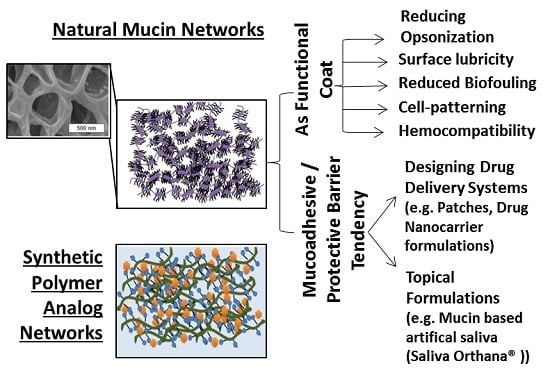
Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications
Abstract
:1. Introduction
2. Natural Mucin Network Structure, Formation and Molecular Properties
2.1. Molecular Properties
2.2. Mucin Network Structural Characteristics
3. Natural Mucin Network Function
3.1. Mucin Network’s Role in Microbial Regulation
4. Mucin Networks as Physical Permeation Barrier for Biofunctional Molecules
5. Critical Role of Mucin Network in Impacting Diverse Bioapplications
5.1. In Designing Drug Delivery Systems
5.2. Mucin as a Bio-Functional Coat
5.3. Mucin in Drug Delivery and Tissue Engineering Applications
6. In Modeling Importance and Need for Rigorous Characterization
7. Polymer Network as Synthetic Mucin Analog
7.1. Developing Polymer-Based Biofunctional Structures
7.2. Polymeric Network: Role as a Synthetic Mucin Analog
7.3. Significance of Mucus Mimetic Systems
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, D.F. Airway goblet cells: Responsive and adaptable front-line defenders. Eur. Respir. J. 1994, 7, 1690–1706. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.A.; Levine, M.J.; Tabak, L.A.; Reddy, M.S. Specificity of salivary-bacterial interactions. 2. Evidence for a lectin on streptococcus sanguis with specificity for a NeuAc-α-2,3Ga1-β-1,3Ga1nac sequence. Biochem. Biophys. Res. Commun. 1982, 106, 390–396. [Google Scholar] [CrossRef]
- Kim, Y.; Dalhaimer, P.; Christian, D.A.; Discher, D.E. Polymeric worm micelles as nano-carriers for drug delivery. Nanotechnology 2005, 16, S484–S491. [Google Scholar] [CrossRef] [PubMed]
- Bradway, S.D.; Bergey, E.J.; Jones, P.C.; Levine, M.J. Oral mucosal pellicle. Adsorption and transpeptidation of salivary components to buccal epithelial cells. Biochem. J. 1989, 261, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Kaditi, E.; Mountrichas, G.; Pispas, S.; Demetzos, C. Block copolymers for drug delivery nano systems (DDnSs). Curr. Med. Chem. 2012, 19, 5088–5100. [Google Scholar] [CrossRef] [PubMed]
- Praud, A.; Bootzeek, O.; Blache, Y. Synthesis of polymerizable vinyltriazoles: Development of an optimized one-pot strategy starting from 4-bromobutyne. Green Chem. 2013, 15, 1138–1141. [Google Scholar] [CrossRef]
- Bae, S.J.; Choi, H.; Choi, J.S. Synthesis of polymerizable amphiphiles with basic oligopeptides for gene delivery application. Polymer Korea 2013, 37, 94–99. [Google Scholar] [CrossRef]
- Prakobphol, A.; Levine, M.J.; Tabak, L.A.; Reddy, M.S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr. Res. 1982, 108, 111–122. [Google Scholar] [CrossRef]
- Tabak, L.A. In defense of the oral cavity: The protective role of the salivary secretions. Pediatr. Dent. 2006, 28, 110–117. [Google Scholar] [PubMed]
- Hill, H.D., Jr.; Reynolds, J.A.; Hill, R.L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J. Biol. Chem. 1977, 252, 3791–3798. [Google Scholar] [PubMed]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Hagerstrom, H. Pharmaceutical applications of mucoadhesion for the non-oral routes. J. Pharm. Pharmacol. 2005, 57, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bradway, S.D.; Bergey, E.J.; Scannapieco, F.A.; Ramasubbu, N.; Zawacki, S.; Levine, M.J. Formation of salivary-mucosal pellicle: The role of transglutaminase. Biochem. J. 1992, 284 Pt 2, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Authimoolam, S.P.; Vasilakes, A.L.; Shah, N.M.; Puleo, D.A.; Dziubla, T.D. Synthetic oral mucin mimic from polymer micelle networks. Biomacromolecules 2014, 15, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloid Surf. B 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Houver, G.J.; Frank, R.M. Ultrastructural study of human dental plaque. Inf. Dent. 1971, 53, 4191–4200. [Google Scholar] [PubMed]
- Lie, T. Scanning and transmission electron microscope study of pellicle morphogenesis. Scand. J. Dent. Res. 1977, 85, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Lendenmann, U.; Grogan, J.; Oppenheim, F.G. Saliva and dental pellicle—A review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.N.; Chasan, B.; Bansil, R.; Turner, B.S.; Bhaskar, K.R.; Afdhal, N.H. Atomic force microscopy reveals aggregation of gastric mucin at low ph. Biomacromolecules 2005, 6, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Krasieva, T.; Tang, S.; Ahn, Y.; Kim, C.S.; Vu, D.; Chen, Z.; Wilder-Smith, P. Optical approach to the salivary pellicle. J. Biomed. Opt. 2009, 14. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the muc2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliver. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Khanvilkar, K.; Donovan, M.D.; Flanagan, D.R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001, 48, 173–193. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [PubMed]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Ermund, A.; Johansson, M.E.V.; Schutte, A.; Hansson, G.C.; Sjovall, H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G430–G438. [Google Scholar] [CrossRef] [PubMed]
- Morgenroth, K.; Bolz, J. Morphological features of the interaction between mucus and surfactant on the bronchial-mucosa. Respiration 1985, 47, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.V.; Rogers, D.F. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology 2016, 97, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Bansil, R.; Bhaskar, K.R.; Turner, B.S.; LaMont, J.T.; Niu, N.; Afdhal, N.H. pH-dependent conformational change of gastric mucin leads to sol–gel transition. Biophys. J. 1999, 76, 1250–1258. [Google Scholar] [CrossRef]
- Brunelli, R.; Papi, M.; Arcovito, G.; Bompiani, A.; Castagnola, M.; Parasassi, T.; Sampaolese, B.; Vincenzoni, F.; de Spirito, M. Globular structure of human ovulatory cervical mucus. FASEB J. 2007, 21, 3872–3876. [Google Scholar] [CrossRef] [PubMed]
- Menarguez, M.; Pastor, L.M.; Odeblad, E. Morphological characterization of different human cervical mucus types using light and scanning electron microscopy. Hum. Reprod. 2003, 18, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.; Sheehan, J.K.; Knight, D.; Richardson, P.S.; Thornton, D.J. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins muc5ac and muc5b. Biochem. J. 2002, 361, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Round, A.N.; Berry, M.; McMaster, T.J.; Stoll, S.; Gowers, D.; Corfield, A.P.; Miles, M.J. Heterogeneity and persistence length in human ocular mucins. Biophys. J. 2002, 83, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Allen, A.; Leonard, A.J.; Sellers, L.A. The mucus barrier. Its role in gastroduodenal mucosal protection. J. Clin. Gastroenterol. 1988, 10, S93–S98. [Google Scholar] [CrossRef] [PubMed]
- Ermund, A.; Schutte, A.; Johansson, M.E.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the peyer’s patches. Am. J. Physiol. Gastrointest. Liver. Physiol. 2013, 305, G341–G347. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Dawes, C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, S.S.; Padgett, J.L.; Yudin, A.I.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001, 81, 1930–1937. [Google Scholar] [CrossRef]
- Allen, A.; Flemstrom, G.; Garner, A.; Kivilaakso, E. Gastroduodenal mucosal protection. Physiol. Rev. 1993, 73, 823–857. [Google Scholar] [PubMed]
- Kirch, J.; Schneider, A.; Abou, B.; Hopf, A.; Schaefer, U.F.; Schneider, M.; Schall, C.; Wagner, C.; Lehr, C.M. Optical tweezers reveal relationship between microstructure and nanoparticle penetration of pulmonary mucus. Proc. Natl. Acad. Sci. USA 2012, 109, 18355–18360. [Google Scholar] [CrossRef] [PubMed]
- Tinanoff, N.; Glick, P.L.; Weber, D.F. Ultrastructure of organic films on the enamel surface. Caries Res. 1976, 10, 19–32. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Marteau, P.R. Probiotics and prebiotics: Effects on diarrhea. J. Nutr. 2007, 137, 803S–811S. [Google Scholar] [PubMed]
- Matsui, H.; Wagner, V.E.; Hill, D.B.; Schwab, U.E.; Rogers, T.D.; Button, B.; Taylor, R.M.; Superfine, R.; Rubinstein, M.; Iglewski, B.H.; et al. A physical linkage between cystic fibrosis airway surface dehydration and pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 2006, 103, 18131–18136. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D. Microbes and you: Normal flora. Available online: http://www.scq.ubc.ca/microbes-and-you-normal-flora/ (accessed on 20 August 2006).
- Chao, C.C.; Stuebben, A.M.; Butala, S.M. Characterization of ocular mucus extracts by crossed immunoelectrophoretic techniques. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1127–1135. [Google Scholar]
- Holly, F.J.; Lemp, M.A. Tear physiology and dry eyes. Surv. Ophthalmol. 1977, 22, 69–87. [Google Scholar] [CrossRef]
- Mantelli, F.; Argueso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Keay, L.; Jalbert, I.; Naduvilath, T.J.; Sweeney, D.F.; Holden, B.A. Mucin balls with wear of conventional and silicone hydrogel contact lenses. Optom. Vis. Sci. 2003, 80, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Szczotka-Flynn, L.; Benetz, B.A.; Lass, J.; Albright, M.; Gillespie, B.; Kuo, J.; Fonn, D.; Sethi, A.; Rimm, A. The association between mucin balls and corneal infiltrative events during extended contact lens wear. Cornea 2011, 30, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hopcraft, M.S.; Tan, C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010, 55, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sasportas, L.S.; Hosford, D.N.; Sodini, M.A.; Waters, D.J.; Zambricki, E.A.; Barral, J.K.; Graves, E.E.; Brinton, T.J.; Yock, P.G.; le, Q.T.; et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, E37–E51. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Ship, J.A. Dry mouth and its effects on the oral health of elderly people. J. Am. Dent. Assoc. 2007, 138, 15s–20s. [Google Scholar] [CrossRef] [PubMed]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar] [PubMed]
- Kavanagh, K.; Dowd, S. Histatins: Antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2004, 56, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.R.; Garzino-Demo, A.; Meiller, T.F.; Meeks, V.; Jabra-Rizk, M.A. Salivary histatin-5 and oral fungal colonisation in HIV+ individuals. Mycoses 2009, 52, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, R.; Bobek, L.A. Antifungal activity of human salivary mucin-derived peptide, muc7 12-mer, in a murine model of oral candidiasis. J. Pept. Res. 2005, 66, 82–89. [Google Scholar] [CrossRef]
- Lin, A.L.; Johnson, D.A.; Patterson, T.F.; Wu, Y.; Lu, D.L.; Shi, Q.; Yeh, C.K. Salivary anticandidal activity and saliva composition in an HIV-infected cohort. Oral Microbiol. Immunol. 2001, 16, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jainkittivong, A.; Lin, A.L.; Johnson, D.A.; Langlais, R.P.; Yeh, C.K. Salivary secretion, mucin concentrations and candida carriage in HIV-infected patients. Oral Dis. 2009, 15, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Fidel, P.L., Jr.; Thunayyan, A.A.; Varlotta, S.; Meiller, T.F.; Jabra-Rizk, M.A. Impaired histatin-5 levels and salivary antimicrobial activity against in hiv infected individuals. J. AIDS Clin. Res. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.D. Lung mucus: A clinician’s view. Eur. Respir. J. 1997, 10, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Clamp, J.R.; Ene, D. The gastric-mucosal barrier. Method Find Exp. Clin. 1989, 11, 19–25. [Google Scholar]
- Allen, A.; Flemstrom, G. Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005, 288, C1–C19. [Google Scholar] [CrossRef] [PubMed]
- Crampton, J.R. Gastroduodenal mucus and bicarbonate—The defensive zone. Q. J. Med. 1988, 67, 269–272. [Google Scholar] [PubMed]
- Juntunen, M.; Kirjavainen, P.V.; Ouwehand, A.C.; Salminen, S.J.; Isolauri, E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 2001, 8, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Liu, X.W.; Liu, J.P.; Yang, X.Y.; Lu, F.G. Ulcerative colitis as a polymicrobial infection characterized by sustained broken mucus barrier. World J. Gastroenterol. 2014, 20, 9468–9475. [Google Scholar] [PubMed]
- Vaughan, E.E.; Mollet, B. Probiotics in the new millennium. Die Nahr. 1999, 43, 148–153. [Google Scholar] [CrossRef]
- Cremonini, F.; di Caro, S.; Nista, E.C.; Bartolozzi, F.; Capelli, G.; Gasbarrini, G.; Gasbarrini, A. Meta-analysis: The effect of probiotic administration on antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2002, 16, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Ther. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Martinez, E.G.; Gregorio, G.V.; Dans, L.F. Probiotics for treating acute infectious diarrhoea. Sao Paulo Med. J. 2011, 129, 185. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Lieleg, C.; Bloom, J.; Buck, C.B.; Ribbeck, K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 2012, 13, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Elstein, M. Cervical mucus: Its physiological role and clinical significance. Adv. Exp. Med. Biol. 1982, 144, 301–318. [Google Scholar] [PubMed]
- Becher, N.; Adams Waldorf, K.; Hein, M.; Uldbjerg, N. The cervical mucus plug: Structured review of the literature. Acta Obstet. Gynecol. Scand. 2009, 88, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Boukari, H.; Brichacek, B.; Stratton, P.; Mahoney, S.F.; Lifson, J.D.; Margolis, L.; Nossal, R. Movements of HIV-virions in human cervical mucus. Biomacromolecules 2009, 10, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Chretien, F.C. Ultrastructure and variations of human cervical mucus during pregnancy and the menopause. Acta Obstet. Gynecol. Scand. 1978, 57, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Authimoolam, S.P.; Lakes, A.L.; Puleo, D.A.; Dziubla, T.D. Layer-by-layers of polymeric micelles as a biomimetic drug-releasing network. Macromol. Biosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nieuw Amerongen, A.V.; Oderkerk, C.H.; Driessen, A.A. Role of mucins from human whole saliva in the protection of tooth enamel against demineralization in vitro. Caries Res. 1987, 21, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, H.S.; White, L.; Kuckhahn, K.; Gerlach, T.T.; Deliyski, D.D. Vocal fold mucus aggregation in persons with voice disorders. J. Commun. Disord. 2012, 45, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.L.; Krahnke, J.S.; Kim, V. Clinical issues of mucus accumulation in copd. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 139–150. [Google Scholar]
- Izuhara, K.; Ohta, S.; Shiraishi, H.; Suzuki, S.; Taniguchi, K.; Toda, S.; Tanabe, T.; Yasuo, M.; Kubo, K.; Hoshino, T.; et al. The mechanism of mucus production in bronchial asthma. Curr. Med. Chem. 2009, 16, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Verghese, M.W.; Kesimer, M.; Schwab, U.E.; Randell, S.H.; Sheehan, J.K.; Grubb, B.R.; Boucher, R.C. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J. Immunol. 2005, 175, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, W.E.; Doyle, R.J.; Taylor, K.G. Hydrophobic interactions and the adherence of streptococcus sanguis to hydroxylapatite. Infect. Immun. 1982, 38, 637–644. [Google Scholar] [PubMed]
- Tabak, L.A.; Levine, M.J.; Mandel, I.D.; Ellison, S.A. Role of salivary mucins in the protection of the oral cavity. J. Oral Pathol. 1982, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McBride, B.C.; Gisslow, M.T. Role of sialic acid in saliva-induced aggregation of streptococcus sanguis. Infect. Immun. 1977, 18, 35–40. [Google Scholar] [PubMed]
- Gibbons, R.J. Bacterial adhesion to oral tissues: A model for infectious diseases. J. Dent. Res. 1989, 68, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Loomis, R.E.; Prakobphol, A.; Levine, M.J.; Reddy, M.S.; Jones, P.C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch. Biochem. Biophys. 1987, 258, 452–464. [Google Scholar] [CrossRef]
- Levine, M.J.; Reddy, M.S.; Tabak, L.A.; Loomis, R.E.; Bergey, E.J.; Jones, P.C.; Cohen, R.E.; Stinson, M.W.; Al-Hashimi, I. Structural aspects of salivary glycoproteins. J. Dent. Res. 1987, 66, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Jachak, A.; Lai, S.K.; Hida, K.; Suk, J.S.; Markovic, N.; Biswal, S.; Breysse, P.N.; Hanes, J. Transport of metal oxide nanoparticles and single-walled carbon nanotubes in human mucus. Nanotoxicology 2012, 6, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Coaker, H. Development and in vitro evaluation of slippery nanoparticles for enhanced diffusion through native mucus. Nanomedicine UK 2014, 9, 382–383. [Google Scholar]
- Crater, J.S.; Carrier, R.L. Barrier properties of gastrointestinal mucus to nanoparticle transport. Macromol. Biosci. 2010, 10, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Li, L.D.; Crouzier, T.; Sarkar, A.; Dunphy, L.; Han, J.; Ribbeck, K. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys. J. 2013, 105, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. Engl. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, W.M.; Radomsky, M.L.; Whaley, K.J.; Cone, R.A. Antibody diffusion in human cervical-mucus. Biophys. J. 1994, 66, 508–515. [Google Scholar] [CrossRef]
- Chen, A.; McKinley, S.A.; Wang, S.; Shi, F.; Mucha, P.J.; Forest, M.G.; Lai, S.K. Transient antibody-mucin interactions produce a dynamic molecular shield against viral invasion. Biophys. J. 2014, 106, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, K.M.; Malykhina, O.; Stieh, D.J.; Hope, T.J. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS ONE 2013, 8, e76176. [Google Scholar] [CrossRef] [PubMed]
- Cu, Y.; Saltzman, W.M. Drug delivery: Stealth particles give mucus the slip. Nat. Mater. 2009, 8, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Tang, B.C.; Wang, Y.Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Karavas, E.; Georgarakis, E.; Bikiaris, D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur. J. Pharm. Biopharm. 2006, 64, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ramineni, S.K.; Cunningham, L.L., Jr.; Dziubla, T.D.; Puleo, D.A. Competing properties of mucoadhesive films designed for localized delivery of imiquimod. Biomater. Sci. 2013, 1, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Montisci, M.J.; Dembri, A.; Durrer, C.; Duchene, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Larhed, A.W.; Artursson, P.; Grasjo, J.; Bjork, E. Diffusion of drugs in native and purified gastrointestinal mucus. J. Pharm. Sci. 1997, 86, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Larhed, A.W.; Artursson, P.; Bjork, E. The influence of intestinal mucus components on the diffusion of drugs. Pharm. Res 1998, 15, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Sawant, K.K. Mucoadhesive microspheres: A promising tool in drug delivery. Curr. Drug Deliv. 2008, 5, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.; Shrestha, N.; Shahbazi, M.A.; Fonte, P.; Makila, E.M.; Salonen, J.J.; Hirvonen, J.T.; Granja, P.L.; Santos, H.A.; Sarmento, B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials 2014, 35, 9199–9207. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Y.; Jian, J.; Song, S. Self-aggregated nanoparticles based on amphiphilic poly(lactic acid)-grafted-chitosan copolymer for ocular delivery of amphotericin B. Int. J. Nanomed. 2013, 8, 3715–3728. [Google Scholar]
- Khutoryanskaya, O.V.; Morrison, P.W.; Seilkhanov, S.K.; Mussin, M.N.; Ozhmukhametova, E.K.; Rakhypbekov, T.K.; Khutoryanskiy, V.V. Hydrogen-bonded complexes and blends of poly(acrylic acid) and methylcellulose: Nanoparticles and mucoadhesive films for ocular delivery of riboflavin. Macromol. Biosci. 2014, 14, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, J.; Vermeire, A.; Adriaens, E.; Remon, J.P.; Ludwig, A. Evaluation of a mucoadhesive tablet for ocular use. J. Control. Release 2001, 77, 333–344. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.K.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.; Pham, J.T.H.; Wang, D.W.; Brownlow, B.; Elbayoumi, T.A. Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. Int. J. Nanomed. 2015, 10, 1569–1584. [Google Scholar]
- Sharma, N.; Kulkarni, G.T.; Sharma, A.; Bhatnagar, A.; Kumar, N. Natural mucoadhesive microspheres of Abelmoschus esculentus polysaccharide as a new carrier for nasal drug delivery. J. Microencapsul. 2013, 30, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Kim, G.; Desai, K.G.; Patel, H.; Olsen, K.F.; Curtis-Fisk, J.; Tocce, E.; Jordan, S.; Schwendeman, S.P. Feasibility investigation of cellulose polymers for mucoadhesive nasal drug delivery applications. Mol. Pharm. 2015, 12, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Devkar, T.B.; Tekade, A.R.; Khandelwal, K.R. Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using delonix regia gum as a natural mucoadhesive polymer. Colloids Surf. B Biointerfaces 2014, 122, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Alpar, H.O.; Somavarapu, S.; Atuah, K.N.; Bramwell, V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Montaseri, H.; Tafaghodi, M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf. B Biointerfaces 2010, 81, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, M.; Sakon, K.; Kinoshita, W.; Makino, Y. Enhanced pulmonary absorption following aerosol administration of mucoadhesive powder microspheres. J. Control. Release 2001, 77, 117–129. [Google Scholar] [CrossRef]
- Liu, X.B.; Ye, J.X.; Quan, L.H.; Liu, C.Y.; Deng, X.L.; Yang, M.; Liao, Y.H. Pulmonary delivery of scutellarin solution and mucoadhesive particles in rats. Eur. J. Pharm. Biopharm. 2008, 70, 845–852. [Google Scholar] [CrossRef] [PubMed]
- De Araujo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Acarturk, F. Mucoadhesive vaginal drug delivery systems. Recent Pat. Drug Deliv. Formul. 2009, 3, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, F.; Koffi, A.; Guyot, M.; Millet, P. Comparative in vitro–in vivo study of two quinine rectal gel formulations. Int. J. Pharm. 2004, 280, 151–162. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium–chitosan microspheres for rectal administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Ofokansi, K.C.; Adikwu, M.U.; Okore, V.C. Preparation and evaluation of mucin-gelatin mucoadhesive microspheres for rectal delivery of ceftriaxone sodium. Drug Dev. Ind. Pharm. 2007, 33, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Pathak, K.; Bali, V. Therapeutic potential of mucoadhesive drug delivery systems—An updated patent review. Recent Pat. Drug Deliv. Formul. 2010, 4, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Thasneem, Y.M.; Rekha, M.R.; Sajeesh, S.; Sharma, C.P. Biomimetic mucin modified plga nanoparticles for enhanced blood compatibility. J. Colloid Interface Sci. 2013, 409, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Janairo, R.R.R.; Zhu, Y.Q.; Chen, T.; Li, S. Mucin covalently bonded to microfibers improves the patency of vascular grafts. Tissue Eng. A 2014, 20, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, T.; Karlsson Ott, M.; Carlsson, J.; Feiler, A.; Caldwell, K.D. Potential use of mucins as biomaterial coatings. Ii. Mucin coatings affect the conformation and neutrophil-activating properties of adsorbed host proteins—Toward a mucosal mimic. J. Biomed. Mater. Res. A 2009, 91, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ardehali, R.; Caldwell, K.D.; Valint, P. Mucin coating on polymeric material surfaces to suppress bacterial adhesion. Colloid Surf. B 2000, 17, 229–239. [Google Scholar] [CrossRef]
- Crouzier, T.; Jang, H.; Ahn, J.; Stocker, R.; Ribbeck, K. Cell patterning with mucin biopolymers. Biomacromolecules 2013, 14, 3010–3016. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Fath, K.; Henricus, M.; Banerjee, I. Self-assembly and growth of smart cell-adhesive mucin-bound microtubes. Soft Matter 2009, 7, 21–36. [Google Scholar] [CrossRef]
- Chen, X.; Lee, G.S.; Zettl, A.; Bertozzi, C.R. Biomimetic engineering of carbon nanotubes by using cell surface mucin mimics. Angew. Chem. Int. Ed. Engl. 2004, 43, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Drug, E.; Landesman-Milo, D.; Belgorodsky, B.; Ermakov, N.; Frenkel-Pinter, M.; Fadeev, L.; Peer, D.; Gozin, M. Enhanced bioavailability of polyaromatic hydrocarbons in the form of mucin complexes. Chem. Res. Toxicol. 2011, 24, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Belgorodsky, B.; Drug, E.; Fadeev, L.; Hendler, N.; Mentovich, E.; Gozin, M. Mucin complexes of nanomaterials: First biochemical encounter. Small 2010, 6, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Lasky, L.A.; Singer, M.S.; Dowbenko, D.; Imai, Y.; Henzel, W.J.; Grimley, C.; Fennie, C.; Gillett, N.; Watson, S.R.; Rosen, S.D. An endothelial ligand for l-selectin is a novel mucin-like molecule. Cell 1992, 69, 927–938. [Google Scholar] [CrossRef]
- Wahrenbrock, M.; Borsig, L.; le, D.; Varki, N.; Varki, A. Selectin-mucin interactions as a probable molecular explanation for the association of trousseau syndrome with mucinous adenocarcinomas. J. Clin. Investig. 2003, 112, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Aigner, S.; Sthoeger, Z.M.; Fogel, M.; Weber, E.; Zarn, J.; Ruppert, M.; Zeller, Y.; Vestweber, D.; Stahel, R.; Sammar, M.; et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 1997, 89, 3385–3395. [Google Scholar] [PubMed]
- Chen, S.H.; Dallas, M.R.; Balzer, E.M.; Konstantopoulos, K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J. 2012, 26, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.D.; Julian, J.; Lessey, B.A.; Prakobphol, A.; Fisher, S.J. Muc1 is a scaffold for selectin ligands in the human uterus. Front. Biosci. 2006, 11, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Beckwitt, C.H.; Ribbeck, K. Mucin multilayers assembled through sugar–lectin interactions. Biomacromolecules 2012, 13, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Crouzier, T.; Lim, R.M.; Ribbeck, K.; Beppu, M.M.; Pitombo, R.N.M.; Cohen, R.E.; Rubner, M.F. Sugar-mediated disassembly of mucin/lectin multi layers and their use as pH-tolerant, on-demand sacrificial layers. Biomacromolecules 2014, 15, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Jangdey, M.S. Lectin conjugated gastroretentive multiparticulate delivery system of clarithromycin for the effective treatment of helicobacter pylori. Mol. Pharm. 2009, 6, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Adebisi, A.O.; Conway, B.R. Lectin-conjugated microspheres for eradication of helicobacter pylori infection and interaction with mucus. Int. J. Pharm. 2014, 470, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Z.Y.; Xu, Y.L.; Li, Y.H.; An, T.Z.; Su, Z.H.; Peng, B.; Lin, Y.; Wang, Q. Construction of glycoprotein multilayers using the layer-by-layer assembly technique. J. Mater. Chem. 2012, 22, 17954–17960. [Google Scholar] [CrossRef]
- Svensson, O.; Lindh, L.; Cardenas, M.; Arnebrant, T. Layer-by-layer assembly of mucin and chitosan—Influence of surface properties, concentration and type of mucin. J. Colloid Interface Sci. 2006, 299, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.E.; Solodukhina, N.M.; Nilsson, L.; Nikitin, M.P.; Nikitin, P.I.; Zubov, V.P.; Vikhrov, A.A. Binding of mucin to water-soluble and surface-grafted boronate-containing polymers. Polym. Sci. Ser. A 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Ding, Z.B.; Guan, Y.; Zhang, Y.; Zhu, X.X. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 2009, 5, 2302–2309. [Google Scholar] [CrossRef]
- Vissink, A.; Schaub, R.M.; van Rijn, L.J.; Gravenmade, E.J.; Panders, A.K.; Vermey, A. The efficacy of mucin-containing artificial saliva in alleviating symptoms of xerostomia. Gerodontology 1987, 6, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Blixt-Johansen, G.; Ek, A.C.; Ganowiak, W.; Granerus, A.K.; von Schenck, H.; Unosson, M.; Wiesel, K. Improvement of oral mucosa with mucin containing artificial saliva in geriatric patients. Arch. Gerontol. Geriatr. 1992, 14, 193–201. [Google Scholar] [CrossRef]
- S-Gravenmade, E.J.; Roukema, P.A.; Panders, A.K. The effect of mucin-containing artificial saliva on severe xerostomia. Int. J. Oral Surg. 1974, 3, 435–439. [Google Scholar] [CrossRef]
- Duxbury, A.J.; Thakker, N.S.; Wastell, D.G. A double-blind cross-over trial of a mucin-containing artificial saliva. Br. Dent. J. 1989, 166, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Sgravenmade, E.J.; Panders, A.K.; Vermey, A.; Petersen, J.K.; Visch, L.L.; Schaub, R.M.H. A clinical comparison between commercially available mucin-containing and cmc-containing saliva substitutes. Int. J. Oral Surg. 1983, 12, 232–238. [Google Scholar] [CrossRef]
- Vissink, A.; Waterman, H.A.; Sgravenmade, E.J.; Panders, A.K.; Vermey, A. Rheological properties of saliva substitutes containing mucin, carboxymethylcellulose or polyethylenoxide. J. Oral Pathol. Med. 1984, 13, 22–28. [Google Scholar] [CrossRef]
- Davies, A.N.; Daniels, C.; Pugh, R.; Sharma, K. A comparison of artificial saliva and pilocarpine in the management of xerostomia in patients with advanced cancer. Palliat. Med. 1998, 12, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.P.; Bagg, J.; Baxter, W.P.; Aitchison, T.C. Clinical trial of a mucin-containing oral spray for treatment of xerostomia in hospice patients. Palliat. Med. 1997, 11, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.V.; David, L.; Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Gniewek, P.; Kolinski, A. Coarse-grained modeling of mucus barrier properties. Biophys. J. 2012, 102, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vilar, J.; Mabolo, R. Gel-forming mucins. Notions from in vitro studies. Histol. Histopathol. 2007, 22, 455–464. [Google Scholar] [PubMed]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.A.; Sinko, P.J. Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin. J. Appl. Polym. Sci. 1997, 63, 1481–1492. [Google Scholar] [CrossRef]
- Sakuma, S.; Sudo, R.; Suzuki, N.; Kikuchi, H.; Akashi, M.; Hayashi, M. Mucoadhesion of polystyrene nanoparticles having surface hydrophilic polymeric chains in the gastrointestinal tract. Int. J. Pharm. 1999, 177, 161–172. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Fukada, H.; Inui, T. Synthesis and binding properties of peptidomimetics based on a dendritic polymer. Polym. J. 2013, 45, 339–345. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2007, 19, 906. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electro spinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.H.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine UK 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, B.K.; Zin, W.C. Supramolecular structures from rod-coil block copolymers. Chem. Rev. 2001, 101, 3869–3892. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.J.; Lim, Y.B. Self-assembled filamentous nanostructures for drug/gene delivery applications. Expert Opin. Drug Deliv. 2010, 7, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Discher, D. Synthetic filamentous phages from self-assembling biocompatible diblock copolymers. Abstr. Pap. Am. Chem. Soc. 2005, 229, U970. [Google Scholar]
- Kim, T.H.; Mount, C.W.; Dulken, B.W.; Ramos, J.; Fu, C.J.; Khant, H.A.; Chiu, W.; Gombotz, W.R.; Pun, S.H. Filamentous, mixed micelles of triblock copolymers enhance tumor localization of indocyanine green in a murine xenograft model. Mol. Pharm. 2012, 9, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Dalhaimer, P.; Engler, A.J.; Parthasarathy, R.; Discher, D.E. Targeted worm micelles. Biomacromolecules 2004, 5, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Dalhaimer, P.; Discher, D.E. Targeted worm micelles for drug delivery. Abstr. Pap. Am. Chem. Soc. 2004, 228, U495. [Google Scholar]
- Discher, D.E.; Dalhaimer, P.; Engler, A.J.; Parthasarthy, R. Targeted worm-like micelles. Abstr. Pap. Am. Chem. Soc. 2005, 230, U4026–U4027. [Google Scholar]
- Geng, Y.; Dalhaimer, P.; Cai, S.S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Ilies, M.A.; Simone, E.; Zaitsev, S.; Kim, Y.; Cai, S.; Mahmud, A.; Dziubla, T.; Muro, S.; Discher, D.E.; et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano 2011, 5, 6991–6999. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Sanwaria, S.; Srivastava, R.; Nandan, B.; Horechyy, A.; Stamm, M.; Chen, H.L. Hairy polymer nanofibers via self-assembly of block copolymers. J. Mater. Chem. 2012, 22, 25102–25107. [Google Scholar] [CrossRef]
- Mahalingam, A.; Jay, J.I.; Langheinrich, K.; Shukair, S.; McRaven, M.D.; Rohan, L.C.; Herold, B.C.; Hope, T.J.; Kiser, P.F. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials 2011, 32, 8343–8355. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, D.G.; Li, W.J.; Gao, Y.; Chen, H.B.; Li, H.M. Phenylboronate-diol crosslinked polymer gels with reversible sol–gel transition. Polymer 2011, 52, 4268–4276. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Wu, Z.M.; Zhang, X.G.; Sun, L.; Li, C.X. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological ph. Soft Matter 2014, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Liu, M.N.; Chen, H.B.; Xu, J.; Gao, Y.; Li, H.M. Phenylboronate-diol crosslinked polymer/SWCNT hybrid gels with reversible sol–gel transition. Polym. Adv. Technol. 2014, 25, 233–239. [Google Scholar] [CrossRef]
- Cook, M.T.; Smith, S.L.; Khutoryanskiy, V.V. Novel glycopolymer hydrogels as mucosa-mimetic materials to reduce animal testing. Chem. Commun. 2015, 51, 14447–14450. [Google Scholar] [CrossRef] [PubMed]
- Rabuka, D.; Parthasarathy, R.; Lee, G.S.; Chen, X.; Groves, J.T.; Bertozzi, C.R. Hierarchical assembly of model cell surfaces: Synthesis of mucin mimetic polymers and their display on supported bilayers. J. Am. Chem. Soc. 2007, 129, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- Rabuka, D.; Forstner, M.B.; Groves, J.T.; Bertozzi, C.R. Noncovalent cell surface engineering: Incorporation of bioactive synthetic glycopolymers into cellular membranes. J. Am. Chem. Soc. 2008, 130, 5947–5953. [Google Scholar] [CrossRef] [PubMed]
- Godula, K.; Rabuka, D.; Nam, K.T.; Bertozzi, C.R. Synthesis and microcontact printing of dual end-functionalized mucin-like glycopolymers for microarray applications. Angew. Chem. Int. Ed. Engl. 2009, 48, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Godula, K.; Bertozzi, C.R. Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins. J. Am. Chem. Soc. 2012, 134, 15732–15742. [Google Scholar] [CrossRef] [PubMed]
- Authimoolam, S.P.; Puleo, D.A.; Dziubla, T.D. Affinity based multilayered polymeric self-assemblies for oral wound applications. Adv. Healthc. Mater. 2013, 2, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Fiegel, J. Synthetic tracheal mucus with native rheological and surface tension properties. J. Biomed. Mater. Res. A 2014, 102, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Groo, A.C.; Mircheva, K.; Bejaud, J.; Ailhas, C.; Panaiotov, I.; Saulnier, P.; Ivanova, T.; Lagarce, F. Development of 2D and 3D mucus models and their interactions with mucus-penetrating paclitaxel-loaded lipid nanocapsules. Pharm. Res. 2014, 31, 1753–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burruano, B.T.; Schnaare, R.L.; Malamud, D. Synthetic cervical mucus formulation. Contraception 2002, 66, 137–140. [Google Scholar] [CrossRef]
- Burruano, B.T.; Schnaare, R.L.; Malamud, D. In vitro test to evaluate the interaction between synthetic cervical mucus and vaginal formulations. AAPS PharmSciTech 2004, 5, E17. [Google Scholar] [PubMed]
- Wang, W.; Chance, D.L.; Mossine, V.V.; Mawhinney, T.P. Raft-based tri-component fluorescent glycopolymers: Synthesis, characterization and application in lectin-mediated bacterial binding study. Glycoconj. J. 2014, 31, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.J. Intervention with bacterial adhesion by multivalent carbohydrates. Med. Res. Rev. 2007, 27, 796–816. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Chabre, Y.M.; Roy, R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chem. Eur. J. 2008, 14, 7490–7499. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.; Gerken, T.A.; Jentoft, N. Role of glycosylation on the conformation and chain dimensions of o-linked glycoproteins: Light-scattering studies of ovine submaxillary mucin. Biochemistry 1989, 28, 5525–5536. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.J.; Herzberg, M.C.; Levine, M.S.; Ellison, S.A.; Stinson, M.W.; Li, H.C.; Vandyke, T. Specificity of salivary-bacterial interactions—Role of terminal sialic-acid residues in interaction of salivary glycoproteins with streptococcus-sanguis and streptococcus-mutans. Infect. Immun. 1978, 19, 107–115. [Google Scholar] [PubMed]
- Stinson, M.W.; Levine, M.J.; Cavese, J.M.; Prakobphol, A.; Murray, P.A.; Tabak, L.A.; Reddy, M.S. Adherence of streptococcus-sanguis to salivary mucin bound to glass. J. Dent. Res. 1982, 61, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.J.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Developing synthetic mucosa-mimetic hydrogels to replace animal experimentation in characterisation of mucoadhesive drug delivery systems. Soft Matter 2011, 7, 9620–9623. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, C.P.; Lee, Y.D. Biomimetic porous scaffolds made from poly(l-lactide)-g-chondroitin sulfate blend with poly(l-lactide) for cartilage tissue engineering. Biomacromolecules 2006, 7, 2200–2209. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Authimoolam, S.P.; Dziubla, T.D. Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications. Polymers 2016, 8, 71. https://doi.org/10.3390/polym8030071
Authimoolam SP, Dziubla TD. Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications. Polymers. 2016; 8(3):71. https://doi.org/10.3390/polym8030071
Chicago/Turabian StyleAuthimoolam, Sundar P., and Thomas D. Dziubla. 2016. "Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications" Polymers 8, no. 3: 71. https://doi.org/10.3390/polym8030071
APA StyleAuthimoolam, S. P., & Dziubla, T. D. (2016). Biopolymeric Mucin and Synthetic Polymer Analogs: Their Structure, Function and Role in Biomedical Applications. Polymers, 8(3), 71. https://doi.org/10.3390/polym8030071