar]
- He, J.; Wu, X.; Xie, Y.; Gao, Y.; McClements, D.J.; Zhang, L.; Zou, L.; Liu, W.; He, J.; Wu, X.; et al. Capsaicin encapsulated in W/O/W double emulsions fabricated via ethanol-induced pectin gelling: Improvement of bioaccessibility and reduction of irritation. Int. J. Biol. Macromol. 2023, 235, 123899. [Google Scholar] [CrossRef]
- Bu, X.; Ji, N.; Dai, L.; Dong, X.; Chen, M.; Xiong, L.; Sun, Q. Self-assembled micelles based on amphiphilic biopolymers for delivery of functional ingredients. Trends Food Sci. Technol. 2021, 114, 386–398. [Google Scholar] [CrossRef]
- Bao, C.; Li, Z.; Liang, S.; Hu, Y.; Wang, X.; Fang, B.; Wang, P.; Chen, S.; Li, Y. Microneedle Patch Delivery of Capsaicin-Containing α-Lactalbumin Nanomicelles to Adipocytes Achieves Potent Anti-Obesity Effects. Adv. Funct. Mater. 2021, 31, 2011130. [Google Scholar] [CrossRef]
- Zuo, C.; Zhang, H.; Liang, S.; Teng, W.; Bao, C.; Li, D.; Hu, Y.; Wang, Q.; Li, Z.; Li, Y. The alleviation of lipid deposition in steatosis hepatocytes by capsaicin-loaded α-lactalbumin nanomicelles via promoted endocytosis and synergetic multiple signaling pathways. J. Funct. Foods 2021, 79, 104396. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Mu, H.; Liu, Y.; Zhang, Y.; Wang, Q.; Quintero, L.E.E.; Li, X.; Chen, S.; Gong, Y.; et al. The stability and spicy taste masking effect of capsaicin loaded α-lactalbumin micelles formulated in defatted cheese. Food Funct. 2022, 13, 12258–12267. [Google Scholar] [CrossRef]
- Du, M.; Zhou, L.; Wang, X.; Zhang, Z.; Chen, H.; Zhu, G.; Zhang, G. Synthesis and encapsulation of 1, 4-butanediol esters as energy storage phase change materials for overheating protection of electronic devices. J. Energy Storage 2022, 53, 105239. [Google Scholar] [CrossRef]
- Cui, F.; Zhang, H.; Wang, D.; Tan, X.; Li, X.; Li, Y.; Li, J.; Li, T. Advances in the preparation and application of microencapsulation to protect food functional ingredients. Food Funct. 2023, 14, 6766–6783. [Google Scholar] [CrossRef]
- Kulig, D.; Bobak, Ł.; Jarmoluk, A.; Szmaja, A.; Król-Kilińska, Ż.; Zimoch-Korzycka, A. Effect of Chemical Degradation of Sodium Alginate on Capsaicin Encapsulation. Molecules 2023, 28, 7844. [Google Scholar] [CrossRef]
- Cui, S.-F.; Wang, J.-W.; Li, H.-F.; Fang, R.; Yu, X.; Lu, Y.-J. Microencapsulation of Capsaicin in Chitosan Microcapsules: Characterization, Release Behavior, and Pesticidal Properties against Tribolium castaneum (Herbst). Insects 2023, 14, 27. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.; Liang, S.; Lu, Y.; Zheng, J.; Zhang, G.; Li, W.; Jiang, H. Encapsulation of Capsaicin in Whey Protein and OSA-Modified Starch Using Spray-Drying: Physicochemical Properties and Its Stability. Foods 2022, 11, 612. [Google Scholar] [CrossRef]
- Razzak, M.A.; Cho, S.-J. Physicochemical and functional properties of capsaicin loaded cricket protein isolate and alginate complexes. J. Colloid. Interface Sci. 2023, 641, 653–665. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Higuchi, Y. One-Step Encapsulation of Capsaicin into Chitosan–Oleic Acid Complex Particles: Evaluation of Encapsulation Ability and Stability. Polymers 2022, 14, 2163. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, J.; Liu, Y.; Wang, S. Development of pea protein isolate-based complexes as a novel delivery system for capsaicin. Food Hydrocoll. 2024, 149, 109542. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhao, R.; Tian, Z.; Zhang, L.; Yu, Q.; Chen, C. Effect of pomegranate peel extract on the reduction of heterocyclic amines and quality characteristics of mutton meatballs. Food Qual. Saf. 2023, 7, fyac073. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Sathiyaseelan, A.; Saravanakumar, K.; Hu, X.; Wang, M.-H. Edible treatments of Capsicum extracts inactivate the microbial contaminations to improve the quality of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). J. Food Process. Preserv. 2020, 44, e14977. [Google Scholar] [CrossRef]
- Ehsanur Rahman, S.M.; Islam, S.; Pan, J.; Kong, D.; Xi, Q.; Du, Q.; Yang, Y.; Wang, J.; Oh, D.-H.; Han, R. Marination ingredients on meat quality and safety—A review. Food Qual. Saf. 2023, 7, fyad027. [Google Scholar] [CrossRef]
- Jeong, K.-J.; Seo, J.-K.; Ahamed, Z.; Su Lee, Y.; Yang, H.-S. Paprika extract as a natural antioxidant in cold-stored pork patties: Effect on oxidative stability and heterocyclic amines inhibition. Food Chem. X 2023, 20, 100936. [Google Scholar] [CrossRef]
- Oh, Y.-N.; Choi, H.-Y.; Kim, Y.-B.; Hong, S.-G.; Kim, H.-Y. Effect of paprika powder on the antioxidant capacity of emulsion-type sausages. Food Sci. Anim. Resour. 2024, 44, 1126. [Google Scholar] [CrossRef]
- Zaher, H.; El-Sherbiny, H.; El-Khateeb, A.Y.; Elkenany, R.; Zakaria, A. Preservative Effect of Selected Natural Flavoring Spices and Their Extracts on Microbial Quality of Meatballs During Cold Storage. Egypt. J. Vet. Sci. 2023, 54, 997–1014. [Google Scholar] [CrossRef]
- Wu, R.; Xie, Y.; Zhao, L.; Fu, C.; He, W.; Guo, D.; Xu, W.; Yi, Y.; Wang, H. Effect mechanism of capsaicin and dihydrocapsaicin in chili on the oxidative stability of myoglobin in duck meat. J. Sci. Food Agric. 2024, 104, 6799–6808. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Su, X.; Yang, Z.; Tan, K.B.; Chen, J.; Huang, J.; Li, Q. Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, F.; Xu, W.; Han, X. Enhanced antibacterial performance of gelatin/chitosan film containing capsaicin loaded MOFs for food packaging. Appl. Surf. Sci. 2020, 510, 145418. [Google Scholar] [CrossRef]
- Rather, J.A.; Kaur, G.; Shah, I.A.; Majid, D.; Makroo, H.A.; Dar, B.N. Sustainable gelatin-based packaging with nanoemulsified chilli seed oil for enhancing poultry meat preservation: An eco-friendly approach. Food Chem. Adv. 2024, 5, 100761. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Hamishehkar, H.; Ehsani, A.; Ghasempour, Z.; Moghaddas Kia, E. Applications of capsaicin in food industry: Functionality, utilization and stabilization. Crit. Rev. Food Sci. Nutr. 2023, 63, 4009–4025. [Google Scholar] [CrossRef]
- Jooyandeh, H.; Alizadeh Behbahani, B. Development of a probiotic low-fat set yogurt containing concentrated sweet pepper extract. Food Sci. Nutr. 2024, 12, 4656–4666. [Google Scholar] [CrossRef]
- Goktas, H.; Baycar, A.; Konar, N.; Yaman, M.; Sagdic, O. Using paprika extract in chocolate spread and white compound chocolate: Effects on color stability and bioavailability. J. Food Meas. Charact. 2023, 17, 3403–3412. [Google Scholar] [CrossRef]
- Kim, Y.K. Quality Improvement of the chicken sausage with pepper seed (Capsicum annuum L.). Curr. Res. Nutr. Food Sci. 2020, 8, 829–836. [Google Scholar] [CrossRef]
- Avci, E.; Tekin-Cakmak, Z.H.; Ozgolet, M.; Karasu, S.; Kasapoglu, M.Z.; Ramadan, M.F.; Sagdic, O. Capsaicin Rich Low-Fat Salad Dressing: Improvement of Rheological and Sensory Properties and Emulsion and Oxidative Stability. Foods 2023, 12, 1529. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin—The major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Nutr. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Merritt, J.C.; Richbart, S.D.; Moles, E.G.; Cox, A.J.; Brown, K.C.; Miles, S.L.; Finch, P.T.; Hess, J.A.; Tirona, M.T.; Valentovic, M.A. Anti-cancer activity of sustained release capsaicin formulations. Pharmacol. Ther. 2022, 238, 108177. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Andretta, E.; Costa, A.; Ventura, E.; Quintiliani, M.; Damiano, S.; Giordano, A.; Morrione, A.; Ciarcia, R. Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients 2024, 16, 3758. [Google Scholar] [CrossRef] [PubMed]
- Vel, S.K.; Ramakrishnan, A.; Sindya, J.; Rajanathadurai, J.; Perumal, E. Evaluation of cytotoxic and anti-cancer potential of capsaicin on lung cancer cell line: An in vitro investigation. Cureus 2024, 16, e68119. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.D.; Iadarola, M.J. Standing out from the crowd in treating COVID-19. Med. Drug Discov. 2020, 6, 100034. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Wang, Y.-C.; Lin, Y.W.; Chou, S.-Y.; Chen, S.-F.; Liu, L.T.; Wu, Y.-T.; Kuo, C.-J.; Chen, T.S.-S.; Juang, S.-H. Synthesis and evaluation of isatin derivatives as effective SARS coronavirus 3CL protease inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 3058–3062. [Google Scholar] [CrossRef]
- Cortés-Ferré, H.E.; Guajardo-Flores, D.; Romero-De La Vega, G.; Gutierrez-Uribe, J.A. Recovery of Capsaicinoids and Other Phytochemicals Involved With TRPV-1 Receptor to Re-valorize Chili Pepper Waste and Produce Nutraceuticals. Front. Sustain. Food Syst. 2021, 4, 588534. [Google Scholar] [CrossRef]
- Nahama, A.; Ramachandran, R.; Cisternas, A.F.; Ji, H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A review. Med. Drug Discov. 2020, 5, 100033. [Google Scholar] [CrossRef]




| Model Organism | Treatment | Results | References |
|---|---|---|---|
| BALB/c mice | Mouse peritoneal macrophages were isolated and activated for 24 h with 1, 2, 5, or 100 μg/mL of CAP and 1 μg/mL of LPS. | Decreased release cytokines that were conductive to the inflammatory response, including IL-6, TNF-α, and NO. | [14] |
| IPEC-J2 cell line | After 2 weeks of culturing IPECJ2 cells, they were incubated with different concentrations of CAP ranging from 0 to 300 uM for 24 h. | Reduced LPS-induced protein expression of extracellular signaling-related kinase 1/2 and p65. | [15] |
| Human Umbilical Vein Endothelial Cells (HUVECs) | For 30 min, HUVECs were exposed to CAP or dihydrocapsaicin at several doses (0, 5, 25, and 50 µM), respectively. | Reduced gene expression and secretion of pro-inflammatory cytokines induced by LPS via the TLR4/NF-κB signaling pathway. | [16] |
| RAW264.7 cells | Cultivated RAW264.7 cells for 24 h, then treated with different concentrations of silymarin and CAP. | Nutrient transport protein mRNA abundance was upregulated (e.g., Na+/glucose cotransporter 1). | [17] |
| Male Wistar | For seven days, intraperitoneal injections of CAP (50 mg/kg) and steroids (10 mg/kg) were given to rats. | Mitigated the activation of NF-κB and its molecular targets in endothelial cells mediated by TNF-α. | [18] |
| Human-induced pluripotent stem cell (iPSC)-derived cardiomyocytes (hiPSC-CM) | Incubated cells with media containing the agonist CAP (10 μM). | Significantly reduced monocytes’ adherence to the surface of endothelial cells. | [19] |
| Male and female rat pups, five days old (P5) | Intraperitoneal injections of 0.2, 1, and 5 mg/kg of CAP were given to P5 rat pups. | Induced NO production. | [20] |
| Model Organism | CAP Dosage | Mechanism of Action | References |
|---|---|---|---|
| Wistar female rats, aged three to four months | 5–50 µM | Shielded the antioxidant enzyme SOD from oxidative damage brought on by radiation. Inhibition of the endogenous antioxidant GSH depletion caused by radiation. | [21] |
| Male C57BL/6 J mice | 0.4 mg/day for 15 days | Enhancing the antioxidant capacity of the intestine via the pathways TRPV1/PKA/UCP2 and Keap1/Nrf2. | [22] |
| Male adult (8 weeks old) Wistar rats | 2 mg/k bw | Enhancement of Nrf2 protein expression and antioxidant activity of SOD and CAT enzymes in the serum. | [24] |
| Female Sprague–Dawley rats | 150–1500 µg/k bw | Increased hepatic GSH values. Reduced serum nitric oxide and protected the liver and lungs from LPS-induced tissue damage. | [25] |
| Male Kunming mice | 7.5 mg/k bw | Reduced malondialdehyde levels and increased expression of glutathione peroxidase, SOD, and CAT in the liver. | [26] |
| Model Organism | Treatment | Mechanism of Action | Results | References |
|---|---|---|---|---|
| Diethylnitrosamine-induced hepatocellular carcinoma model in rats | Low-dose group: 1 mg/kg liposomal CAP twice a week High dose group: 2 mg/kg liposomal CAP, subcutaneous injection for 4–6 weeks. | Hepatocellular carcinogenesis was decreased when the SIRT1/SOX2 signaling pathway was used to inhibit the stemness of HPCs. | Reduced SIRT1 and SOX2 protein levels. Apoptosis in HepG2 cells. | [33] |
| Cell line MDA-MB-231 | Cells were grown at distinct CAP doses (0, 10, 50, 100, and 200 uM) and at 37 °C with 5% CO2 for 48 h. | Reduced CDK8 expression and caused G2/M cell cycle arrest through the restraint of the Wnt/β-catenin/PI3K/CDK8/Akt signaling path. | G2/M cell cycle blocked. The levels of CDK8, PI3K, and Akt expression were lowered. Wnt and β-conjugated protein expression was downregulated. | [35] |
| A549 cell line H1299 cell line H2009 cell line H23 cell line | Injections of CAP (20, 50, or 100 µM) treatments. | Inhibiting the mitochondrial respiration of lung cancer cells reduced ATP production and the accumulation of HIF-1α, thereby suppressing cancer cell proliferation. | Inhibited the buildup of HIF-1α protein. ATP synthesis in cellular mitochondria was reduced and, therefore, cancer cell growth was inhibited. | [36] |
| Anaplastic thyroid cancer cell 8505C | Cells received treatment with 50–200 uM CAP for 24 h. | Through a TRPV1-mediated mechanism, CAP caused mitochondrial calcium excess and death in anaplastic thyroid carcinoma (ATC) cells. | Mesenchymal thyroid cancer cells exhibited both apoptosis and mitochondrial calcium excess. | [37] |
| Human breast cancer cell lines (MCF-7 and MDA-MB-231) | After 24 h of cell attachment culture, treat with different concentrations of CAP. | By suppressing the NF-kB signaling pathway mediated by FBI-1, CAP impeded the growth of breast cancer cells. | Decreased expression of the proteins FBI-1, Ki-67, Bcl-2, and survivin. Inhibited cell proliferation. | [38] |
| Syrian hamsters | Control group: oral cancer was triggered by DMBA alone, excluding CAP. Experimental group: tumor induction with dimethylbenzanthracene followed by CAP application in the digestive tract. | Reduced the expression of the proteins Bcl-2 and ki-67, which in turn triggered apoptosis and stopped the development of cancer cells. | Encourage the death of malignant cells. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Xie, J.; Mei, J. Recent Advances in the Applications and Studies of Polysaccharide-, Protein-, and Lipid-Based Delivery Systems in Enhancing the Bioavailability of Capsaicin—A Review. Polymers 2025, 17, 1196. https://doi.org/10.3390/polym17091196
Qiu X, Xie J, Mei J. Recent Advances in the Applications and Studies of Polysaccharide-, Protein-, and Lipid-Based Delivery Systems in Enhancing the Bioavailability of Capsaicin—A Review. Polymers. 2025; 17(9):1196. https://doi.org/10.3390/polym17091196
Chicago/Turabian StyleQiu, Xiang, Jing Xie, and Jun Mei. 2025. "Recent Advances in the Applications and Studies of Polysaccharide-, Protein-, and Lipid-Based Delivery Systems in Enhancing the Bioavailability of Capsaicin—A Review" Polymers 17, no. 9: 1196. https://doi.org/10.3390/polym17091196
APA StyleQiu, X., Xie, J., & Mei, J. (2025). Recent Advances in the Applications and Studies of Polysaccharide-, Protein-, and Lipid-Based Delivery Systems in Enhancing the Bioavailability of Capsaicin—A Review. Polymers, 17(9), 1196. https://doi.org/10.3390/polym17091196