Abstract
Amongst functional macromolecules, the combination of polymers and dyes is a research field of great potential with regard to high-performance materials. Accordingly, colored polymers have become increasingly important as materials for miscellaneous technical applications in recent years while also being a major part of everyday life. For instance, dye-containing polymers are nowadays widely applied in medicine, painting industries, analytics and gas separation processes. Since these applications are obviously connected to the dye’s nature, which is incorporated into the corresponding polymers, the affinity of certain polymers to dyes is exploited in wastewater work-ups after (textile) dyeing procedures. In this review, we wish to point out the great importance of dye-containing polymers, with a comprehensive scope and a focus on azo, triphenylmethane, indigoid, perylene and anthraquinone dyes. Since a large number of synthetic approaches towards the preparation of such materials can be found in the literature, an elaborated overview of different preparation techniques is given as well.
1. Introduction
Natural and synthetic dyes are compounds of great interest since they play an important role in our everyday life. The broad variety of technical and industrial applications, which includes “classical” utilizations like dyeing of textiles and other consumer goods as well as rather new usages such as laser dyes and dyes for organic light emitting diodes (OLEDs), liquid crystal (LC) displays, optical data storage and fluorescent labeling, has produced a great deal of research in this field. The main driving force is the constant demand for improved dyeing efficiency [1,2] or photochemical/photophysical properties [3,4], while also focusing on eco-friendly procedures [5,6], reduced toxicity [7,8], and decreased production costs [9]. A promising approach to fulfilling these requirements is the combination of dyes and polymeric materials, which will be highlighted in this review. The great advantage of such systems is the controllability of many features like solubility, stability, and toxicity through appropriate choice of polymeric material.
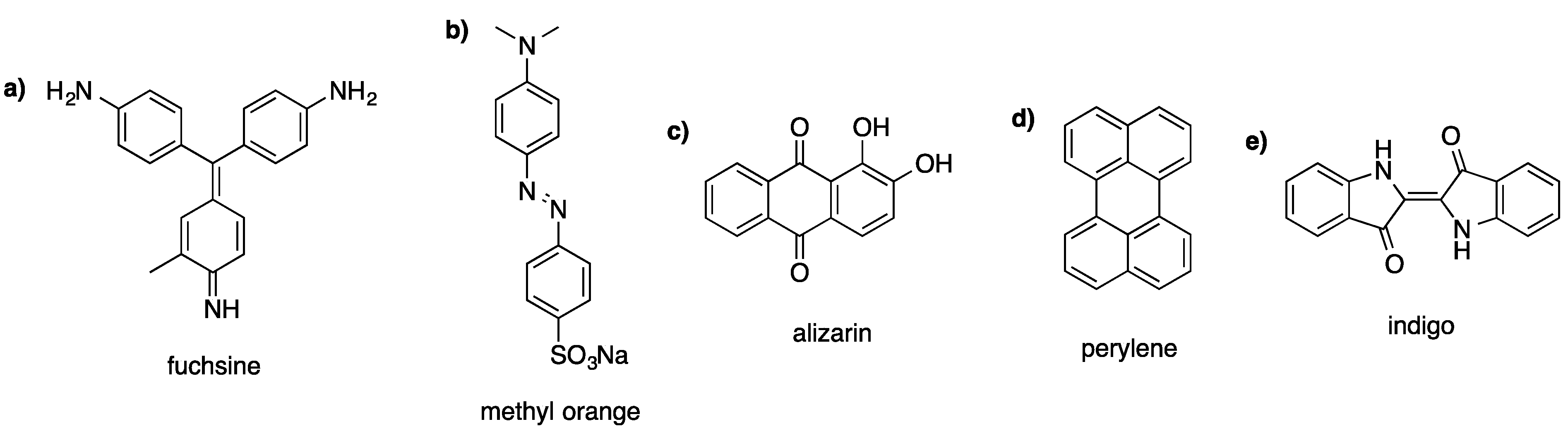
Widely applied and interesting representatives amongst the large number of dye categories are triphenylmethane, azo, anthraquinone, perylene, and indigoid dyes (see Figure 1). Due to the fact that these compounds cover a large spectrum of applications, they are the main focus of this article.
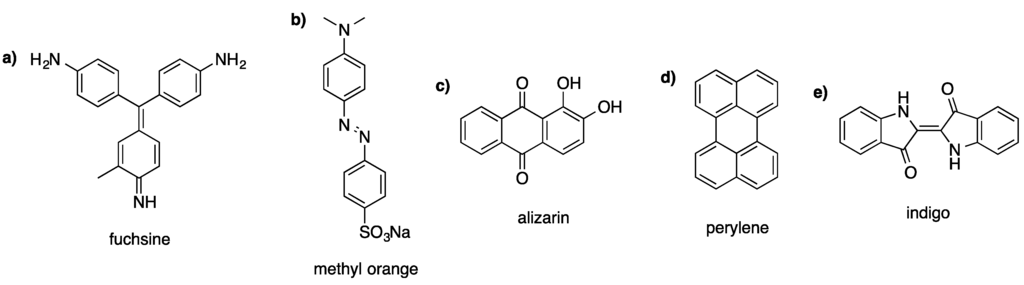
Figure 1.
Characteristic representatives of (a) triphenylmethane dyes, (b) azo dyes, (c) anthraquinone dyes, (d) perylene dyes, and (e) indigoid dyes.
Figure 1.
Characteristic representatives of (a) triphenylmethane dyes, (b) azo dyes, (c) anthraquinone dyes, (d) perylene dyes, and (e) indigoid dyes.
Triphenylmethane dyes owe their importance to their cheapness and brilliance of color with typical shades of red, violet, blue, and green [10]. The major application of these stains is their use in the textile industry for dyeing nylon, wool, silk, cotton, etc., in the paper and leather industries, and in the food and cosmetics industries [11]. Their high dyeing efficiency and the low light fastness are considered the major benefits of these stains. The underlying structures of triphenylmethane dyes are the colorless compounds triphenylmethane and triphenylcarbinol, whose conversion into dyes is achieved through the introduction of amino- or hydroxyl-groups stabilizing the cationic charge that serves as the chromophore. Depending on the resulting substitution pattern, monoamino-, diamino-, triamino-, and hydroxyl-triphenylmethane dyes are differentiated [12]. Another important application of some triphenylmethane dyes is their use as indicator dyes due to their pH sensitivity, which is derived from their constitution. Some important examples of this class of dyes are phenolphthalein, fuchsine, and fluorescein.
Azo dyes are numerically the most important class of dyes since more than 50% of all dyes listed in the Color Index are azo dyes. Covering all shades of color, azo dyes are used for dyeing textiles, paper, leather, rubber or even foodstuffs. Some examples of indicators, drugs, and histological staining agents are known as well. Since there are no naturally occurring azo dye derivatives, their synthesis usually involves two steps: diazotation and azo coupling, the latter of which requires a nucleophilic or anionoid center present in the coupling partner [13]. Regarding the spatial arrangement of the azo group, two configurations—cis and trans—are possible, with trans being the more stable one [14]. The UV-induced conversion to the cis-configuration is a well-investigated phenomenon that allows these compounds to be used for optical storage [15,16,17].
Another valuable class of dyes is represented by perylenes, belonging to the group of oligo(peri)naphthalene (rylene) chromophores. Due to their excellent physical and chemical properties, these compounds are widely used as laser markers, sensitizers in photovoltaic devices, and fluorescent labels [18]. The basic chromophores of these stains are perylene and the photochemically more stable perylenetetracarboxdiimide. Since the latter derivative is of outstanding value for single molecule spectroscopy, it serves as a key structure for a variety of chromophores [19]. Extension of the perylene motif with additional naphthalene units leads to larger chromophores such as terrylene and quaterrylene, which also offer a large number of applications [20].
Stains belonging to the class of anthraquinone-based dyes are prepared via introduction of various substituents to anthraquinone, which is readily available via oxidation of anthracene. Additionally, the rare mineral hoelite is known to be a natural source of anthraquinone. Due to their high color fastness and stability, anthraquinone dyes are important compounds in printing processes and textile dyeing [21,22,23]. An interesting aspect is their potential use in cross-dyeing processes since they exhibit a high affinity to silk and wool while leaving cellulose fibers unaffected [24]. Depending on the substitution patterns, some anthraquinone dyes also show biological/medical activity [25]. For instance, mutagenic, laxative, and antifungal derivatives are known [26,27,28]. Furthermore, the electronic properties of these dyes make them valuable candidates for photosensitizers and solar energy storage devices [29].
Indigo is one of the oldest natural dyes and has been used in the textile industry as a vat dye for more than 5000 years [30,31]. Many natural indigo derivatives like indirubin are known and can be found, e.g., in plants and slugs. Adolf von Bayer first described the synthesis of indigo starting from isatin. Nowadays, use of the precursor indoxyl, which can be obtained from the reaction of aniline, formaldehyde, and sodium cyanide is preferred [32]. The indigoid chromophore consists of two keto functions and a double bond in α-position and the overall planarity of the molecules causes a low solubility in water and most organic solvents [33]. The most important indigo derivatives, tyrian purple, indirubin, and indigo carmine, are used as food colorants (E132), as anti-cancer drugs and in coloration processes [34].
In the following article, we wish to give an overview of the different synthetic routes that lead to polymer-dye conjugates and typical applications of such materials.
2. Preparation Techniques
The binding modes leading to the formation of dye-polymer conjugates can be either covalent or non-covalent in nature. While the first approach obviously requires the formation of covalent bonds, non-covalent binding can occur through different kinds of interactions such as ionic and dipole–dipole interactions or through the formation of inclusion complexes.
2.1. Non-Covalent Attachment
2.1.1. Sugar-Based Polymers
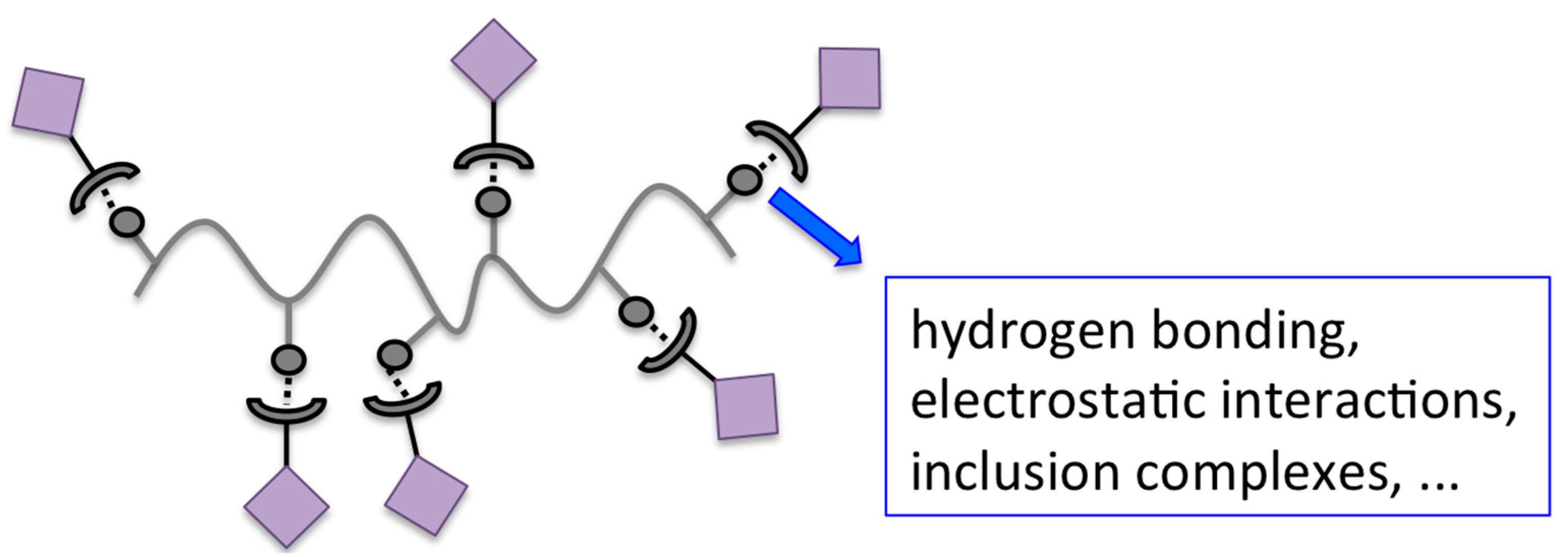
A schematic illustration of the non-covalent dye binding to polymeric materials can be found in Figure 2.
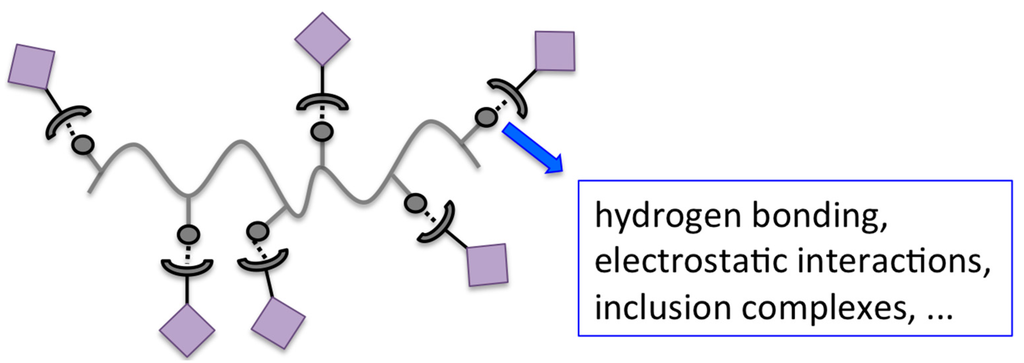
Figure 2.
Schematic illustration of non-covalent dye binding to polymers.
Figure 2.
Schematic illustration of non-covalent dye binding to polymers.
Due to the large number of polar substituents that enable the formation of dipolar interactions with adequate substrates, sugar-based (macro)molecules are suitable materials for the supramolecular attachment of dyes. Such oligo-/polysaccharides can be obtained from natural products (e.g., starch, cellulose, chitosane) or from chemical linkage of monomeric subunits and are therefore readily accessible.
The efficient adsorption of anionic azo dyes bearing sulfonate moieties to starch and β-cyclodextrin polymers was reported [35,36]. In the underlying studies, the polymers were prepared by cross-linking of β-cyclodextrin and starch, respectively, with hexamethylene diisocyanate. For both types of polymers, the main effects resulting in adsorption of the dyes were found to be hydrogen bonds formed between hydroxyl and amine groups located at polymers and the sulfonate moieties of the azo dyes. Additionally, cyclodextrins are known to form inclusion complexes with several azo dyes, and the formation of host guest complexes was therefore expected to contribute to the dye sorption of corresponding cyclodextrin-based polymers. The formation of such supramolecular complexes was verified, but a strong pH dependence was found and the whole effect was found to be inferior to the hydrogen bonding. Furthermore, the efficiency of calix[4]arene-based polymers for azo dye sorption was published [36]. Analogously to the cyclodextrin polymers, p-tert-butylcalix[4]arene-based polymers were prepared via condensation of the monomeric building blocks forming oligomeric structures. Compared to β-cyclodextrin polymers, lower affinity of the simple p-tert-butylcalix[4]arene-based oligomer with anionic azo dyes was determined. A drastic increase in adsorbance was observed when p-tert-butylcalix[4]arene oligomers bearing crown-6 functionalities on the lower rim were utilized. This led to the assumption that the ion pair complexation of sodium ions and the sulfonated azo dyes plays an important role during dye binding. Employing alkylamine derivatives of calix[4]arene polymers proved to be a promising approach as well [37]. Reaction with p-dibromo-xylene results in the formation of copolymers showing a strong affinity to anionic azo dyes. In this case, hydrogen bonds and electrostatic interactions formed between the amino residues and the hydroxyl groups of the azo dyes and the calix[4]arene units interactions are the most important effects leading to the dye adsorption.
Moreover, β-cyclodextrin polymers exhibited efficient adsorption of cationic dyes [38]. The polymers were prepared by cross-linking of β-cyclodextrin with epichlorhydrin in the presence of carboxymethyl cellulose. Adsorption measurements with the basic dyes Astrazone Blue, Crystal Violet, and Rhodamine B were performed at pH 8 at which the carboxyl groups attached to the cellulose units are negatively charged. The results obtained therefrom lead to the assumption that electrostatic interactions between the carboxylate moieties and the cationic dyes contribute to a chemisorption binding process. A structurally different β-cyclodextrin-based polymer was introduced by modification of N-vinylpyrrolidone [39]. In this study, propargyl functionalized N-vinylpyrrolidone was reacted with β-cyclodextrin azide in a Cu(I)-mediated azide-alkyne click reaction. The resulting β-cyclodextrin bearing monomer was then copolymerized with N-vinylpyrrolidone under free radical conditions yielding a polymer functionalized with 10 mol% β-cyclodextrin. With the intention to investigate the complexation capacity of the polymer bound β-cyclodextrin moieties, adsorption experiments with the indicator dye phenolphthalein were performed. The complexation of phenolphthalein by β-cyclodextrins is a well-investigated phenomenon. In basic solution the dominant species of the molecule is the ring-opened dianion. Upon addition of β-cyclodextrin, a complexation-induced re-lactonization of the molecule takes place and the resulting color change from pink to colorless allows for simple monitoring of the complexation efficiency [39,40]. In another study, doping of ethyl cellulose with different triphenylmethane dyes was achieved through dissolving of the polymer in an acetone/ethanol mixture and the subsequent addition of 1 wt.%–35 wt.% of Ethyl Violet and Crystal Violet, respectively [41]. Solid films of these composites were prepared via spin coating method and further investigated regarding non-linear optical properties.
2.1.2. Synthetic Polymers
Besides the sugar based materials discussed above, several other polymers were found to adsorb dye molecules through ionic or dipol–dipol interactions. For instance, polymers formed via electrostatic self-assembly were described [42]. Fiber-like polymeric materials were formed from the combination of a positively charged perylenediimide derivative and a negatively charged copper-phthalocyanide derivative. Helical stacking of both compounds leads to the polymeric structures, which are stabilized through a combination of charge transfer interactions and Coulomb coupling. Another system that also involves electrostatic interactions consists of poly(acrylic acid-co-acylamide) hydrogels and the cationic dye methyl violet [43]. Different molar ratios of both monomers were employed and hydrogel synthesis occurred via gamma-irradiation. Aqueous dye solutions were added and dye diffusion into the hydrogels was studied. A distinct correlation between dye uptake and pH value was found, since the protonation of methyl violet and acrylic acid are strongly pH dependent. The impregnation of poly(methyl methacrylate) (PMMA) with the azo dyes Disperse Red 1 and Disperse Orange 25 solved in supercritical carbon dioxide was investigated in 2003 [44]. Supercritical carbon dioxide serves as a good alternative for water in transporting the dye molecules, while acting as a swelling agent for the polymer at the same time. Adsorption of the dyes to the polymer was found to occur via hydrogen bonding or dipole–dipole interactions; however, it was revealed that relatively strong dye–dye interactions hinder the diffusion process. A solution to this problem was found by using a dye mixture. In that case, the intramolecular dye–dye interactions dominate the dye–polymer interactions, and accordingly, the diffusion rate is higher compared to the pure dyes. PMMA was employed for the non-covalent incorporation of dyes as well [45]. For this, PMMA beads were prepared via a surfactant-free emulsion polymerization technique. To the reaction mixture consisting of methyl methacrylate, water, toluene, and initiator, different fluorescent dyes were added. The resulting dye containing beads were further used for the formation of photonic crystals. In another study, the inclusion of the betaine dye 2,6-diphenyl-4-(2,4,6-triphenylpyridino)phenolate (DTPP, Reichardt’s Dye) and Cresol Red, respectively, into poly(vinylalcohol)-borax networks was investigated [46]. For this purpose, aqueous and alcoholic solutions of the dyes, suitable surfactants, poly(vinylalcohol), and borax were combined and allowed to stand for several hours. The resulting transparent hydrogels showed reversible thermochromic behavior. Since the conduction of several control experiments (e.g., temperature-dependent measurements in aqueous solution in the absence and presence of poly(vinylalcohol) and the surfactants) did not indicate any thermochromic behavior, the authors assumed that the micro-environment inside the polymer network plays a crucial role in the observed thermochromism. According to their findings, polymer-dye hydrogen bonds stabilize the colorless phenol form of the dye, while heating results in weakening of the hydrogen bonds, which triggers the dye to undergo a structural change to the colored phenolate forms. A different kind of thermochromic behavior can be referred to the temperature-dependent assembly and disassembly of dye aggregates. In this specific case, the emission characteristics of dye molecules in the aggregated state differ from those in solution. For instance, this was demonstrated for the dispersion of perylene derivatives into poly(vinyl alcohol) (PVA) matrices. By inclusion of N,N'-bis-(2-(1-piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic acid diimide dichloride (PZPER) in PVAs, the temperature dependent formation of dye aggregates causing shifts in emission/absorption spectra was observed. The polarity of the surrounding media was shown to influence the aggregation behavior as well, as shown for PZPER embedded into more hydrophobic poly(ethylene-co-vinyl alcohol) copolymers [47]. Aside from temperature and polarity changes, mechanical stimuli can affect the aggregate formation as well. The so-called mechanochromic polymers represent a highly important class of dye containing polymers that can be obtained through the non-covalent inclusion of dye molecules. Several examples can be found in which apolar matrices such as poly(ethylene) are employed. Due to the lack of polymer–dye interactions, the preparation of such dye-polymer dispersions requires special techniques such as melt-extrusion in order to prevent phase separation of both compounds [48]. For instance, oligo(phenylenevinylene) dyes showing the formation of excimers were embedded into poly(ethylene) films [49]. The ability of the polymer matrix to break up these aggregates was found to be mainly determined by the dye aggregate size, the crystallinity of the polymer matrix, and the strain rate. Hydrophobically modified perylene derivatives were also found to be suitable candidates for forming aggregates in poly(ethylene) matrices that are responsive to mechanical stimuli [50].
2.2. Covalent Attachment
A large number of synthetic routes can be found in the literature that allow for the covalent emplacement of dye molecules in polymeric materials. These can generally be categorized under the following approaches:
- (1)
- Polymerization of colored monomers;
- (2)
- Polycondensation or (cross)coupling reactions of adequate dyes/dye derivatives;
- (3)
- Polymer-analogous attachment of dye molecules to preformed polymers;
- (4)
- Preparation of high molecular weight derivatives of single chromophores (e.g., via grafting onto mechanism).
An overview of the different polymerization techniques and the accessible materials will be given in the following paragraphs. The preparation of polymeric dyes for technical and industrial applications is mainly conducted via routes 1–3 since the corresponding materials contain a higher dye concentration and therefore a higher tinctorial strength than the high molecular weight derivatives of single chromophores [51].
2.2.1. Polymerization of Colored Monomers
The conversion of dye molecules into polymerizable derivatives followed by their (co)polymerization is a valuable approach for the preparation of dye-containing polymers. The general procedure is illustrated in Figure 3.
Figure 3.
Schematic illustration of the preparation of polymeric dyes via copolymerization of colored monomers.
Figure 3.
Schematic illustration of the preparation of polymeric dyes via copolymerization of colored monomers.
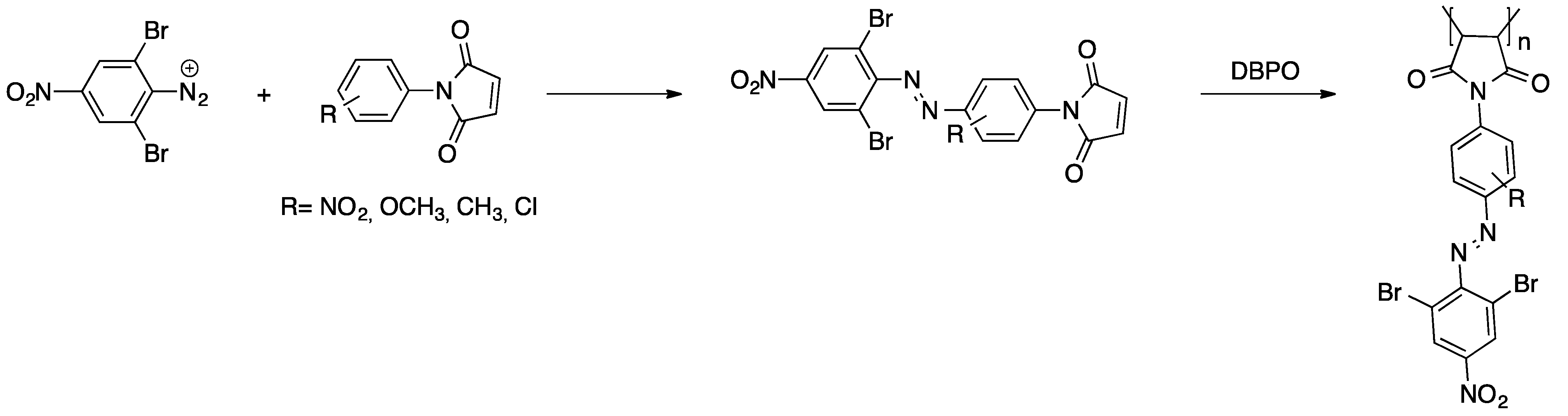
Several examples of azo dyes being converted into radically polymerizable compounds are known. An interesting example is acrylated azo dyes for materials applied in non-linear optics [52]. In the first reaction step, the azo dye was prepared via azo coupling of 6-(methyl(phenyl)amino)hexan-1-ol and a 4-nitrobenzenediazonium salt. Subsequently, this dye was (meth)acrylated through treatment with acryloyl chloride and methacryloyl chloride, repectively. Copolymerization with methyl methacrylate as well as homopolymerization of each dye monomer yielded linear soluble polymers. A different approach is the performance of azo coupling with N-aryl maleimides for the generation of radically polymerizable azo dye derivatives [53,54]. For the preparation of structurally different azo dyes, 2,6-dibromo-4-nitroaniline (see Scheme 1) and 2-amino-3,5-bis(ethoxycarbonyl)-4-methylthiophene were converted into the corresponding diazonium salts. These were subsequently coupled with several N-aryl maleimides yielding the desired dye monomers that readily polymerize under free radical conditions.
The preparation of a methacrylate-based azo dye monomer within three reaction steps was addressed in another survey (see Scheme 2) [55]. In the first step, tyramine was methacrylated by treatment with methacrylic anhydride. Consecutively, diazotation of 3-aminopyridine was conducted. The product obtained therefrom was then coupled with the methacrylated tyramine. The resulting azo dye monomer was incorporated into polymers through copolymerization with N,N-dimethylacrylamide. Furthermore, the copolymer was studied regarding its complexation behavior in presence of copper ions.
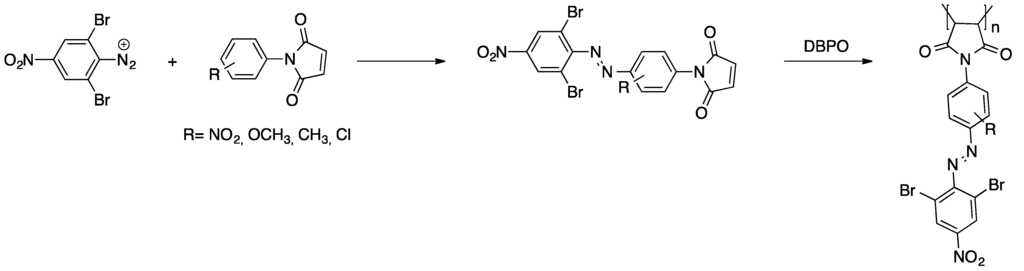
Scheme 1.
Synthesis of azo dye bearing N-aryl maleimides and their free radical polymerization [53].
Scheme 1.
Synthesis of azo dye bearing N-aryl maleimides and their free radical polymerization [53].
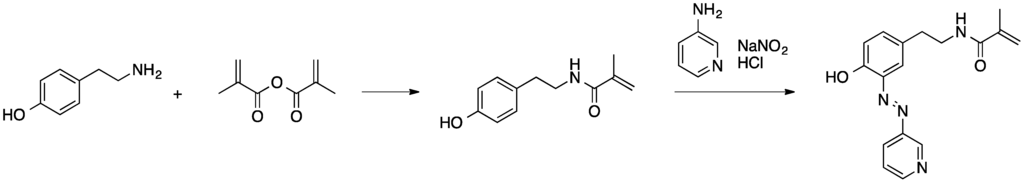
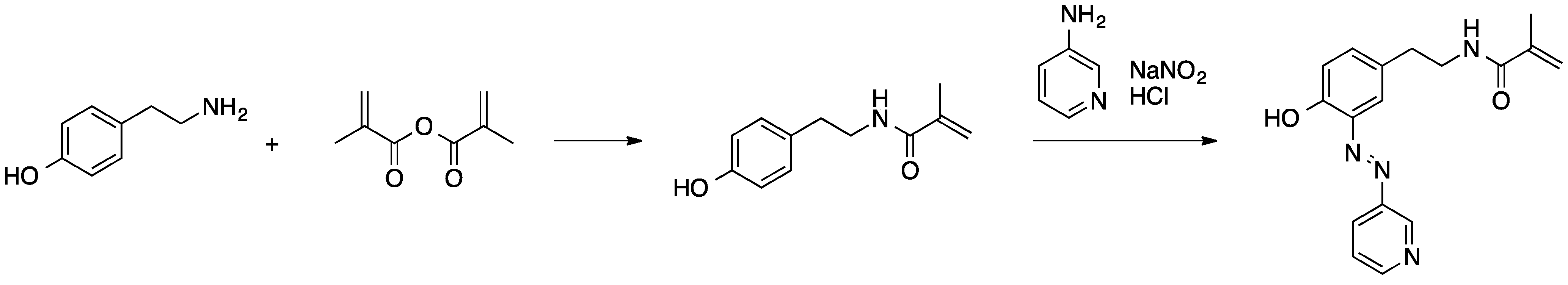
Scheme 2.
Synthesis of a polymerizable azo dye derivative [55].
Scheme 2.
Synthesis of a polymerizable azo dye derivative [55].
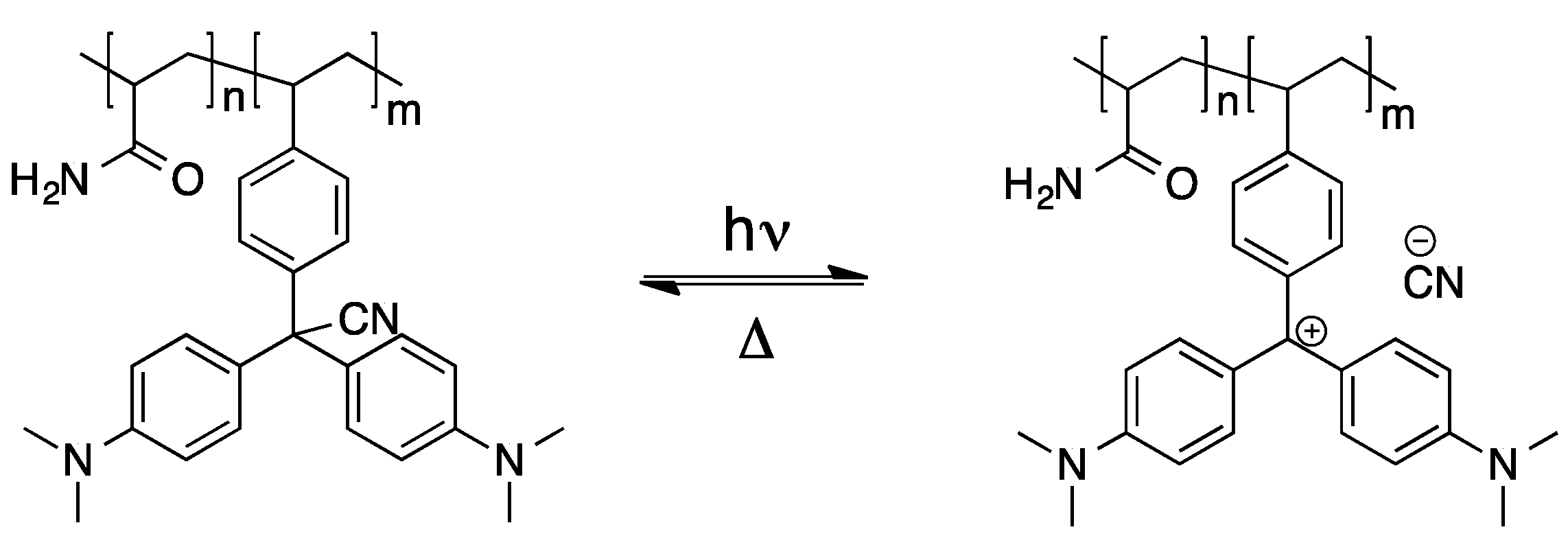
Regarding the polymerization of triphenylmethane dyes and potential applications thereof, an interesting study was published, in which acrylamide, N,N-methylenebisacrylamide, vinyl-substituted leucohydroxide and leucocyanide, respectively, were copolymerized [56]. The gels obtained therefrom undergo a large reversible deformation upon irradiation with UV-light, which can be referred to the light-induced dissociation of the leuco-derivatives (see Scheme 3). Additionally, the resulting triphenylmethyl cations cause a strong colorization of the polymeric materials.
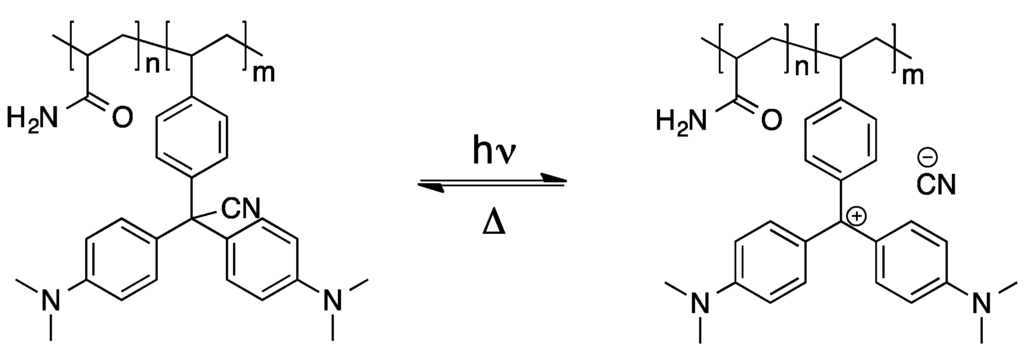
Scheme 3.
Dissociation of triphenylmethane leucocyanide upon UV radiation [56].
Scheme 3.
Dissociation of triphenylmethane leucocyanide upon UV radiation [56].
As another example of polymerizable triphenylmethane dyes, a radically polymerizable phenolphthalein derivative was obtained through reaction of phenolphthalein with N-(hydroxymethyl)acrylamide [57,58,59]. The latter compound possesses a relatively high reactivity towards nucleophilic attacks, which enables condensation with electron-rich aromatic substances under Friedel-Crafts-like conditions. The versatility of the reaction products, namely a monofunctional monomer as well as a bifunctional cross-linker, was demonstrated in several publications.
Furthermore, the preparation of different radically polymerizable anthraquinone derivatives of blue, red, and green color was introduced (see Figure 4) [60]. Mixing of these fundamental dyes enables a broad color spectrum to be covered. The different dye derivatives were prepared starting from 5,8-dichloro-1,4-dihydroxyanthraquinone, 1,4-dichloroanthraquinone and 1-chloroanthraquinone, respectively. As a first step, the compounds were reacted with a hydroxy-functionalized amine in a nucleophilic aromatic substitution reaction. The free aliphatic hydroxy groups were then methacrylated by treatment with methacryloyl chloride or methacrylic anhydride, respectively. The bifunctional cross-linkers and the monofunctional monomer obtained were polymerized with different methacrylate-based comonomers and the resulting polymers were found to be stable against UV radiation.
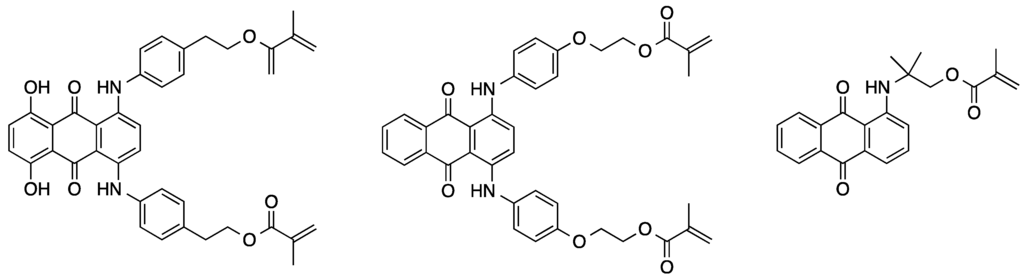
Figure 4.
Chemical structures of several polymerizable anthraquinone derivatives [60].
Figure 4.
Chemical structures of several polymerizable anthraquinone derivatives [60].
Several further examples of polymerizable dye derivatives belonging to different classes of dyes were described. For instance, the covalent emplacement of laser dyes into polymeric materials offers a variety of advantages such as improved handling and increased lasing efficiency. This was shown for 2-(2'-hydroxyphenyl)benzimidazole derivatives bearing allyloxycarbonyl groups that allow for radical polymerization [61]. Methyl methacrylate was found to be the best comonomer for the preparation of such solid dye materials due to the excellent transparency of the resulting polymers in the spectral range of interest. The synthesis of a polymeric donor–acceptor energy transfer system obtained from polymerization of monomeric derivatives of coumarin and bipyridine was also published [62]. Addition of ruthenium(II) to the polymeric materials resulted in the formation of energy transfer complexes with the coumarin moieties and the ruthenium complexes being the active centers. A solvatochromic and thermoresponsive N-isopropylacrylamide (NIPAM) based copolymer was prepared through incorporation of 4[(E)-2-(4-pyridinyl)ethenyl)phenol [63]. In basic solution, the dye was found to show the same solvatochromic behavior as described in literature for the low-molecular weight analog. 4[(E)-2-(4-pyridinyl)ethenyl)phenol was prepared through condensation of p-hydroxybenzaldehyde and 4-methylpyridine. The monomeric derivative of this compound was then obtained from treatment with 1-(chloromethyl)-4-vinylbenzene subsequent to protection of the phenolic hydroxyl group with triisopropylsilane. Deprotection was conducted after copolymerization of the monomer with NIPAM. NIPAM-based (co)polymers are well-known for their thermoresponsive behavior that can be observed as a phase transition from soluble to insoluble upon reaching a certain temperature, the so-called lower critical solution temperature. Since this phase transition causes the expulsion of water molecules from the polymer coils, it induces the solvatochromic color change.
2.2.2. Polycondensation and (Cross)coupling Reactions of Adequate Dye Derivatives
The great potential of polycondensation (schematic illustration, see Figure 5) has been demonstrated in several studies. For instance, several azo dyes and an anthraquinone dye were reacted with sebacoyl chloride in interfacial polycondensation reactions [64]. The polymers showed the same absorption maxima in UV/Vis spectra compared to their low-molecular weight analogues, but the maxima were found to shift when there were changes in the solvent systems.
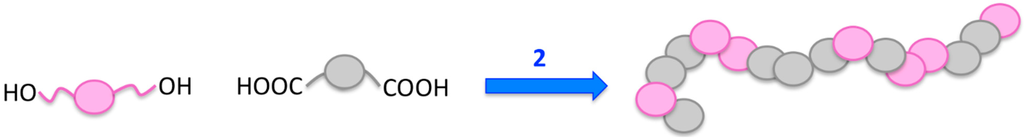
Figure 5.
General procedure of polycondensation for the preparation of dye-containing polymers.
Figure 5.
General procedure of polycondensation for the preparation of dye-containing polymers.
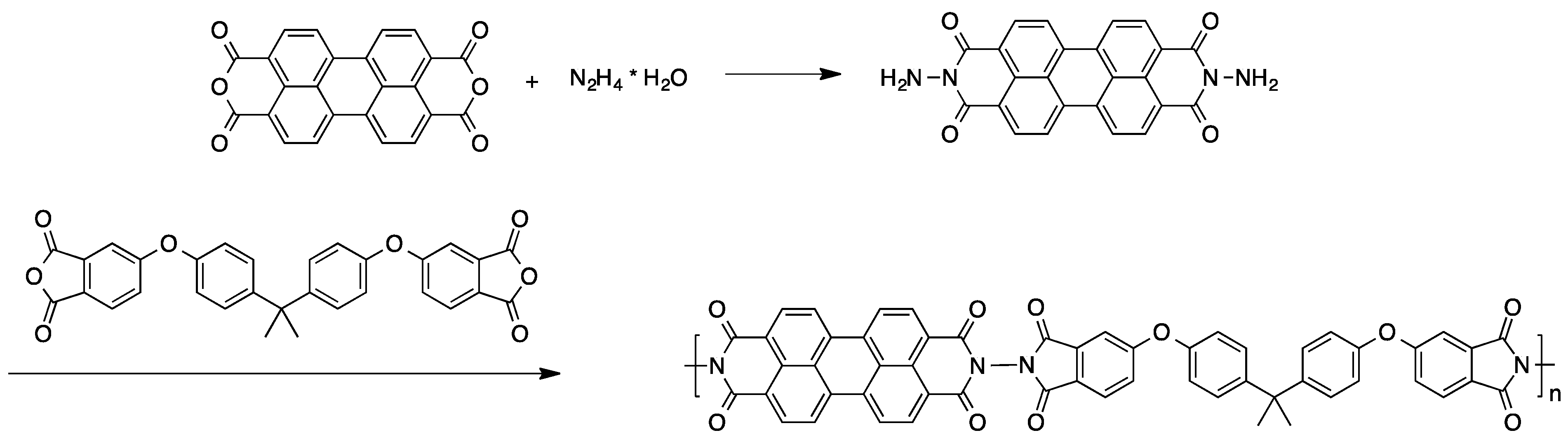
A valuable approach for obtaining high molecular weight perylene-containing copolymers was described in another survey [65]. Treatment of 3,4,9,10-perylenetetradicarboxylic dianhydride with hydrazine monohydrate gave the perylene-based monomer, which was then reacted with Bisphenol A dianhydride (see Scheme 4). In some cases, an additional diamine was added to the reaction mixture. The polycondensation of these compounds yields red polymeric materials.
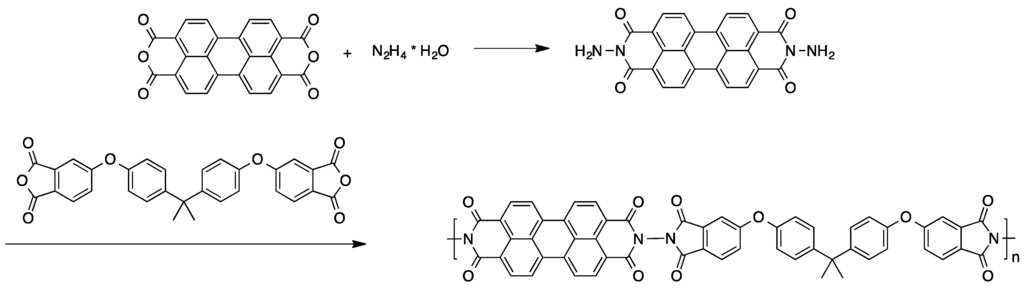
Scheme 4.
Preparation of perylene-containing polymers via polycondensation [65].
Scheme 4.
Preparation of perylene-containing polymers via polycondensation [65].
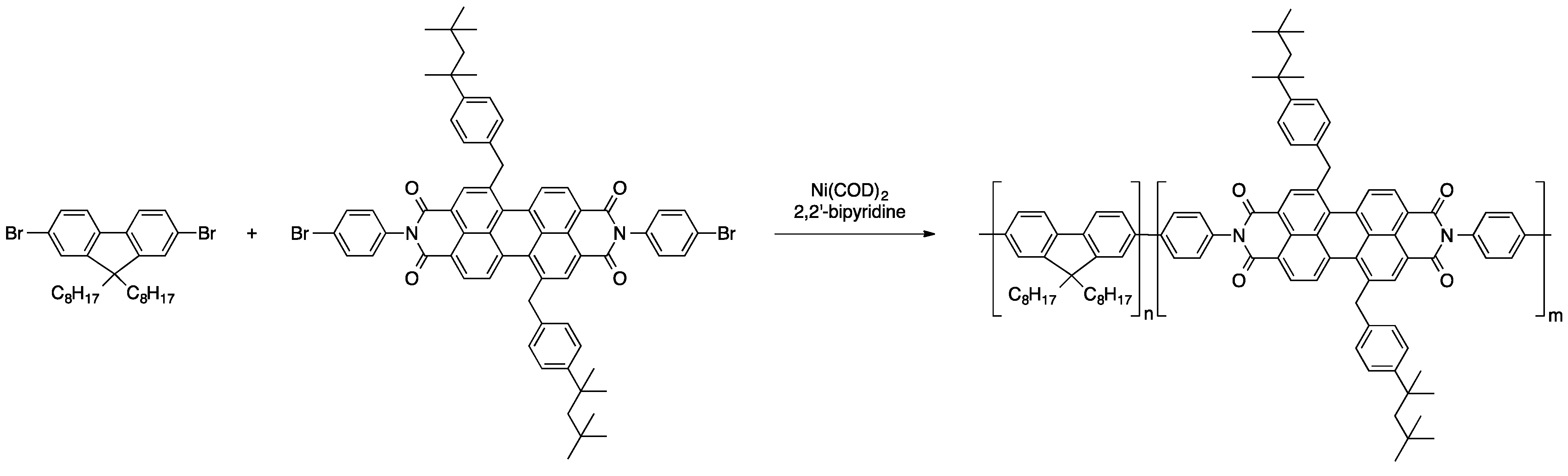
Another common way of introducing perylene dyes to polymeric materials is the coupling of dihalogenated derivatives with adequate comonomers that results in the formation of copolymers. A variety of such copolymers, in which the perylene moieties were either a part of the polymer backbone, pendant side groups, or served as end capping units at the polymer chain ends, was presented [66]. For instance, the emplacement of perylene dyes into the polymer backbone in a statistical fashion was achieved via Yamamoto polymerization of bisbrominated perylene derivatives derivatives and dibromodioctylfluorene (see Scheme 5). Through this nickel-mediated reaction, polymers of an average molecular weight of about 63,500 g/mol were obtained. A large spectrum of colors was covered through variation of the perylene derivatives.
Besides Yamamoto polymerization, Sonogashira and Suzuki coupling are versatile methods for the synthesis of difluoroboraindacene containing polymers as well. In one study, a di-iodinated derivative of difluoroboraindacene was prepared [67]. Homopolymerization of this molecule was conducted according to the protocol of Yamamoto, while use of 1,4-diethynylbenzene and benzene-(1,4)-diboronic acid enabled copolymerization via Sonogashira and Suzuki coupling, respectively.
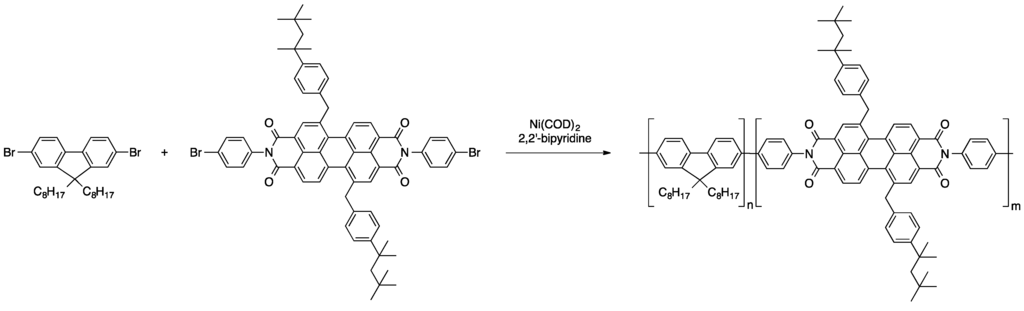
Scheme 5.
Yamamoto polymerization of dibromodioctylfluorene and a dibromoperylene bisamide derivative [66].
Scheme 5.
Yamamoto polymerization of dibromodioctylfluorene and a dibromoperylene bisamide derivative [66].
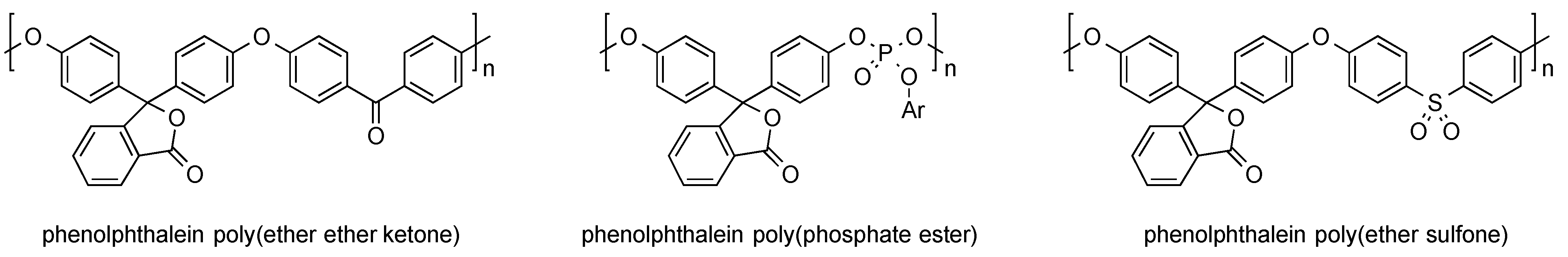
A large number of studies dealing with the incorporation of phenolphthalein into polymers via polycondensation indicate the great potential of such materials. For instance, several examples can be found in which the indicator dye was reacted with hydroxyl group bearing compounds. Typical materials derived this way are poly(ether ether ketones), poy(phosphate ester), and poly(ether sulfone), as can be seen in Figure 6.
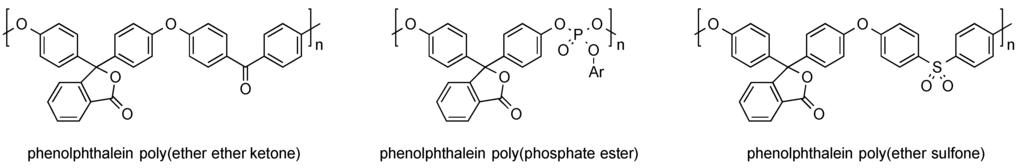
Figure 6.
Examples of phenolphthalein-containing polymers obtained from polycondensation reactions.
Figure 6.
Examples of phenolphthalein-containing polymers obtained from polycondensation reactions.
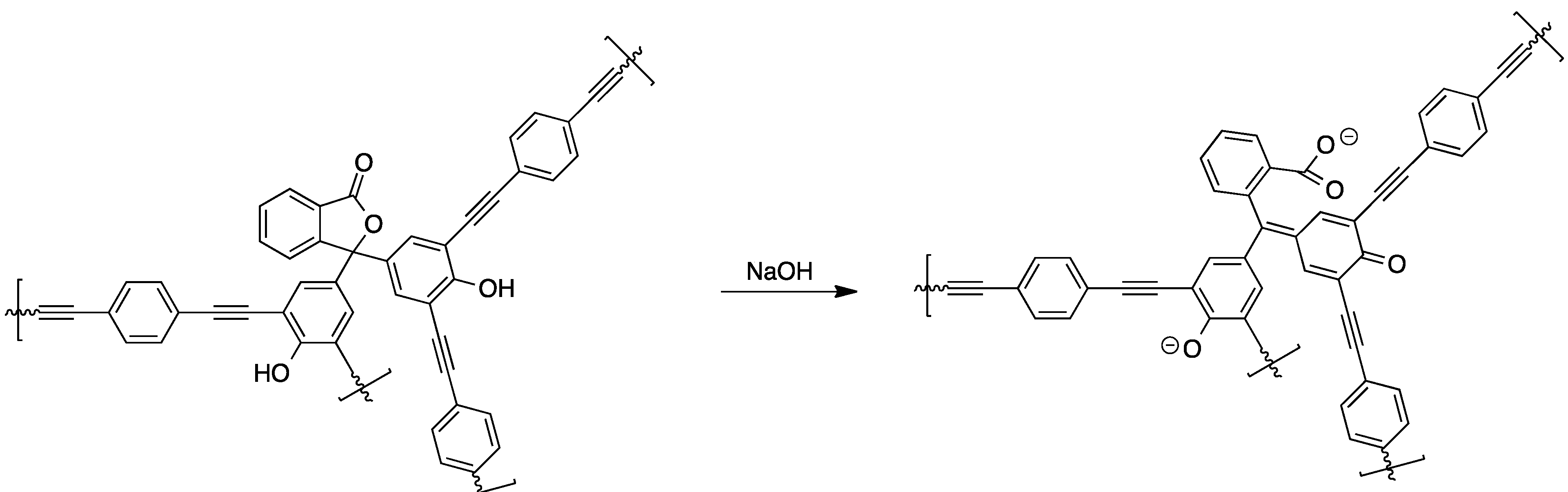
The reaction of phenolphthalein and related compounds with aliphatic and aromatic diacids yields the corresponding linear polycondensation products. For instance, this was shown for the condensation of phenolphthalein with isophthaloyl chloride and phosgene, respectively [68]. Furthermore, the preparation of poly(phosphate esters) from dichlorophosphoridates and phenolphthalein has been described in another study [69]. A very valuable compound is linear phenolphthalein-based poly(ether ether sulfone) since it is commercially available and enables the preparation of membranes. The versatility of this material was verified in several studies by preparing pH-sensitive membranes from phenolphthalein poly(ether sulfone). In one approach, poly(methyl methacryate) brushes were grafted onto a membrane consisting of phenolphthalein poly(ether sulfone) and poly(acrylonitrile) membrane surface, yielding pH-sensitive membranes [70,71]. As another example of phenolphthalein-based materials, polymeric materials in which the molecule’s pH sensitivity was kept intact were synthesized [72]. For this, the tetrabromo-derivative of phenolphthalein was reacted with 1,4-diethynylbenzene under Sonogashira cross-coupling conditions, yielding pH-switchable porous networks suitable for gas sorption applications (see Scheme 6).
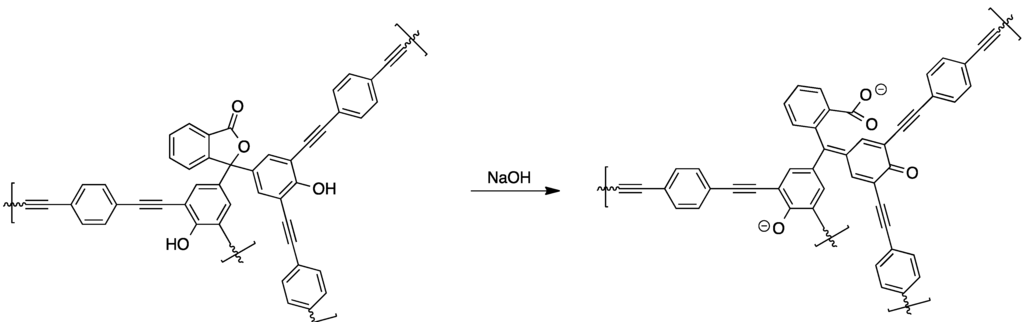
Scheme 6.
Chemical structure and pH-response of phenolphthalein-based microporous networks prepared via Sonogashira cross-coupling [72].
Scheme 6.
Chemical structure and pH-response of phenolphthalein-based microporous networks prepared via Sonogashira cross-coupling [72].
2.2.3. Polymer-Analogous Attachment of Dye Molecules to Preformed Polymers
The polymer-analogous attachment of dyes requires the presence of functional side groups bound to the polymeric backbone that can readily react with functional groups of small molecules (see Figure 7).
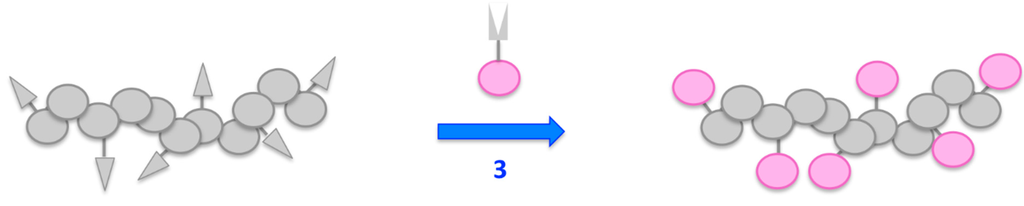
Figure 7.
Schematic illustration of the polymer analogous attachment of dye molecules.
Figure 7.
Schematic illustration of the polymer analogous attachment of dye molecules.
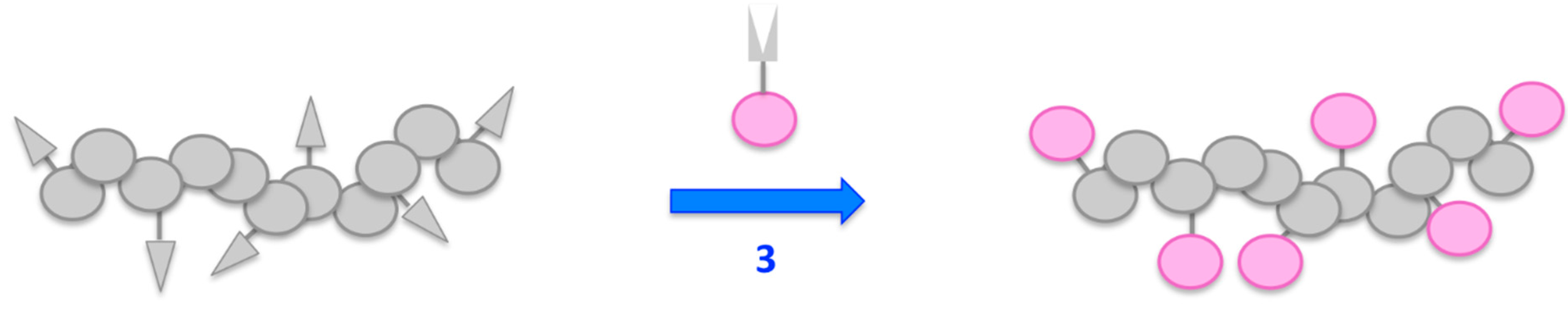
These small molecules can either be the dyes themselves, or a building block that enables the stepwise construction and attachment of a chromophore. Depending on the molecular structure of the dye as well as the nature of the reactive side group, this attachment can result in notable changes of the chromophoric system.
A useful route for the polymer analogous attachment of azo dyes to soluble catalyst carrying polymers was developed [73]. The polymeric backbone was synthesized from copolymerization of NIPAM and N-acryloxysuccinimide, which yielded linear polymers bearing active ester side groups. These were reacted with a catalyst derivative and amino-terminated methyl orange resulting in the formation of polymeric catalysts, including a colored “phase tag”.
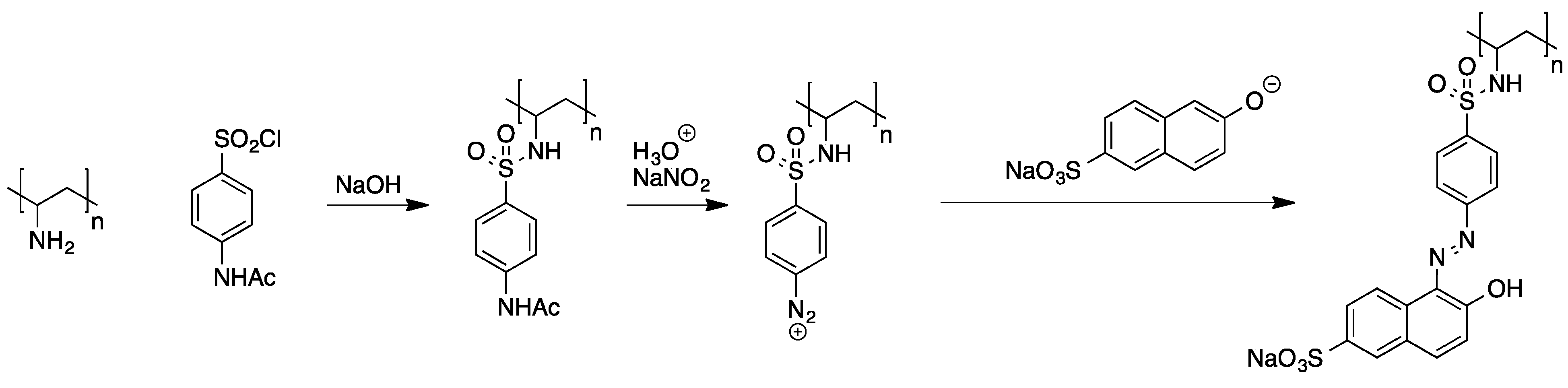
A variety of examples describing the attachment of azo dyes to preformed polymers have been published [51]. Polyelectrophiles such as poly(epichlorhydrin) and poly(chloromethylstyrene) were found to generally allow for tethering of nucleophilic building blocks that can be further converted to azo dyes, but the main problem with these compounds is their tendency towards cross-linking. Using polynucelophiles instead, the efficient attachment of N-acetylsulfanilyl chloride to poly(vinylamine) was demonstrated. The polymeric azo dyes were then obtained from subsequent deprotection and diazotation of the sulfanilic acid moieties followed by azo coupling with adequate aromatic compounds (see Scheme 7).
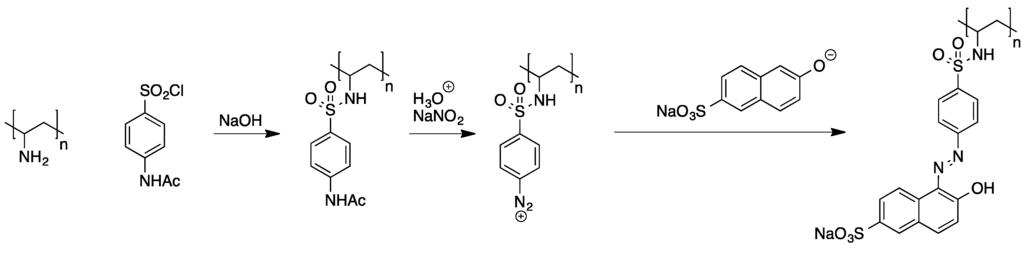
Scheme 7.
Stepwise attachment of an azo dye to poly(vinylamine) [51].
Scheme 7.
Stepwise attachment of an azo dye to poly(vinylamine) [51].
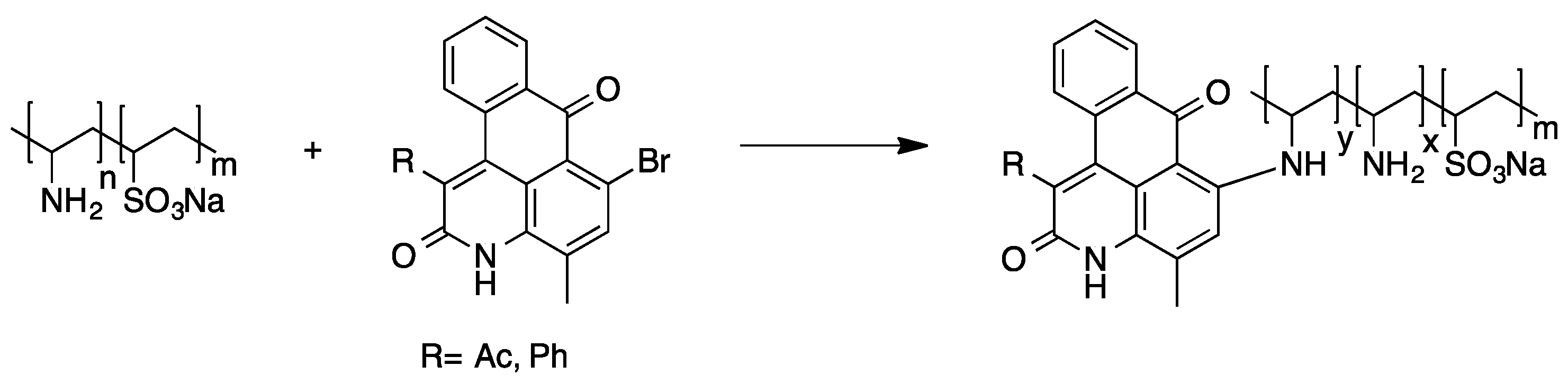
Poly(vinylamine) was found to be a suitable starting material for the attachment of anthraquinone dyes as well. The reaction of 1-bromoanthraquinone derivatives with poly(vinylamine) under Ullmann condensation conditions was used for the preparation of intensely colored polymers (see Scheme 8). Regarding the low solubility of some anthraquinone derivatives, it was found that the use of a vinyl sulfonate containing copolymer enabled the transfer of even highly hydrophobic polymer bound dyes into aqueous media [74].
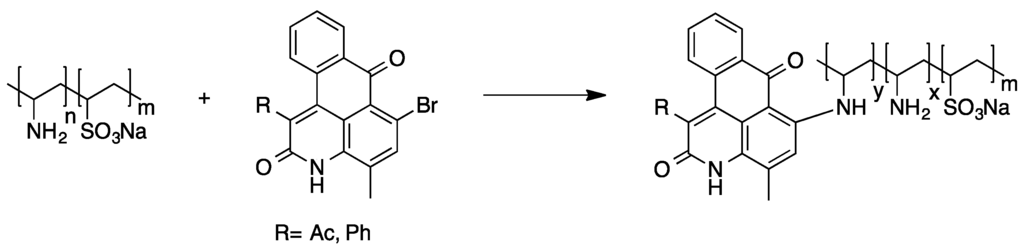
Scheme 8.
Polymer-analogous reaction of poly(vinylamine) and an anthraquinone dye [74].
Scheme 8.
Polymer-analogous reaction of poly(vinylamine) and an anthraquinone dye [74].
Further, the linkage of a triphenylmethane dye to polystyrene resins for the preparation of polymeric singlet oxygen sensitizers was investigated [75]. In the corresponding study, chloromethylated styrene-co-divinylbenzene beads were reacted with Bengal Rose. This dye molecule contains a carboxylate as well as phenoxide groups that readily react with the chloromethylene units upon heating and therefore allows for tethering to the beads.
2.2.4. Preparation of High Molecular Weight Derivatives of Single Chromophores
For industrial purposes, a class of macromolecule-functionalized dyes was developed my Milliken Chemicals. These dyes, sold under the name Versatint®, are high molecular weight derivatives of stains used for textile dyeing and offer unique properties such as good protection against fugitivity problems [76]. The materials are obtained via grafting polymerization of ethylene oxide onto the dye molecules. Resulting therefrom, single chromophores bearing one or more poly(ethylene glycol) grafts whose molecular weight can be adjusted to any desired value, were obtained [51]. Besides industrial applications, some purposes like the construction of well-defined polymer architectures require the introduction of a single dye molecule to a polymer.
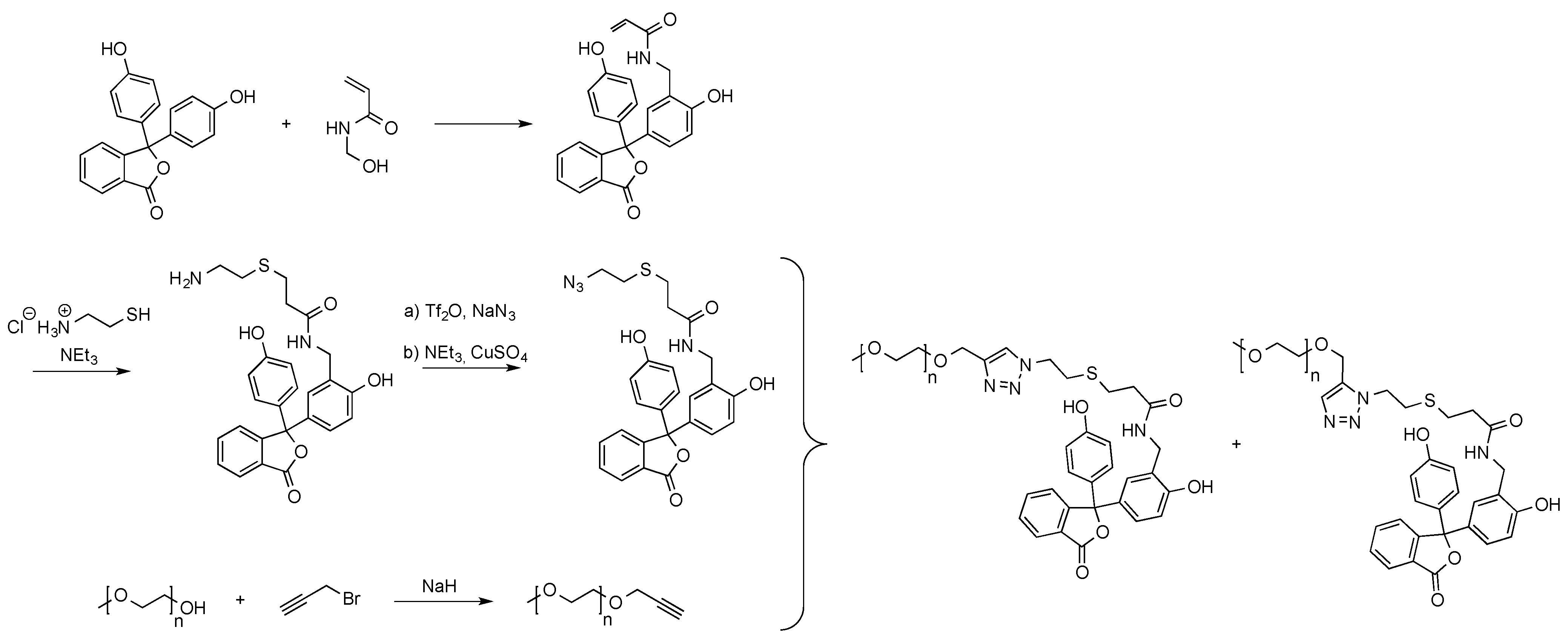
One example, in which a phenolphthalein derivative served as the end capping of poly(ethylene glycol), enabling the formation of supramolecular star copolymers, was recently published (see Scheme 9) [77]. For this, an acrylamide functionalized phenolphthalein monomer was reacted with cysteamine hydrochloride. The molecule’s primary amino group obtained from the thiol-Michael addition was then converted into an azide functionality facilitating the employment of the molecule in azide-alkyne click reactions. The polymeric alkyne counter part was obtained from the reaction of propargyl bromide with α-methoxy poly(ethylene glycol).
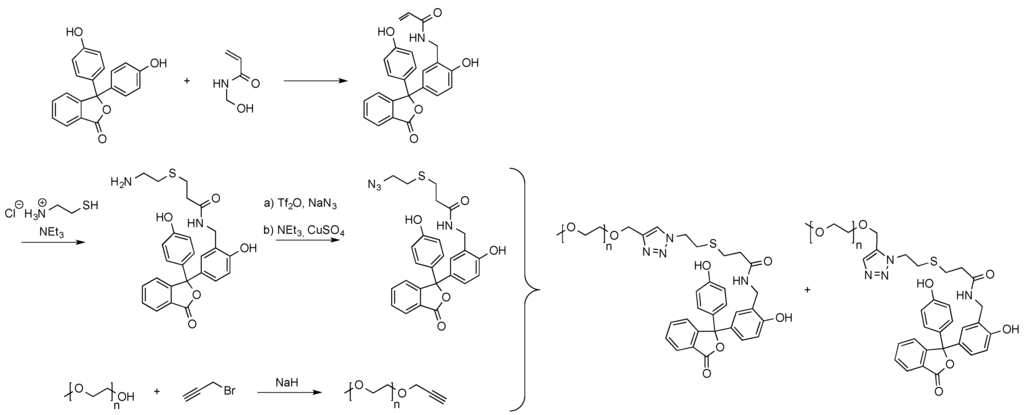
Scheme 9.
End group functionalization of α-methoxy poly(ethylene glycol) with phenolphthalein [77].
Scheme 9.
End group functionalization of α-methoxy poly(ethylene glycol) with phenolphthalein [77].
The synthesis of poly(NIPAM) bearing covalently attached azo dye end groups was achieved through a different approach (see Scheme 10) [78]. The polymerization of NIPAM was conducted in presence of 3-mercapropropionic acid as chain transfer agent yielding acid end group functionalized poly(NIPAM). Conversion to the corresponding acid chloride and subsequent condensation with N,N-dimethyl-[4-(4'-aminophenylazo)phenyl]amine resulted in the formation of the desired azo dye functionalized polymers.
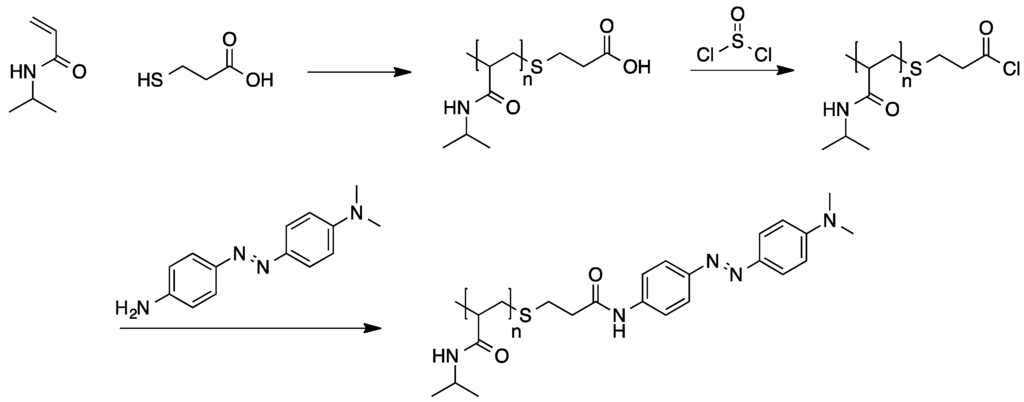
Scheme 10.
End group functionalization of poly(N-isopropylacrylamide) (PNIPAM) with an azo dye [78].
Scheme 10.
End group functionalization of poly(N-isopropylacrylamide) (PNIPAM) with an azo dye [78].
3. Applications
Synthetic dyes and especially polymer linked dyes are of growing interest for technical and medical applications due to their versatile properties and increasing environmental consciousness. The advantages over low-molecular compounds seem to be not only the reduced toxicity or the possibility of better recovery and reusability but also improved quality characteristics such as high color fastness in textile dyeing. Therefore, the typical applications of dye-containing polymers are not limited to color impression in paintings or textile dyeing. Moreover, utilization of dye properties in medicine, for analytical purposes or as optical sensors in chemical research, for example, is of great interest. Thereby, the dye does not need to be covalently attached to the polymer, since supramolecular interactions play an important role in dye-polymer chemistry as well. Conversely, the affinity of certain dyes to specific polymers and their non-covalent attachment can also be used for purification processes like waste water extraction. Below, typical applications of dye containing polymers are summarized and discussed in consideration of their properties and linkage to the polymer.
3.1. Waste Water Treatment
Dyes are valuable materials not only in the textile industry but also in the manufacturing of paper, plastics, cosmetics, medicine and biology. Hence, the environment is also being more and more affected by the steadily increasing frequency of dye pollutants, which induce unwanted color contaminations and sometimes have toxic effects to humans and animals [11,79]. Thus, effective dye extraction from waste water is becoming more important. Plenty of physical, chemical and biological methods such as ozonation, fungal decolorization and degradation, membrane-filtration, activated carbon usage or electrochemical techniques like coagulation have been developed and optimized in recent years [80,81,82]. Regardless, adsorption and the formation of inclusion complexes still seem to be the most effective and cheapest methods for the purification of effluents. One reason is the high stability of synthetic dyes due to their aromatic structure, which for example impedes degradation. Native adsorbents such as biopolymers, especially, are a good alternative to expensive and, in some cases, unselective purification techniques.
For instance, polysaccharide-based materials can be a cheap and effective alternative to commonly used systems [83,84]. They exhibit a high binding capacity and specificity while being non-toxic, stable and renewable. A notable example is represented by calix[4]arenes which have a high removal ability for selected water-soluble azo dyes [36]. Furthermore, biopolymers like starch and chitin are abundant and also modifiable to produce selective and biodegradable compounds like cyclodextrin and chitosan. The latter compound is a linear polycation with high affinity to reactive dyes like the anthraquinone and azo derivatives reactive blue 2 and reactive red 2 that are commonly used in textile dyeing and chemical research. Cross-linking of the polymers results in the formation of beads, which improves their handling. Current cross-linking agents for cyclodextrin, starch, and chitosan are epichlorhydrin, glutaraldehyde or ethylene glycol diglycidyl ether. In several publications epichlorhydrin cross-linked cyclodextrine and starch, as well as glutaraldehyde cross-linked chitosan, showed a high affinity, selectivity and capacity towards anthraquinone and azo dye pollutants in waste water [85,86,87]. For instance, chitosan has both chelating and complexing properties contributing to interactions with dyes. Furthermore, due to its reactive groups, it allows for the generation of intermolecular hydrogen bonds, cross-linking, or chemical activation. The only disadvantages in using chitosan are the variability in its characteristics, as the distribution of the acetyl groups along the backbone cannot be controlled, and its pH sensitivity [88]. Therefore, it is not suitable for cationic dyes if not modified. Chitosan can also be used for the adsorption of indigo carmine, an indigo derivative which is used as food additive and pH and redox indicator [89]. Different dyes and other pollutants in waste water as well as a suitable adsorbent with the associated adsorption capacities were listed by G. Crini in 2005 [83].
Polymers are not only used for waste water purification processes with dye pollutants. Due to the dyes’ affinity to polymers and several low-molecular compounds, or rather metal ions, dye-containing polymers can be used for the detection and determination of metal pollutants in industrial waste waters. The increasing use of lead in industry causes a high contamination of effluent and therefore produces hazards to human health. Early studies revealed the high selectivity of specific anthraquinone derivatives to lead ions [90,91]. Correspondingly, anthraquinone based poly(vinyl chloride) (PVC) membranes in which the anthraquinone is covalently attached to the polymer are of great value for lead determination in waste water. Figure 8 shows anthraquinone monomers, which can be copolymerized with vinyl chloride to form PVC membranes and show a high affinity to Pb2+ ions.
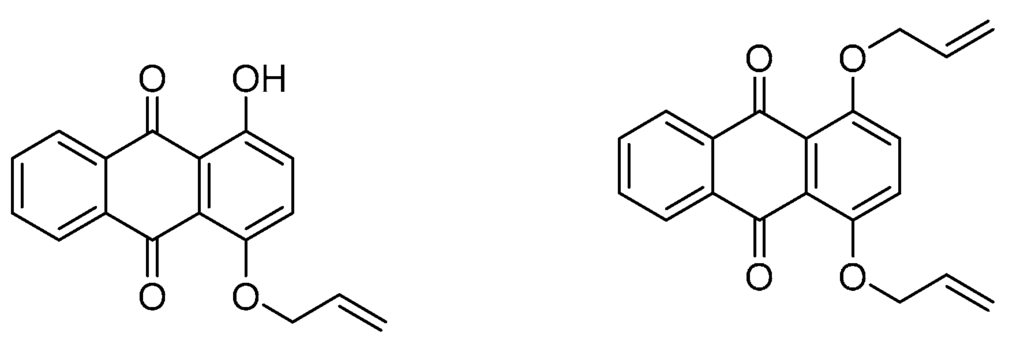
Figure 8.
Anthraquinone monomers used in poly(vinyl chloride) (PVC) membranes for lead waste water purification [92].
Figure 8.
Anthraquinone monomers used in poly(vinyl chloride) (PVC) membranes for lead waste water purification [92].
Such membranes can be used in a wide concentration range of pH values and can be used without any changes in effectiveness for at least 4 months. The effective pH range is 4.7 to 6.8 with ion concentrations of 2.5 × 10−6–1.0 × 10−2 M [92].
3.2. Paintings and Textiles
The best-known applications of dyes for hundreds of years are painting and colorization of textiles. These applications refer more to the color impression of the compounds than the chemical characteristics, whereby every dye class like azo, anthraquinone, indigo and triphenylmethane is represented. Most dyeing processes are restricted to non-covalent attachment to textile fibers like nylon, wool, silk and cotton. Covalent dye-polymer systems are also known, often offering even better characteristics. The most important dye-polymer systems in textile industry and paintings are described below.
Indigo is known as one of the oldest natural dyes in the world, having been used for more than 5000 years in the painting and textile industry [30,31]. Due to its low solubility in most solvents, the applications are limited in terms of use as a vat dye and pigment. Regardless, polymeric indigo derivatives are known that show a high resistance towards heat and solvents due to their low solubility and stability. Therefore, they can be used as high quality fibers and films [93]. Nowadays, indigo is still used for blue jeans but has been gradually replaced by different dye classes with diversified colors as indigo derivatives are limited to blue shades. Frequently used stains in the textile industry are anthraquinone derivatives. Due to the influence on the optical characteristics of the compounds from the position and nature of the substituents in the anthraquinone building block, it is possible to synthesize derivatives of almost every color. While electron-attracting groups hardly change the maximum of adsorption, the spectral displacement to the visible region increases with the electron-donating character of the substituent. Thus, various anthraquinone derivatives can be used as dyes, e.g., mordant, vat dyes or in the printing processes. Anthraquinone derivatives like β-thio or β-phenol substituted 1,8-dihydroxy-4,5-diaminoanthraquinone exhibited a high affinity for polymer fibers like polyesters and produce a blue-greenish color [94]. Their high color fastness and also their stability against heat and oxidizing agents make anthraquinone dyes no longer conceivable. Anthraquinone sulfonic acid derivatives, in some cases bearing further amino groups, are important compounds for dyeing processes due to their high affinity towards silk and wool while cellulose fibers leave unaffected. This feature allows for cross-dyeing processes for unions of mixed materials [24]. Similar high affinities to textile fibers are shown by triphenylmethane and azo dyes. Synthetic fibers like nylon and poly(acrylonitrile) modified nylon are effectively dyed as well as natural cotton and silk [11,95].
A much higher fastness to light, washing and solvents can be achieved by using polymer-linked dyes. Anthraquinone can be modified to enable the synthesis of dye-containing polyamides, polyesters, polyureas or poly(acrylonitrile). These polymers can show both a certain color impression and improved properties such as enhanced thermal stability. Furthermore, due to the vat dye character of anthraquinone, it can be transferred from an insoluble form into a soluble form by reduction. This gives the opportunity to wet spin the anthraquinone polymer into fibers and make it insoluble by oxidation [96]. Also, reactive dyes are important compounds for dyeing processes occurring via covalent attachment of the dye to the textile fibers. Typically, these dyes bear reactive groups like bromo or chloro substituents. For instance, the conversion of C.I. Disperse Yellow 23 into a reactive derivative can be achieved through modification with 1,3,5-trichloro-2,4,6-triazine or 2-bromoacrylic acid [97]. Conduction of the dyeing process in supercritical CO2 enables the activation of functional groups such as amino, hydroxyl or thiol groups that are present in natural fibers like cotton or protein fibers. This enables readily attaching the reactive dyes via covalent bond without further pre-treatment of the textile fibers. The more traditional way of dyeing cotton fibers is called “exhaust dyeing” and consists of two steps. First, the dye is sulphonated by the addition of sodium sulphonate in the presence of salt. Second, the dye is covalently fixed onto the cotton fiber by use of alkali (sodium carbonate) [98,99]. For improving the dyeing performance of reactive dyes on cotton, the fibers can be pre-treated with amino-group-bearing dendrimers like AstramolTM [100]. Technically, the dye is then attached to the dendrimer, which allows enhanced dyeing efficiency and color strength since primary amino groups are easier to activate than the hydroxyl-groups in the cotton fiber.
3.3. Medicine
Dyes play an important role in medical applications because of both their physicochemical properties and color impression. Since this often involves applications inside the human body, it is quite important to use non-toxic derivatives. To skirt that problem, polymers are a good alternative to low-molecular dyes for in vivo applications like polymer-linked water-soluble rylene dyes for cell staining. Perylene-dicarboxmonoimide, perylene-tetracarboxdiimide and benzoylterrylen-3,4-dicarboximid were modified with a single poly(ethylene oxide) chain to obtain polymeric rylene dyes which are suitable for staining cellular membranes (Chinese hamster ovarian cell line, bronchial carcinoma cell line and ovarian carcinoma cell line). The special feature of these polymeric materials is their ability to indicate the polarity of their environment due to their high fluorescence in nonpolar solvents and a much lower fluorescence in polar medium caused by aggregation [20].
Another in vivo application of polymer-attached dyes in medicine could be the therapy of rheumatoid arthritis. In this case the dye is instead needed for polymer tracking in the body, as it is used as a fluorescence dye (atto680). Therefore, a copolymer consisting of N-(2-hydroxypropyl)methacrylamide) and N-(3-azidopropyl)methacrylamide is used which allows a postmodification with the fluorescence dye by azide-alkyne click-reaction. Tests with murine models of rheumatoid arthritis proofed that the polymer has a high accumulation in the inflamed joints. The synthesized polymer is hydrophilic and exhibited size-dependent blood circulation. High molecular-weight polymers (54 kDa) had a very good bioavailability and could still be detected 24 hours after injection [101,102].
Very application-oriented examples of polymers in medicine are artificial iris implants, which are used to treat iris defects by improving vision and reducing glare. Therefore, it is necessary to use polymer-attached dyes which can be polymerized and cross-linked into blanks and afterwards sharpened to the required iris form. Suitable monomers with a broad spectrum of synthesizable colors are methacrylated anthraquinone dyes. They can be synthesized in blue, green and red colors and afterwards copolymerized with 2-hydroxyethylmethacrylate and tetrahydrofurfuryl methacrylate and cross-linked with ethylene glycol dimethacrylate. By mixing different anthraquinone monomers almost every color can be generated [60]. The broad spectrum of colors and iris implants resulting from a red and a blue anthraquinone monomer are pictured in Figure 9
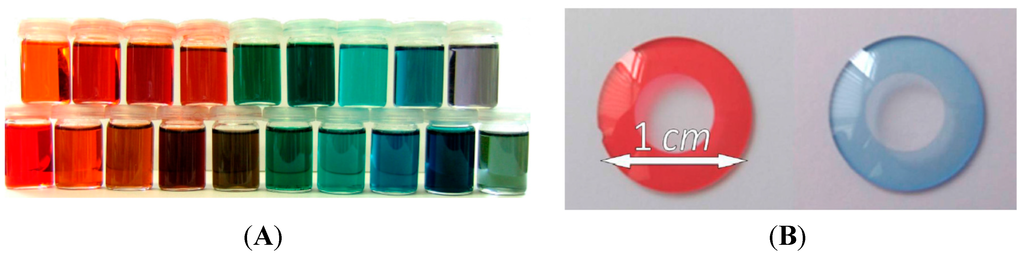
Figure 9.
The broad color spectrum of mixed anthraquinone monomers (A) and the resulting iris implants after copolymerizing, crosslinking and sharpening the anthraquinone derivatives (B) [60]. Reprinted with the permission from Beilstein Journal of Organic Chemistry, 2013, 9, 453–459.
Figure 9.
The broad color spectrum of mixed anthraquinone monomers (A) and the resulting iris implants after copolymerizing, crosslinking and sharpening the anthraquinone derivatives (B) [60]. Reprinted with the permission from Beilstein Journal of Organic Chemistry, 2013, 9, 453–459.
3.4. Photochemistry
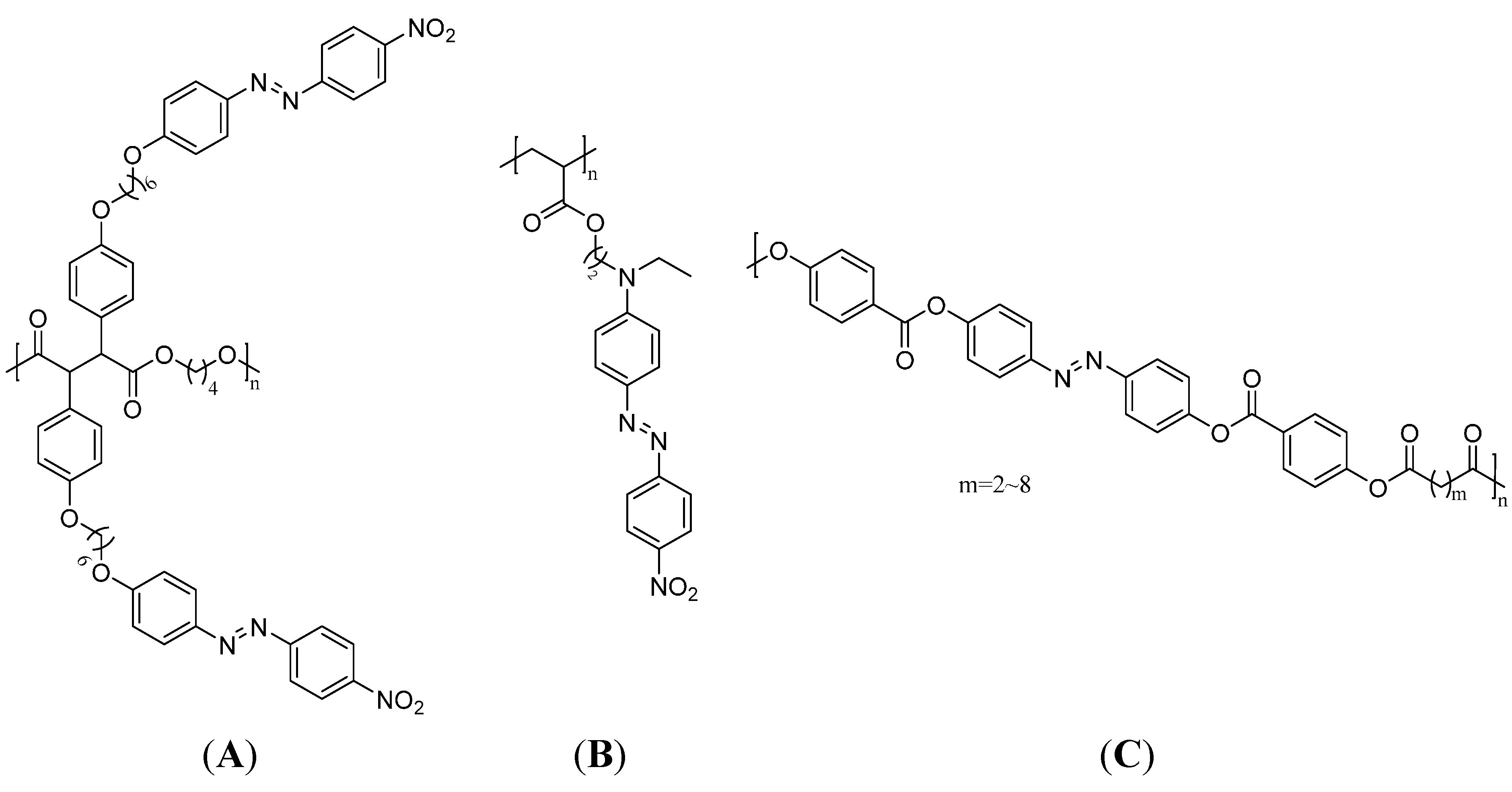
Azo dyes are one of the most important dye classes in the world since many of them exhibit nonlinear optical characteristics, liquid crystallinity, light-induced dichroism, and birefringence [103]. These properties are complemented by their ability to switch from the more stable trans-configuration into the cis-configuration upon exposure to UV-radiation or visible light. Therefore, they find various applications in photochemistry such as optical storage devices [16,104,105]. Polymers for this application can either be main-chain or side-chain azo polymers and have the ability to orientate the modified azobenzene groups perpendicular to the direction of the irradiated linearly polarized light. This so-called Weigert effect induces birefringence of the materials [106,107]. To reverse this process, the azobenzene polymer can either be irradiated with circularly polarized light to induce a photochemical cis-trans-isomerization or the isomerization can occur thermally. Thereby, the original disorder in the molecule is reconstituted [15]. Hence, the stability of the cis-configuration plays an important role for the desired application. For instance, aromatic azobenzene compounds have quite good stabilities in the cis-configuration and are able to store light energy as a chemical structure change. The optical storage can be either holographic or digital. Suitable polymers are compounds with rigid mesogenic groups combined with flexible spacers to form liquid crystalline azo polymers with a good ability of reorientation upon radiation. While main-chain liquid crystalline azo polymers are mostly polyesters, the side chain compounds can have ester, amide or phosphazene backbones. Recently, new copolymers with pendant azo-benzene chromophores that can be used as memory devices have been established. The azo dye was functionalized with a polymerizable maleimide group and copolymerized with styrene to yield an active layer with rewritable flash memory performance [108]. Additional, cross-linked azo-polymers are known; however, cross-linking seems to depress the azo groups’ isomerization ability. Azo dye doped polymers like poly(methyl methacrylate) can also be used as optical data storage media, but in these cases the chemical linking is negligible [109]. Some important side chain and main chain azo polymers are shown in Figure 10.
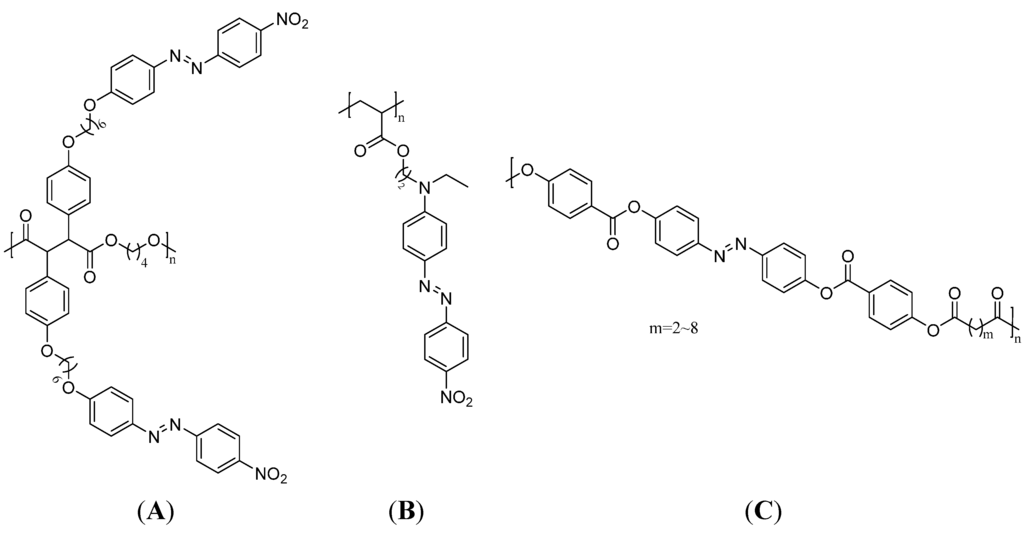
Figure 10.
A side-chain liquid crystalline azo polymer (A), a side-chain azo polymer without liquid crystallinity (B) and a main-chain liquid crystalline azo polymer (C) [103,104].
Figure 10.
A side-chain liquid crystalline azo polymer (A), a side-chain azo polymer without liquid crystallinity (B) and a main-chain liquid crystalline azo polymer (C) [103,104].
Further applications derived from the liquid crystallinity and the trans-cis-isomerization are for example passive optical elements like optical sensors and controlled optical and photooptical media.
Another advantage of azo polymers is their nonlinear optical behavior. Therefore, they can be used as modulators or optical frequency doublers and switches while showing good mechanical characteristics and processability. Polymers used for the preparation of nonlinear optical azopolymers are—amongst others—polyacrylates, polyesters and polysilanes.
3.5. Optical Sensors
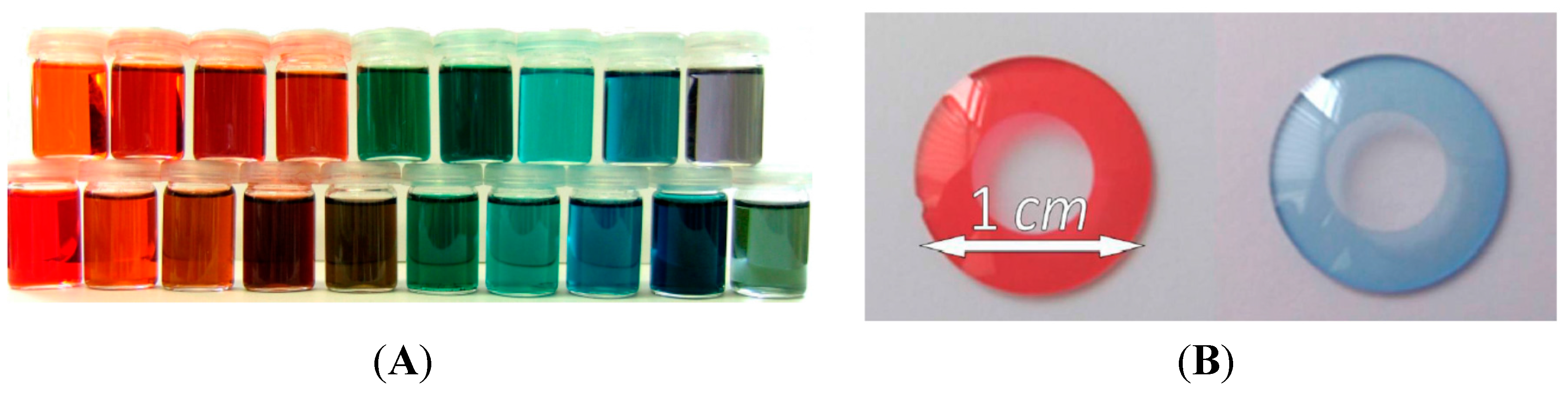
Besides medicine, paintings, waste water treatment, and photochemistry, polymer-attached dyes have various other applications, for instance in electrochemistry or chemical research. One example of polymer-attached dyes, which refer to the optical characteristics of the dye, is polymer-attached phenolphthalein that can be used as pH-sensitive material for analytical purposes. The triphenylmethane-based indicator dye phenolphthalein is colorless within a pH range of 0–8.2 and turns violet at higher pH values. One disadvantage of this dye is its insolubility in water. By copolymerization of an acrylated phenolphthalein derivative with hydrophilic monomers such as acrylic acid or N-(isopropyl)acrylamide, water-soluble polymers with pH sensitivity can be obtained. Moreover, the diacrylated phenolphthalein derivative can be used as a cross-linker to form superabsorbent and insoluble polymers while still being pH-sensitive. Therefore, these polymers are suggested for reusable materials for analytical purposes to indicate basic medium [57]. The color change of a cross-linked phenolphthalein-containing polymer from acidic to basic medium and back is shown in Figure 11.
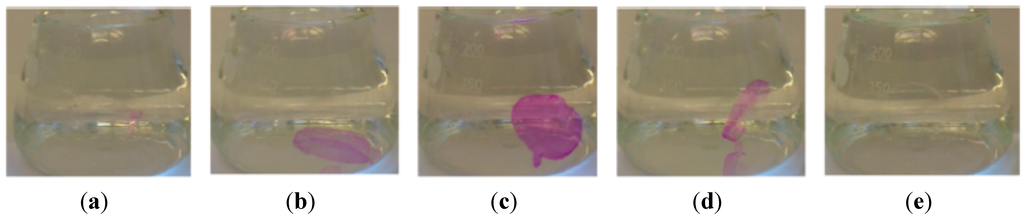
Figure 11.
Cross-linked phenolphthalein containing polymer blank in distilled water (a), after adding NaOH solution (b), over pH 10 (c), after adding HCl (d) and again in neutral solution (e) [57]. Reprinted with the permission from Macromolecules, 2012, 45, 5343–5346. Copyright 2012 American Chemical Society.
Figure 11.
Cross-linked phenolphthalein containing polymer blank in distilled water (a), after adding NaOH solution (b), over pH 10 (c), after adding HCl (d) and again in neutral solution (e) [57]. Reprinted with the permission from Macromolecules, 2012, 45, 5343–5346. Copyright 2012 American Chemical Society.
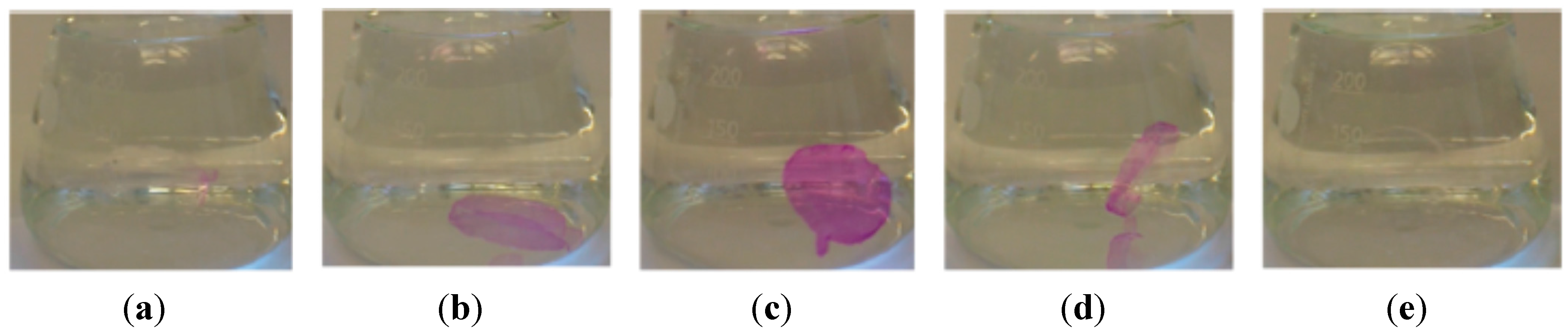
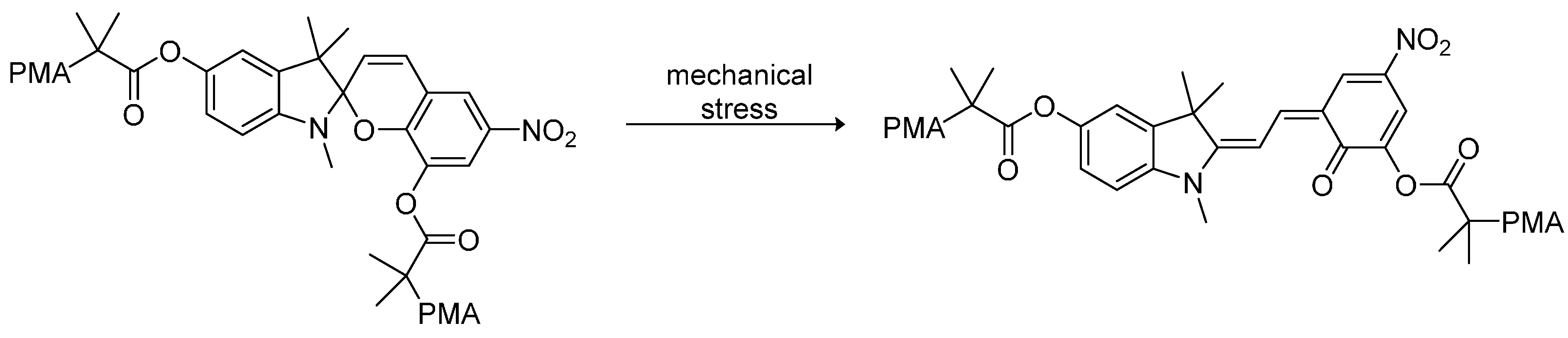
Since the late 1990s, chromogenic (e.g., mechanochromic, photochromic, and thermochromic) materials represent a quite new field of dye-containing polymers. The advantage of those materials is their combination of the viscoelastic properties of thermoplastic polymers and the optical response to changes in temperature, visible light or mechanic deformation of the dye attached to the polymer. In general, dye-polymer blends in which the bonding is supramolecular (e.g., hydrogen bonding, van der Waals forces, and dipole–dipole interactions) and covalent linkage of the dye to the polymer backbone are distinguished. Dyes used for supramolecular chromogenic polymers are, for instance, oligo(p-phenylene vinylene) derivatives (OPV derivatives), bis(benzoxazolyl) stilbene (BBS), and perylene bisimides as well as some triphenylmethane dyes like cresol red [46,48]. These dyes are so-called “aggregachromic” dyes. For their use in mechanochromic materials the formation of dye aggregates accompanied by the simultaneous interaction with the polymer matrix is required. Therefore, many dyes are functionalized with alkyl chains to balance both interactions. The mechanism of mechanochromic polymers is based on breaking up the dye aggregates via mechanical deformation. Hence, the dye mixes with the polymer matrix, which induces a color change due to shifted emission maxima. Perylene derivatives offer interesting optoelectronic and redox properties and are therefore often used as dyes in chromogenic polymers. For instance, doping of poly(vinyl alcohol) (PVA) with N,N'-bis(2(1-piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic acid diimide dichloride (PZPER), results in polymeric materials containing large perylene aggregates formed through strong hydrogen bonds [47]. The disaggregation can be induced either by mechanical deformation or by thermal stimuli and can be followed as the partial recovery of the luminescence from the PVA-doped film. Films containing 0.5 wt.% of the dye were found to show the first distinct shifts in their emission spectra at 55 °C. However, the observed shifts in the emission spectra revealed that the disaggregation is driven to completion when approaching the glass transition temperature (Tg) of the poly(vinyl alcohol). Similar changes in the emission spectra were observed for cyano-OPVs mixed with linear low-density polyethylene (LLDPE) upon mechanical deformation [110]. Further ore, dyes with “aggregation-induced emission” were found. For such compounds, the emission intensity is affected by the formation of aggregates and can be controlled by external stimuli. For instance, some dyes show a remarkably higher emission in the aggregated state than in solution, which allows for switching the emission properties through thermic or mechanic stimuli. Thus, potential applications for mechanochromic blends are data storage devices, security plastics and papers, or mechanosensors and pressure sensors [111].
Regarding functionalized dyes that are covalently attached to the polymer backbone, color change due to external stimuli is based on constitutional changes in the chromophore, like isomerization or scission. In most cases, the dye undergoes a ring-opening reaction induced by thermic or mechanic forces, which requires the presence of mechanically or thermally labile bonds. A noted example for polymer-dye systems in chromogenic materials are spiropyrans [112,113]. Mechanical stress such as ultrasound induces the ring-opening from the spiropyran to the merocyanine form, which induces a color change of the polymer from colorless to pink. Exposure to ambient light enables reversing this process. For this system, the highest efficiency was found when the spiropyran was emplaced in-between two polymerchains such as poly(methyl acrylate) (PMA) or poly(methyl methacrylate) (PMMA). The stress-induced transformation is shown in Figure 12.
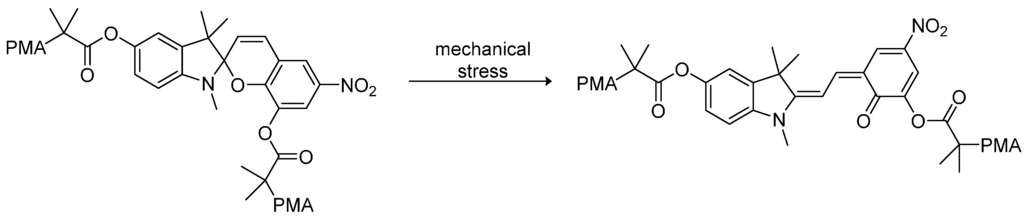
Figure 12.
The ring-opening reaction of a spiropyran-poly(methyl acrylate) (PMA) polymer due to mechanical stress [112].
Figure 12.
The ring-opening reaction of a spiropyran-poly(methyl acrylate) (PMA) polymer due to mechanical stress [112].
Besides the PMA-spiropyran, various studies on other dye-polymer systems were performed. The ring-opening polymerization of ε-caprolactone with an indolinospiropyran diol yielded photochromic poly(ε-caprolactone) [114]. The corresponding materials showed blue emission upon mechanical stress due to the conversion to the merocyanine derivative via ring-opening reaction. The covalent integration of cyano-OPVs into thermoplastic polyurethanes led to chromogenic materials with an excimer emission in the unstreched state and a fluorescence color change by mechanical deformation [49]. Overall, both covalent and supramolecular chromogenic dye-polymer systems have great potential for further technological applications, particularly as sensors for external stimuli like temperature, the chemical environment, and mechanical stress.
3.6. Electrochemical and Optoelectronic Applications
Nowadays, also electrochemistry is more and more affected by the polymer chemistry since high molecular structures offer good processability and modifiability, while giving the opportunity to combine the advantages of polymeric compounds and the properties of low molecular substances. Many inorganic compounds with good capacities and cyclabilities lose value due to their solubility in organic solvents and bad sustainability [115]. However, organic materials are both sustainable and quite simple in their synthetic routes. For instance, anthraquinone-based polymers are excellent candidates for high-performance cathode materials. For this, poly(anthraquinoyl sulfide) is a common polymer, which is based on a thioether bridge bond and for this reason stable and completely insoluble in any solvent. It shows good cyclability and also capability while not generating oxidizing substances during the charge process, which is a safety problem in many transition metal oxide-based cathodes [116].
Another application of dye-containing polymers in electrochemistry is the coating of electrodes with polypyrrole films and use of indigo carmine as the counter ion. Polypyrrole is a conducting polymer with the advantages of good stability and simple preparation. Therefore, it can be used as a modified electrode for a biosensor, electrocatalyst or electrochemically controlled release device. Incorporating indigo-carmine as a counter ion is advantageous due to its good electroactivity and optical properties, as it is a redox indicator, which shows a color change in redox reactions [117].
A quite new field of interest in polymers combined with dyes is organic light-emitting diodes (OLEDs) or rather polymer light-emitting diodes (PLEDs), which can be used as a full-color, high-efficiency and low-drive voltage material for flat-panel displays [118]. These polymers are required to be uniform and smooth amorphous films without exhibiting pinholes and to be thermally stable without crystallizing [119]. These diodes use two electrodes between which one or more semiconducting organic layers with either fluorescence or phosphorescence capability are placed [120]. The advantages of using, for example, polymer-attached dyes instead of inorganic LEDs are the low costs of production, their good processability and their high chemical and electrical stability. Furthermore, the optical and electrical properties can be changed easily by modifying the polymer structure. For example, perylene-containing polyfluorenes can be obtained through copolymerization and the emission color can be tuned over the whole visible region by synthesizing either main-chain copolymers or attaching the perylene dye to the polymer backbone as a side-chain or endcapping group. Therefore, red, green and blue emitting compounds with great light fastness, stability and energy transfer can be obtained with quite high durability and brightness [66]. Besides the described perylene-containing polyfluorenes, various other perylene materials for organic light-emitting diodes are listed in the literature, though their improvement is still in progress [121,122].
4. Conclusions
The most important synthetic routes as well as the major applications of dye-containing polymers have been presented. As reviewed in this article, either covalently or supramolecularly polymer-attached dyes find various applications in many fields for up-to-date issues like organic light-emitting diodes, waste water treatment, medical utilizations such as iris implants or in vivo applications, and electrochemistry. Non-covalent connection of the dye to the polymer can be ionic, dipole-dipole driven or caused by inclusion complexes. Sugar-based polymers like oligo-/polysaccharides, such as cross-linked β-cyclodextrin or even calix[4]arene compounds, are quite important for the adsorption of either cationic or anionic azo and anthraquinone dyes in, for example, dye waste water extraction. Covalent attachment can be achieved through polymerization of colored monomers, polycondensation or polymer-analogous attachment either in the main chain or as an end group. According to the number of publications, colored monomers are usually synthesized through (meth)acrylation, as for many azo, anthraquinone and triphenylmethane dyes, whereas polycondensation and polymer-analogous reactions require suitable functional groups in the monomeric or polymeric building blocks.
Although dye-containing polymers already present a considerable field of compounds for contemporary applications, there is a constant demand for further improvement and research, especially to optimize progress in terms of technology, sustainability and environmental consciousness. For instance, there are various in vivo applications in which the usage of a dye containing polymers is reasonable and should be pursued, like treatment for rheumatoid arthritis as reviewed in this article. Furthermore, technological issues like organic light-emitting diodes represent a crucial topic of further research, as polymeric compounds are eco-friendly and cheaper while showing improved properties.
Author Contributions
Helmut Ritter initiated and supervised writing of the article. All three authors developed the general concept. Carolin Fleischmann and Melanie Lievenbrück contributed equally in doing the literature survey and writing the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abou-Okeil, A.; El-Shafie, A.; El Zawahry, M.M. Ecofriendly laccase–hydrogen peroxide/ultrasound-assisted bleaching of linen fabrics and its influence on dyeing efficiency. Ultrason. Sonochem. 2010, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Lewis, D.; Broadbent, P. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Color. Technol. 2008, 124, 186–194. [Google Scholar] [CrossRef]
- Baptista, M.S.; Indig, G.L. Effect of BSA binding on photophysical and photochemical properties of triarylmethane dyes. J. Phys. Chem. B 1998, 102, 4678–4688. [Google Scholar] [CrossRef]
- Mula, S.; Ray, A.K.; Banerjee, M.; Chaudhuri, T.; Dasgupta, K.; Chattopadhyay, S. Design and development of a new pyrromethene dye with improved photostability and lasing efficiency: Theoretical rationalization of photophysical and photochemical properties. J. Org. Chem. 2008, 73, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Nazarpoor, K.; Karimi, L. Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J. Clean. Prod. 2011, 19, 1045–1051. [Google Scholar] [CrossRef]
- Sulak, M.T.; Yatmaz, H.C. Removal of textile dyes from aqueous solutions with eco-friendly biosorbent. Desalin. Water Treat. 2012, 37, 169–177. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Robra, K.-H.; Cavaco-Paulo, A.; Gübitz, G.M. Decolorization and detoxification of textile dyes with a laccase from trametes hirsuta. Appl. Environ. Microb. 2000, 66, 3357–3362. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pavlostathis, S.G. Decolorization and toxicity of reactive anthraquinone textile dyes under methanogenic conditions. Water Res. 2004, 38, 1838–1852. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green chemistry. Environ. Impact Assess. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Duxbury, D.F. The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 1993, 93, 381–433. [Google Scholar] [CrossRef]
- Azmi, W.; Sani, R.K.; Banerjee, U.C. Biodegradation of triphenylmethane dyes. Enzyme Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 2, pp. 707–710. [Google Scholar]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 4, pp. 409–464. [Google Scholar]
- Bergmann, E.; Engel, L.; Sándor, S. Beiträge zur kenntnis der doppelten bindung, ii.: Über die räumliche konfiguration der aromatischen azokörper. Ber. Dtsch. Chem. Ges. 1930, 63, 2572–2575. [Google Scholar]
- Kumar, G.S.; Neckers, D. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P.; Ho, M.-S.; Barrett, C. Azo polymers for reversible optical storage.6. Poly[4-[2-(methacryloyloxy)ethyl]azobenzene]. Macromolecules 1995, 28, 4179–4183. [Google Scholar] [CrossRef]
- Natansohn, A.; Xie, S.; Rochon, P. Azo polymers for reversible optical storage. 2. Poly[4'-[[2-(acryloyloxy)ethyl]ethylamino]-2-chloro-4-nitroazobenzene]. Macromolecules 1992, 25, 5531–5532. [Google Scholar]
- Kohl, C.; Becker, S.; Müllen, K. Bis(rylenedicarboximide)-a,d-1,5-diaminoanthraquinones as unique infrared absorbing dyes. Chem. Commun. 2002, 2778–2779. [Google Scholar] [CrossRef]
- Herrmann, A.; Müllen, K. From industrial colorants to single photon sources and biolabels: The fascination and function of rylene dyes. Chem. Lett. 2006, 35, 978–985. [Google Scholar] [CrossRef]
- Weil, T.; Abdalla, M.A.; Jatzke, C.; Hengstler, J.; Müllen, K. Water-soluble rylene dyes as high-performance colorants for the staining of cells. Biomacromolecules 2005, 6, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Epolito, W.; Lee, Y.; Bottomley, L.; Pavlostathis, S. Characterization of the textile anthraquinone dye reactive blue 4. Dyes Pigments 2005, 67, 35–46. [Google Scholar] [CrossRef]
- Aspland, J.R. Textile Dyeing and Coloration; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Schaetzer, H.; Raisin, H.; Mausezahl, D. Anthraquinone Dyes, Dyeing, Printing, Textiles. U.S. Patent 4,396,393, 8 February 1983. [Google Scholar]
- Venkataraman, K. The Chemistry of Synthetic Dyes; Academic Press: New York, NY, USA, 2012; Volume 4, pp. 1–548. [Google Scholar]
- Johnson, M.G.; Kiyokawa, H.; Tani, S.; Koyama, J.; Morris-Natschke, S.L.; Mauger, A.; Bowers-Daines, M.M.; Lange, B.C.; Lee, K.-H. Antitumor agents—CLXVII. Synthesis and structure-activity correlations of the cytotoxic anthraquinone 1,4-bis-(2,3-epoxypropylamino)-9, 10-anthracenedione, and of related compounds. Bioorgan. Med. Chem. 1997, 5, 1469–1479. [Google Scholar]
- Agarwal, S.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Oshio, H.; Kawamura, N. Quantitative analysis of the laxative components in rhubarb by high performance liquid chromatography. Jpn. J. Pharmacogn. 1985, 39, 131–138. [Google Scholar] [CrossRef]
- Brown, J.P.; Brown, R.J. Mutagenesis by 9,10-anthraquinone derivatives and related compounds in salmonella typhimurium. Mutat. Res. 1976, 40, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.N. Absorption and emission spectroscopy and photochemistry of 1,10-anthraquinone derivatives: A review. J. Photochem. Photobiol. 1990, 53, 141–167. [Google Scholar] [CrossRef]
- Rondão, R.; de Melo, J.S.; Schaberle, F.; Voss, G. Excited state characterization of a polymeric indigo. Phys. Chem. Chem. Phys. 2012, 14, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Truckmeier, S. Naturfarbstoffe: Farben mit geschichte. Chem. Unserer Zeit 2003, 37, 402–409. [Google Scholar] [CrossRef]
- W. Kratzert, R.P. Farbstoffe; Quelle & Meyer: Heidelberg, Germany, 1981. [Google Scholar]
- Friedländer, P. Über indigoide farbstoffe. Ber. Dtsch. Chem. Ges. 1908, 29, 359–374. [Google Scholar]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.M.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D. Indirubin, the active constituent of a chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, E.Y.; Sezgin, M.; Yilmaz, A.; Yilmaz, M. Synthesis of β-cyclodextrin and starch based polymers for sorption of azo dyes from aqueous solutions. Bioresour. Technol. 2008, 99, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Yilmaz, E.; Yilmaz, M.; Bartsch, R.A. Removal of azo dyes from aqueous solutions using calix[4]arene and β-cyclodextrin. Dyes Pigments 2007, 74, 54–59. [Google Scholar] [CrossRef]
- Akceylan, E.; Bahadir, M.; Yilmaz, M. Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J. Hazard. Mater. 2009, 162, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigments 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Trellenkamp, T.; Ritter, H. Poly(N-vinylpyrrolidone) bearing covalently attached cyclodextrin via click-chemistry: Synthesis, characterization, and complexation behavior with phenolphthalein. Macromolecules 2010, 43, 5538–5543. [Google Scholar] [CrossRef]
- Taguchi, K. Transient binding of phenolphthalein-β-cyclodextrin complex: An example of induced geometrical distortion. J. Am. Chem. Soc. 1986, 108, 2705–2709. [Google Scholar] [CrossRef]
- Ramos-Ortiz, G.; Maldonado, J.; Meneses-Nava, M.; Barbosa-García, O.; Olmos, M.; Cha, M. Third-harmonic generation performance of organic polymer films doped with triphenylmethane derivative dyes. Opt. Mater. 2007, 29, 636–641. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, S.H.; Antonietti, M.; Böttcher, C.; Faul, C.F. Synthesis of supramolecular polymers by ionic self-assembly of oppositely charged dyes. Chem. Eur. J. 2005, 11, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Şolpan, D.; Duran, S.; Saraydin, D.; Güven, O. Adsorption of methyl violet in aqueous solutions by poly (acrylamide-co-acrylic acid) hydrogels. Radiat. Phys. Chem. 2003, 66, 117–127. [Google Scholar] [CrossRef]
- Ngo, T.T.; Liotta, C.L.; Eckert, C.A.; Kazarian, S.G. Supercritical fluid impregnation of different azo-dyes into polymer: In situ UV/Vis spectroscopic study. J. Supercrit. Fluids 2003, 27, 215–221. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R.; Maka, T.; Romanov, S.G.; Sotomayor Torres, C.M. Dye-containing polymer beads as photonic crystals. Chem. Mater. 2000, 12, 2508–2512. [Google Scholar] [CrossRef]
- Seeboth, A.; Kriwanek, J.; Vetter, R. The first example of thermochromism of dyes embedded in transparent polymer gel networks. J. Mater. Chem. 1999, 9, 2277–2278. [Google Scholar] [CrossRef]
- Donati, F.; Pucci, A.; Ruggeri, G. Temperature and chemical environment effects on the aggregation extent of water soluble perylene dye into vinyl alcohol-containing polymers. Phys. Chem. Chem. Phys. 2009, 11, 6276–6282. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.R.; Burnworth, M.; Khariwala, D.; Hiltner, A.; Mather, P.T.; Simha, R.; Weder, C. Deformation-induced color changes in mechanochromic polyethylene blends. Macromolecules 2007, 40, 2400–2408. [Google Scholar] [CrossRef]
- Donati, F.; Pucci, A.; Cappelli, C.; Mennucci, B.; Ruggeri, G. Modulation of the optical response of polyethylene films containing luminescent perylene chromophores. J. Phys. Chem. B 2008, 112, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D. Polymeric dyes. Aldrichim. Acta 1981, 14, 23–29. [Google Scholar]
- Robello, D.R. Linear polymers for nonlinear optics. I. Polyacrylates bearing aminonitro-stilbene and-azobenzene dyes. J. Polym. Sci. Pol. Chem. 1990, 28, 1–13. [Google Scholar]
- Maradiya, H.R.; Patel, V.S. Synthesis, characterization and application of monomeric and polymeric dyes based on N-arylmaleimides. High Perform. Polym. 2000, 12, 335–348. [Google Scholar] [CrossRef]
- Maradiya, H.R.; Patel, V.S. Studies of novel monomeric and polymeric azo disperse dyes. J. Appl. Polym. Sci. 2002, 84, 1380–1389. [Google Scholar] [CrossRef]
- Retzmann, N.; Maatz, G.; Ritter, H. Host–guest-driven color change in water: Influence of cyclodextrin on the structure of a copper complex of poly((4-hydroxy-3-(pyridin-3-yldiazenyl)phenethyl) methacrylamide-co-dimethylacrylamide). Beilstein J. Org. Chem. 2014, 10, 2480–2483. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Kungwatchakun, D. Photoresponsive polymers. Mechanochemistry of polyacrylamide gels having triphenylmethane leuco derivatives. Macromol. Rapid Comm. 1984, 5, 829–832. [Google Scholar]
- Fleischmann, C.; Cheng, J.; Tabatabai, M.; Ritter, H. Extended applicability of classical phenolphthalein: Color changing polymeric materials derived from pH-sensitive acrylated phenolphthalein derivatives. Macromolecules 2012, 45, 5343–5346. [Google Scholar] [CrossRef]
- Fleischmann, C.; Ritter, H. Color indicator for supramolecular polymer chemistry: Phenolphthalein-containing thermo-and pH-sensitive N-(isopropyl) acrylamide copolymers and β-cyclodextrin complexation. Macromol. Rapid Comm. 2013, 34, 1085–1089. [Google Scholar] [CrossRef]
- Hetzer, M.; Fleischmann, C.; Schmidt, B.V.; Barner-Kowollik, C.; Ritter, H. Visual recognition of supramolecular graft polymer formation via phenolphthalein–cyclodextrin association. Polymer 2013, 54, 5141–5147. [Google Scholar] [CrossRef]
- Dollendorf, C.; Kreth, S.K.; Choi, S.W.; Ritter, H. Polymerization of novel methacrylated anthraquinone dyes. Beilstein J. Org. Chem. 2013, 9, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sastre, R.; Costela, A. Polymeric solid-state dye lasers. Adv. Mater. 1995, 7, 198–202. [Google Scholar] [CrossRef]
- Serin, J.; Schultze, X.; Adronov, A.; Fréchet, J.M. Synthesis and study of the absorption and luminescence properties of polymers containing Ru(BpyMe2)32+ chromophores and coumarin laser dyes. Macromolecules 2002, 35, 5396–5404. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Color change of N-isopropylacrylamide copolymer bearing reichardts dye as optical sensor for lower critical solution temperature and for host–guest interaction with β-cyclodextrin. J. Am. Chem. Soc. 2007, 129, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
- Shiba, M.; Hiramasu, H.; Nakano, H.; Kawano, Y.; Shigeri, Y.; Kondo, T. Synthesis of high polymers with a light absorption band in the visible region by interfacial polycondensation reaction. Polym. J. 1973, 4, 366–371. [Google Scholar] [CrossRef]
- Ghassemi, H.; Hay, A.S. Red pigmentary polyimides from N,N'-diamino-3,4,9,10-perylenetetracarboxylic acid bisimide. Macromolecules 1994, 27, 4410–4412. [Google Scholar] [CrossRef]
- Ego, C.; Marsitzky, D.; Becker, S.; Zhang, J.; Grimsdale, A.C.; Müllen, K.; MacKenzie, J.D.; Silva, C.; Friend, R.H. Attaching perylene dyes to polyfluorene: Three simple, efficient methods for facile color tuning of light-emitting polymers. J. Am. Chem. Soc. 2003, 125, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroglu, F.E.; Alexander, S.C.; Ji, D.; Prusty, D.K.; Borsch, M.; Herrmann, A. Poly(BODIPY)s: A new class of tunable polymeric dyes. Macromolecules 2009, 42, 6529–6536. [Google Scholar] [CrossRef]
- Morgan, P. Linear condensation polymers from phenolphthalein and related compounds. J. Polym. Sci. 1964, 2, 437–459. [Google Scholar] [CrossRef]
- Kishore, K.; Kannan, P. Synthesis, spectral, thermal, and flammability studies of phenolphthalein polyphosphate esters. J. Polym. Sci. Pol. Chem. 1990, 28, 3481–3486. [Google Scholar] [CrossRef]
- Wang, M.; An, Q.-F.; Wu, L.-G.; Mo, J.-X.; Gao, C.-J. Preparation of pH-responsive phenolphthalein poly(ether sulfone) membrane by redox-graft pore-filling polymerization technique. J. Membrane Sci. 2007, 287, 257–263. [Google Scholar] [CrossRef]
- Wang, M.; Wu, L.-G.; Zheng, X.-C.; Mo, J.-X.; Gao, C.-J. Surface modification of phenolphthalein poly(ether sulfone) ultrafiltration membranes by blending with acrylonitrile-based copolymer containing ionic groups for imparting surface electrical properties. J. Colloid Interface Sci. 2006, 300, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kiskan, B.; Antonietti, M.; Weber, J. Teaching new tricks to an old indicator: pH-Switchable, photoactive microporous polymer networks from phenolphthalein with tunable CO2 adsorption power. Macromolecules 2012, 45, 1356–1361. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Osburn, P.L.; Li, C. Soluble polymer-supported catalysts containing azo dyes. Org. Lett. 2002, 4, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.J.; Otteson, K.M.; Wang, P.C.; Wingard, R.E., Jr. Soluble functional polymers. 2. Utilization of water-insoluble chromophores in water-soluble polymeric dyes. Macromolecules 1978, 11, 320–324. [Google Scholar]
- Schaap, A.P.; Thayer, A.L.; Blossey, E.C.; Neckers, D.C. Polymer-based sensitizers for photooxidations. II. J. Am. Chem. Soc. 1975, 97, 3741–3745. [Google Scholar] [CrossRef]
- Milliken Chemical. Available online: http://millikenchemical.com/versatint-water-soluble-colorant/ (accessed on 21 February 2015).
- Fleischmann, C.; Wöhlk, H.; Ritter, H. End group functionalization of poly(ethylene glycol) with phenolphthalein: Towards star-shaped polymers based on supramolecular interactions. Beilstein J. Org. Chem. 2014, 10, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Maatz, G.; Maciollek, A.; Ritter, H. Cyclodextrin-induced host–guest effects of classically prepared poly(NIPAM) bearing azo-dye end groups. Beilstein J. Org. Chem. 2012, 8, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Alinsafi, A.; Khemis, M.; Pons, M.; Leclerc, J.; Yaacoubi, A.; Benhammou, A.; Nejmeddine, A. Electro-coagulation of reactive textile dyes and textile wastewater. Chem. Eng. Process. 2005, 44, 461–470. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-S.; Chen, M.-L. Decolourization of dye-containing solutions by electrocoagulation. J. Appl. Electrochem. 1994, 24, 785–790. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Studies on adsorption of dyes on β-cyclodextrin polymer. Bioresource Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Vebrel, J.; Knorr, M.; Sauvin, G.; Conte, E. Starch-Modified Filters Used for the Removal of Dyes from Waste Water; Macromolecular Symposia; Wiley Online Library: Weinheim, Gemany, 2003; pp. 165–172. [Google Scholar]
- Chiou, M.; Li, H. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Progr. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Torres, J.D.; Faria, E.A.; Dias, S.C.L. Comparative adsorption studies of indigo carmine dye on chitin and chitosan. J. Colloid Interface Sci. 2004, 277, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, N.; Khojasteh, Z.; Sharghi, H.; Shamsipur, M. Lead ion-selective membrane electrodes based on some recently synthesized 9,10-anthraquinone derivatives. Anal. Chim. Acta 1998, 360, 203–208. [Google Scholar] [CrossRef]
- Reza Pouretedal, H.; Forghaniha, A.; Sharghi, H.; Shamsipur, M. Lead-selective membrane potentiometric sensor based on a recently synthesized bis(anthraquinone) sulfide derivative. Anal. Lett. 1998, 31, 2591–2605. [Google Scholar] [CrossRef]
- Rahmani, A.; Barzegar, M.; Shamsipur, M.; Sharghi, H.; Mousavi, M. New potentiometric membrane sensors responsive to Pb(ii) based on some recently synthesized 9,10-anthraquinone derivatives. Anal. Lett. 2000, 33, 2611–2629. [Google Scholar] [CrossRef]
- Voss, G.; Drechsler, M.; Eller, S.; Gradzielski, M.; Gunzelmann, D.; Mondal, S.; van Smaalen, S.; Voertler, C.S. Polymeric colorants: Statistical copolymers of indigo building blocks with defined structures. Helv. Chim. Acta 2009, 92, 2675–2697. [Google Scholar] [CrossRef]
- Peters, A.T. 1,5-Dihydroxy-4,8-diamino-and 1,8-dihydroxy-4,5-diaminoanthraquinone-β-ethers and thioethers. Blue dyes for synthetic polymer fibres. J. Chem. Technol. Biot. 1990, 48, 135–143. [Google Scholar]
- Dixit, B.C.; Patel, H.M.; Desai, D.J.; Dixit, R.B. Studies on dyeing performance of novel acid azo dyes and mordent acid azo dyes based on 2,4-dihydroxybenzophenone. E-J. Chem. 2009, 6, 315–322. [Google Scholar] [CrossRef]
- Asquith, R.S.; Blair, H.S.; Crangle, A.A.; Riordan, E. Self-coloured polymers based on anthraquinone residues. J. Soc. Dyers Colour 1977, 93, 114–125. [Google Scholar] [CrossRef]
- Schmidt, A.; Bach, E.; Schollmeyer, E. The dyeing of natural fibres with reactive disperse dyes in supercritical carbon dioxide. Dyes Pigments 2003, 56, 27–35. [Google Scholar] [CrossRef]
- Lewis, D.M.; Vo, L.T. Dyeing cotton with reactive dyes under neutral conditions. Color. Technol. 2007, 123, 306–311. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Burkinshaw, S.; Mignanelli, M.; Froehling, P.; Bide, M. The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dyes Pigments 2000, 47, 259–267. [Google Scholar] [CrossRef]
- Ebbesen, M.F.; Bienk, K.; Deleuran, B.W.; Howard, K.A. Extended blood circulation and joint accumulation of a p(HPMA-co-AzMA)-based nanoconjugate in a murine model of rheumatoid arthritis. Mol. Cell. Ther. 2014, 2, 29. [Google Scholar] [CrossRef]
- Ebbesen, M.F.; Schaffert, D.H.; Crowley, M.L.; Oupický, D.; Howard, K.A. Synthesis of click-reactive hpma copolymers using raft polymerization for drug delivery applications. J. Polym. Sci. Pol. Chem. 2013, 51, 5091–5099. [Google Scholar] [CrossRef]
- Xie, S.; Natansohn, A.; Rochon, P. Recent developments in aromatic azo polymers research. Chem. Mater. 1993, 5, 403–411. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P.; Gosselin, J.; Xie, S. Azo polymers for reversible optical storage. 1. Poly[4'-[[2-(acryloyloxy)ethyl]ethylamino]-4-nitroazobenzene]. Macromolecules 1992, 25, 2268–2273. [Google Scholar]
- Xu, G.; Yang, Q.G.; Si, J.; Liu, X.; Ye, P.; Li, Z.; Shen, Y. Application of all-optical poling in reversible optical storage in azopolymer films. Opt. Commun. 1999, 159, 88–92. [Google Scholar] [CrossRef]
- Weigert, F. Über einen neuen effekt der strahlung. Z. Phys. 1921, 5, 410–427. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Xia, X.; Ge, J.; Xu, Q.; Lu, J. Flash memory effects based on styrene/maleimiade copolymers with pendant azobenzene chromophores. Eur. Polym. J. 2011, 47, 1160–1167. [Google Scholar] [CrossRef]
- Pham, V.P.; Galstyan, T.; Granger, A.; Lessard, R.A. Novel azo dye-doped poly (methyl methacrylate) films as optical data storage media. Jpn. J. Appl. Phys. 1997, 36, 429. [Google Scholar] [CrossRef]
- Crenshaw, B.R.; Weder, C. Deformation-induced color changes in melt-processed photoluminescent polymer blends. Chem. Mater. 2003, 15, 4717–4724. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G. Mechanochromic polymer blends. J. Mater. Chem. 2011, 21, 8282–8291. [Google Scholar] [CrossRef]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-linked addition polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, G.; Wong, B.M.; McElhanon, J.R. Stress sensing in polycaprolactone films via an embedded photochromic compound. ACS App. Mat. Interfaces 2010, 2, 1594–1600. [Google Scholar] [CrossRef]
- Le Gall, T.; Reiman, K.H.; Grossel, M.C.; Owen, J.R. Poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene): A new organic polymer as positive electrode material for rechargeable lithium batteries. J. Power Sources 2003, 119, 316–320. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Anthraquinone based polymer as high performance cathode material for rechargeable lithium batteries. Chem. Commun. 2009, 448–450. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, S.; Han, S.; Yang, Z.; Ye, L.; Yang, T. Organic light-emitting diodes with azo films as electrodes. Synth. Met. 2000, 114, 251–254. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Müller, D.C.; Nothofer, H.-G.; Scherf, U.; Neher, D.; Bräuchle, C.; Meerholz, K. Improving the performance of doped π-conjugated polymers for use in organic light-emitting diodes. Nature 2000, 405, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Benning, S.; Kitzerow, H.-S.; Bock, H.; Achard, M.-F. Fluorescent columnar liquid crystalline 3,4,9,10-tetra-(N-alkoxycarbonyl)-perylenes. Liq. Cryst. 2000, 27, 901–906. [Google Scholar] [CrossRef]
- Ranke, P.; Bleyl, I.; Simmerer, J.; Haarer, D.; Bacher, A.; Schmidt, H. Electroluminescence and electron transport in a perylene dye. Appl. Phys. Lett. 1997, 71, 1332–1334. [Google Scholar] [CrossRef]
- Li, L.; Guan, M.; Cao, G.; Li, Y.; Zeng, Y. Highly efficient and stable organic light-emitting diodes employing MoO3-doped perylene-3,4,9,10-tetracarboxylic dianhydride as hole injection layer. Appl. Phys. A 2010, 99, 251–254. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
