(Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules
Abstract
:1. Introduction
2. TEM Preparation Techniques
2.1. Preparation of Stained Samples for TEM
2.2. Preparation of Vitrified Samples for CryoTEM
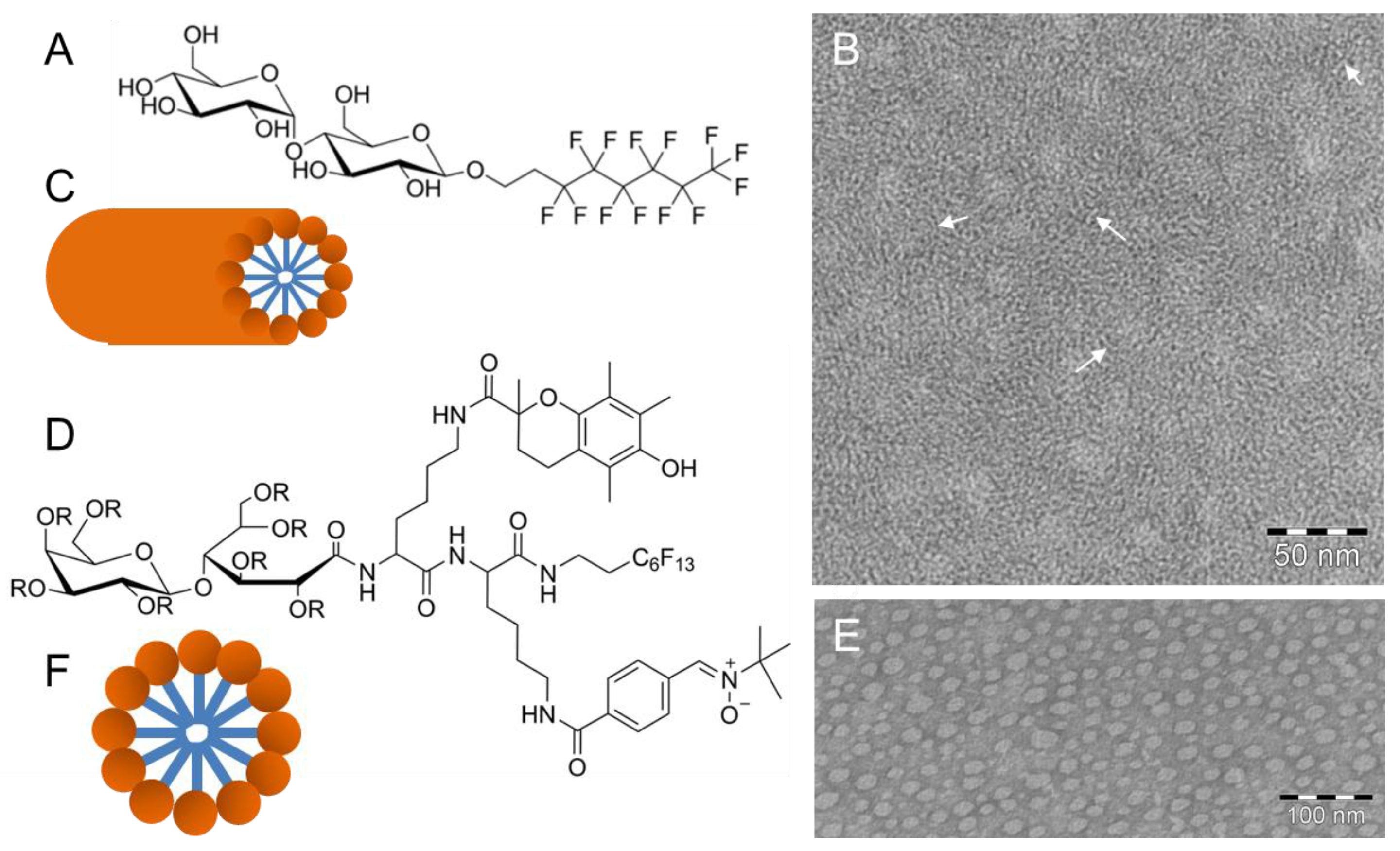
3. (Cryo)TEM of Self-Assembled Fluorinated Amphiphiles
4. (Cryo)TEM of Phospholipid Model Membranes Interacting with Amphiphilic Bolalipids, Amphiphilic T-Shaped Molecules, and X-Shaped Bolapolyphiles
4.1. Phospholipid Membranes and Amphiphilic Bolalipids
4.2. Phospholipid Membranes and Amphiphilic T-Shaped Molecules
4.3. Phospholipid Membranes and X-Shaped Bolapolyphiles
5. (Cryo)TEM of Phospholipid Model Membranes Interacting with Amphiphilic Macromolecules
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The fluid-mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric lipid membranes: Towards more realistic model systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Scheres, S.H.W. Cryo-EM: A unique tool for the visualization of Macromolecular complexity. Mol. Cell 2015, 58, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Leiro, R.; Scheres, S.H.W. Unravelling biological macromolecules with cryo-electron microscopy. Nature 2016, 537, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. The resolution revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Cryo-EM enters a new era. eLife 2014, 3, e03678. [Google Scholar] [CrossRef] [PubMed]
- Binshtein, E.; Ohi, M.D. Cryo-electron microscopy and the amazing race to atomic resolution. Biochemistry 2015, 54, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.G. Structural biology in situ—The potential of subtomogram averaging. Curr. Opin. Struct. Biol. 2013, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E. The development of cryo-EM into a mainstream structural biology technique. Nat. Methods 2016, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, B. How good can cryo-EM become? Nat. Methods 2016, 13, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Brilot, A.F.; Chen, J.Z.; Cheng, A.; Pan, J.; Harrison, S.C.; Potter, C.S.; Carragher, B.; Henderson, R.; Grigorieff, N. Beam-induced motion of vitrified specimen on holey carbon film. J. Struct. Biol. 2012, 177, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Harapin, J.; Eibauer, M.; Medalia, O. Structural analysis of supramolecular assemblies by cryo-electron tomography. Structure 2013, 21, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. Single-particle cryo-EM at crystallographic resolution. Cell 2015, 161, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.; Arheit, M.; Kowal, J.; Zeng, X.; Stahlberg, H. Single particle 3D reconstruction for 2D crystal images of membrane proteins. J. Struct. Biol. 2014, 185, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Rigort, A.; Bäuerlein, F.J.B.; Villa, E.; Eibauer, M.; Laugks, T.; Baumeister, W.; Plitzko, J.M. Focused ion beam micromachining of eukaryotic cells for cryoelectron tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Schaffer, M.; Plitzko, J.M.; Baumeister, W. Opening windows into the cell: Focused-ion-beam milling for cryo-elctron tomography. Curr. Opin. Struct. Biol. 2013, 23, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, W.; Schorb, M.; Welsch, S.; Picco, A.; Kaksonen, M.; Briggs, J.A.G. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J. Cell Biol. 2011, 192, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Correlative cryo-electron tomography and optical microscopy of cells. Curr. Opin. Struct. Biol. 2013, 23, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Kim, Y.-K.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.-S.; Jakli, A.; Lavrentovich, O.D.; Tamba, M.-G.; Kohlmeier, A.; et al. Direct observation liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microscopy Res. Tech. 2014, 77, 754–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Finger, S.; Hause, G.; Blume, A. Morphological changes of bacterial model membrane vesicles. Eur. J. Lipid Sci. Technol. 2014, 116, 1228–1233. [Google Scholar] [CrossRef]
- Danino, D. Cryo-TEM of soft molecular assemblies. Curr. Opin. Colloid Interface Sci. 2012, 17, 316–329. [Google Scholar] [CrossRef]
- Blume, A.; Drescher, S.; Graf, G.; Köhler, K.; Meister, A. Self-assembly of different single-chain bolaphospholipids and their miscibility with phospholipids or classical amphiphiles. Adv. Colloid Interface Sci. 2014, 208, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.F.; Thordarson, P. Polymersomes with asymmetric membranes based on readily accessible di- and triblock copolymers synthesized via SET-LRP. ACS Macro Lett. 2016, 5, 1172–1175. [Google Scholar] [CrossRef]
- Daum, B.; Auerswald, A.; Gruber, T.; Hause, G.; Balbach, J.; Kühlbrandt, W.; Meister, A. Supramolecular organization of the human N-BAR domain in shaping the sarcolemma membrane. J. Struct. Biol. 2016, 194, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R. Negative staining of thinly spread biological particulates. In Methods in Molecular Biology; Hajibagheri, N., Ed.; Springer: New York, NY, USA, 1999; Volume 117, pp. 13–30. [Google Scholar]
- Booth, D.S.; Avila-Sakar, A.; Cheng, Y. Visualizing proteins and macromolecular complexes by negative stain EM: From grid preparation to image acquisition. J. Vis. Exp. 2011, 58, e3227. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Roos, C.; Djalali, R.; Theingans, O.; Maskos, M.; Schmidt, M. Application of the negative staining technique to both aqueous and organic solvent solutions of polymer particles. Micron 1999, 30, 289–298. [Google Scholar] [CrossRef]
- Bremer, A.; Henn, C.; Engel, A.; Baumeister, W.; Aebi, U. Has negative staining still a place in biomacromolecular electron microscopy? Ultramicroscopy 1992, 46, 85–111. [Google Scholar] [CrossRef]
- De Carlo, S.; Harris, J.R. Negative staining and cryo-negative staining of macromolecules and viruses for TEM. Micron 2011, 42, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ohi, M.; Li, Y.; Cheng, Y.; Walz, T. Negative staining and image classification—Powerful tools in modern electron microscopy. Biol. Proced. Online 2004, 6, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlova, E.V.; Saibil, H.R. Structural analysis of macromolecular assemblies by electron microscopy. Chem. Rev. 2011, 111, 7710–7748. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Horne, R.W. Negative staining: A brief assessment of current technical benefits, limitations and future possibilities. Micron 1994, 25, 5–13. [Google Scholar] [CrossRef]
- Drescher, S.; Garamus, V.M.; Garvey, C.J.; Meister, A.; Blume, A. Aggregation behaviour of a single-chain, phenylene-modified bolalipid and its miscibility with classical phospholipids. Beilstein J. Org. Chem. 2017, 13, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D. Wechselwirkungen X-Förmiger Polyphiler Moleküle Mit Phospholipiden in Modellmembranen. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2015. [Google Scholar]
- Adrian, M.; Dubochet, J.; Lepault, J.; McDowall, A.W. Cryo-electron microscopy of viruses. Nature 1984, 308, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloids Surf. A 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Kourkoutis, L.F.; Plitzko, J.M.; Baumeister, W. Electron microscopy of biological materials at the nanometer scale. Annu. Rev. Mater. Res. 2012, 42, 33–58. [Google Scholar] [CrossRef]
- Frederik, P.M.; Hubert, D.H.W. Cryoelectron microscopy of liposomes. Methods Enzymol. 2005, 391, 431–448. [Google Scholar] [PubMed]
- Resch, G.P.; Brandstetter, M.; Pickl-Herk, A.M.; Königsmaier, L.; Wonesch, V.I.; Urban, E. Immersion freezing of biological specimens: Rationale, principles, and instrumentation. Cold Spring Harb. Protoc. 2011, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Resch, G.P.; Brandstetter, M.; Königsmaier, L.; Urban, E.; Pickl-Herk, A.M. Immersion freezing of suspended particles and cells for cryo-electron microscopy. Cold Spring Harb. Protoc. 2011, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Dobro, M.J.; Melanson, L.A.; Jensen, G.J.; McDowall, A.W. Plunge freezing for electron cryomicroscopy. Methods Enzymol. 2010, 481, 63–82. [Google Scholar] [PubMed]
- Dubochet, J.; Lepault, J.; Freeman, R.; Berriman, J.A.; Homo, J.-C. Electron microscopy of frozen water and aqueous solutions. J. Microsc. 1982, 128, 219–237. [Google Scholar] [CrossRef]
- Friedrich, H.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. Imaging of self-assembled structures: Interpretation of TEM and cryo-TEM images. Angew. Chem. Int. Ed. 2010, 49, 7850–7858. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Andersson, M.; Hammarström, L.; Edwards, K. Effect of bilayer phase transitions on vesicle structure and its influence on the kinetics of viologen reduction. J. Phys. Chem. 1995, 99, 14531–14538. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Gustafsson, J. Cryotransmission electron microscopy of thin vitrified samples. Curr. Opin. Colloid Interface Sci. 1996, 1, 270–278. [Google Scholar] [CrossRef]
- Kunitake, T.; Okahata, Y.; Yasunami, S. Formation and enhanced stability of fluoroalkyl bilayer membranes. J. Am. Chem. Soc. 1982, 104, 5547–5549. [Google Scholar] [CrossRef]
- Krafft, M.P. Controlling phospholipid self-assembly and film properties using highly fluorinated components—Fluorinated monolayers, vesicles, emulsions and microbubbles. Biochimie 2012, 94, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Motegi, T.; Morita, K.; Takagi, T.; Amii, H.; Kanamori, T.; Sonoyama, M.; Tero, R. Lateral diffusion and molecular interaction in a bilayer membrane consisting of partially fluorinated phospholipids. Langmuir 2016, 32, 10712–10718. [Google Scholar] [CrossRef] [PubMed]
- Mahrhauser, D.-S.; Reznicek, G.; Kotisch, H.; Brandstetter, M.; Nagelreiter, C.; Kwizda, K.; Valenta, C. Semi-solid fluorinated-DPPC liposomes: Morphological, rheological and thermic properties as well as examination of the influence of a model drug on their skin permeation. Int. J. Pharm. 2015, 486, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Schiedknecht, L.; Marie, P.; Giulieri, F.; Schmutz, M.; Poulain, N.; Nakache, E. Fluorinated vesicles allow intrabilayer polymerization of a hydrophobic monomer, yielding polymerized microcapsules. Langmuir 2001, 17, 2872–2877. [Google Scholar] [CrossRef]
- Santaella, C.; Vierling, P.; Riess, J.G.; Gulik-Krzywicki, T.; Gulik, A.; Monasse, G. Polymeric phase behavior of perfluoroalkylated phosphatidylcholines. Biochim. Biophys. Acta 1994, 1190, 25–39. [Google Scholar] [CrossRef]
- Guedj, C.; Pucci, B.; Zarif, L.; Coulomb, C.; Riess, J.G.; Pavia, A.A. Vesicles and other supramolecular systems from biocompatible synthetic glycolipids with hydrocarbon and/or fluorocarbon chains. Chem. Phys. Lipids 1994, 72, 153–173. [Google Scholar] [CrossRef]
- Hirsh, D.J.; Lazaro, N.; Wright, L.R.; Boggs, J.M.; McIntosh, T.J.; Schaefer, J.; Blazyk, J. A new monofluorinated phosphatidylcholine forms interdigitated bilayers. Biophys. J. 1998, 75, 1858–1868. [Google Scholar] [CrossRef]
- Toimil, P.; Davina, R.; Sabin, J.; Prieto, G.; Sarmiento, F. Influence of temperature on the colloidal stability of the F-DPPC and DPPC liposomes induced by lanthanum ions. J. Colloid Interface Sci. 2012, 367, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.W.H. Investigations of the Potential of Synthetic Phospholipids as Membrane Mimics: Interactions with Amphiphilic and Polyphilic Block Copolymers. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2016. [Google Scholar]
- Sanii, B.; Szmodis, A.W.; Bricarello, D.A.; Oliver, A.E.; Parikh, A.N. Frustrated phase transformations in supported, interdigitating lipid bilayers. J. Phys. Chem. B 2010, 114, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Riess, J.G. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie 1998, 80, 489–514. [Google Scholar] [CrossRef]
- Krafft, M.P. Strasbourg’s SOFFT team—Soft functional systems self-assembled from perfluoroalkylated molecular components. J. Fluorine Chem. 2012, 134, 90–102. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Trybala, A.; Starov, V.; Matar, O.; Ivanova, N. Fluoro- vs. hydrocarbon sufactants: Why do they differ in wetting performance? Adv. Colloid Interface Sci. 2014, 210, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabaud, E.; Barthélémy, P.; Mora, N.; Popot, J.-L.; Pucci, B. Stabilization of integral membrane proteins in aqueous solution using fluorinated surfactants. Biochimie 1998, 80, 515–530. [Google Scholar] [CrossRef]
- Polidori, A.; Presset, M.; Lebaupain, F.; Améduri, B.; Popot, J.-L.; Breyton, C.; Pucci, B. Fluorinated and hemifluorinated surfactants derived from maltose: Synthesis and application to handling membrane proteins in aqueous solution. Bioorg. Med. Chem. Lett. 2006, 16, 5827–5831. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Unger, S.; Keller, S.; Bonneté, F.; Ebel, C.; Pucci, B.; Breyton, C.; Durand, G. Micellar and biochemical properties of a propyl-ended fluorinated surfactant designed for membrane-protein study. J. Colloid Interface Sci. 2015, 445, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Polidori, A.; Raynal, S.; Barret, L.-A.; Dahani, M.; Barrot-Ivolot, C.; Jungas, C.; Frotscher, E.; Keller, S.; Ebel, C.; Breyton, C.; et al. Sparingly fluorinated maltoside-based surfactants for membrane-protein stabilization. New J. Chem. 2016, 40, 5364–5378. [Google Scholar] [CrossRef]
- Breyton, C.; Pucci, B.; Popot, J.-L. Amphiopols and fluorinated surfactants: Two alternatives to detergents for studying membrane proteins in vivo. In Methods in Molecular Biology; Mus-Veteau, I., Ed.; Springer: New York, NY, USA, 2010; Volume 601, pp. 219–245. [Google Scholar]
- Frotscher, E.; Danielczak, B.; Vargas, C.; Meister, A.; Durand, G.; Keller, S. A fluorinated detergent for membrane-protein applications. Angew. Chem. Int. Ed. 2015, 54, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Rosselin, M.; Meyer, G.; Guillet, P.; Cheviet, T.; Walther, G.; Meister, A.; Hadjipavlou-Litina, D.; Durand, G. Divalent amino-acid-based amphiphilic antioxidants: Synthesis, self-assembling properties, and biological evaluation. Bioconjugate Chem. 2016, 27, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Choteau, F.; Durand, G.; Ranchon-Cole, I.; Cercy, C.; Pucci, B. Cholesterol-based a-phenyl-N-tert-butyl nitrone derivatives as antioxidants against light-induced retinal degeneration. Bioorg. Med. Chem. Lett. 2010, 20, 7405–7409. [Google Scholar] [CrossRef] [PubMed]
- Der Mardirossian, C.; Krafft, M.P.; Gulik-Krzywicki, T.; le Maire, M.; Lederer, F. Perfluoroalkylphosphocholines are poor protein-solubilizing surfactants, as tested with neutrophil plasma membrane. Biochimie 1998, 80, 531–541. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Chong, P.L. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Benvegnu, T.; Réthoré, G.; Brard, M.; Richter, W.; Plusquellec, D. Archaeosomes based on novel synthetic tetraether-type lipids for the development of oral delivery systems. Chem. Commun. 2005, 5536–5538. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Barbeau, J.; Lemiègre, L.; Benvegnu, T. Archaeal tetraether bipolar lipids: Structures, functions and applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; Lechner, B.-D.; Garamus, V.M.; Almásy, L.; Meister, A.; Blume, A. The headgroup (a)symmetry strongly determines the aggregation behavior of single-chain phenylene-modified bolalipids and their miscibility with classical phospholipids. Langmuir 2014, 30, 9273–9284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, A.; Blume, A. Single-chain bolaphospholipids: Temperature-dependent self-assembly and mixing behavior with phospholipids. Adv. Planar Lip. Bilayers Liposomes 2012, 16, 93–128. [Google Scholar]
- Meister, A.; Drescher, S.; Garamus, V.M.; Karlsson, G.; Graf, G.; Dobner, B.; Blume, A. Temperature-dependent self-assembly and mixing behavior of symmetrical single-chain bolaamphiphiles. Langmuir 2008, 24, 6238–6246. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; Meister, A.; Garamus, V.M.; Hause, G.; Garvey, C.J.; Dobner, B.; Blume, A. Phenylene bolaamphiphiles: Influence of the substitution pattern on the aggregation behavior and the miscibility with classical phospholipids. Eur. J. Lipid Sci. Technol. 2014, 116, 1205–1216. [Google Scholar] [CrossRef]
- Tschierske, K. Liquid crystal engineering—New complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Baumeister, U.; Pelzl, G.; Das, M.K.; Zeng, X.B.; Ungar, G.; Tschierske, C. Carbohydrate rod conjugates: Ternary rod-coil molecules forming complex liquid crystal structures. J. Am. Chem. Soc. 2005, 127, 16578–16591. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, P.; Achilles, A.; Hoffmann, C.-V.; Lechner, B.-D.; Meister, A.; Tschierske, C.; Saalwächter, K.; Edwards, K.; Blume, A. A T-shaped amphiphilic molecule forms closed vesicles in water and bicelles in mixtures with a membrane lipid. J. Phys. Chem. B 2012, 116, 4871–4878. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, C.; Achilles, A.; Scholz, S.; Rüger, J.; Bacia, K.; Saalwächter, K.; Kressler, J.; Blume, A. Binding of amphiphilic and triphilic block copolymers to lipid model membranes: The role of perfluorinated moieties. Soft Matter 2014, 10, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, P.; Prehm, M.; Glettner, B.; Pelz, K.; Baumeister, U.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. X-shaped polyphilics: Liquid crystal honeycombs with single-molecule walls. Chem. Commun. 2008, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebert, H.; Lechner, B.D.; Lange, F.; Achilles, A.; Bärenwald, R.; Poppe, S.; Blume, A.; Saalwächter, K.; Tschierske, C.; et al. Dendritic domains with hexagonal symmetry formed by X-shaped bolapolyphiles in lipid membranes. Chem. Eur. J. 2015, 21, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D.; Ebert, H.; Prehm, M.; Werner, S.; Meister, A.; Hause, G.; Beerlink, A.; Saalwächter, K.; Bacia, K.; Tschierske, C.; et al. Temperature-dependent in-plane structure formation of an X-shaped bolapolyphile within lipid bilayers. Langmuir 2015, 31, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Hädicke, A.; Blume, A. Interactions of pluronic block copolymers with lipid vesicles depend on lipid phase and pluronic aggregation state. Colloid Polym. Sci. 2014, 293, 267–276. [Google Scholar] [CrossRef]
- Tribet, C.; Vial, F. Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter 2008, 4, 68–81. [Google Scholar] [CrossRef]
- Mansfeld, U.; Hoeppener, S.; Kempe, K.; Schumers, J.-M.; Gohy, J.-F.; Schubert, U.S. Tuning the morphology of triblock terpoly(2-oxazoline)s containing a 2-phenyl-2-oxazoline block with varying fluorine content. Soft Matter 2013, 9, 5966–5974. [Google Scholar] [CrossRef]
- Kaberov, L.I.; Verbraeken, B.; Hruby, M.; Riabtseva, A.; Kovacik, L.; Kereiche, S.; Brus, J.; Stepanek, P.; Hoogenboom, R.; Filippov, S.K. Novel triphilic block copolymers based on poly(2-methyl-2-oxazoline)-block-poly(2-octyl-2-oxazoline) with different terminal perfluoroalkyl fragments: Synthesis and self-assembly behaviour. Eur. Polym. J. 2017, 88, 645–655. [Google Scholar] [CrossRef]
- Kyeremateng, S.O.; Amado, E.; Blume, A.; Kressler, J. Synthesis of ABC and CABAC triphilic block copolymers by ATRP Combined with ‘click’ chemistry. Macromol. Rapid Commun. 2008, 29, 1140–1146. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated surfactants. In Surface Sience Series; No. 50; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Rossi, S.; Karlsson, G.; Ristori, S.; Martini, G.; Edwards, K. Aggregate structures in a dilute aqueous dispersion of a fluorinated/hydrogenated surfactant system. A cryo-transmission electron microscopy study. Langmuir 2001, 17, 2340–2345. [Google Scholar] [CrossRef]
- Scholtysek, P.; Li, Z.; Kressler, J.; Blume, A. Interactions of DPPC with semitelechelic poly(glycerol methacrylate)s with perfluoroalkyl end groups. Langmuir 2012, 28, 15651–15662. [Google Scholar] [CrossRef] [PubMed]
- Scholtysek, P. Chirale und Achirale Polymere in Wechselwirkung mit Phospholipid-Monolayern und -Bilayern. Ph.D. Thesis, MLU Halle-Wittenberg, Halle, Germany, 2014. [Google Scholar]
- Knowles, T.J.; Finka, R.; Smith, C.; Lin, Y.-P.; Dafforn, T.; Overduin, M. Membrane proteins solublilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009, 131, 7484–7485. [Google Scholar] [CrossRef] [PubMed]
- Dörr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schaefer, M.; van Walree, C.A.; Killian, J.A. The styrene-maleic acid copolymer: A versatile tool in membrane research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Grethen, A.; Oluwole, A.O.; Danielczak, B.; Vargas, C.; Keller, S. Thermodynamics of nanodisc formation mediated by styrene/maleic acid (2:1) copolymer. Sci. Rep. 2017, 7, 11517. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Arenas, R.; Danielczak, B.; Martel, A.; Porcar, L.; Bryton, C.; Ebel, C.; Keller, S. Fast collisional lipid transfer among polymer-bounded nanodiscs. Sci. Rep. 2017, 7, 45875. [Google Scholar] [CrossRef] [PubMed]
- Scheidelaar, S.; Koorengevel, M.C.; Pardo, J.D.; Meeldijk, J.D.; Breukink, E.; Killian, J.A. Molecular model for the solubilization of membranes into nanodisks by styrene maleic acid copolymers. Biophys. J. 2015, 108, 279–290. [Google Scholar]
- Vargas, C.; Cuevas Arenas, R.; Frotscher, E.; Keller, S. Nanoparticle self-assembly in mixtures of phospholipids with styrene/maleic acid copolymers or fluorinated surfactants. Nanoscale 2015, 7, 20685. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Danielszak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew. Chem. Int. Ed. 2017, 56, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, size-tunable self-assembly of polymer-lipid bilayer nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 11466–11470. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meister, A.; Blume, A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers 2017, 9, 521. https://doi.org/10.3390/polym9100521
Meister A, Blume A. (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers. 2017; 9(10):521. https://doi.org/10.3390/polym9100521
Chicago/Turabian StyleMeister, Annette, and Alfred Blume. 2017. "(Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules" Polymers 9, no. 10: 521. https://doi.org/10.3390/polym9100521
APA StyleMeister, A., & Blume, A. (2017). (Cryo)Transmission Electron Microscopy of Phospholipid Model Membranes Interacting with Amphiphilic and Polyphilic Molecules. Polymers, 9(10), 521. https://doi.org/10.3390/polym9100521