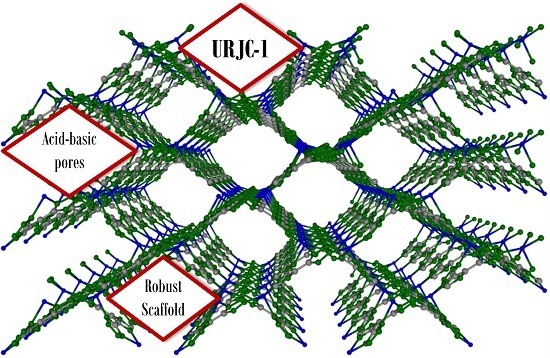
3.1. Physicochemical Characterization of URJC-1 Material
The crystalline structure of URJC-1 material was solved by the single crystal X-ray diffraction technique resulting in a three-dimensional non-interpenetrated
utp or (10,3)-d topological network [
29]. A molar composition of Cu(HIT)(DMF)
0.5 was determined for the as-synthesized material. The asymmetric unit of the URJC-1 material comprises a copper ion linked to one ligand (HIT) and one DMF solvent molecule (
Figure 2A). The copper ion is connected to five nitrogen atoms belonging to tetrazole and imidazole rings of the HIT ligand and one oxygen atom of a carbonyl group of the DMF molecule (
Figure 2B). This copper ion exhibits a distorted octahedral CuN
5O geometry confirmed by the planar Cu–N distances in the range of 1.999(8)–2.031(10) Å for N1, N2, N5, and N7, and axial Cu–N10 and Cu–O distances of 2.171(10) Å and 2.340(2) Å, respectively. The bond angles of the metal environment are in the wide range of 77.8(7)–103.37(4)°. Concerning the HIT ligand coordination (
Figure 2C), three copper ions are connected to one HIT molecule. Two coppers are linked to nitrogen atoms of tetrazole rings (N7 and N10) and imidazole and tetrazole rings (N1 and N5, respectively). The third copper atom is connected to nitrogen N2 atom of a tetrazole ring. The crystal data found for the as-synthesized URJC-1 material are:
Mr = 314.25; orthorhombic, Pn21a;
a = 8.6577(6),
b = 9.0478(9),
c = 14.9973(11),
V = 1174.79 Å
3;
Z = 4;
Dc = 1.77 g/cm
3. More details of the structural unit of URJC-1 material were included in
Figure S1 and
Table S1.
Figure 2.
(A) Asymmetric unit of URJC-1 structure; (B) copper coordination; (C) ligand coordination; (D) and three-dimensional view of URJC-1 structure free of solvent. Cu: blue, C: grey, N: green, and O: red.
Figure 2.
(A) Asymmetric unit of URJC-1 structure; (B) copper coordination; (C) ligand coordination; (D) and three-dimensional view of URJC-1 structure free of solvent. Cu: blue, C: grey, N: green, and O: red.
The crystallographic data also evidenced a one-dimensional rhombohedral pore system along the [100] direction with dimensions of
ca. 12.0 × 9.0 Å. The removal of the solvent molecules generated unsaturated copper sites accessible from the porous system, as potential Lewis acid sites. Additionally, non-substituted nitrogen atoms of tetrazole heterocycles also provide Lewis basic sites to the porous structure (see
Figure S2). The three-dimensional view of the packed URJC-1 structure along the [100] axis free of solvent is shown in
Figure 2D.
In order to confirm the purity of the crystalline phase of the URJC-1 material, X-ray diffraction patterns of the bulk powder sample and the simulated pattern from the crystallographic data of the single crystal technique were compared (
Figure 3A). As it can be seen, the location and intensity of the main reflections of both patterns match quite well, endorsing that the crystalline structure resolved by single crystal X-ray diffraction is uniquely present in the bulk sample. SEM micrographs of the as-synthesized URJC-1 material shows sphere crystalline particles with uniform size of
ca. 5–10 μm (
Figure 3B).
Figure 3.
(A) XRD patterns of powder URJC-1 sample and simulated from single-crystal data; (B) SEM micrographs of the as-synthesized URJC-1 sample.
Figure 3.
(A) XRD patterns of powder URJC-1 sample and simulated from single-crystal data; (B) SEM micrographs of the as-synthesized URJC-1 sample.
The porosity of the copper-based MOF material was measured by argon adsorption/desorption isotherms at 87 K (
Figure 4A). The URJC-1 material displayed a characteristic type I adsorption–desorption isotherm of a microporous material with a BET specific surface area of 408 m
2/g and a pore volume of 0.24 cm
3/g at
P/
P0 of 0.998. Additionally, a pore size distribution centered at 14.8 Å was estimated using the NL-DFT method applied for an Ar–carbon kernel at 87 K based on a slit pore model (
Figure 4B). The mean pore size was slightly higher than that found from the crystallography data. Crystal density of the activated sample was experimental measured by He pycnometry resulting 1.45 g/cm
3.
Figure 4.
(A) Ar adsorption–desorption isotherms at 87 K; and (B) pore size distribution estimated by non-local DFT of the activated URJC-1 sample.
Figure 4.
(A) Ar adsorption–desorption isotherms at 87 K; and (B) pore size distribution estimated by non-local DFT of the activated URJC-1 sample.
The copper content of the powder URJC-1 material was also determined by ICP-OES. The metal content of the activated sample was 22.6%. This value is very close to the theoretical content (23.8%) calculated from the crystallographic molecule, excluding coordinated DMF solvent molecules. Thus, it is evident that DMF molecules were successfully removed from the crystalline framework, confirming the presence of unsaturated metal centers with potential activity as Lewis acid sites. Therefore, URJC-1 material appears as a very promising porous material with dual acid and basic sites, due to the co-existence of accessible unsaturated copper centers and non-coordinated nitrogen atoms of the HIT ligand, respectively.
3.2. Thermal and Chemical Stability of URJC-1 Material
Figure 5 shows
in situ temperature-dependence PXRD patterns and TG/DTG analyses in N
2 atmosphere of URJC-1 sample.
In situ PXRD patterns at different temperatures display a remarkable structural integrity of URJC-1 sample up to 325 °C (
Figure 5A). Likewise, TG/DTG analyses also confirm this stability with a remarkable weight loss in the range of 340–380 °C and a maximum DTG peak centered at 350 °C (
Figure 5B), which is associated with the decomposition of the HIT ligand and subsequent structure collapse. It is also noticeable the presence of two other weight losses (
ca. 80 and 160 °C), which are attributed to the elimination of the synthesis solvents, acetonitrile/water and
N,
N-dimethylformamide, respectively.
The chemical stability of URJC-1 material was tested by dispersion of the solid sample in different organic solvents. The solvents were selected according to their different polarity (polarity index of toluene is 2.3 and that of water 10.2).
Figure 6 shows the PXRD patterns of URJC-1 material after dispersion in each solvent during 24 h at room temperature. As it is observed, the crystalline structure remained intact after the chemical stability tests, evidencing a strong robustness of the copper–imidazole/tetrazole framework. Additionally, the URJC-1 material also maintained its integrity when it was immersed in boiling water for 1 h (also shown in
Figure 6).
Figure 5.
(A) In-situ temperature-dependence PXRD patterns and (B) TG/DTG analyses of URJC-1 material.
Figure 5.
(A) In-situ temperature-dependence PXRD patterns and (B) TG/DTG analyses of URJC-1 material.
Figure 6.
PXRD patterns of the URJC-1 material after suspension in different solvents.
Figure 6.
PXRD patterns of the URJC-1 material after suspension in different solvents.
Similar stability tests were also carried out for one of the most extended Cu–carboxylic-based MOF, the commercial HKUST-1 (or Basolite C-300). In this case, the characteristic crystalline phase of HKUST-1 material was drastically modified after 24 h in water, toluene, and methanol, maintaining it only in acetonitrile and nitrobenzene (
Figure 7). Additionally, the structure also collapsed in boiling water after 1 h. These results clearly evidenced a minor structural strength of this MOF structure based on 4-coordinate Cu
2+ with oxygen linkages, as compared to the URJC-1 structure based on 5-coordinate Cu
2+ and nitrogen linkages.
Figure 7.
PXRD patterns of HKUST-1 material after suspension in different solvents.
Figure 7.
PXRD patterns of HKUST-1 material after suspension in different solvents.
Finally, the stability of the URJC-1 material was also studied under strong acidic and basic conditions using HCl and HNO
3 solutions (0.05M, pH = 1.3) and NaOH solution (0.01M, pH = 12).
Figure 8 shows PXRD patterns of URJC-1 samples after 1 h at room temperature, evidencing that the crystalline URJC-1 framework was also stable under acid and basic agents. From these results, it has been attested that the URJC-1 material provides not only a crystalline microporous framework with promising acid and basic properties but also a remarkable thermal and chemical stability, which make it very attractive for catalytic applications.
Figure 8.
PXRD patterns of URJC-1 material after suspension in acid and basic solutions.
Figure 8.
PXRD patterns of URJC-1 material after suspension in acid and basic solutions.
3.3. Catalytic Activity of URJC-1 Material
The activity of the URJC-1 material was assessed in the reaction of anisole acylation with acetyl chloride and in the Knoevenagel condensation of benzaldehyde with malononitrile, being both typical catalytic tests for acid and basic-catalyzed reactions, respectively.
In the case of the acylation of anisole, URJC-1 material was studied and compared with other catalysts typically used for this reaction, like Cu-based MOF material (HKUST-1) and some conventional microporous zeolitic materials (Beta and ZSM-5), using the same operation conditions of previous works [
12,
30].
Figure 9 shows the catalytic performance in terms of anisole conversion and
p-methoxyacetophenone (
p-MAP) yield as the final product for all materials, including a blank experiment with no catalyst. All the catalysts showed a better performance than the blank experiment. Among the tested materials, URJC-1 exhibited the best catalytic activity. Note that URJC-1 has lower surface area (408 m
2/g) than HKUST-1 (708 m
2/g). In these materials, the catalytic activity is related to the active role of unsaturated copper centers as Lewis acid sites. Regarding the zeolitic materials, it was also remarkable not only the higher anisole conversion for Cu-based MOF materials but also the enhancement of
p-MAP yield. Nevertheless, taking into account the reaction rate referred to the number of catalytic sites, also known as turnover frequency (TOF), MOF materials revealed lower values of TOF as compared to zeolitic samples of this work (see
Table S2) [
31]. This fact is probably associated to certain diffusion constraints of the organic reactants to get the metal sites as consequence of stronger interactions of reactants within the metal organic framework. Another hypothesis could be also the different nature of the catalytic sites of assessed samples (aluminum sites in the case of ZSM-5 and BETA materials and copper sites in the case of MOFs). Thus, the higher activity of MOF materials, and in particular of URJC-1, is due to the high concentration of active acid sites per gram of catalyst.
Figure 9.
Catalytic performance of URJC-1 material and other catalytic materials in the acylation of anisole. Normal columns indicate anisole conversion; dense fill columns indicate
p-MAP yield. On the other hand, PRXD of URJC-1 and HKUST-1 materials before and after acylation reaction were performed in order to evaluate the catalyst stability of MOF materials (
Figure 10). Interestingly, the URJC-1 material did not undergo any modification of the main diffraction peaks. In contrast, the structure of Cu-carboxylic MOF material (HKUST-1) collapsed after reaction according to the PXRD patterns of the recovered sample. Therefore, the higher stability of the URJC-1 structure enables the reusability of this material for successive catalytic runs.
Figure 9.
Catalytic performance of URJC-1 material and other catalytic materials in the acylation of anisole. Normal columns indicate anisole conversion; dense fill columns indicate
p-MAP yield. On the other hand, PRXD of URJC-1 and HKUST-1 materials before and after acylation reaction were performed in order to evaluate the catalyst stability of MOF materials (
Figure 10). Interestingly, the URJC-1 material did not undergo any modification of the main diffraction peaks. In contrast, the structure of Cu-carboxylic MOF material (HKUST-1) collapsed after reaction according to the PXRD patterns of the recovered sample. Therefore, the higher stability of the URJC-1 structure enables the reusability of this material for successive catalytic runs.
Figure 10.
PXRD patterns of URJC-1 and HKUST-1 materials before and after the acid catalyzed acylation reaction.
Figure 10.
PXRD patterns of URJC-1 and HKUST-1 materials before and after the acid catalyzed acylation reaction.
The URJC-1 material was also tested as a catalyst in the Knoevenagel condensation of benzaldehyde with malononitrile to produce 2-benzylidenemalononitrile (2-BM). In this case, the catalytic performance of the URJC-1 material was compared to HKUST-1 and UiO-66-NH
2 MOF materials which provide basic sites from oxygen atoms of the metal-carboxylated linkages (HKUST-1) and amino groups of the organic ligand (UiO-66-NH
2).
Figure 11 illustrates the conversion of benzaldehyde for all materials as well as a blank experiment without any catalyst, in the same operation conditions of previous works [
28]. It is remarkable that high selectivity toward 2-BM product (above 99%) was obtained in all cases.
Figure 11.
Catalytic performance of the URJC-1 material and other heterogeneous catalysts in Knoevenagel condensation of benzaldehyde and malononitrile reaction.
Figure 11.
Catalytic performance of the URJC-1 material and other heterogeneous catalysts in Knoevenagel condensation of benzaldehyde and malononitrile reaction.
The basicity of the oxygen atoms from the metal-carboxylated linkages of HKUST-1 material [
32] seems to be too weak to activate the benzaldehyde condensation, leading to conversion of benzaldehyde only slightly greater than the blank experiment. On the other side, UiO-66-NH
2 material enhanced significantly the catalytic performance. This fact has been attributed to the synergetic effect of neighboring structural zirconium acid sites to amino-containing groups of the ligand [
16]. The catalytic performance of the URJC-1 material was even superior to that shown by the UiO-66-NH
2 sample. Herein, the synergetic effect of nitrogen basic sites of the ligand containing tetrazole groups and unsaturated copper acid sites could be also responsible of its remarkable activity [
16].
The turnover frequency parameter was also estimated for the three MOF materials in order to compare the catalytic activity per active site (
Table S3). It has to be noted that this is a gross estimation since the different nature of basic sites for each MOF material and also the assumption of total accessibility of all active sites (non-coordinated nitrogen atoms from tetrazole groups for URJC-1, basic oxygen atoms of the metal–carboxylate linkages for HKUST-1, and amino groups for UiO-66-NH
2. Amino-containing MOF showed the highest TOF value, followed by URJC-1 and, finally, HKUST-1 materials, possibly due to the higher basicity of the amino groups of UiO-66-NH
2 in comparison with the nitrogens from tetrazole groups of URJC-1 or oxygens from carboxylated ligands of HKUST-1.
Similarly to the other catalytic tests, the stability of the three materials was assessed by comparison of PXRD patterns of the fresh and recovered material after reaction (
Figure 12). PXRD patterns of URJC-1 and UiO-66-NH
2 materials revealed good stability under the conditions of Knoevenagel condensation reaction. In contrast, the HKUST-1 material showed a partial collapse of the structure, as shown by the disappearance of some characteristic diffraction peaks of its structure.
Figure 12.
PXRD patterns of URJC-1, UIO-66-NH2, and HKUST-1 materials before and after Knoevenagel condensation of benzaldehyde with malononitrile.
Figure 12.
PXRD patterns of URJC-1, UIO-66-NH2, and HKUST-1 materials before and after Knoevenagel condensation of benzaldehyde with malononitrile.
3.4. Reusability of URJC-1 Material
Reusability of URJC-1 as a catalyst was also assesed in both reactions, carrying out until five consecutive reaction cycles at the same operation conditions described in the experimental section. The structural stability of the materials was evaluated after each reaction cycle by PXRD. The results displayed in
Figure 13A evidence a catalytic activity practically constant in terms of anisole conversion and
p-MAP yield for five reaction cycles of 5 h each. Additionally, PXRD patterns of the catalyst after each reaction cycle confirm the stability of URJC-1 structure, demonstrating again a remarkable chemical stability (
Figure 13B).
Figure 13.
(A) Catalytic performance of URJC-1 in several acylation reaction cycles. Normal columns indicate anisole conversion; dense fill columns indicate p-MAP yield; and (B) X-ray diffraction patterns of fresh and used URJC-1 catalyst after several anisole acylation reaction cycles.
Figure 13.
(A) Catalytic performance of URJC-1 in several acylation reaction cycles. Normal columns indicate anisole conversion; dense fill columns indicate p-MAP yield; and (B) X-ray diffraction patterns of fresh and used URJC-1 catalyst after several anisole acylation reaction cycles.
Regarding the Knoevenagel condensation reaction, the URJC-1 material also maintained its activity, in terms of benzaldehyde conversion, and structural integrity for five consecutive cycles of 4 h each, as it is observed in
Figure 14A,B, respectively.
Figure 14.
(A) Catalytic performance of URJC-1 in several Knoevenagel condensation reaction cycles; and (B) X-ray diffraction patterns of fresh and used URJC-1 catalyst after several Knoevenagel condensation reaction cycles.
Figure 14.
(A) Catalytic performance of URJC-1 in several Knoevenagel condensation reaction cycles; and (B) X-ray diffraction patterns of fresh and used URJC-1 catalyst after several Knoevenagel condensation reaction cycles.