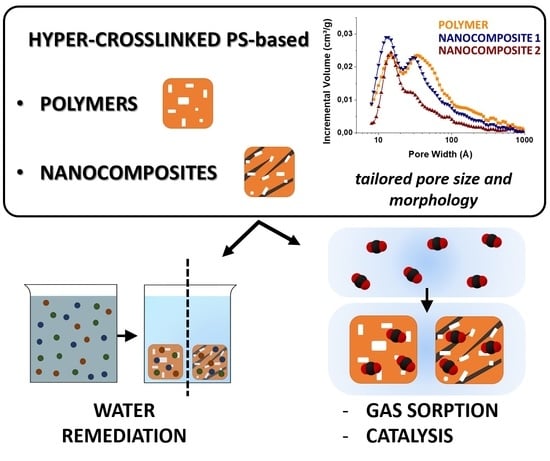
Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review
Abstract
:1. Introduction
2. HCL Polystyrenes
2.1. Precursor Polymers
2.2. Synthetic Routes
2.3. External Crosslinkers, Catalysts and Solvents
2.4. Crosslinking Degree
2.5. Chemical Modification
3. HCL Polystyrene Based Nanocomposites
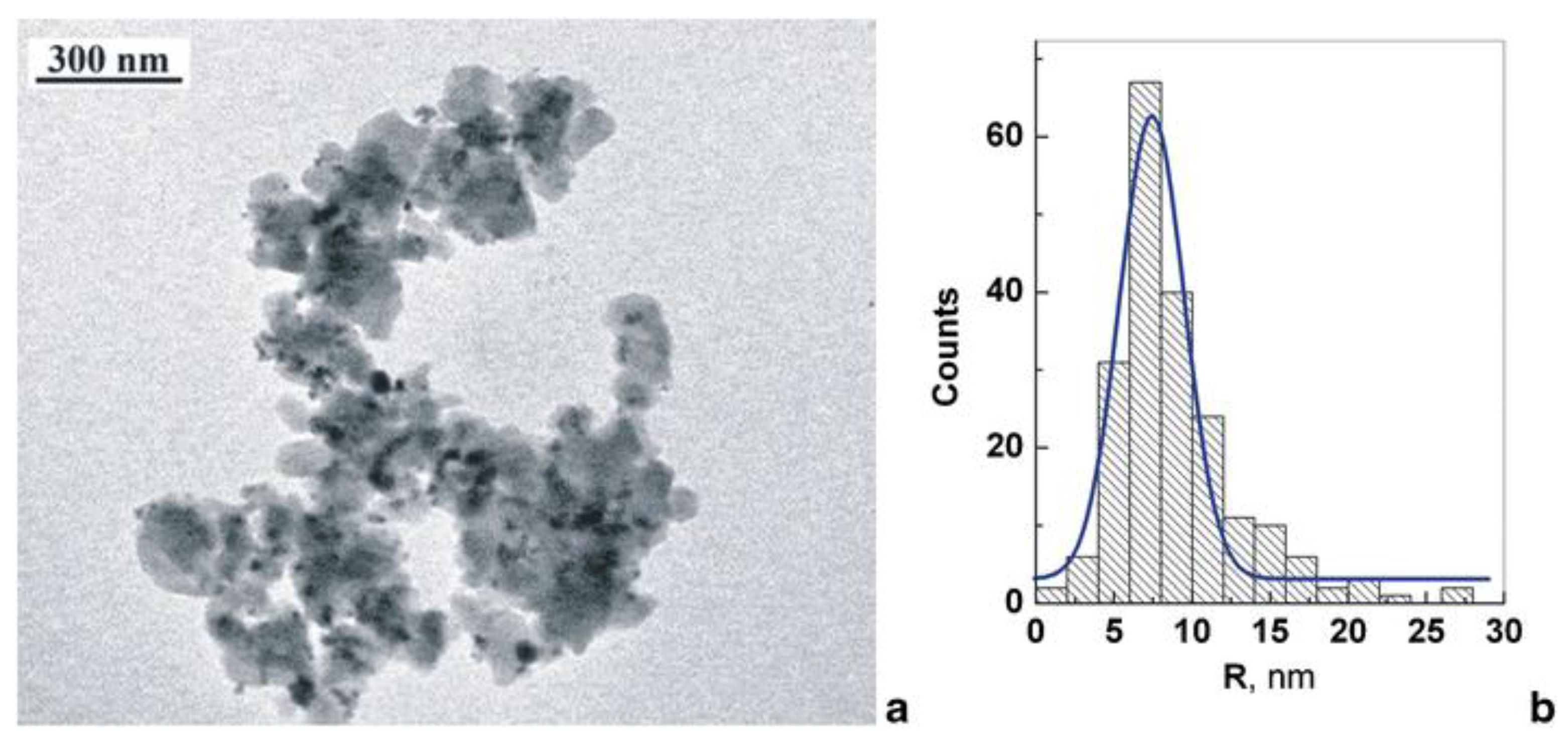
3.1. Nanocomposites Containing Magnetic Nanoparticles
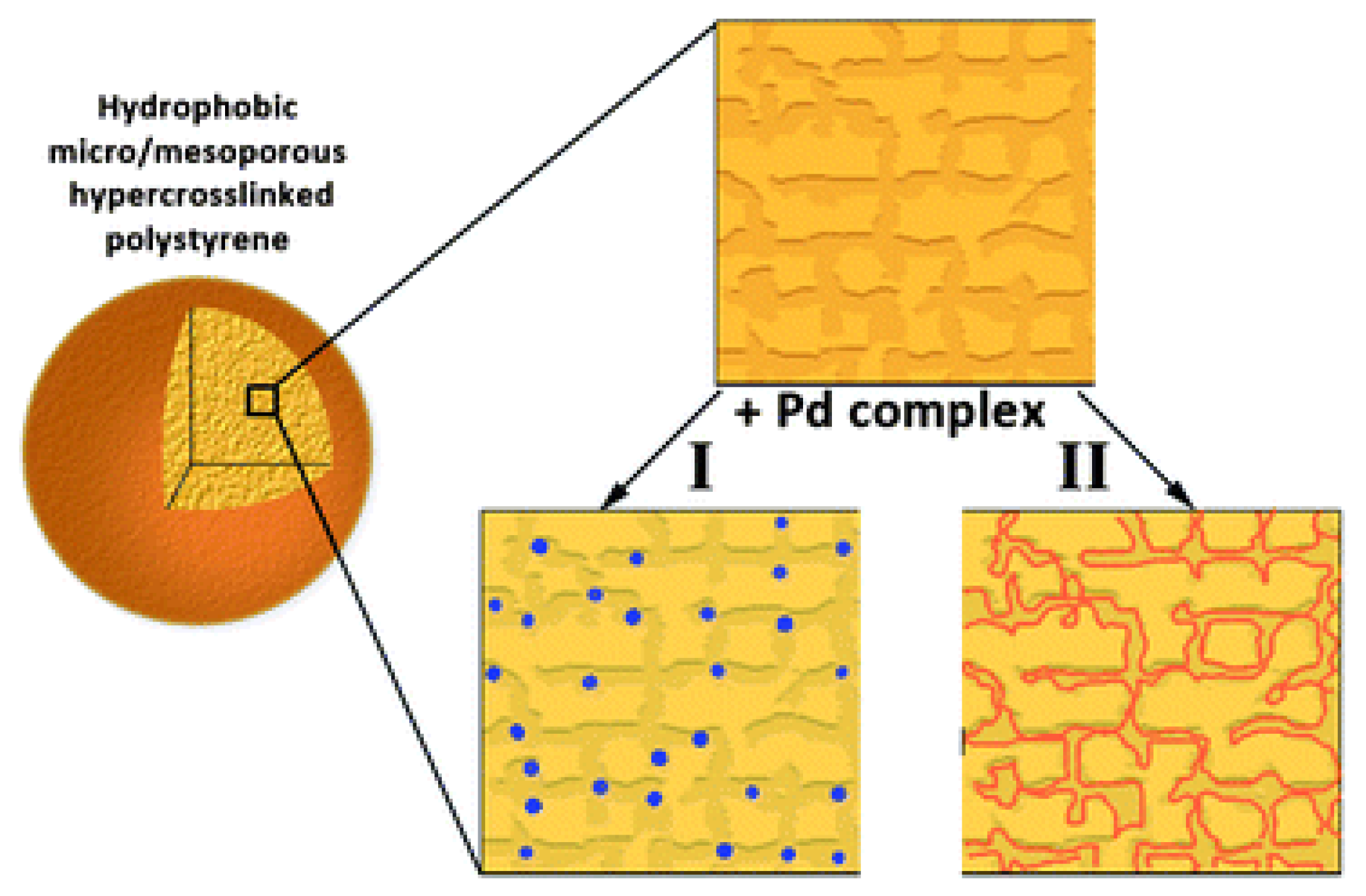
3.2. Nanocomposites Containing Metal Nanoparticles
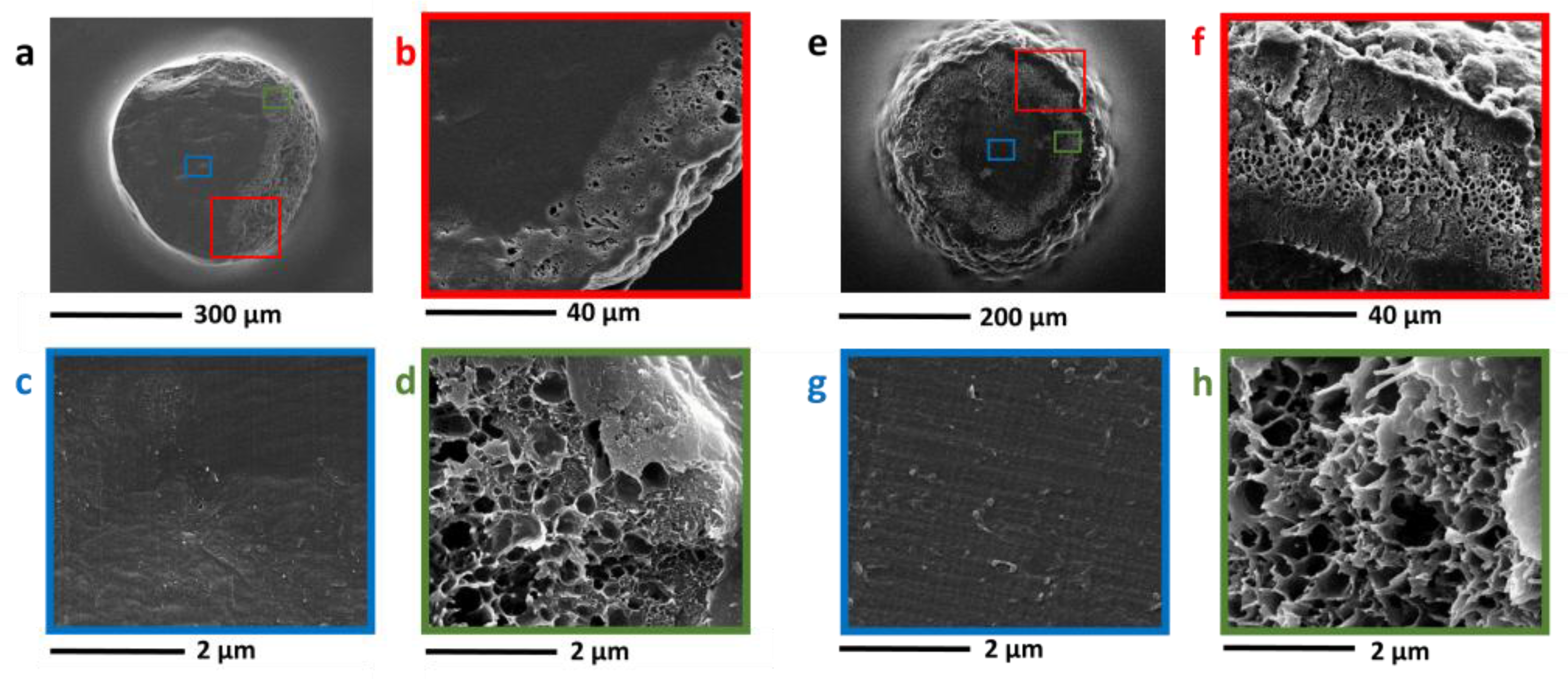
3.3. Nanocomposites Containing Carbon-Based Nanostructured Fillers
4. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Weickert, J.; Dunbar, R.B.; Hesse, H.C.; Wiedemann, W.; Schmidt-Mende, L. Nanostructured organic and hybrid solar cells. Adv. Mater. 2011, 23, 1810–1828. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, J.B.; Peterson, G.W. Metal-organic frameworks for air purification of toxic chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Z.; Ga, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, X.; Xia, L.; Majeed, M.I.; Tan, B. Hollow microporous organic capsules. Sci. Rep. 2013, 3, 2128. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-Polymer Composite Microcapsules Derived from Pickering Emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Svec, F.; Fréchet, J.M. Efficient separation of small molecules using a large surface area hypercrosslinked monolithic polymer capillary column. Anal. Chem. 2010, 82, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, S.; Xu, J.; Ding, Y.; Ouyang, G. Exploitation of a microporous organic polymer as a stationary phase for capillary gas chromatography. Anal. Chim. Acta 2016, 902, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Millini, R. Porous materials in catalysis: Challenges for mesoporous materials. Chem. Soc. Rev. 2013, 42, 3956–3976. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-linked conjugated microporous polymers: Sensing and removal of fluoride ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef] [PubMed]
- Crossland, E.J.W.; Noel, N.; Sivaram, V.; Leijtens, T.; Alexander-Webber, J.A.; Snaith, H.J. Mesoporous TiO2 single crystals delivering enhanced mobility and optoelectronic device performance. Nature 2013, 495, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, X.; Shi, Y.; Wan, C. Fabrication of a superhydrophobic surface from porous polymer using phase separation. Appl. Surf. Sci. 2014, 297, 33–39. [Google Scholar] [CrossRef]
- Everett, D.H. Manual of symbols and terminology for physicochemical quantities and units, appendix II: Definitions, terminology and symbols in colloid and surface chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.W.; Foley, N.J.; Norman, P.R.; Francis, D.C.; Thomas, K.M. Diffusion barriers in the kinetics of water vapor adsorption/desorption on activated carbons. Langmuir 1998, 14, 3858–3864. [Google Scholar] [CrossRef]
- Bonakala, S.; Balasubramanian, S. Structure-property relationships in amorphous microporous polymers. J. Phys. Chem. B 2016, 120, 557–565. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Stöckel, E.; Wu, X.; Trewin, A.; Wood, C.D.; Clowes, R.; Campbell, N.L.; Jones, J.T.A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. High surface area amorphous microporous poly(aryleneethynylene) networks using tetrahedral carbon- and silicon-centred monomers. Chem. Commun. 2009, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Turner, S.R. Hypercrosslinked Polymers: A Review. Polym. Rev. 2017, 1–41. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Chemical functionalization strategies for carbon dioxide capture in microporous organic polymers. Polym. Int. 2013, 62, 345–352. [Google Scholar] [CrossRef]
- Dawson, R.; Stevens, L.A.; Drage, T.C.; Snape, C.E.; Smith, M.W.; Adams, D.J.; Cooper, A.I. Impact of water coadsorption for carbon dioxide capture in microporous polymer sorbents. J. Am. Chem. Soc. 2012, 134, 10741–10744. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.; Svec, F.; Fréchet, J.M.J. Preparation of size-selective nanoporous polymer networks of aromatic rings: Potential adsorbents for hydrogen storage. Chem. Mater. 2008, 20, 7069–7076. [Google Scholar] [CrossRef]
- Woodward, R.T.; Stevens, L.A.; Dawson, R.; Vijayaraghavan, M.; Hasell, T.; Silverwood, I.P.; Ewing, A.V.; Ratvijitvech, T.; Exley, J.D.; Chong, S.Y.; et al. Swellable, water-and acid-tolerant polymer sponges for chemoselective carbon dioxide capture. J. Am. Chem. Soc. 2014, 136, 9028–9035. [Google Scholar] [CrossRef] [PubMed]
- Davankov, V.A.; Rogozhin, V.; Tsyurupa, M.P. Macronet Polystyrene Structures for Ionites and Method of Producing Same. U.S. Patent 3729457, 24 April 1973. [Google Scholar]
- Warshawsky, A.; Deshe, A.; Gutman, R. Safe halomethylation of aromatic polymers via BCME-free long chain haloalkylethers. Polym. Int. 1984, 16, 234–238. [Google Scholar] [CrossRef]
- Sharma, V.; Sahoo, A.; Sharma, Y.; Mohanty, P. Synthesis of nanoporous hypercrosslinked polyaniline (HCPANI) for gas sorption and electrochemical supercapacitor applications. RSC Adv. 2015, 5, 45749–45754. [Google Scholar] [CrossRef]
- Meng, Q.B.; Weber, J. Lignin-based microporous materials as selective adsorbents for carbon dioxide separation. ChemSusChem 2014, 7, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Avolio, R.; Cocca, M.; Gentile, G.; Errico, M.E.; Avella, M.; Carfagna, C.; Ambrogi, V. A versatile synthetic approach toward hyper-cross-linked styrene-based polymers and nanocomposites. Macromolecules 2017, 50, 4132–4143. [Google Scholar] [CrossRef]
- Sherrington, D.C. Preparation, structure and morphology of polymer supports. Chem. Commun. 1998, 2275–2410. [Google Scholar] [CrossRef]
- Veverka, P.; Jerábek, K. Mechanism of hypercrosslinking of chloromethylated styrene–divinylbenzene copolymers. React. Funct. Polym. 1999, 41, 21–25. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, J.E.; Oh, C.G.; Ihm, S.K.; Cortez, J.; Sherrington, D.C. Rapid generation and control of microporosity, bimodal pore size distribution, and surface area in davankov-type hyper-cross-linked resins. Macromolecules 2006, 39, 627–632. [Google Scholar] [CrossRef]
- Davankov, V.; Tsyurupa, M.P. Hypercrosslinked Polymeric Networks and Adsorbing Materials: Synthesis, Properties, Structure, and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 56, pp. 3–354. ISBN 9780444537010. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 152–156. ISBN 978-90-481-6633-6. [Google Scholar]
- Fontanals, N.; Cortés, J.; Galià, M.; Cormack, P.A.G.; Marcé, R.M.; Borrull, F.; Sherrington, D.C. Synthesis of davankov-type hypercrosslinked resins using different isomer compositions of vinylbenzyl chloride monomer, and application in the solid-phase extraction of polar compounds. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1718–1728. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Hypercrosslinked polyanilines with nanoporous structure and high surface area: Potential adsorbents for hydrogen storage. J. Mater. Chem. 2007, 17, 4989–4997. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous, hypercrosslinked polypyrroles: Effect of crosslinking moiety on pore size and selective gas adsorption. Chem. Commun. 2009, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Ratvijitvech, T.; Corker, M.; Laybourn, A.; Khimyak, Y.Z.; Cooper, A.I.; Adams, D.J. Microporous copolymers for increased gas selectivity. Polym. Chem. 2012, 3, 2034–2038. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, V.A. Structure and properties of hypercrosslinked polystyrene—The first representative of a new class of polymer networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Macintyre, F.S.; Sherrington, D.C.; Tetley, L. Synthesis of ultrahigh surface area monodisperse porous polymer nanospheres. Macromolecules 2006, 39, 5381–5384. [Google Scholar] [CrossRef]
- Fontanals, N.; Manesiotis, P.; Sherrington, D.C.; Cormack, P.A.G. Synthesis of spherical ultra-high-surface-area monodisperse amphipathic polymer sponges in the low-micrometer size range. Adv. Mater. 2008, 20, 1298–1302. [Google Scholar] [CrossRef]
- Tong, W.; Lv, Y.; Svec, F. Advantage of nanoporous styrene-based monolithic structure over beads when applied for methane storage. Appl. Energy 2016, 183, 1520–1527. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Kubo, M.; Moteki, T.; Sugawara-Narutaki, A.; Shimojima, A.; Okubo, T. Porous siloxane-organic hybrid with ultrahigh surface area through simultaneous polymerization-destruction of functionalized cubic siloxane cages. J. Am. Chem. Soc. 2011, 133, 13832–13835. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; White, D.; Mason, A.; Liu, D.-J. Porous organic polymers containing carborane for hydrogen storage. Int. J. Energy Res. 2013, 37, 732–740. [Google Scholar] [CrossRef]
- Law, R.; Sherrington, D.C.; Snape, C.; Ando, I.; Kurosu, H. Solid state 13C MAS NMR studies of hyper-cross-linked polystyrene resins. Macromolecules 1996, 29, 6284–6293. [Google Scholar] [CrossRef]
- Gawdzik, B.; Osypiuk, J. Modification of porous poly(styrene-divinylbenzene) beads by Friedel-Crafts reaction. Chromatographia 2001, 54, 323–328. [Google Scholar] [CrossRef]
- Pastukhov, A.V.; Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polystyrene: A polymer in a non-classical physical state. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2324–2333. [Google Scholar] [CrossRef]
- Davankov, V.A.; Rogoshin, S.V.; Tsyurupa, M.P. Macronet isoporous gels through crosslinking of dissolved polystyrene. J. Polym. Sci. 1974, 47, 95–101. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Niu, H.; Bradshaw, D.; Rosseinsky, M.J.; Khimyak, Y.Z.; Campbell, N.L.; Kirk, R.; Stockel, E.; et al. Hydrogen storage in microporous hypercrosslinked organic polymer networks. Chem. Mater. 2007, 19, 2034–2048. [Google Scholar] [CrossRef]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A new strategy to microporous polymers: Knitting rigid aromatic building blocks by external cross-linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Qiao, Z.; Chai, S.; Nelson, K.; Bi, Z.; Chen, J.; Mahurin, S.M.; Zhu, X.; Dai, S. Polymeric molecular sieve membranes via in situ cross-linking of non-porous polymer membrane templates. Nat. Commun. 2014, 5, 3705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, B.; Wood, C.D. Solution-processable hypercrosslinked polymers by low cost strategies: A promising platform for gas storage and separation. J. Mater. Chem. A 2016, 4, 15072–15080. [Google Scholar] [CrossRef]
- Urban, J.; Škeříková, V. Effect of hypercrosslinking conditions on pore size distribution and efficiency of monolithic stationary phases. J. Sep. Sci. 2014, 37, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Saalwachter, K.; Chassé, W.; Sommer, J.U. Structure and swelling of polymer networks: Insights from NMR. Soft Matter 2013, 9, 6587–6593. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davankov, V.A. Hypercrosslinked polystyrene networks with ultimate degrees of crosslinking and their sorption activity. Russ. J. Phys. Chem. 2010, 84, 1937–1942. [Google Scholar] [CrossRef]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. Pore size tuning of poly(styrene-co-vinylbenzyl chloride-codivinylbenzene) hypercrosslinked polymers: Insights from molecular simulations. Polymer 2016, 99, 173–184. [Google Scholar] [CrossRef]
- Lazutin, A.A.; Glagoleva, A.A.; Vasilevskaya, V.V.; Khokhlov, A.R. Computer synthesis of hypercrosslinked polystyrene: All-atom simulations. Low Temp. Phys. 2017, 43, 244–247. [Google Scholar] [CrossRef]
- Trewin, A.; Willock, D.J.; Cooper, A.I. Atomistic simulation of micropore structure, surface area, and gas sorption properties for amorphous microporous polymer networks. J. Phys. Chem. C 2008, 112, 20549–20559. [Google Scholar] [CrossRef]
- Ferrante, F.; Lo Celso, F.; Duca, D. Construction and characterization of models of hypercrosslinked polystyrene. Colloid Polym. Sci. 2012, 290, 1443–1450. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Li, A.; Fei, Z.; Ge, J.; Zhang, Q. Equilibrium adsorption of single component and binary mixtures of aromatic compounds onto a polyfunctional hypercrosslinked polymeric adsorbent. Adsorpt. Sci. Technol. 2004, 22, 13–24. [Google Scholar]
- Cormack, P.A.G.; Davies, A.; Fontanals, N. Synthesis and characterization of microporous polymer microspheres with strong cation-exchange character. React. Funct. Polym. 2012, 72, 939–946. [Google Scholar] [CrossRef]
- Bhunia, S.; Banerjee, B.; Bhaumik, A. A new hypercrosslinked supermicroporous polymer, with scope for sulfonation, and its catalytic potential for the efficient synthesis of biodiesel at room temperature. Chem. Commun. 2015, 51, 5020–5023. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Svec, F. Porous polymer monoliths with large surface area and functional groups prepared via copolymerization of protected functional monomers and hypercrosslinking. J. Chromatogr. A 2013, 1317, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Chen, Q.; Lu, L.-C.; Han, B.-H. Sugar-based micro/mesoporous hypercross-linked polymers with in situ embedded silver nanoparticles for catalytic reduction. Beilstein J. Org. Chem. 2017, 13, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, J.; Tian, L.; Zhang, X. Hierarchical porous interlocked polymeric microcapsules: Sulfonic acid functionalization as acid catalysts. Sci. Rep. 2017, 7, 44178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, S.; Fan, L. Studies progress of preparation, properties and applications of hyper-cross-linked polystyrene networks. J. Mater. Sci. 2007, 42, 7621–7629. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Prakash, A.; Mayo, J.T.; Colvin, V.L. Magnetic separations: From steel plants to biotechnology. Chem. Eng. Sci. 2009, 64, 2510–2521. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Pastukhov, A.V.; Davankov, V.A.; Volkov, V.V.; Amarantov, S.V.; Lubentsova, K.I. Structure and sorption properties of hypercrosslinked polystyrenes and magnetic nanocomposite materials based on them. J. Polym. Res. 2014, 21, 406. [Google Scholar] [CrossRef]
- Tolmacheva, V.V.; Apyari, V.V.; Kochuk, E.V.; Dmitrienko, S.G. Magnetic adsorbents based on iron oxide nanoparticles for the extraction and preconcentration of organic compounds. J. Anal. Chem. 2016, 71, 321–338. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Z.; Shuang, C.; Li, A.; Zhang, M.; Wang, M. Efficient removal of tetracycline by reusable magnetic microspheres with a high surface area. Chem. Eng. J. 2012, 210, 350–356. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Zhou, Q.; Shuang, C.; Zhou, W.; Wang, M. Effect of pore size distribution on tetracycline adsorption using magnetic hypercrosslinked resins. Microporous Mesoporous Mater. 2014, 184, 105–111. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Y.; Zhou, Q.; Shuang, C.; Zhang, M.; Li, A. Preparation of a permanent magnetic hypercrosslinked resin and assessment of its ability to remove organic micropollutants from drinking water. Front. Environ. Sci. Eng. 2015, 9, 96–104. [Google Scholar] [CrossRef]
- Tolmacheva, V.V.; Apyari, V.V.; Ibragimova, B.N.; Kochuk, E.V.; Dmitrienko, S.G.; Zolotov, Y.A. A polymeric magnetic adsorbent based on Fe3O4 nanoparticles and hypercrosslinked polystyrene for the preconcentration of tetracycline antibiotics. J. Anal. Chem. 2015, 70, 1313–1321. [Google Scholar] [CrossRef]
- Tolmacheva, V.V.; Apyari, V.V.; Furletov, A.A.; Dmitrienko, S.G.; Zolotov, Y.A. Facile synthesis of magnetic hypercrosslinked polystyrene and its application in the magnetic solid phase extraction of sulfonamides from water and milk samples before their HPLC determination. Talanta 2016, 152, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, J.; Zhou, L.; Qiu, Y. Magnetic microsphere to remove tetracycline from water: Adsorption, H2O2 oxidation and regeneration. Chem. Eng. J. 2017, 330, 191–201. [Google Scholar] [CrossRef]
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J. Nanoparticles for heterogeneous catalysis: New mechanistic insights. Acc. Chem. Res. 2013, 46, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Nikoshvili, L.; Shimanskaya, E.; Bykov, A.; Yuranov, I.; Kiwi-Minsker, L.; Sulman, E. Selective hydrogenation of 2-methyl-3-butyn-2-ol over Pd-nanoparticles stabilized in hypercrosslinked polystyrene: Solvent effect. Catal. Today 2015, 241, 179–188. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Bronstein, L.M.; Davankov, V.A.; Tsyurupa, M.P.; Solodovnikov, S.P.; Valetsky, P.M.; Wilder, E.A.; Spontak, R.J. Cobalt nanoparticle formation in the pores of hyper-cross-linked polystyrene: Control of nanoparticle growth and morphology. Chem. Mater. 1999, 11, 3210–3215. [Google Scholar] [CrossRef]
- Sidorov, S.N.; Volkov, I.V.; Davankov, V.A.; Tsyurupa, M.P.; Valetsky, P.M.; Bronstein, L.M.; Karlinsey, R.; Zwanziger, J.W.; Matveeva, V.G.; Sulman, E.M.; et al. Platinum-containing hyper-cross-linked polystyrene as a modifier-free selective catalyst for L-sorbose oxidation. J. Am. Chem. Soc. 2001, 123, 10502–10510. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M.; Goerigk, G.; Kostylev, M.; Pink, M.; Khotina, I.A.; Valetsky, P.M.; Matveeva, V.G.; Sulman, E.M.; Sulman, M.G.; Bykov, A.V.; et al. Structure and catalytic properties of Pt-modified hyper-cross-linked polystyrene exhibiting hierarchical porosity. J. Phys. Chem. B 2004, 108, 18234–18242. [Google Scholar] [CrossRef] [Green Version]
- Bykov, A.; Matveeva, V.; Sulman, M.; Valetsky, P.; Tkachenko, O.; Kustov, L.; Bronstein, L.; Sulman, E. Enantioselective catalytic hydrogenation of activated ketones using polymer-containing nanocomposites. Catal. Today 2009, 140, 64–69. [Google Scholar] [CrossRef]
- Doluda, V.Y.; Sulman, E.M.; Matveeva, V.G.; Sulman, M.G.; Lakina, N.V.; Sidorov, A.I.; Valetsky, P.M.; Bronstein, L.M. Kinetics of phenol oxidation over hypercrosslinked polystyrene impregnated with Pt nanoparticles. Chem. Eng. J. 2007, 134, 256–261. [Google Scholar] [CrossRef]
- Sulman, E.M.; Matveeva, V.G.; Doluda, V.Y.; Sidorov, A.I.; Lakina, N.V.; Bykov, A.V.; Sulman, M.G.; Valetsky, P.M.; Kustov, L.M.; Tkachenko, O.P.; et al. Efficient polymer-based nanocatalysts with enhanced catalytic performance in wet air oxidation of phenol. Appl. Catal. B 2010, 94, 200–210. [Google Scholar] [CrossRef]
- Tsvetkova, I.B.; Matveeva, V.G.; Doluda, V.Y.; Bykov, A.V.; Sidorov, A.I.; Schennikov, S.V.; Sulman, M.G.; Valetsky, P.M.; Stein, B.D.; Chen, C.-H.; et al. Pd(II) nanoparticles in porous polystyrene: Factors influencing the nanoparticle size and catalytic properties. J. Mater. Chem. 2012, 22, 6441. [Google Scholar] [CrossRef]
- Sapunov, V.N.; Stepachev, A.A.; Sulman, E.M.; Wärnå, J.; Mäki-Arvela, P.; Sulman, M.G.; Sidorov, A.I.; Stein, B.D.; Murzin, D.Y.; Matveeva, V.G. Stearic acid hydrodeoxygenation over Pd nanoparticles embedded in mesoporous hypercrosslinked polystyrene. J. Ind. Eng. Chem. 2017, 46, 426–435. [Google Scholar] [CrossRef]
- Sulman, E.; Doluda, V.; Dzwigaj, S.; Marceau, E.; Kustov, L.; Tkachenko, O.; Bykov, A.; Matveeva, V.; Sulman, M.; Lakina, N. Catalytic properties of Ru nanoparticles introduced in a matrix of hypercrosslinked polystyrene toward the low-temperature oxidation of d-glucose. J. Mol. Catal. A Chem. 2007, 278, 112–119. [Google Scholar] [CrossRef]
- Protsenko, I.I.; Nikoshvili, L.Z.; Bykov, A.V.; Matveeva, V.G.; Sulman, A.; Sulman, E.M.; Rebrov, E.V. Hydrogenation of levulinic acid using Ru-containing catalysts based on hypercrosslinked polystyrene. Green Process. Synth. 2017, 6, 281–286. [Google Scholar] [CrossRef]
- Sapunov, V.N.; Grigoryev, M.Y.; Sulman, E.M.; Konyaeva, M.B.; Matveeva, V.G. d-Glucose hydrogenation over Ru nanoparticles embedded in mesoporous hypercrosslinked polystyrene. J. Phys. Chem. A 2013, 117, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Sulman, E.M.; Matveeva, V.G.; Manaenkov, O.V.; Filatova, A.E.; Kislitza, O.V.; Doluda, V.Y.; Rebrov, E.V.; Sidorov, A.I.; Shimanskaya, E.I. Cellulose hydrogenolysis with the use of the catalysts supported on hypercrosslinked polystyrene. AIP Conf. Proc. 2016, 1787, 30004. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; Anand, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Gundiah, G.; Govindaraj, A.; Rajalakshmi, N.; Dhathathreyan, K.S.; Rao, C.N.R. Hydrogen storage in carbon nanotubes and related materials. J. Mater. Chem. 2003, 13, 209–213. [Google Scholar] [CrossRef]
- Takagi, H.; Hatori, H.; Soneda, Y.; Yoshizawa, N.; Yamada, Y. Adsorptive hydrogen storage in carbon and porous materials. Mater. Sci. Eng. B 2004, 108, 143–147. [Google Scholar] [CrossRef]
- Poirier, E.; Chahine, R.; Benard, P.; Cossement, D.; Lafi, L.; Melancon, E.; Bose, T.K.; Désilets, S. Storage of hydrogen on single-walled carbon nanotubes and other carbon structures. Appl. Phys. A 2004, 78, 961–967. [Google Scholar] [CrossRef]
- Bacs, R.; Laurent, C.; Morishima, R.; Suzuki, H.; Le Lay, M. Hydrogen storage in high surface area carbon nanotubes produced by catalytic chemical vapor deposition. J. Phys. Chem. B 2004, 108, 12718–12723. [Google Scholar] [CrossRef] [Green Version]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Vecitis, C.D.; Elimelech, M. Electrochemical carbon-nanotube filter performance toward virus removal and inactivation in the presence of natural organic matter. Environ. Sci. Technol. 2012, 46, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ren, W.; Cheng, H.-M. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012, 22, 20197–20202. [Google Scholar] [CrossRef]
- Rubino, R.S.; Takeuchi, E.S. The study of irreversible capacity in lithium-ion anodes prepared with thermally oxidized graphite. J. Power Sources 1999, 81–82, 373–377. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.P.; Robert, K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface area measurement of graphene oxide in aqueous solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.; Tao, X.; Yang, H.; Li, A.; Cheng, R. Influence of the surface structure of graphene oxide on the adsorption of aromatic organic compounds from water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Lama, G.C.; Aprea, P.; Gentile, G.; Lavorgna, M.; Ambrogi, V.; Cerruti, P. Effect of the oxidation degree on self-assembly, adsorption and barrier properties of nano-graphene. Microporous Mesoporous Mater. 2017, 260, 102–115. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, R.; Avolio, R.; Cocca, M.; Gentile, G.; Errico, M.E.; Avella, M.; Carfagna, C.; Ambrogi, V. Synthesis and adsorption study of hyper-crosslinked styrene-based nanocomposites containing multi-walled carbon nanotubes. RSC Adv. 2017, 7, 6865–6874. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Andersson, R.L.; Ström, V.; Gedde, U.W.; Olsson, R.T. Aqueous synthesis of (21̅0) oxygen-terminated defect-free hierarchical ZnO particles and their heat treatment for enhanced reactivity. Langmuir 2016, 32, 11002–11013. [Google Scholar] [CrossRef] [PubMed]
- Salzano de Luna, M.; Castaldo, R.; Altobelli, R.; Gioiella, L.; Filippone, G.; Gentile, G.; Ambrogi, V. Chitosan hydrogels embedding hyper-crosslinked polymer particles as reusable broad-spectrum adsorbents for dye removal. Carbohydr. Polym. 2017, 177, 347–354. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review. Polymers 2017, 9, 651. https://doi.org/10.3390/polym9120651
Castaldo R, Gentile G, Avella M, Carfagna C, Ambrogi V. Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review. Polymers. 2017; 9(12):651. https://doi.org/10.3390/polym9120651
Chicago/Turabian StyleCastaldo, Rachele, Gennaro Gentile, Maurizio Avella, Cosimo Carfagna, and Veronica Ambrogi. 2017. "Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review" Polymers 9, no. 12: 651. https://doi.org/10.3390/polym9120651
APA StyleCastaldo, R., Gentile, G., Avella, M., Carfagna, C., & Ambrogi, V. (2017). Microporous Hyper-Crosslinked Polystyrenes and Nanocomposites with High Adsorption Properties: A Review. Polymers, 9(12), 651. https://doi.org/10.3390/polym9120651