Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells
Abstract
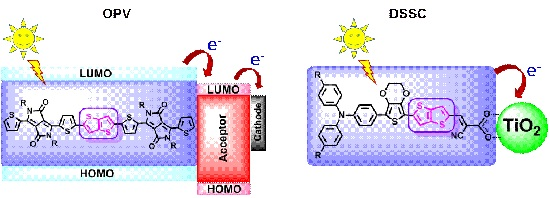
:1. Introduction
2. Organic Photovoltaics (OPV)

2.1. Vacuum-Deposited Fused-Thiophene-Based Small Molecules for OPVs
2.2. Solution-Processed Fused-Thiophene-Based Small Molecules for OPVs

3. Dye-sensitized Solar Cells (DSSCs)

| Cpd. | λmax (nm) | HOMO (eV) | LUMO (eV) | Band gap (eV) | Jsc (mA·cm−2) | Voc (V) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 a | 528 | −5.51 | −3.48 | 2.03 | 11.04 | 0.87 | 0.57 | 5.41 | [13] |
| 2 a | 537 | −5.57 | −3.58 | 1.99 | 12.01 | 0.95 | 0.54 | 6.20 | [13] |
| 3 a | 387 | −5.66 | −2.95 | 2.71 | 9.24 | 0.98 | 0.40 | 3.60 | [14] |
| 4 b | 523 | −5.15 | −3.51 | 1.65 | 7.58 | 0.80 | 0.35 | 2.09 | [15] |
| 5 b | 527 | −5.13 | −3.46 | 1.66 | 7.60 | 0.74 | 0.31 | 1.80 | [15] |
| 6 b | 542 | −5.13 | −3.38 | 1.75 | 6.80 | 0.96 | 0.43 | 2.87 | [16] |
| 7 b | 568 | −5.10 | −3.42 | 1.68 | 5.71 | 0.74 | 0.34 | 1.44 | [17] |
| 8 b | 585 | −5.01 | −3.36 | 1.65 | 3.61 | 0.61 | 0.34 | 0.75 | [17] |
| 9 b | 618 | −5.11 | −3.56 | 1.54 | 9.3 | 0.81 | 0.53 | 4.00 | [19] |
| 10 b | 617 | −4.98 | −3.38 | 1.60 | 5.74 | 0.78 | 0.38 | 1.70 | [20] |
| 11 b | 670 | −5.00 | −3.50 | 1.50 | 7.67 | 0.57 | 0.5 | 2.19 | [23] |
| 12 b | 600 | −5.10 | −3.58 | 1.52 | 6.82 | 0.77 | 0.46 | 2.39 | [24] |

Fused-Thiophene-Based Sensitizers in Dye-Sensitized Solar Cells





| Dye | λmax (nm) | Jsc (mA·cm−2) | Voc (mV) | FF | η (%) | Ref. |
|---|---|---|---|---|---|---|
| 13 | 488 | 15.23 | 560 | 0.730 | 6.23 | [35] |
| 14 | 443 | 15.4 | 610 | 0.700 | 6.57 | [36] |
| 15 | 493 | 11.74 | 661 | 0.764 | 5.93 | [37] |
| 16 | 512 | 13.38 | 664 | 0.736 | 6.54 | [37] |
| 17 | 517 | 13.56 | 673 | 0.745 | 6.80 | [37] |
| 18 | 516 | 14.49 | 693 | 0.702 | 7.05 | [37] |
| 19 | 450 | 20.9 | 687 | 0.639 | 9.18 | [38] |
| 20 | 524 | 15.2 | 720 | 0.733 | 8.02 | [39] |
| 21 | 552 | 16.1 | 803 | 0.759 | 9.80 | [40] |
| 22 | 437 | 7.8 | 600 | 0.705 | 3.30 | [41] |
| 23 | 445 | 6.56 | 629 | 0.737 | 3.02 | [42] |
| 24 | 432 | 5.78 | 688 | 0.718 | 2.87 | [42] |
| 25 | 439 | 5.70 | 685 | 0.717 | 2.82 | [42] |
| 26 | 443 | 8.05 | 756 | 0.733 | 4.50 | [42] |
| 27 | 494 | 14.47 | 670 | 0.630 | 6.11 | [43] |
| 28 | 492 | 7.56 | 567 | 0.630 | 2.70 | [43] |
| 29 | 514 | 13.35 | 777 | 0.749 | 7.80 | [44] |
| 30 | 436 | 11.51 | 670 | 0.731 | 5.67 | [45] |
| 31 | 469 | 13.26 | 732 | 0.723 | 7.00 | [45] |
| 32 | 450 | 13.02 | 570 | 0.720 | 5.34 | [46] |
| 33 | 481 | 13.81 | 590 | 0.680 | 5.65 | [46] |
| 34 | 455 | 16.34 | 640 | 0.740 | 7.83 | [46] |
| 35 | 476 | 15.22 | 580 | 0.700 | 6.21 | [46] |
| 36 | 466 | 17.49 | 700 | 0.700 | 8.70 | [46] |
| 37 | 484 | 15.94 | 660 | 0.670 | 7.04 | [46] |
| 38 | 372 | 9.4 | 710 | 0.750 | 5.10 | [47] |
| 39 | 480 | 15.7 | 690 | 0.740 | 8.00 | [47] |
| 40 | 490 | 17.6 | 710 | 0.720 | 9.10 | [47] |
| 41 | 485 | 15.50 | 727 | 0.746 | 8.40 | [48] |
| 42 | 449 | 9.40 | 644 | 0.680 | 4.11 | [49] |
| 43 | 486 | 14.81 | 688 | 0.690 | 7.03 | [49] |
| 44 | 500 | 11.9 | 900 | 0.710 | 7.60 | [50] |
| 45 | 524 | 8.80 | 560 | 0.690 | 3.40 | [51] |
| 46 | 546 | 4.86 | 520 | 0.690 | 1.72 | [51] |
| 47 | 496 | 8.14 | 580 | 0.700 | 3.30 | [51] |
| 48 | 553 | 3.64 | 500 | 0.680 | 1.23 | [51] |
| 49 | 570 | 2.92 | 440 | 0.520 | 0.66 | [51] |
| 50 | 509 | 12.6 | 610 | 0.660 | 5.03 | [51] |
| 51 | 536 | 9.21 | 540 | 0.660 | 3.26 | [51] |
| 52 | 477 | 12.2 | 640 | 0.680 | 5.31 | [51] |
| 53 | 523 | 7.52 | 530 | 0.660 | 2.65 | [51] |
| 54 | 548 | 6.64 | 510 | 0.650 | 2.17 | [51] |
| 55 | 429 | 12.09 | 620 | 0.670 | 5.02 | [52] |
| 56 | 426 | 12.72 | 610 | 0.640 | 4.99 | [52] |
| 57 | 440 | 13.31 | 620 | 0.620 | 5.15 | [52] |
| 58 | 440 | 9.48 | 570 | 0.660 | 3.54 | [52] |
| 59 | 465 | 13.5 | 715 | 0.650 | 6.27 | [53] |
| 60 | 489 | 14.4 | 697 | 0.730 | 7.30 | [54] |
| 61 | 472 | 7.3 | 570 | 0.650 | 2.76 | [55] |
| 62 | 453 | 12.08 | 680 | 0.760 | 6.24 | [56] |
| 63 | 455 | 12.66 | 690 | 0.760 | 6.63 | [56] |
| 64 | 497 | 11.42 | 600 | 0.610 | 4.18 | [43] |
| 65 | 514 | 13.52 | 620 | 0.620 | 5.19 | [43] |
| 66 | 443 | 6.85 | 570 | 0.700 | 2.69 | [57] |
| 67 | 476 | 9.98 | 580 | 0.650 | 3.72 | [57] |
| 68 | 473 | 6.77 | 600 | 0.700 | 2.82 | [57] |
| 69 | 467 | 6.87 | 514 | 0.660 | 2.34 | [58] |
| 70 | 444 | 8.84 | 522 | 0.630 | 2.92 | [58] |
| 71 | 428 | 13.45 | 618 | 0.679 | 5.64 | [59] |
| 72 | 481 | 15.85 | 589 | 0.671 | 6.11 | [59] |
| 73 | 485 | 16.17 | 595 | 0.663 | 6.38 | [59] |
| 74 | 525 | 14.33 | 734 | 0.760 | 8.00 | [60] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bäuerle, P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. 2012, 51, 2020–2067. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 2012, 6, 591–595. [Google Scholar]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Skabara, P.J. Fused Oligothiophenes. In Handbook of Thiophene-Based Materials; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 219–254. [Google Scholar]
- Yang, Y.S.; Yasuda, T.; Kakizoe, H.; Mieno, H.; Kino, H.; Tateyama, Y.; Adachi, C. High performance organic field-effect transistors based on single-crystal microribbons and microsheets of solution-processed dithieno[3,2-b:2',3'-d]thiophene derivatives. Chem. Commun. 2013, 49, 6483–6485. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Kim, C.; Nguyen, T.Q. Small molecule solution-processed bulk heterojunction solar cells. Chem. Mater. 2010, 23, 470–482. [Google Scholar] [CrossRef]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, H.; Li, Y.; Zhan, X. Thiazole-based organic semiconductors for organic electronics. Adv. Mater. 2012, 24, 3087–3106. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Tian, H. Diketopyrrolopyrrole (DPP)-based materials for organic photovoltaics. Chem. Commun. 2012, 48, 3039–3051. [Google Scholar] [CrossRef]
- Kim, J.; Shim, H.S.; Lee, H.; Choi, M.S.; Kim, J.J.; Seo, Y. Highly efficient vacuum-processed organic solar cells containing thieno[3,2-b]thiophene-thiazole. J. Phys. Chem. C 2014, 118, 11559–11565. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, T.M.; Oh, H.S.; Kim, J.J.; Hong, J.I. Vacuum processable donor material based on dithieno[3,2-b:2[prime or minute],3[prime or minute]-d]thiophene and pyrene for efficient organic solar cells. RSC Adv. 2014, 4, 24453–24457. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.; Yun, S.J. Effects of fused thiophene bridges in organic semiconductors for solution-processed small-molecule organic solar cells. Bull. Korean Chem. Soc. 2013, 34, 2148–2154. [Google Scholar] [CrossRef]
- Deng, D.; Shen, S.; Zhang, J.; He, C.; Zhang, Z.; Li, Y. Solution-processable star-shaped photovoltaic organic molecule with triphenylamine core and thieno[3,2-b]thiophene–dicyanovinyl arms. Org. Electron. 2012, 13, 2546–2552. [Google Scholar] [CrossRef]
- Deng, D.; Yang, Y.; Zhang, J.; He, C.; Zhang, M.; Zhang, Z.G.; Zhang, Z.; Li, Y. Triphenylamine-containing linear D–A–D molecules with benzothiadiazole as acceptor unit for bulk-heterojunction organic solar cells. Org. Electron. 2011, 12, 614–622. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zhao, G.; Liu, Y.; Li, Y. Poly(3,6-dihexyl-thieno[3,2-b]thiophene vinylene): Synthesis, field-effect transistors, and photovoltaic properties. Macromolecules 2008, 41, 9760–9766. [Google Scholar] [CrossRef]
- Choi, Y.S.; Jo, W.H. A strategy to enhance both VOC and JSC of A–D–A type small molecules based on diketopyrrolopyrrole for high efficient organic solar cells. Org. Electron. 2013, 14, 1621–1628. [Google Scholar] [CrossRef]
- Huang, J.; Jia, H.; Li, L.; Lu, Z.; Zhang, W.; He, W.; Jiang, B.; Tang, A.; Tan, Z.A.; Zhan, C.; et al. Fine-tuning device performances of small molecule solar cells via the more polarized DPP-attached donor units. Phys. Chem. Chem. Phys. 2012, 14, 14238–14242. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Walker, B.; Nguyen, T.Q. A low band gap, solution processable oligothiophene with a diketopyrrolopyrrole core for use in organic solar cells. J. Phys. Chem. C 2008, 112, 11545–11551. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Yuan, M.; Okamoto, K.; Luscombe, C.K. Oligoselenophene derivatives functionalized with a diketopyrrolopyrrole core for molecular bulk heterojunction solar cells. ACS Appl. Mater. Interfaces 2011, 3, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, C.; Walker, B.; Nguyen, T.Q.; Seo, J.H. Morphology control of solution processable small molecule bulk heterojunction solar cellsviasolvent additives. RSC Adv. 2012, 2, 2232–2234. [Google Scholar] [CrossRef]
- Yu, Q.C.; Fu, W.F.; Wan, J.H.; Wu, X.F.; Shi, M.M.; Chen, H.Z. Evaluation of heterocycle-modified pentathiophene-based molecular donor materials for solar cells. ACS Appl. Mater. Interfaces 2014, 6, 5798–5809. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ning, Z.; Fu, Y.; Tian, H. Improvement of dye-sensitized solar cells: What we know and what we need to know. Energy Environ. Sci. 2010, 3, 1170–1181. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Numata, Y.; Han, L. Highly efficient dye-sensitized solar cells: Progress and future challenges. Energy Environ. Sci. 2013, 6, 1443–1464. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem., Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Zhang, Q.; Wu, W.; Pei, H.; Liu, B.; Tian, H. Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J. Org. Chem. 2008, 73, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, W. Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Jiang, S.; Cha, M.; Zhou, G.; Wang, Z.S. Thiophene-bridged double D–π–A dye for efficient dye-sensitized solar cell. Chem. Mater. 2012, 24, 3493–3499. [Google Scholar] [CrossRef]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.P.; Ruiz Delgado, M.C.; Casado, J.; Hernández, V.; Kim, O.K.; Woo, H.Y.; López Navarrete, J.T. Electronic modulation of dithienothiophene (DTT) as π-Center of D–π–D chromophores on optical and redox properties: Analysis by UV−Vis−NIR and Raman spectroscopies combined with electrochemistry and quantum chemical DFT calculations. J. Am. Chem. Soc. 2004, 126, 13363–13376. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Jiang, K.J.; Shao, K.F.; Yang, L.M. Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 2006. [Google Scholar] [CrossRef]
- Li, G.; Jiang, K.J.; Li, Y.F.; Li, S.L.; Yang, L.M. Efficient structural modification of triphenylamine-based organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 11591–11599. [Google Scholar] [CrossRef]
- Xu, M.; Li, R.; Pootrakulchote, N.; Shi, D.; Guo, J.; Yi, Z.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P. Energy-level and molecular engineering of organic D–π–A sensitizers in dye-sensitized solar cells. J. Phys. Chem. C 2008, 112, 19770–19776. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kim, D.Y.; Seo, Y. Resonant multiple light scattering for enhanced photon harvesting in dye-sensitized solar cells. Adv. Mater. 2014, 26, 5192–5197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Bai, Y.; Li, R.; Shi, D.; Wenger, S.; Zakeeruddin, S.M.; Gratzel, M.; Wang, P. Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 92–95. [Google Scholar] [CrossRef]
- Zhang, G.; Bala, H.; Cheng, Y.; Shi, D.; Lv, X.; Yu, Q.; Wang, P. High efficiency and stable dye-sensitized solar cells with an organic chromophore featuring a binary π-conjugated spacer. Chem. Commun. 2009. [Google Scholar] [CrossRef]
- Katono, M.; Bessho, T.; Meng, S.; Humphry-Baker, R.; Rothenberger, G.; Zakeeruddin, S.M.; Kaxiras, E.; Grätzel, M. D–π–A dye system containing cyano-benzoic acid as anchoring group for dye-sensitized solar cells. Langmuir 2011, 27, 14248–14252. [Google Scholar] [CrossRef] [PubMed]
- Katono, M.; Bessho, T.; Wielopolski, M.; Marszalek, M.; Moser, J.E.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. Influence of the anchoring modes on the electronic and photovoltaic properties of D−π–A dyes. J. Phys. Chem. C 2012, 116, 16876–16884. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, M.; Sun, S.; Shi, Y.; Ma, Z.; Sun, Z.; Xue, S. Synthesis and photovoltaic properties of organic sensitizers containing electron-deficient and electron-rich fused thiophene for dye-sensitized solar cells. Tetrahedron 2012, 68, 5375–5385. [Google Scholar] [CrossRef]
- Wang, M.; Xu, M.; Shi, D.; Li, R.; Gao, F.; Zhang, G.; Yi, Z.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. High-performance liquid and solid dye-sensitized solar cells based on a novel metal-free organic sensitizer. Adv. Mater. 2008, 20, 4460–4463. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, J.Y.; Lee, D.H.; Ko, M.J. Novel D–π–A organic dyes with thieno[3,2-b]thiophene-3,4-ethylenedioxythiophene unit as a π-bridge for highly efficient dye-sensitized solar cells with long-term stability. ACS Appl. Mater. Interfaces 2014, 6, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.; Choi, H.; Choi, H.; Lee, C.W.; Kang, M.S.; Song, K.; Nazeeruddin, M.K.; Ko, J. Molecular engineering of efficient organic sensitizers incorporating a binary π-conjugated linker unit for dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 14646–14653. [Google Scholar] [CrossRef]
- Choi, H.; Raabe, I.; Kim, D.; Teocoli, F.; Kim, C.; Song, K.; Yum, J.H.; Ko, J.; Nazeeruddin, M.K.; Grätzel, M. High molar extinction coefficient organic sensitizers for efficient dye-sensitized solar cells. Chem.Eur. J. 2010, 16, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Paek, S.; Lim, K.; Kim, C.; Kang, M.S.; Song, K.; Ko, J. Molecular engineering of organic sensitizers for highly efficient gel-state dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 8226–8233. [Google Scholar] [CrossRef]
- Cai, S.; Tian, G.; Li, X.; Su, J.; Tian, H. Efficient and stable DSSC sensitizers based on substituted dihydroindolo[2,3-b]carbazole donors with high molar extinction coefficients. J. Mater. Chem. A 2013, 1, 11295–11305. [Google Scholar] [CrossRef]
- Zong, X.; Liang, M.; Chen, T.; Jia, J.; Wang, L.; Sun, Z.; Xue, S. Efficient iodine-free dye-sensitized solar cells employing truxene-based organic dyes. Chem. Commun. 2012, 48, 6645–6647. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chou, H.H.; Tsai, M.C.; Chen, S.Y.; Lin, J.T.; Yao, C.F.; Chen, K. Thieno[3,4-b]thiophene-based organic dyes for dye-sensitized solar cells. Chem. Eur. J. 2012, 18, 5430–5437. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yen, Y.S.; Hsu, Y.C.; Chou, H.H.; Lin, J.T. Organic dyes incorporating the dithieno[3,2-b:2',3'-d]thiophene moiety for efficient dye-sensitized solar cells. Org. Lett. 2009, 12, 16–19. [Google Scholar] [CrossRef]
- Kozma, E.; Concina, I.; Braga, A.; Borgese, L.; Depero, L.E.; Vomiero, A.; Sberveglieri, G.; Catellani, M. Metal-free organic sensitizers with a sterically hindered thiophene unit for efficient dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 13785–13788. [Google Scholar] [CrossRef]
- Kwon, T.H.; Armel, V.; Nattestad, A.; MacFarlane, D.R.; Bach, U.; Lind, S.J.; Gordon, K.C.; Tang, W.; Jones, D.J.; Holmes, A.B. Dithienothiophene (DTT)-based dyes for dye-sensitized solar cells: Synthesis of 2,6-Dibromo-DTT. J. Org. Chem. 2011, 76, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, X.; Chen, R.; Zhang, R.; Hagfeldt, A.; Sun, L. Effect of different dye baths and dye-structures on the performance of dye-sensitized solar cells based on triphenylamine dyes. J. Phys. Chem. C 2008, 112, 11023–11033. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, H.S.; Yun, S.J. Synthesis of organic dye containing an alkylenesulfanyl-bridged bithienyl π-linker and its use in dye-sensitized solar cells. J. Photochem. Photobiol. A 2014, 275, 47–53. [Google Scholar] [CrossRef]
- Shellaiah, M.; Fang, H.P.; Lin, Y.L.; Hsu, Y.C.; Lin, J.T.S.; Lin, H.C. Synthesis of metal-free organic dyes containing tris(dodecyloxy)phenyl and dithienothiophenyl units and a study of their mesomorphic and photovoltaic properties. Tetrahedron 2013, 69, 2124–2130. [Google Scholar] [CrossRef]
- Chen, R.; Yang, X.; Tian, H.; Wang, X.; Hagfeldt, A.; Sun, L. Effect of tetrahydroquinoline dyes structure on the performance of organic dye-sensitized solar cells. Chem. Mater. 2007, 19, 4007–4015. [Google Scholar] [CrossRef]
- Akhtaruzzaman, M.; Menggenbateer; Islam, A.; El-Shafei, A.; Asao, N.; Jin, T.; Han, L.; Alamry, K.A.; Kosa, S.A.; Asiri, A.M.; et al. Structure–property relationship of different electron donors: Bovel organic sensitizers based on fused dithienothiophene π-conjugated linker for high efficiency dye-sensitized solar cells. Tetrahedron 2013, 69, 3444–3450. [Google Scholar] [CrossRef]
- Qin, H.; Wenger, S.; Xu, M.; Gao, F.; Jing, X.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. An organic sensitizer with a fused dithienothiophene unit for efficient and stable dye-sensitized solar cells. J. Am. Chem. Soc. 2008, 130, 9202–9203. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chiang, Y.J.; Kim, C.; Guo, Y.J.; Chen, S.Y.; Liang, Y.J.; Huang, Y.W.; Hu, T.S.; Lee, G.H.; Facchetti, A.; Marks, T.J. One-pot [1 + 1 + 1] synthesis of dithieno[2,3-b:3',2'-d]thiophene (DTT) and their functionalized derivatives for organic thin-film transistors. Chem. Commun. 2009, 14, 1846–1848. [Google Scholar] [CrossRef]
- Youn, J.; Chen, M.C.; Liang, Y.J.; Huang, H.; Ortiz, R.P.; Kim, C.; Stern, C.; Hu, T.S.; Chen, L.H.; Yan, J.Y.; et al. Novel semiconductors based on functionalized benzo[d,d']thieno[3,2-b;4,5-b'] dithiophenes (BTDTs) and the effects of thin film growth conditions on organic field effect transistor performance. Chem. Mater. 2010, 22, 5031–5041. [Google Scholar] [CrossRef]
- Youn, J.; Huang, P.Y.; Huang, Y.W.; Chen, M.C.; Lin, Y.J.; Huang, H.; Ortiz, R.P.; Stern, C.; Chung, M.C.; Feng, C.Y.; et al. Versatile α,ω-Disubstituted Tetrathienoacene Semiconductors for High Performance Organic Thin-Film Transistors. Adv. Funct. Mater. 2012, 22, 48–60. [Google Scholar] [CrossRef]
- Cheng, S.S.; Huang, P.Y.; Ramesh, M.; Chang, H.C.; Chen, L.M.; Yeh, C.M.; Fung, C.L.; Wu, M.C.; Liu, C.C.; Kim, C.; et al. Solution-processed small-molecule bulk heterojunction ambipolar transistors. Adv. Funct. Mater. 2014, 24, 2057–2064. [Google Scholar] [CrossRef]
- Youn, J.; Huang, P.Y.; Zhang, S.; Liu, C.W.; Vegiraju, S.; Prabakaran, K.; Stern, C.; Kim, C.; Chen, M.C.; Facchetti, A.; et al. Functionalized benzothieno[3,2-b]thiophenes (BTTs) for high performance organic thin-film transistors (OTFTs). J. Mater. Chem. C 2014, 2, 7599–7607. [Google Scholar] [CrossRef]
- Chen, M.C.; Vegiraju, S.; Huang, C.M.; Huang, P.Y.; Prabakaran, K.; Yau, S.L.; Chen, W.C.; Peng, W.T.; Chao, I.; Kim, C.; et al. Asymmetric fused thiophenes for field-effect transistors: crystal structure–film microstructure–transistor performance correlations. J. Mater. Chem. C 2014, 2, 8892–8902. [Google Scholar] [CrossRef]
- Zhou, N.; Lin, H.; Lou, S.J.; Yu, X.; Guo, P.; Manley, E.F.; Loser, S.; Hartnett, P.; Huang, H.; Wasielewski, M.R.; et al. Morphology-performance relationships in high-efficiency all-polymer solar cells. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Lou, S.J.; Smith, J.; Tice, D.B.; Hennek, J.W.; Ortiz, R.P.; Navarrete, J.T.L.; Li, S.; Strzalka, J.; et al. Polymer solar cells with enhanced fill factors. Nat. Photon. 2013, 7, 825–833. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, X.; Ortiz, R.P.; Li, S.; Zhang, S.; Chang, R.P.; Facchetti, A.; Marks, T.J. Bithiophene imide and benzodithiophene copolymers for efficient inverted polymer solar cells. Adv. Mater. 2012, 24, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, N.; Lou, S.J.; Hennek, J.W.; Ponce Ortiz, R.; Butler, M.R.; Boudreault, P.L.; Strzalka, J.; Morin, P.O.; Leclerc, M.; et al. Bithiopheneimide-dithienosilole/dithienogermole copolymers for efficient solar cells: Information from structure-property-device performance correlations and comparison to thieno[3,4-c]pyrrole-4,6-dione analogues. J. Am. Chem. Soc. 2012, 134, 18427–18439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Lee, B.; Timalsina, A.; Guo, P.; Yu, X.; Marks, T.J.; Facchetti, A.; Chang, R.P. Cross-linkable molecular hole-transporting semiconductor for solid-state dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16967–16975. [Google Scholar] [CrossRef]
- Zhou, N.; Buchholz, D.B.; Zhu, G.; Yu, X.; Lin, H.; Facchetti, A.; Marks, T.J.; Chang, R.P. Ultraflexible polymer solar cells using amorphous zinc indium tin oxide transparent electrodes. Adv. Mater. 2014, 26, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.D.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P. Air stable molecular semiconducting iodosalts for solar cell applications: Cs2sni6, as a hole conductor. J. Am. Chem. Soc. 2014. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, N.; Ortiz, R.P.; Chen, Z.; Loser, S.; Zhang, S.; Guo, X.; Casado, J.; López Navarrete, J.T.; Yu, X.; et al. Alkoxy-functionalized thienyl-vinylene polymers for field-effect transistors and all-polymer solar cells. Adv. Funct. Mater. 2014, 24, 2782–2793. [Google Scholar] [CrossRef]
- Harschneck, T.; Zhou, N.; Manley, E.F.; Lou, S.J.; Yu, X.; Butler, M.R.; Timalsina, A.; Turrisi, R.; Ratner, M.A.; Chen, L.X.; et al. Substantial photovoltaic response and morphology tuning in benzo[1,2-b:6,5-b']dithiophene (bbdt) molecular donors. Chem. Commun. 2014, 50, 4099–4101. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; Yau, S.L.; Kim, C.; Lee, W.-H.; Chen, M.-C. Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells. Polymers 2014, 6, 2645-2669. https://doi.org/10.3390/polym6102645
Kumaresan P, Vegiraju S, Ezhumalai Y, Yau SL, Kim C, Lee W-H, Chen M-C. Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells. Polymers. 2014; 6(10):2645-2669. https://doi.org/10.3390/polym6102645
Chicago/Turabian StyleKumaresan, Prabakaran, Sureshraju Vegiraju, Yamuna Ezhumalai, Shueh Lin Yau, Choongik Kim, Wen-Hsi Lee, and Ming-Chou Chen. 2014. "Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells" Polymers 6, no. 10: 2645-2669. https://doi.org/10.3390/polym6102645