Poly(propylene fumarate) Composite Scaffolds for Bone Tissue Engineering: Innovation in Fabrication Techniques and Artificial Intelligence Integration
Abstract
1. Introduction
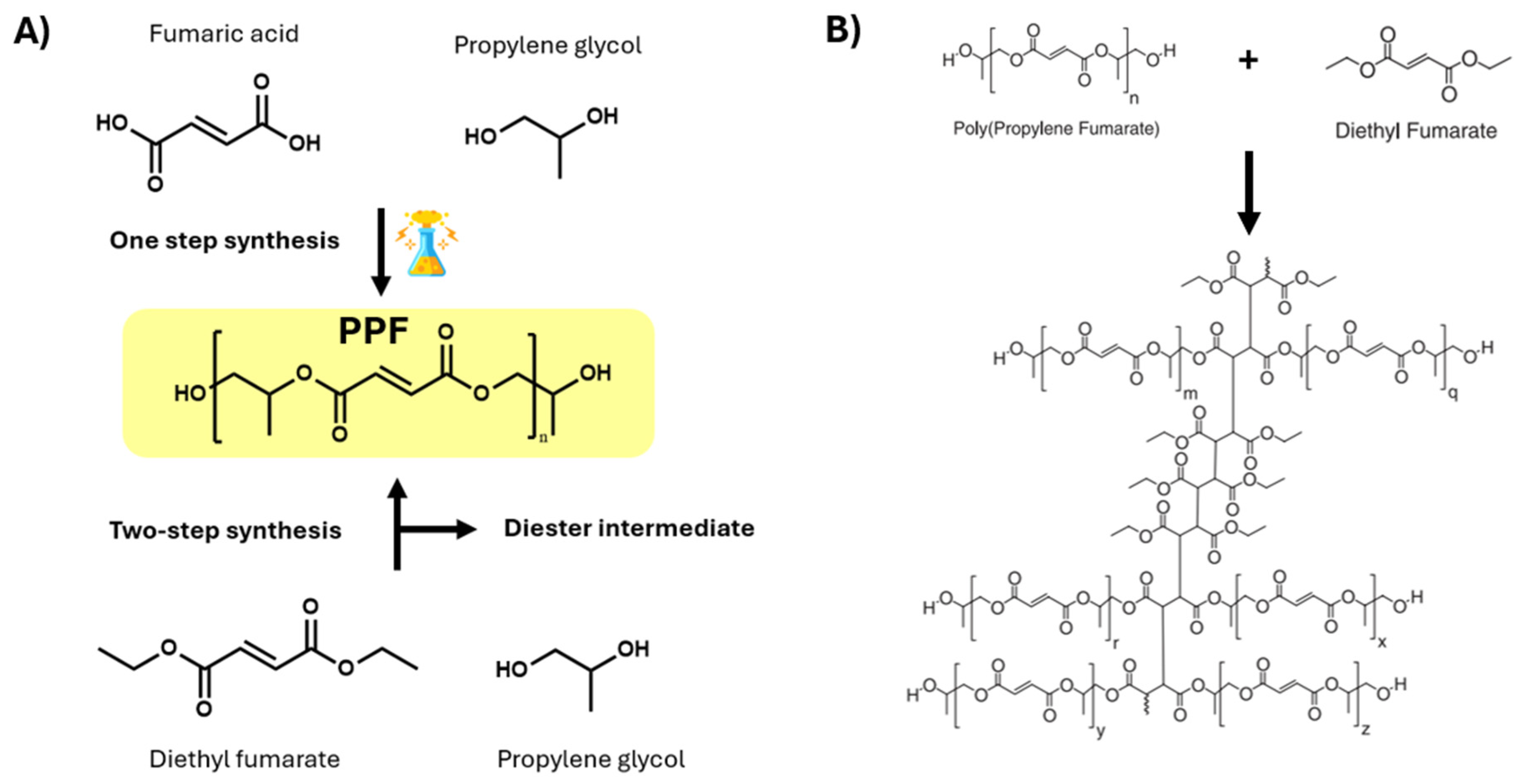
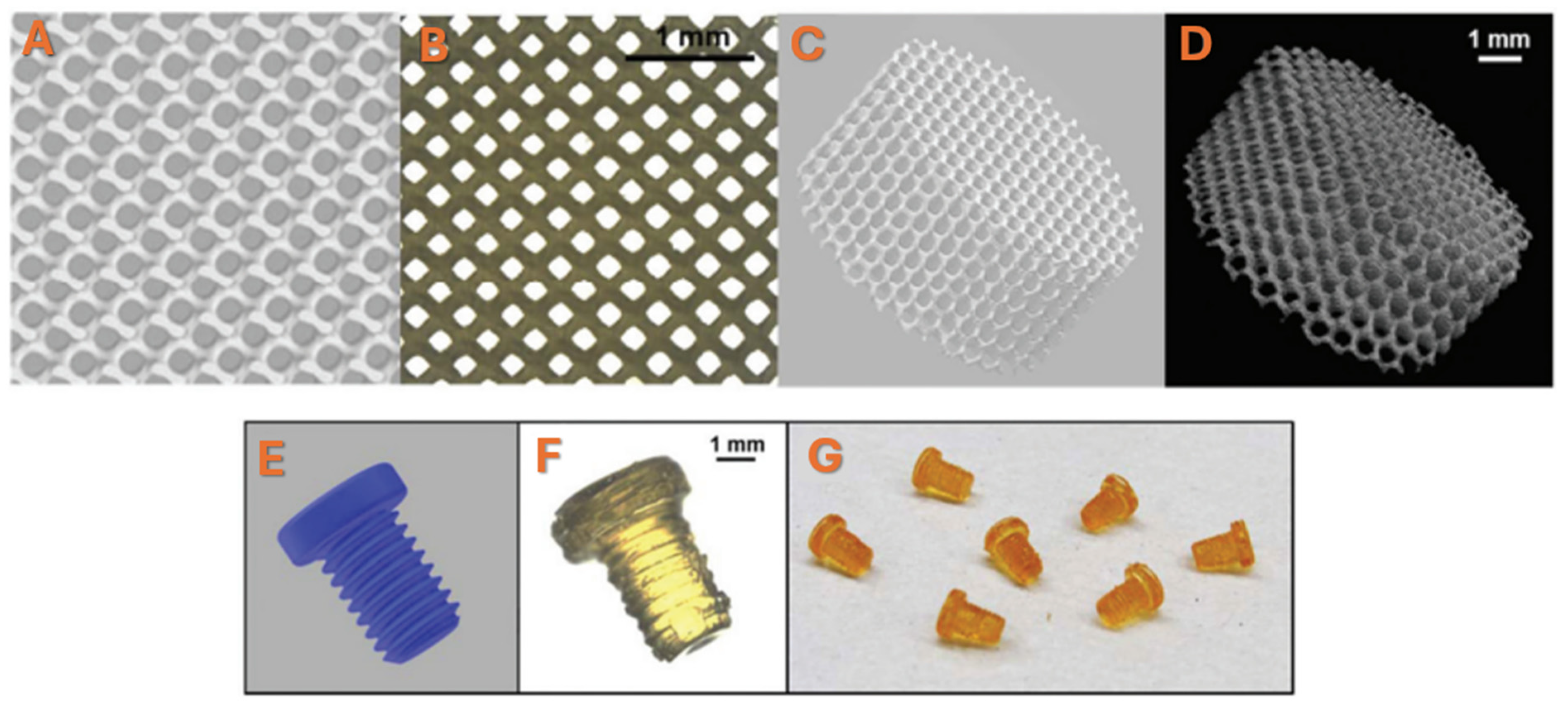
2. Fabrication of Scaffolds for Tissue Engineering Based on PPF
3. Synthesis of Bone Tissue Engineering Composite Scaffolds Based on PPF
- Inorganic Substances
- Carbonaceous Nanostructures
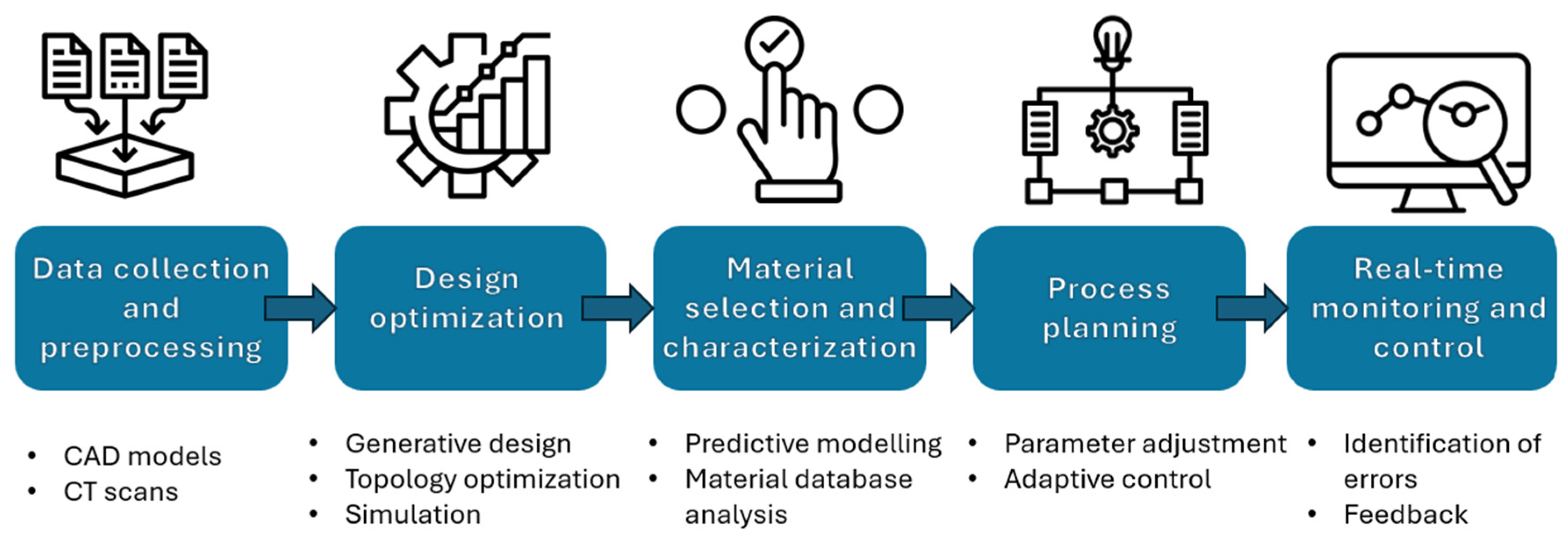
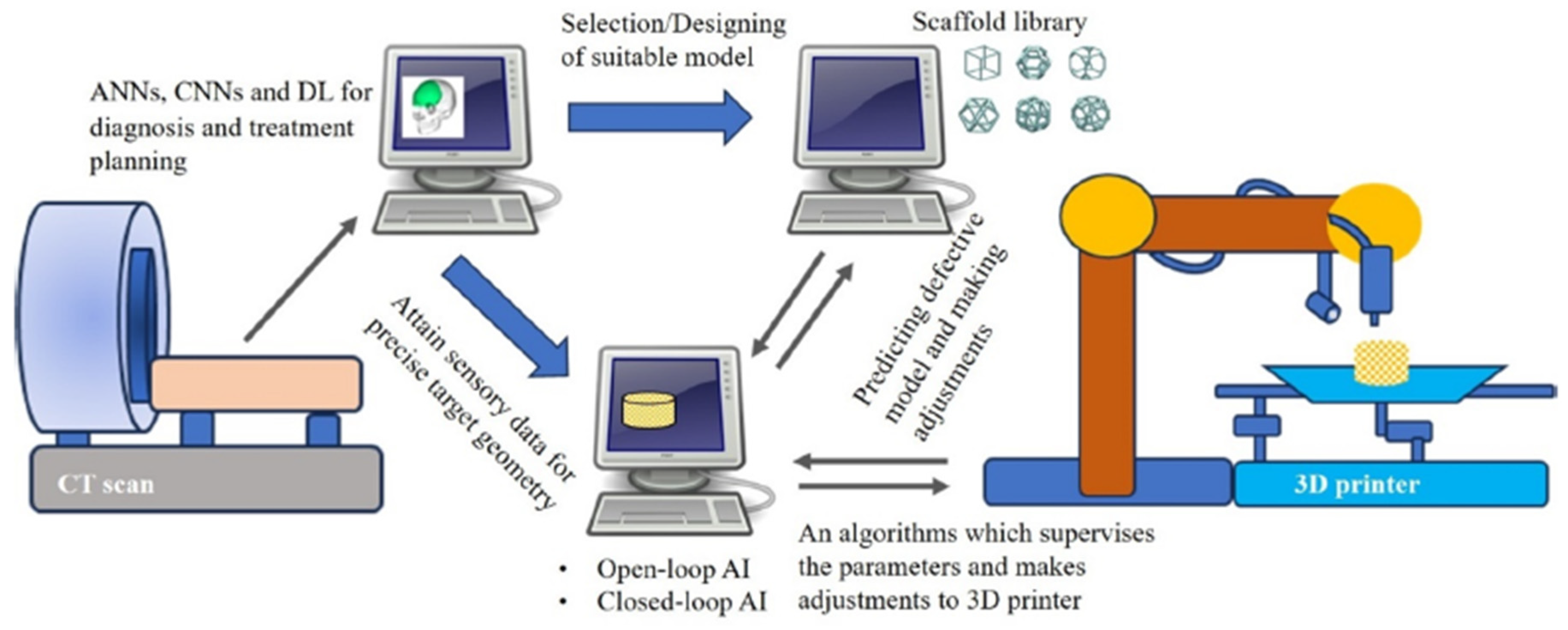
4. Future Directions Integrating AI
5. Discussions
6. Conclusions
- Advantages such as biocompatibility and biodegradability to nontoxic products following non-enzymatic hydrolysis of the crosslinking polymer makes PPF a good candidate for medical field, especially for bone tissue engineering.
- Traditional methods like porogen leaching and gas foaming have limitations in controlling essential scaffold parameters, while advanced processing methods such as stereolithography (SLA), microstereolithography (µSLA), and digital light processing (DLP) offer higher resolution and control over scaffold architecture.
- Additive manufacturing techniques enable the fabrication of scaffolds with complex architectures and tailored mechanical properties.
- Post-processing conditions are crucial for maintaining mechanical strength, as demonstrated by the significant drop in properties without adequate post-processing.
- Despite the numerous advantages provided by the 3D printing techniques, there are still deficiencies and drawbacks that need to be considered, mostly coming from the high viscosity of PPF and weak inherent mechanical properties, which limit its processability and use in load-bearing applications.
- Blending PPF with other synthetic or natural polymers or reinforcing it with inorganic and organic substances represents a valuable solution to developing advanced composite formulations to overcome the main limitations.
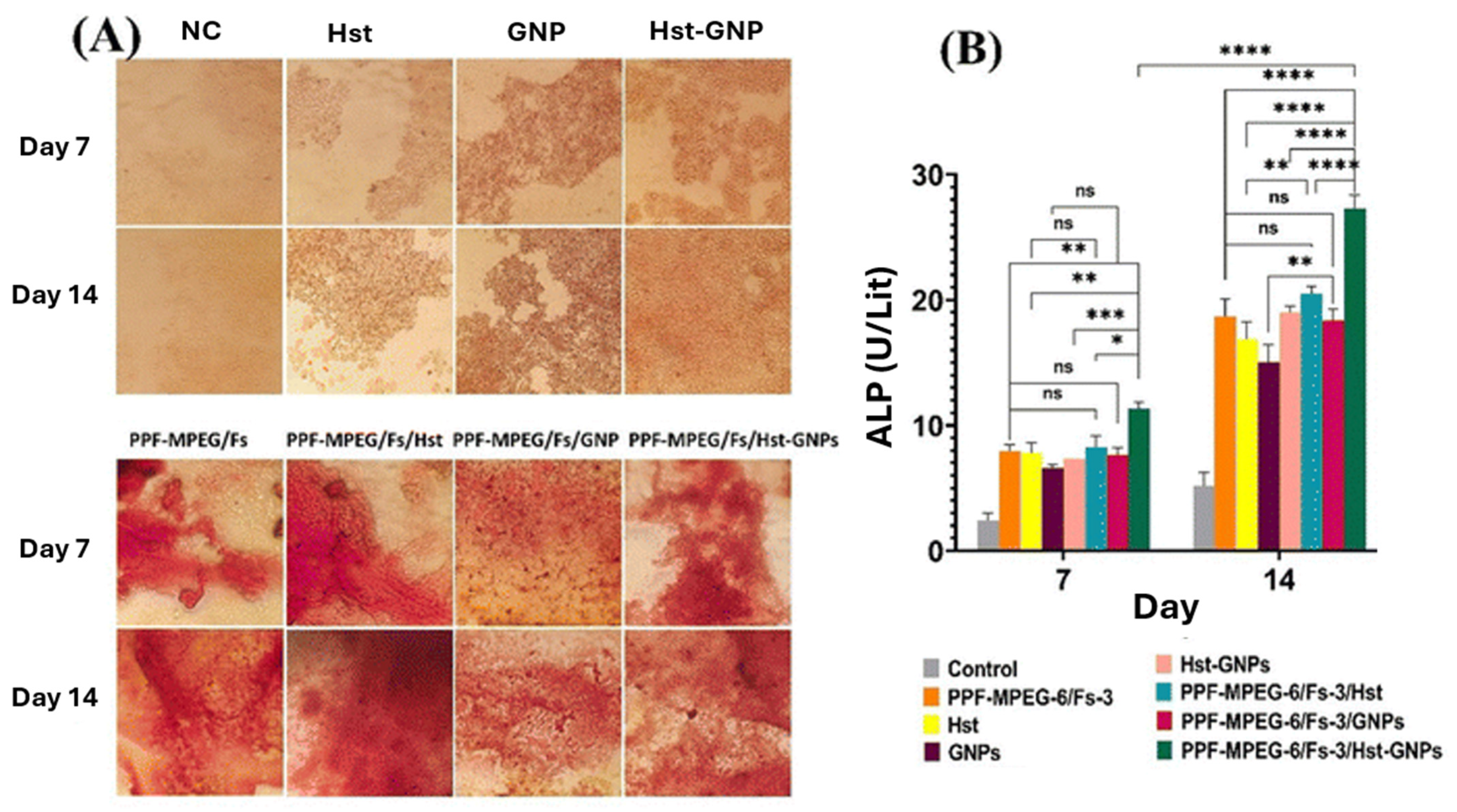
- Amongst the inorganic reinforcing agents, calcium phosphate enhances bone regeneration and maintains scaffold porosity; hydroxyapatite significantly improves compressive strength and osteoconductivity, leading to well-defined layers and linked pores in 3D-printed scaffolds.
- Bioactive glass composites show good bioactivity and stable mechanical properties over a 12-week degradation period, while boron nitride nanotubes (BNNTs) substantially enhance the mechanical properties of PPF, outperforming other reinforcing agents, such as single-walled carbon nanotubes (SWCNTs).
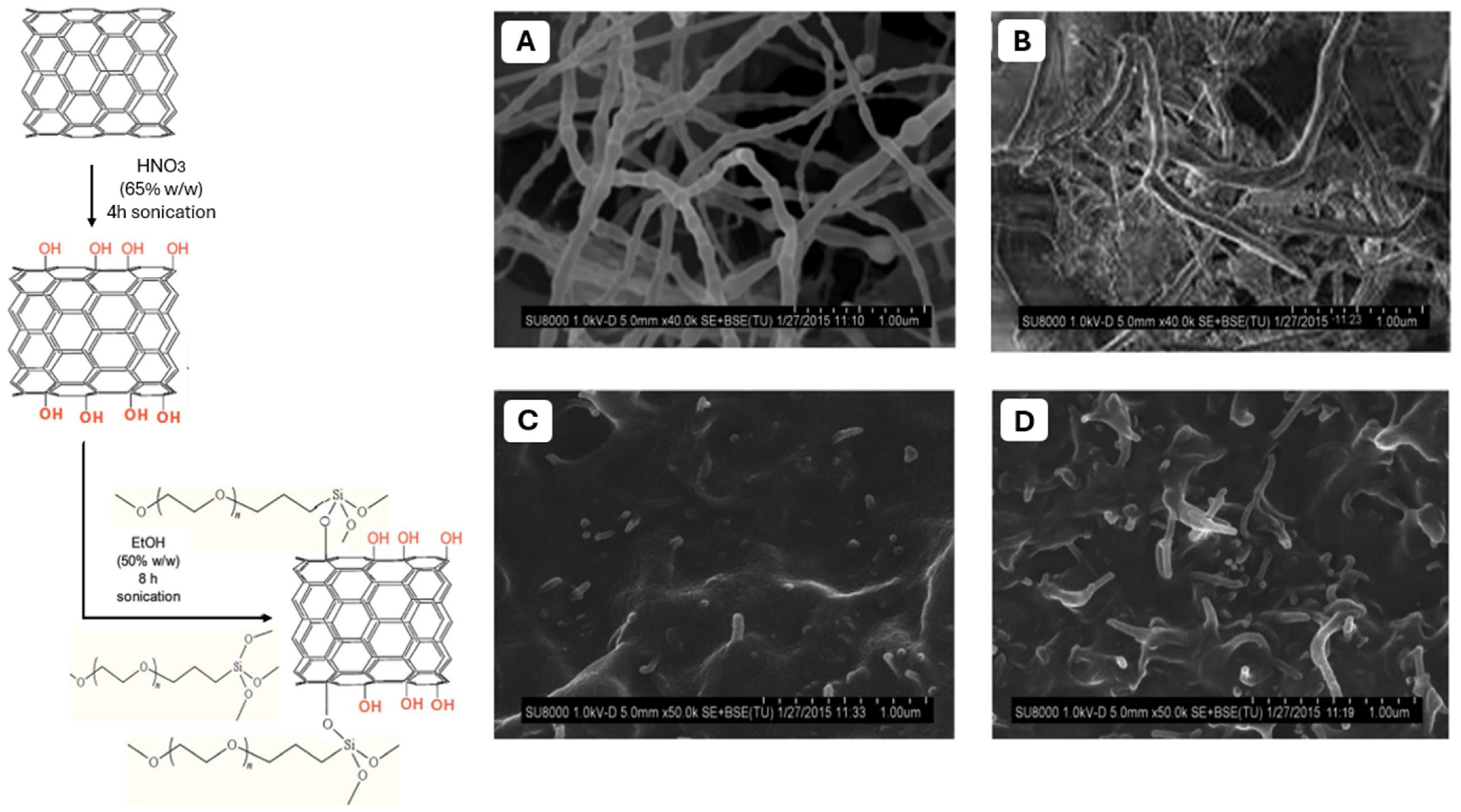
- In the case of carbonaceous nanostructure PPF-based composites, functionalization proved to be an effective approach to reach optimum mechanical and biological activity of the final materials.
- Graphene oxide nanoribbons and nanoplatelets exhibit high reinforcement potential due to their shape and surface area, surpassing traditional carbon nanotubes in some cases. The reinforcement efficiency of nanoparticles alongside PPF depends on their shape, with nanoplatelets generally providing the most significant reinforcement, followed by nanoribbons and nanotubes. However, considering all these aspects, reinforced PPF can achieve mechanical properties comparable to natural bone, maintaining biocompatibility and promoting cell adhesion and growth.
- By focusing on these prospects, bone tissue engineering can fully utilize AI to advance scaffold production and characterization. This novel approach will not only speed up discovery and development but will also make tissue-engineering structures more effective, dependable, and patient-specific, paving the way for game-changing therapeutic applications.
7. Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Ferraz, M.P. An Overview on the Big Players in Bone Tissue Engineering: Biomaterials, Scaffolds and Cells. Int. J. Mol. Sci. 2024, 25, 3836. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue engineered scaffolds in regenerative medicine. World J. Plast. Surg. 2014, 3, 3. [Google Scholar]
- Kim, Y.-H.; Vijayavenkataraman, S.; Cidonio, G. Biomaterials and scaffolds for tissue engineering and regenerative medicine. BMC Methods 2024, 1, 2. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766. [Google Scholar] [CrossRef]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, K.M.; van der Boon, T.-B.; Devia-Rodriguez, R.; Schuurmann, R.-L.; Sjollema, J.; van Huizen, L.; De Vries, J.-M.; van Rijn, P. Recent regulatory developments in EU Medical Device Regulation and their impact on biomaterials translation. Bioeng. Transl. Med. 2025, 10, e10721. [Google Scholar] [CrossRef]
- ISO 10993-1:2009; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. International Organization for Standardization: Geneva, Switzerland, 2009.
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Zarei, M.; Dargah, M.S.; Azar, M.H.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly(lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Pramanik, S.; Kharche, S.; More, N.; Ranglani, D.; Singh, G.; Kapusetti, G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Reg. 2023, 4, 193–204. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.-S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Comp. Part. B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-Hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Barman, J.; Babu, P.J. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Dong, B.; Yang, P.; Ji, C.; Li, Y.; Ma, J.; Ma, X. Understanding the relationship between pore structure and properties of triply periodic minimal surface bone scaffolds. J. Mater. Sci. Mater. Med. 2025, 36, 6. [Google Scholar] [CrossRef]
- He, S.; Zhu, J.; Jing, Y.; Long, S.; Tang, L.; Cheng, L.; Shi, Z. Effect of 3D-Printed Porous Titanium Alloy Pore Structure on Bone Regeneration: A Review. Coatings 2024, 14, 253. [Google Scholar] [CrossRef]
- Pamula, E.; Filová, E.; Bacáková, L.; Lisá, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. Part A 2009, 89, 432–443. [Google Scholar] [CrossRef]
- Murugan, S.; Parcha, S.R. Fabrication techniques involved in developing the composite scaffolds PCL/HA nanoparticles for bone tissue engineering applications. J. Mater. Sci. Mater. Med. 2021, 32, 93. [Google Scholar] [CrossRef]
- Osgouei, M.A.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High Strength Porous PLA Gyroid Scaffolds Manufactured via Fused Deposition Modeling for Tissue-Engineering Applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Lu, L.; Jabbari, E.; Currier, B.L.; Yaszemski, M.J. Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding. Tissue Eng. 2006, 12, 2801–2811. [Google Scholar] [CrossRef]
- Kasper, F.K.; Tanahashi, K.; Fisher, J.P.; Mikos, A.G. Synthesis of poly(propylene fumarate). Nat. Protoc. 2009, 4, 518–525. [Google Scholar] [CrossRef]
- Peter, S.J.; Suggs, L.J.; Yaszemski, M.J.; Engel, P.S.; Mikos, A.G. Synthesis of poly(propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. J. Biomater. Sci. Polym. Ed. 1999, 10, 363–373. [Google Scholar] [CrossRef]
- Peter, S.J.; Yaszemski, M.J.; Suggs, L.J.; Payne, R.G.; Langer, R.; Hayes, W.C.; Unroe, M.R.; Alemany, L.B.; Engel, P.S.; Mikos, A.G. Characterization of partially saturated poly(propylene fumarate) for orthopaedic application. J. Biomater. Sci. Polym. Ed. 1997, 8, 893–904. [Google Scholar] [CrossRef]
- Kamel, N.A.; Abou-Aiaad, T.H.; Iskander, B.A.; Khalil, S.-H.; Mansour, S.H.; Abd-El-Messieh, S.L.; Abd-El-Nour, K.N. Biophysical Studies on Bone Cement Composites Based on Polyester Fumarate. J. Appl. Polym. Sci. 2009, 116, 876–885. [Google Scholar] [CrossRef]
- Peter, S.J.; Kim, P.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J. Biomed. Mater. Res. 1999, 44, 314–321. [Google Scholar] [CrossRef]
- Fisher, J.P.; Dean, D.; Mikos, A.G. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials 2002, 23, 4333–4343. [Google Scholar] [CrossRef]
- Fisher, J.P.; Timmer, M.D.; Holland, T.A.; Dean, D.; Engel, P.S.; Mikos, A.G. Photoinitiated cross-linking of the biodegradable polyester poly(propylene fumarate). Part. I. Determination of network structure. Biomacromolecules 2003, 4, 1327–1334. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.Z.; Dean, D. Poly(propylene fumarate)-based materials: Synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P. Bone grafts in dental Medicine: An Overview of autografts, allografts and synthetic materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials 1996, 17, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M. Tissue Engineering Bionanocomposites Based on Poly(propylene fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.D.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)–diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Shahabi, S.; Rezaei, Y.; Moztarzadeh, F.; Najafi, F. In vitro degradation and bioactivity of poly(propylene fumarate)/bioactive glass sintered microsphere scaffolds for bone tissue engineering. Sci. Eng. Comp. Mater. 2016, 23, 245–256. [Google Scholar] [CrossRef]
- Nettleton, K.; Luong, D.; Kleinfehn, A.P.; Savariau, L.; Premanandan, C.; Becker, M.L. Molecular Mass-Dependent Resorption and Bone Regeneration of 3D Printed PPF Scaffolds in a Critical-Sized Rat Cranial Defect Model. Adv. Healthc. Mater. 2019, 8, e1900646. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Kuang, T. Oriented Porous Polymer Scaffolds in Tissue Engineering: A Comprehensive Review of Preparation Strategies and Applications. Macromol. Mater. Eng. 2023, 309, 2300246. [Google Scholar] [CrossRef]
- Salerno, A.; Oliviero, M.; Maio, E.D.; Iannace, S. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D printing method for bone tissue engineering scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Liu, X.; Schreiber, A.C.; Astudillo Potes, M.D.; Dashtdar, B.; Hamouda, A.M.; Rezaei, A.; Elder, B.D.; Lu, L. Bone Enzyme-Responsive Biodegradable Poly(propylene fumarate) and Polycaprolactone Polyphosphoester Dendrimer Cross-Linked via Click Chemistry for Bone Tissue Engineering. Biomacromolecules 2025, 26, 835–847. [Google Scholar] [CrossRef]
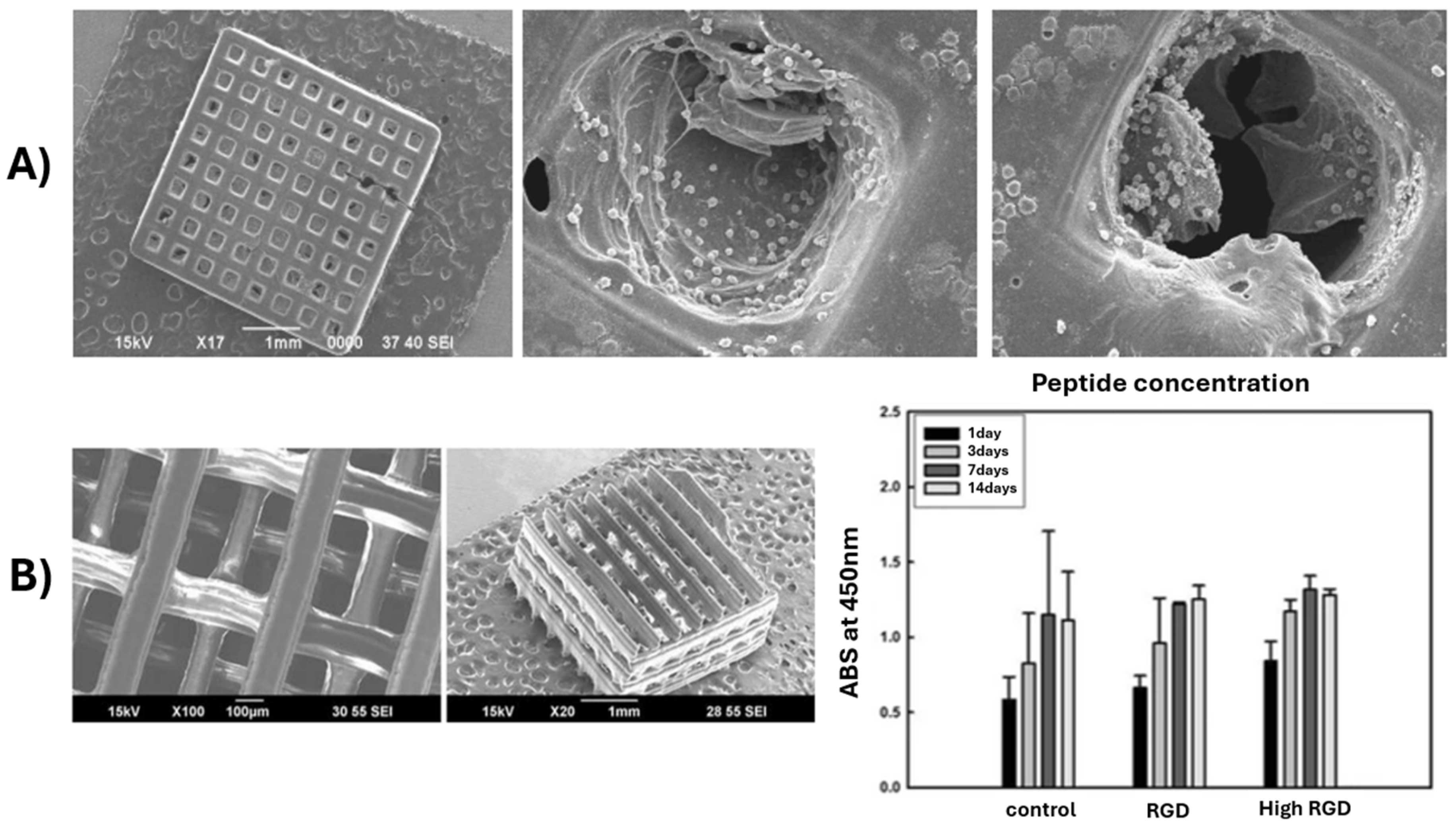
- Lee, J.W.; Ahn, G.S.; Kim, D.S.; Cho, D.-W. Development of Nano- and Microscale Composite 3D Scaffolds Using PPF/DEF-HA and Micro-Stereolithography. Microelectron. Eng. 2009, 86, 1465–1467. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, J.H.; Kim, D.S.; Lim, G.; Cho, D.-W. Estimation of Cell Proliferation by Various Peptide Coating at the PPF/DEF 3D Scaffold. Microelectron. Eng. 2009, 86, 1451–1454. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.Q.; He, X.H.; Cui, Y.D.; Rehman Lashari, N.U.; Guo, D.G.; Zheng, J. In vitro biocompatiability and mechanical properties of bone adhesive tape composite based on poly(butyl fumarate)/poly(propylene fumarate)-diacrylate networks. J. Mech. Behav. Biomed. Mater. 2022, 126, 105049. [Google Scholar] [CrossRef]
- Dadsetan, M.; Guda, T.; Runge, M.B.; Mijares, D.; LeGeros, R.Z.; LeGeros, J.P.; Silliman, D.T.; Lu, L.; Wenke, J.C.; Brown Baer, P.R.; et al. Effect of calcium phosphate coating and rhBMP-2 on bone regeneration in rabbit calvaria using poly(propylene fumarate) scaffolds. Acta Biomater. 2015, 18, 9–20. [Google Scholar] [CrossRef]
- Xu, H.; Liao, H.; Liu, X.; Miller, A.L., II; Elder, B.D.; Lu, L. Spinal fusion of biodegradable poly(propylene fumarate) and poly(propylene fumarate-co-caprolactone) copolymers in rabbits. J. Orthop. 2024, 48, 52–59. [Google Scholar] [CrossRef]
- Li, W.; Cheng, X.; Wang, Y.; Wang, S. Projection Printing of Scaffolds with Shape Recovery Capacity and Simultaneously Improved Stiffness and Toughness Using an Ultra-Fast-Curing Poly(propylene Fumarate)/Hyperbranched Additive Resin. Addit. Manuf. 2021, 48, 102446. [Google Scholar] [CrossRef]
- Qayoom, I.; Prasad, A.; Srivastava, E.; Fazili, K.M.; Nussler, A.K.; Kumar, A. Organic-inorganic composite of polypropylene fumarate and nanohydroxyapatite as carrier of antibiotics for the treatment of bone infections. Biomater. Adv. 2024, 157, 213714. [Google Scholar] [CrossRef] [PubMed]
- Mukhtarkhanov, M.; Perveen, A.; Talamona, D. Application of Stereolithography Based 3D Printing Technology in Investment Casting. Micromachines 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Arifin, N.; Sudin, I.; Nagadiman, N.-A.; Ishak, M.-A. A comprehensive review of Biopolymer fabrication in additive manufacturing processing for 3 D-Tissue-Engineering Scaffolds. Polymers 2022, 14, 2119. [Google Scholar] [CrossRef]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Tomaschke, A.; Kleinjan, E.; Muralidhran, A.; Pascual-Garrido, C.; McLeod Robert, R.R.; Ferguson, V.L.; Bryant, S.J. A Stereolithography-based 3D printed hybrid scaffold for in Situ defect repair. Macromol. Biosci. 2018, 18, 1700267. [Google Scholar] [CrossRef]
- Sun, C.; Fang, N.; Wu, D.M.; Zhang, X. Projection micro-stereolithography using digital micro-mirror dynamic mask. Sens. Actuators A Phys. 2005, 121, 113–120. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Yoo, J.J.; Lee, S.J.; Kim, M.S. 3D digital light process bioprinting: Cutting-edge platforms for resolution of organ fabrication. Mater. Today Bio 2024, 29, 101284. [Google Scholar] [CrossRef]
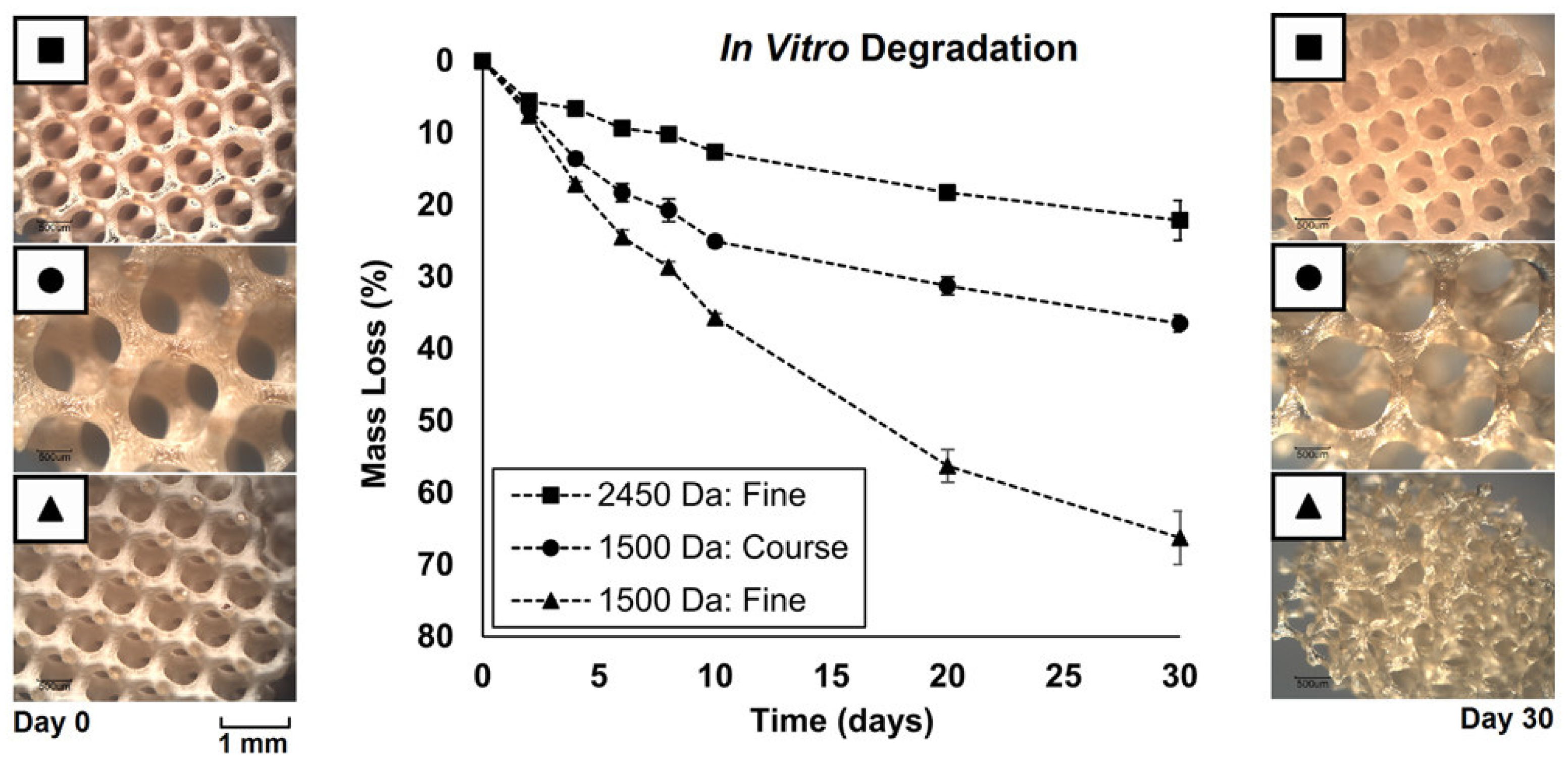
- Walker, J.M.; Bodamer, E.; Krebs, O.; Luo, Y.; Kleinfehn, A.; Becker, M.L.; Dean, D. Effect of Chemical and Physical Properties on the In Vitro Degradation of 3D Printed High Resolution Poly(propylene fumarate) Scaffolds. Biomacromolecules 2017, 18, 1419–1425. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, B.; Lim, G.B. Scaffold Fabrication with Biodegradable Poly(propylene fumarate) Using Microstereolithography. Key Eng. Mater. 2007, 342, 141–144. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.W.; Jung, J.; Lim, G. Evaluation of cell proliferation and differentiation on a poly(propylene fumarate) 3D scaffold treated with functional peptides. J. Mater. Sci. 2011, 46, 5282–5287. [Google Scholar] [CrossRef]
- Luo, Y.; Dolder, C.K.; Walker, J.M.; Mishra, R.; Dean, D.; Becker, M.L. Synthesis and Biological Evaluation of Well-Defined Poly(propylene fumarate) Oligomers and Their Use in 3D Printed Scaffolds. Biomacromolecules 2016, 17, 690–697. [Google Scholar] [CrossRef]
- Verisqa, F.; Cha, J.R.; Nguyen, L.; Kim, H.W.; Knowles, J.C. Digital Light Processing 3D Printing of Gyroid Scaffold with Isosorbide-Based Photopolymer for Bone Tissue Engineering. Biomolecules 2022, 12, 1692. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Abbasi, M.; Váz, P.; Silva, J.; Martins, P. Head-to-Head Evaluation of FDM and SLA in Additive Manufacturing: Performance, Cost, and Environmental Perspectives. Appl. Sci. 2025, 15, 2245. [Google Scholar] [CrossRef]
- Grzebieluch, W.; Grajzer, M.; Mikulewicz, M. Comparative Analysis of Fused Deposition Modeling and Digital Light Processing Techniques for Dimensional Accuracy in Clear Aligner Manufacturing. Med. Sci. Monit. 2023, 29, e940922. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Shah, D.M.; Morris, J.; Plaisted, T.A.; Amirkhizi, A.V.; Hansen, C.J. Highly filled resins for DLP-based printing of low density, high modulus materials. Addit. Manuf. 2021, 37, 101736. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Le Fer, G.; Luo, Y.; Becker, M.L. Poly(propylene fumarate) stars, using architecture to reduce the viscosity of 3D printable resins. Polym. Chem. 2019, 10, 4655–4664. [Google Scholar] [CrossRef]
- Luo, Y.; Le Fer, G.; Dean, D.; Becker, M.L. 3D Printing of Poly(propylene fumarate) Oligomers: Evaluation of Resin Viscosity, Printing Characteristics and Mechanical Properties. Biomacromolecules 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Zeinali, R.; Del Valle, L.J.; Torras, J.; Puiggalí, J. Recent Progress on Biodegradable Tissue Engineering Scaffolds Prepared by Thermally-Induced Phase Separation (TIPS). Int. J. Mol. Sci. 2021, 22, 3504. [Google Scholar] [CrossRef]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Lee Miller, A., II; Waletzki, B.E.; Yaszemski, M.J.; Lu, L. Novel porous poly(propylene fumarate-co-caprolactone) scaffolds fabricated by thermally induced phase separation. J. Biomed. Mater. Res. Part A 2017, 105, 226–235. [Google Scholar] [CrossRef]
- Kim, C.; Talac, R.; Lu, L.; Moore, M.; Currier, B.; Yaszemski, M. Characterization of porous injectable poly-(propylene fumarate)-based bone graft substitute. J. Biomed. Mater. Res. Part A 2008, 85, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wang, S.; Dadsetan, M.; Yaszemski, M.J.; Lu, L. Enhanced Cell Ingrowth and Proliferation through Three-Dimensional Nanocomposite Scaffolds with Controlled Pore Structures. Biomacromolecules 2010, 11, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Xu, W.; Biesinger, M.; Charpentier, P. Synthesis and characterization of novel TiO2-poly(propylene fumarate) nanocomposites for bone cementation. J. Mater. Chem. B 2014, 2, 5145–5156. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Yang, F.; Wang, J.; Liu, C. Poly (propylene fumarate)/β-calcium phosphate composites for enhanced bone repair. Biomed. Mater. 2019, 14, 045002. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Giambini, H.; Rezaei, A.; Liu, X.; Lee Miller, A., II; Waletzki, B.E.; Lu, L. Poly(Propylene Fumarate)-Hydroxyapatite Nanocomposite Can Be a Suitable Candidate for Cervical Cages. J. Biomech. Eng. 2018, 140, 1010091–1010098. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Yang, D.A.; Zhang, N.; Ji, C.G.; Zhu, L.; Zhang, T. Poly(propylene fumarate)/(calcium sulphate/β-tricalcium phosphate) composites: Preparation, characterization and in vitro degradation. Acta Biomater. 2009, 5, 628–635. [Google Scholar] [CrossRef]
- Fekiac, J.J.; Krbata, M.; Kohutiar, M.; Janik, R.; Kakosova, L.; Breznicka, A.; Eckert, M.; Mihus, P. Comprehensive Review: Optimization of Epoxy Composites, Mechanical Properties, & Technological Trends. Polymers 2025, 17, 271. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.Y.; Ji, M.H.; Guo, D.G.; He, X.H.; Lashari, N.-R.; Zheng, J. Development and properties of UV-cured poly (propylene fumarate)/hydroxyapatite composites coatings as potential application for bone adhesive tape. J. Appl. Polym. Sci. 2022, 139, 52289. [Google Scholar] [CrossRef]
- Trachtenberg, J.E.; Placone, J.K.; Smith, B.T.; Fisher, J.P.; Mikos, A.G. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J. Biomater. Sci. Polym. Ed. 2017, 28, 532–554. [Google Scholar] [CrossRef]
- Klein, T.R.; Kirillova, A.; Gall, K.; Becker, M.L. Influence of post-processing on the properties of 3D-printed poly(propylene fumarate) star polymer hydroxyapatite nanocomposites. RSC Appl. Polym. 2023, 1, 73–81. [Google Scholar] [CrossRef]
- Kamel, N.A.; Mansour, S.H.; Abd-El-Messieh, S.L.; Khalil, W.A.; Abd-El Nour, K.N. Biophysical properties of PPF/HA nanocomposites reinforced with natural bone powder. Adv. Mater. Res. 2015, 4, 145. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, N.; Yaszemski, M.J.; Lu, L. Effects of composite formulation on the mechanical properties of biodegradable poly(propylene fumarate)/bone fiber scaffolds. Int. J. Polym. Sci. 2010, 2010, 270273. [Google Scholar] [CrossRef]
- Zaghian, M.; Varshosaz, J.; Rostami, M.; Mirian, M. A forsterite-reinforced polypropylene fumarate/methoxy polyethylene glycol-hydrogel enriched with flavonoid nanoparticles enhances osteoconductivity. Mater. Adv. 2024, 5, 4324–4344. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Mechanical properties of poly(propylene fumarate)/nanotube composites: Experimental results and theoretical predictions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 383, 012001. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. PEGylated boron nitride nanotube-reinforced poly(propylene fumarate) nanocomposite biomaterials. RSC Adv. 2016, 6, 79507–79519. [Google Scholar] [CrossRef]
- Karfarma, M.; Esnaashary, M.H.; Rezaie, H.R.; Javadpour, J.; Naimi-Jamal, M.R. Poly(propylene fumarate)/magnesium calcium phosphate injectable bone composite: Effect of filler size and its weight fraction on mechanical properties. Proc. Inst. Mech. Eng. Part H 2019, 233, 1165–1174. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef]
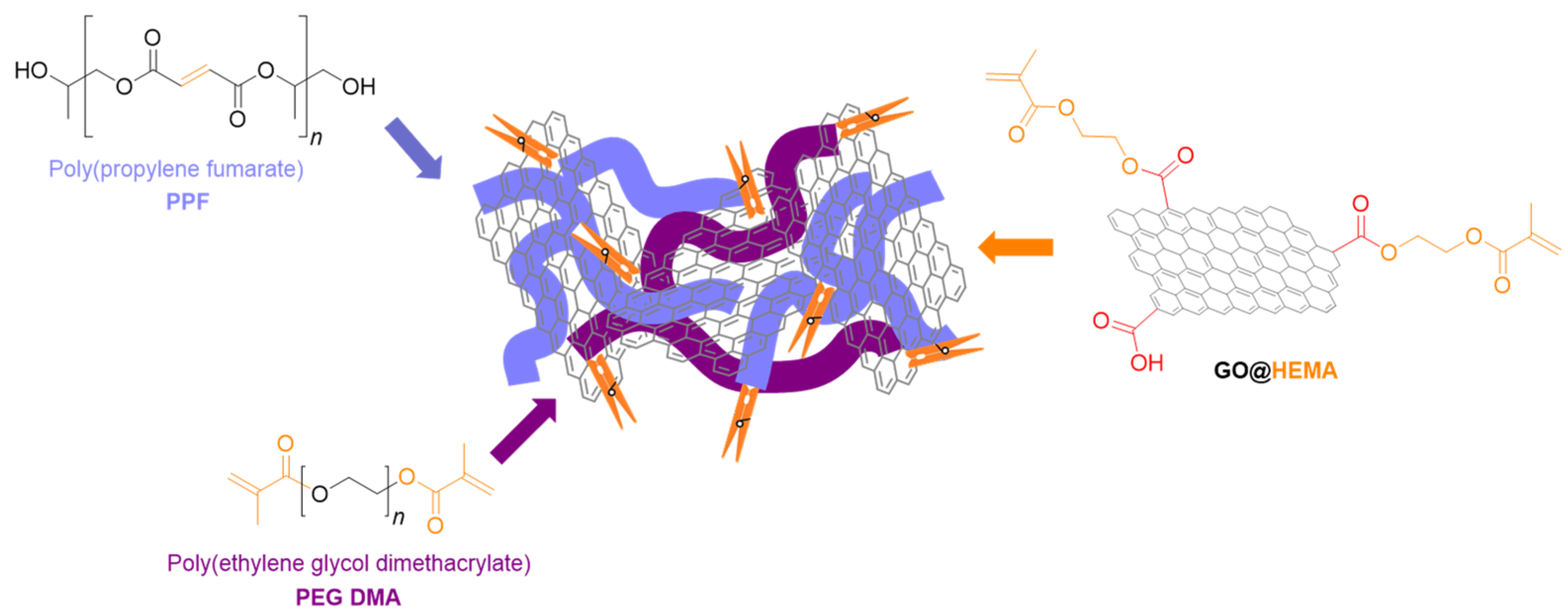
- Vasile, E.; Pandele, A.M.; Andronescu, C.; Selaru, A.; Dinescu, S.; Costache, M.; Hanganu, A.; Raicopol, M.D.; Teodorescu, M. Hema-Functionalized Graphene Oxide: A Versatile Nanofiller for Poly(Propylene Fumarate)-Based Hybrid Materials. Sci. Rep. 2019, 9, 18685. [Google Scholar] [CrossRef]
- Pandele, A.M.; Selaru, A.; Dinescu, S.; Costache, M.; Vasile, E.; Dascălu, C.; Raicopol, M.D.; Teodorescu, M. Synthesis and evaluation of poly(propylene fumarate)-grafted graphene oxide as nanofiller for porous scaffolds. J. Mater. Chem. B 2023, 11, 8241–8250. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(propylene fumarate)/Polyethylene Glycol-Modified Graphene Oxide Nanocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Lin, L.; Kasper, F.K.; Qin, Y.X.; Mikos, A.G.; Sitharaman, B. Two-Dimensional Nanostructure-Reinforced Biodegradable Polymeric Nanocomposites for Bone Tissue Engineering. Biomacromolecules 2013, 14, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hudson, J.L.; Spicer, P.P.; Tour, J.M.; Krishnamoorti, R.; Mikos, A.G. Rheological behaviour and mechanical characterization of injectable poly(propylene fumarate)/single-walled carbon nanotube composites for bone tissue engineering. Nanotechnology 2005, 16, S531. [Google Scholar] [CrossRef]
- Shi, X.; Sitharaman, B.; Pham, Q.P.; Liang, F.; Wu, K.; Edward Billups, W.; Wilson, L.J.; Mikos, A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 4078–4090. [Google Scholar] [CrossRef] [PubMed]
- Farshid, B.; Lalwani, G.; Sitharaman, B. Cytotoxicity of Polypropylene Fumarate Nanocomposites used in Bone Tissue Engineering. In Proceedings of the 2013 39th Annual Northeast Bioengineering Conference, New York, NY, USA, 5–7 April 2013; pp. 119–120. [Google Scholar]
- Kolomenskaya, E.; Butova, V.; Poltavskiy, A.; Soldatov, A.; Butakova, M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines 2024, 12, 76. [Google Scholar] [CrossRef]
- Meyer, T.A.; Ramirez, C.; Tamasi, M.J.; Gormley, A.J. A User’s Guide to Machine Learning for Polymeric Biomaterials. ACS Polym. Au 2023, 3, 141–157. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Torkashvand, M.; Bavisi, M.; Aldaghi, N.; Alizadeh, A. Recent advances in artificial intelligent strategies for tissue engineering and regenerative medicine. Skin Res. Technol. 2024, 30, e70016. [Google Scholar] [CrossRef]
- Jeznach, O.; Tabakoglu, S.; Zaszczynska, A.; Sajikiewicz, P. Review on machine learning application in tissue engineering: What has been done so far? Application areas, challenges, and perspectives. J. Mater. Sci. 2024, 59, 21222–21250. [Google Scholar]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in artificial intelligence for drug delivery and development: A comprehensive review. Comput. Biol. Med. 2024, 178, 108702. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. AI in diagnostic imaging: Revolutionising accuracy and efficiency. Comp. Methods Programs Biomed. Update 2024, 5, 100146. [Google Scholar] [CrossRef]
- Taherdoost, H.; Ghofrani, A. AI′s role in revolutionizing personalized medicine by reshaping pharmacogenomics and drug therapy. Intell. Pharm. 2024, 2, 643–650. [Google Scholar]
- Sufyan, M.; Shokat, Z.; Ashfaq, U.A. Artificial intelligence in cancer diagnosis and therapy: Current status and future perspective. Comput. Biol. Med. 2023, 165, 107356. [Google Scholar] [CrossRef]
- Ibrahimi, S.; D’Andrea, L.; Gastaldi, D.; Rivolta, M.W.; Vena, P. Machine Learning approaches for the design of biomechanically compatible bone tissue engineering scaffolds. Comput. Methods Appl. Mech. Eng. 2024, 423, 116842. [Google Scholar] [CrossRef]
- Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar]
- López-Flores, F.J.; Ornelas-Guillén, J.A.; Pérez-Nava, A. Data-driven machine learning approach for modeling the production and predicting the characteristics of aligned electrospun nanofibers. Ind. Eng. Chem. Res. 2024, 63, 9904–9913. [Google Scholar] [CrossRef]
- Li, F.; Han, J.; Cao, T. Design of self-assembly dipeptide hydrogels and machine learning via their chemical features. Proc. Natl. Acad. Sci. USA 2019, 116, 11259–11264. [Google Scholar] [CrossRef]
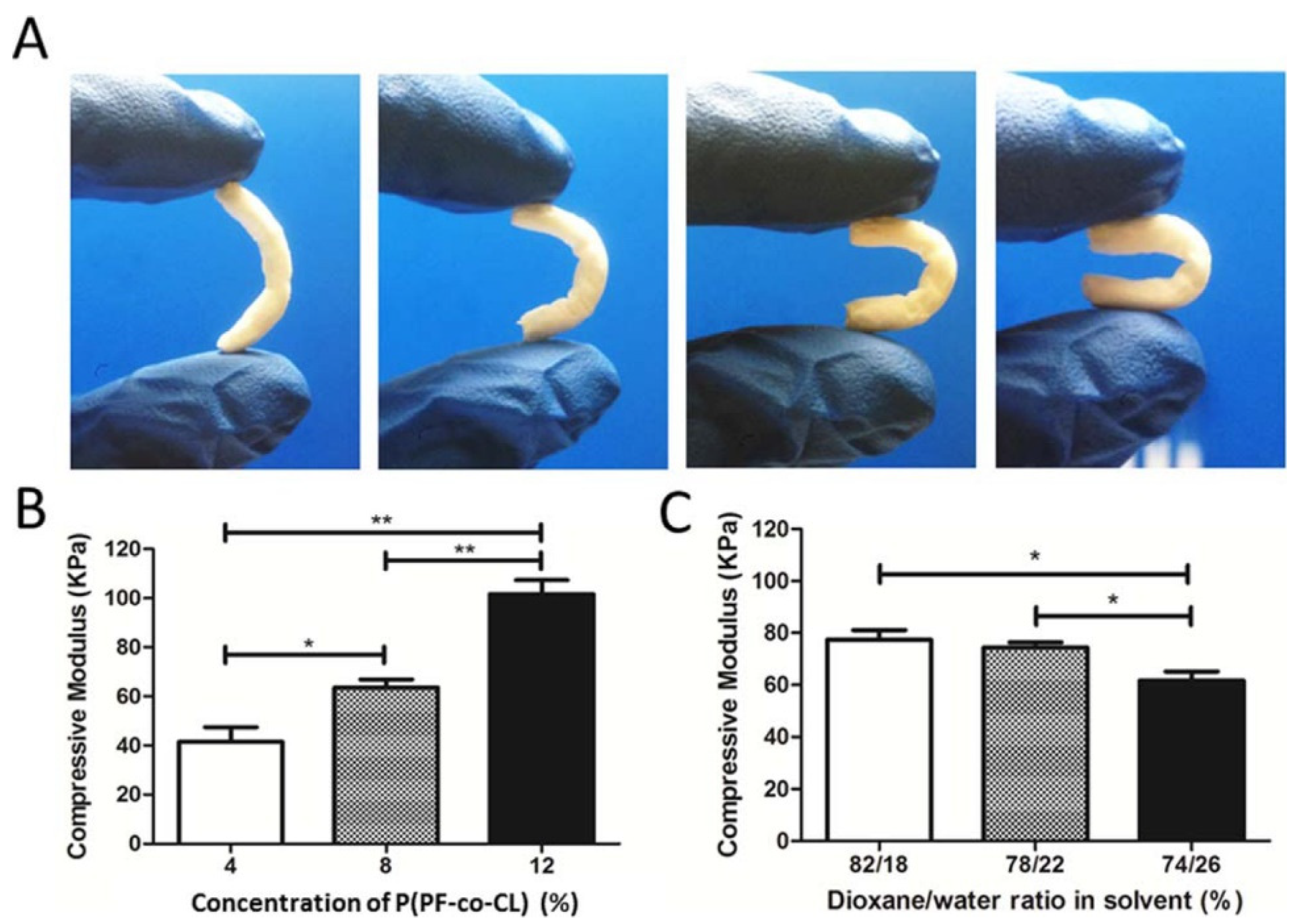
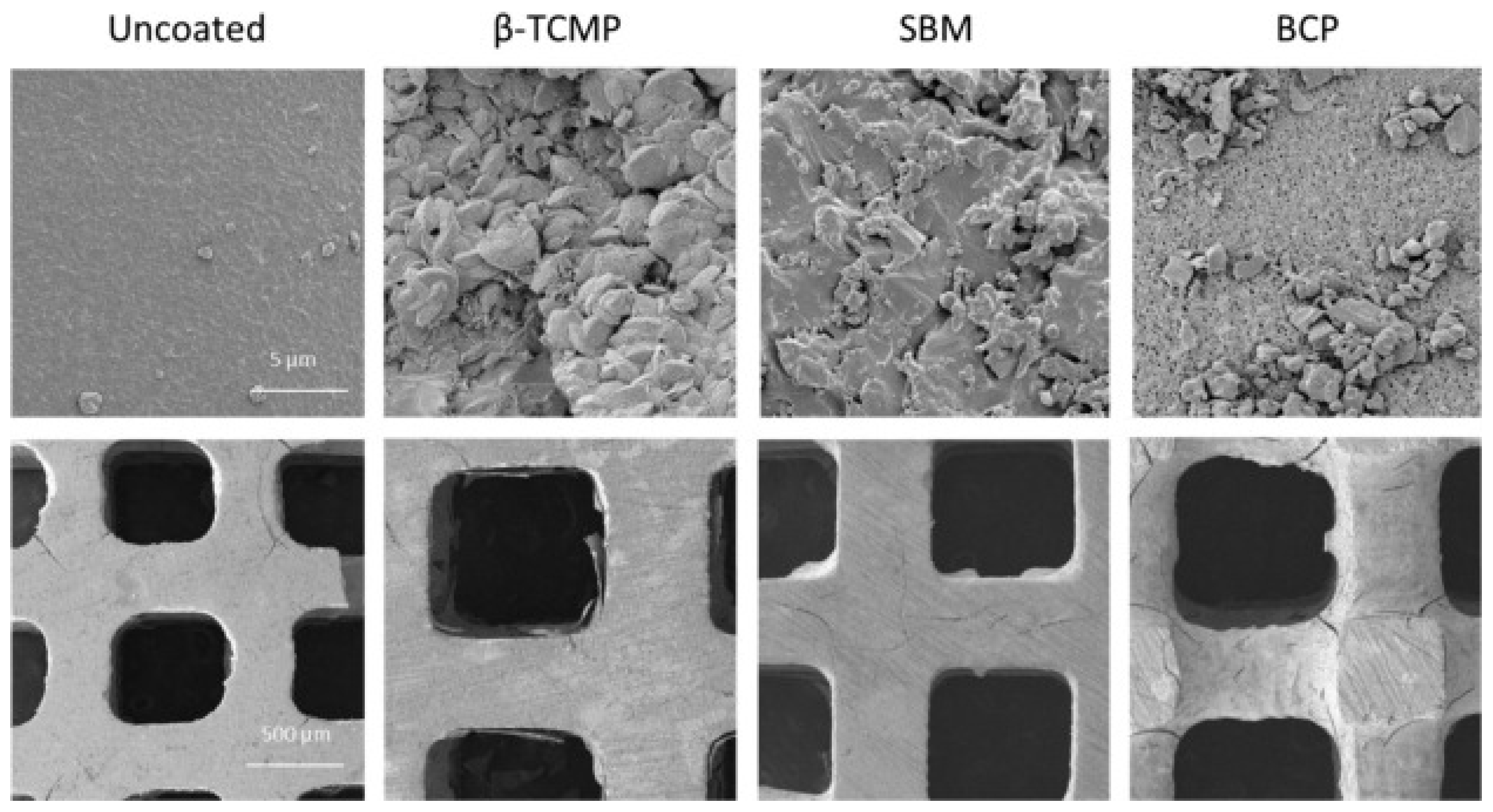
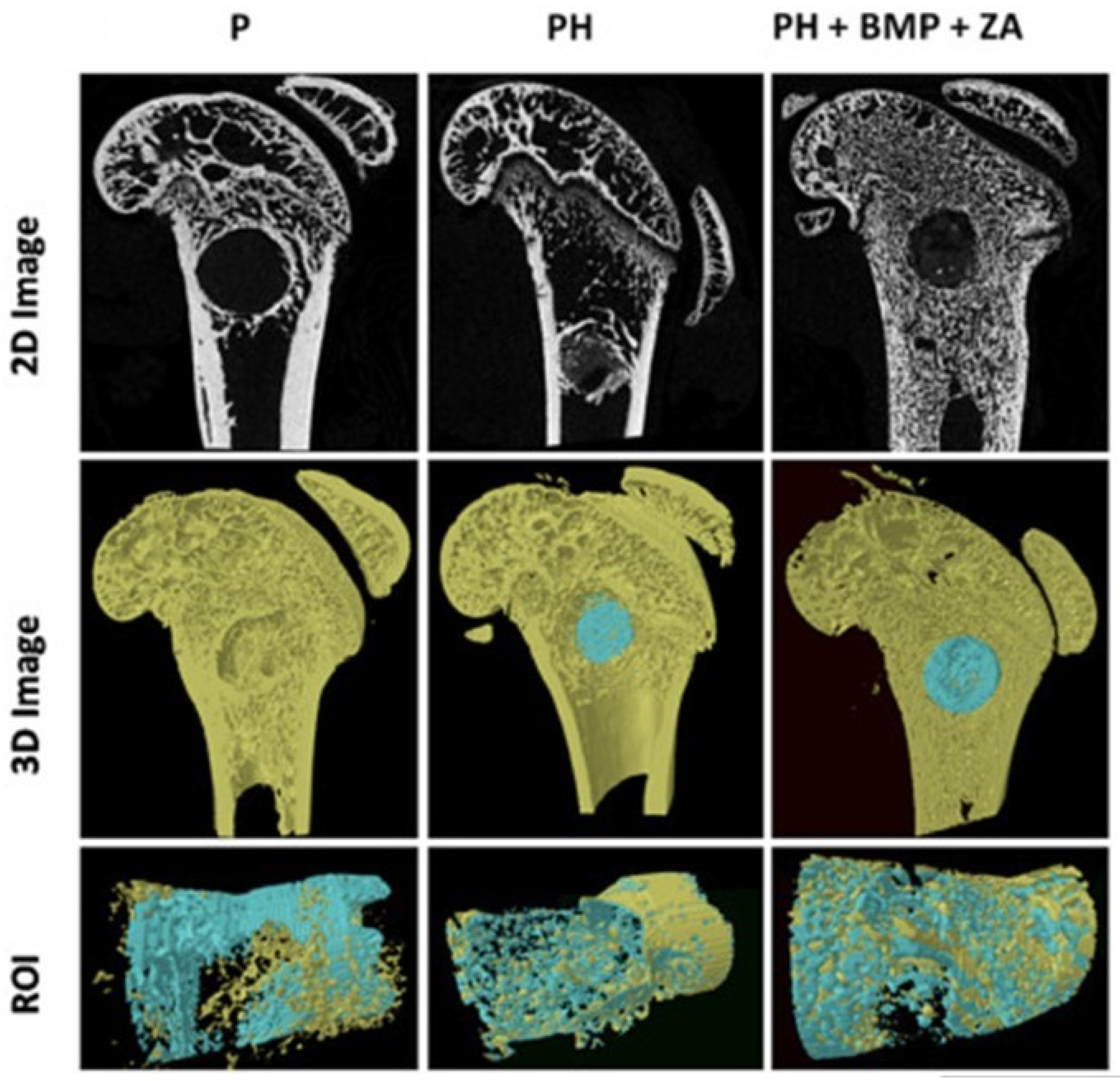
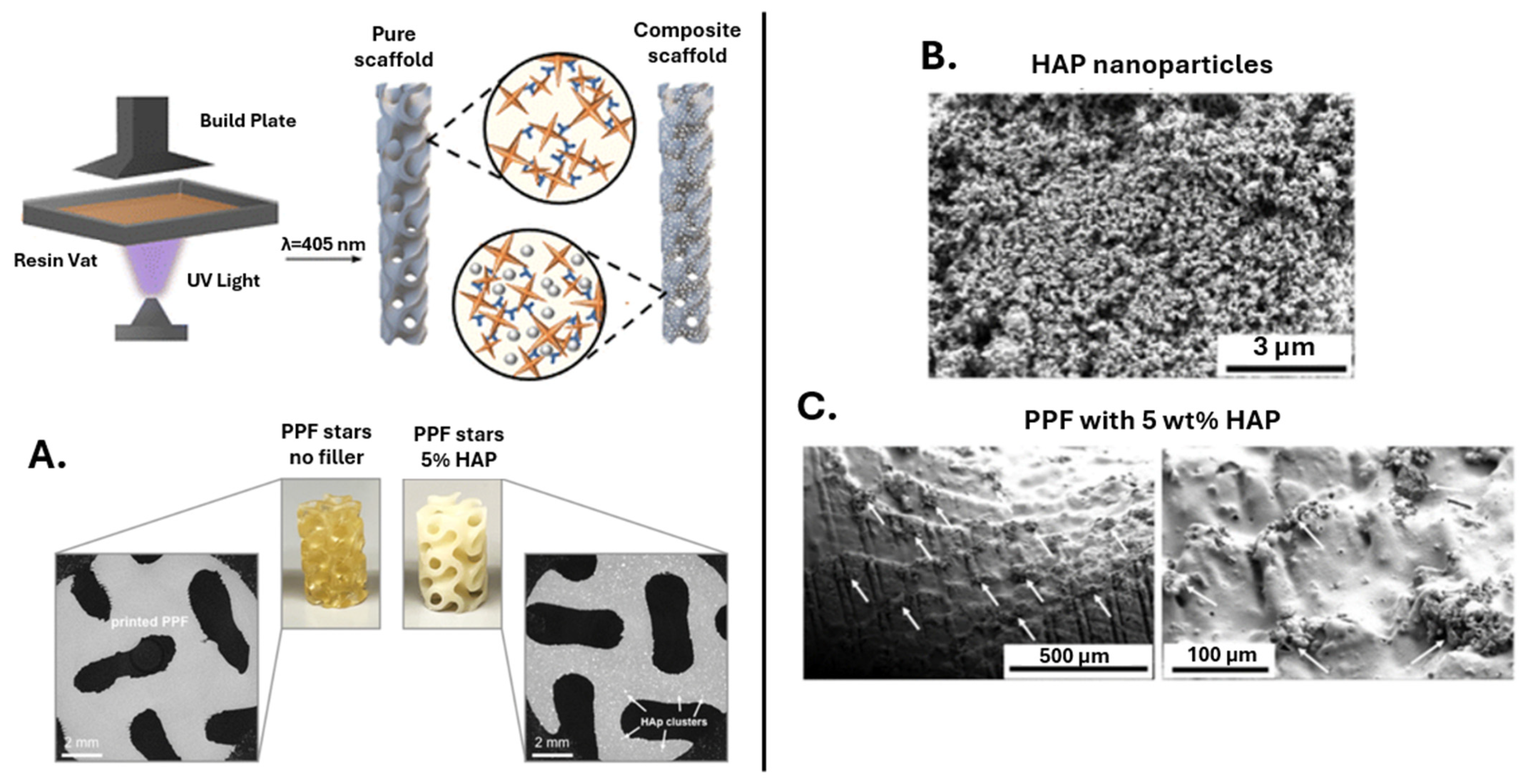



| Polymer | Porosity | Pore Size | Ref. |
|---|---|---|---|
| PLGA | ~83% | 180–600 μm | [31] |
| PCL | 70–93% | 300–600 μm | [18,32] |
| PLA | 86–90% | N/A | [33] |
| PPF | ~50% | 300–900 μm | [34] |
| Material Formulation | Fabrication Technique | Challenges | Key Properties | Ref. |
|---|---|---|---|---|
| PPF/DEF and BAPO | 3D printing-stereolithography | Shrinkage upon polymerization | Compressive modulus ~80–140 MPa Pore size: 446–913 µm Porosity: 35–70% | [42] |
| PPF and PCL polyphosphoester (PPE) | Solvent casting | Reproducibility and consistency | Biocompatibility, enzyme-responsive degradation, stem cell differentiation | [53] |
| PPF/DEF-hydroxyapatite (HA) and BAPO | 3D printing-micro-stereolithography | Shrinkage upon post curing ~20% | Pore size: 330–360 μm HA facilitate cell adhesion and proliferation | [54] |
| PPF/DEF | 3D printing-micro-stereolithography | Scaffolds shrank 25% after post curing; hydrophobic surfaces | Functionalization with peptides improve cell adhesion and proliferation | [55] |
| Poly(butyl fumarate)/PPF-DA, nano-HAP composites | Injection molding | Cell viability after 5 days; control over the crosslinking process; | Compressive strengths of 4–12 MPa and compressive modulus of 100–500 MPa; cell viability between 81–90% after one day of incubation | [56] |
| PPF/DEF and calcium phosphates | 3D printing-stereolithography | Calcium phosphate microstructure affects binding capacity for bone-related proteins | Scaffolds coated with calcium phosphates show good osteointegration and osteoconductivity and sustain the release of recombinant human bone morphogenetic protein-2 (rhBMP-2) | [57] |
| PPF and PPF-co-caprolactone copolymer | Injection molding | Polymerization time of 15–30 min; maximum crosslinking temperature between 38–42 °C | Porosity of 50%; high degradation rate and osteogenesys for copolymer in comparison with PPF | [58] |
| PPF and hyperbranched polyester acrylate (HPA) | 3D printing-projection micro-stereolithography (PμSL) | Post-curing process | Lower shrinkage, higher stiffness and toughness; porosity 80–85%; printing resolution of 50 µm and ultra-fast printing speed of 18 cm/h | [59] |
| Carboxy terminal-PPF/DEF, BAPO, AND nanohydroxyapatite (nHAP) | Solvent casting | Reproducibility and consistency | In vivo infection model demonstrated that composite scaffolds loaded with antibiotics reduce the bacterial burden at the infection site. | [60] |
| 3D Printing Technique | Printing Speed | Printing Resolution XY | Advantages | Limitations |
|---|---|---|---|---|
| SLA | Moderate (laser traces each point) | ~50–140 µm | High surface smoothness (~2 μm roughness); great for complex geometri es; slower than DLP | 90% success rate; post-processing operations |
| DLP | Fast (entire layer cured at once) | ~35–100 µm | High resolution and speed; ideal for micro-scale scaffolds, accurate pore geometry; suitable to develop bone tissue substitutes | Limited to photopolymers; post-processing |
| FDM | Varies, generally slower for detailed prints due to layer-by-layer deposition of material | ~100–300 µm | Cost-effective, easy to use; lower resolution, not suitable for intricate bone scaffolds | Prone to failure due to nozzle clogs; 80% success rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Necolau, M.I.; Ionita, M.; Pandele, A.M. Poly(propylene fumarate) Composite Scaffolds for Bone Tissue Engineering: Innovation in Fabrication Techniques and Artificial Intelligence Integration. Polymers 2025, 17, 1212. https://doi.org/10.3390/polym17091212
Necolau MI, Ionita M, Pandele AM. Poly(propylene fumarate) Composite Scaffolds for Bone Tissue Engineering: Innovation in Fabrication Techniques and Artificial Intelligence Integration. Polymers. 2025; 17(9):1212. https://doi.org/10.3390/polym17091212
Chicago/Turabian StyleNecolau, Madalina I., Mariana Ionita, and Andreea M. Pandele. 2025. "Poly(propylene fumarate) Composite Scaffolds for Bone Tissue Engineering: Innovation in Fabrication Techniques and Artificial Intelligence Integration" Polymers 17, no. 9: 1212. https://doi.org/10.3390/polym17091212
APA StyleNecolau, M. I., Ionita, M., & Pandele, A. M. (2025). Poly(propylene fumarate) Composite Scaffolds for Bone Tissue Engineering: Innovation in Fabrication Techniques and Artificial Intelligence Integration. Polymers, 17(9), 1212. https://doi.org/10.3390/polym17091212








