Comprehensive Study on Chemical and Hot Press-Treated Silver Nanowires for Efficient Polymer Solar Cell Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
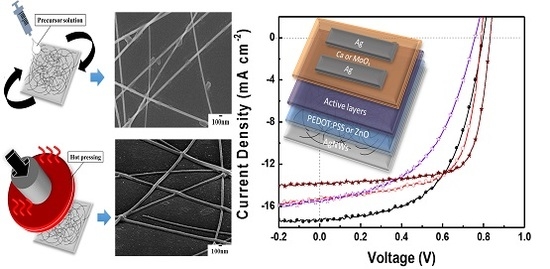
2.2. Modification and Processing of AgNWs
2.3. Device Fabrication
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kettle, J.; Bristow, N.; Gethin, D.T.; Tehrani, Z.; Moudam, O.; Li, B.; Katz, E.A.; dos Reis Benatto, G.A.; Krebs, F.C. Printable luminescent down shifter for enhancing efficiency and stability of organic photovoltaics. Sol. Energy Mater. Sol. Cells 2016, 144, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Pastorelli, F.; Schmidt, T.M.; Hösel, M.; Søndergaard, R.R.; Jørgensen, M.; Krebs, F.C. The organic power transistor: Roll-to-roll manufacture, thermal behavior, and power handling when driving printed electronics. Adv. Eng. Mater. 2016, 18, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Emmott, C.J.M.; Moia, D.; Sandwell, P.; Ekins-Daukes, N.; Hösel, M.; Lukoschek, L.; Amarasinghe, C.; Krebs, F.C.; Nelson, J. In-situ, long-term operational stability of organic photovoltaics for off-grid applications in africa. Sol. Energy Mater. Sol. Cells 2016, 149, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Kim, H.-J.; Kim, C.S.; Jeong, J.-H.; Cho, C.; Lee, J.-Y.; Jin, S.-H.; Choi, D.-G.; Kim, D.-H. ITO-free highly bendable and efficient organic solar cells with Ag nanomesh/ZnO hybrid electrodes. J. Mater. Chem. A 2015, 3, 65–70. [Google Scholar] [CrossRef]
- Triambulo, R.E.; Cheong, H.G.; Park, J.W. All-solution-processed foldable transparent electrodes of Ag nanowire mesh and metal matrix films for flexible electronics. Org. Electron. 2014, 15, 2685–2695. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.R.; Rathmell, A.R.; Chen, Z.F.; Stewart, I.E.; Wiley, B.J. Metal nanowire networks: The next generation of transparent conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef] [PubMed]
- Nam, V.B.; Lee, D. Copper nanowires and their applications for flexible, transparent conducting films: A review. Nanomaterials 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jimenez, C.; Brechet, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: Effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating simulations and experiments to predict sheet resistance and optical transmittance in nanowire films for transparent conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Zhang, D.; Cheng, J.Q.; Liu, J.; Mao, J.; Choy, W.C.H. Locally welded silver nano-network transparent electrodes with high operational stability by a simple alcohol-based chemical approach. Adv. Funct. Mater. 2015, 25, 4211–4218. [Google Scholar] [CrossRef]
- Chang, J.H.; Chiang, K.M.; Kang, H.W.; Chi, W.J.; Chang, J.H.; Wu, C.I.; Lin, H.W. A solution-processed molybdenum oxide treated silver nanowire network: A highly conductive transparent conducting electrode with superior mechanical and hole injection properties. Nanoscale 2015, 7, 4572–4579. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Song, T.B.; Bob, B.; Zhu, R.; Yang, Y. Solution-processed flexible transparent conductors composed of silver nanowire networks embedded in indium tin oxide nanoparticle matrices. Nano Res. 2012, 5, 805–814. [Google Scholar] [CrossRef]
- Zilberberg, K.; Gasse, F.; Pagui, R.; Polywka, A.; Behrendt, A.; Trost, S.; Heiderhoff, R.; Gorrn, P.; Riedl, T. Highly robust indium-free transparent conductive electrodes based on composites of silver nanowires and conductive metal oxides. Adv. Funct. Mater. 2014, 24, 1671–1678. [Google Scholar] [CrossRef]
- Khaligh, H.H.; Goldthorpe, I.A. Hot-rolling nanowire transparent electrodes for surface roughness minimization. Nanoscale Res. Lett. 2014, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.T.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Larios-Lopez, L.; Liu, C.; Garcia-Gutierrez, D.; Camacho-Bragado, A.; Yacaman, M.J. Corrosion at the nanoscale: The case of silver nanowires and nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Jiu, J.T.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Choi, D.Y.; Kang, H.W.; Sung, H.J.; Kim, S.S. Annealing-free, flexible silver nanowire-polymer composite electrodes via a continuous two-step spray-coating method. Nanoscale 2013, 5, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Hau, S.K.; Yip, H.L.; Zou, J.Y.; Jen, A.K.Y. Indium tin oxide-free semi-transparent inverted polymer solar cells using conducting polymer as both bottom and top electrodes. Org. Electron. 2009, 10, 1401–1407. [Google Scholar] [CrossRef]
- Jorgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Pingree, L.S.C.; MacLeod, B.A.; Ginger, D.S. The changing face of pedot: Pss films: Substrate, bias, and processing effects on vertical charge transport. J. Phys. Chem. C 2008, 112, 7922–7927. [Google Scholar] [CrossRef]
- So, F.; Kondakov, D. Degradation mechanisms in small-molecule and polymer organic light-emitting diodes. Adv. Mater. 2010, 22, 3762–3777. [Google Scholar] [CrossRef] [PubMed]
- Mayousse, C.; Celle, C.; Fraczkiewicz, A.; Simonato, J.P. Stability of silver nanowire based electrodes under environmental and electrical stresses. Nanoscale 2015, 7, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Ghosh, D.S.; Mkhitaryan, V.; Pruneri, V. Hybrid transparent conductive film on flexible glass formed by hot-pressing graphene on a silver nanowire mesh. ACS Appl. Mater. Interfaces 2013, 5, 11756–11761. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Hsu, P.C.; Chen, G.C.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.X.; Guo, Y.F.; Lin, L.; Zhou, Y.; et al. Roll-to-roll encapsulation of metal nanowires between graphene and plastic substrate for high-performance flexible transparent electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Leem, D.S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.C.; de Mello, J.C. Efficient organic solar cells with solution-processed silver nanowire electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Yoo, T.H.; Lim, J.W.; Sang, B.I.; Lim, D.S.; Choi, W.K.; Hwang, D.K.; Oh, Y.J. Enhanced light scattering and trapping effect of ag nanowire mesh electrode for high efficient flexible organic solar cell. Small 2015, 11, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Peng, Y.-J.; Chen, C.-P. Simple structured polyetheramines, Jeffamines, as efficient cathode interfacial layers for organic photovoltaics providing power conversion efficiencies up to 9.1%. J. Mater. Chem. A 2017, 5, 10424–10429. [Google Scholar] [CrossRef]





| Voc (V) | Jsc (mA cm−2) | FF | PCE (%) | Rs (Ω cm2) | Rsh (Ω cm2) | |
|---|---|---|---|---|---|---|
| Glass/AgNWs/PEDOT:PSS/P3HT:PC60BM/Ca/Al | 0.51 | 9.21 | 0.54 | 2.54 | 3.8 | 465 |
| Glass/HP-AgNWs/PEDOT:PSS/P3HT:PC60BM/Ca/Al | 0.54 | 8.98 | 0.61 | 2.96 | 3.5 | 505 |
| Glass/ITO/PEDOT:PSS/PffBT4T-2OD:PC71BM/Ca/Al | 0.73 | 14.68 | 0.50 | 5.36 | 3.8 | 325 |
| Glass/AgNWs/PEDOT:PSS/PffBT4T-2OD:PC71BM/Ca/Al | 0.66 | 15.18 | 0.42 | 4.21 | 13.6 | 179 |
| Glass/HP-AgNWs/PEDOT:PSS/PffBT4T-2OD:PC71BM/Ca/Al | 0.70 | 17.71 | 0.43 | 5.33 | 14.5 | 233 |
| PET/HP-AgNWs/PEDOT:PSS/PffBT4T-2OD:PC71BM/Ca/Al | 0.68 | 16.62 | 0.45 | 5.09 | 12.6 | 203 |
| Glass/ITO/ZnO/PTB7-Th: PC71BM/MoO3/Ag | 0.79 | 15.26 | 0.67 | 8.03 | 3.3 | 540 |
| Glass/HP-AgNWs/ZnO/PTB7-Th: PC71BM/MoO3/Ag | 0.80 | 17.17 | 0.57 | 7.83 | 6.0 | 574 |
| Glass/ITO/ZnO/PffBT4T-2OD:PC71BM/MoO3/Ag | 0.73 | 13.82 | 0.71 | 7.16 | 2.3 | 603 |
| Glass/HP-AgNWs/ZnO/PffBT4T-2OD:PC71BM/MoO3/Ag | 0.76 | 15.59 | 0.49 | 5.81 | 7.0 | 298 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-Y.; Ting, Y.-J.; Chung, C.-L.; Tsai, T.-W.; Chen, C.-P. Comprehensive Study on Chemical and Hot Press-Treated Silver Nanowires for Efficient Polymer Solar Cell Application. Polymers 2017, 9, 635. https://doi.org/10.3390/polym9110635
Yu Y-Y, Ting Y-J, Chung C-L, Tsai T-W, Chen C-P. Comprehensive Study on Chemical and Hot Press-Treated Silver Nanowires for Efficient Polymer Solar Cell Application. Polymers. 2017; 9(11):635. https://doi.org/10.3390/polym9110635
Chicago/Turabian StyleYu, Yang-Yen, Yo-Jen Ting, Chung-Lin Chung, Tzung-Wei Tsai, and Chih-Ping Chen. 2017. "Comprehensive Study on Chemical and Hot Press-Treated Silver Nanowires for Efficient Polymer Solar Cell Application" Polymers 9, no. 11: 635. https://doi.org/10.3390/polym9110635
APA StyleYu, Y.-Y., Ting, Y.-J., Chung, C.-L., Tsai, T.-W., & Chen, C.-P. (2017). Comprehensive Study on Chemical and Hot Press-Treated Silver Nanowires for Efficient Polymer Solar Cell Application. Polymers, 9(11), 635. https://doi.org/10.3390/polym9110635