Aerogels from Chitosan Solutions in Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
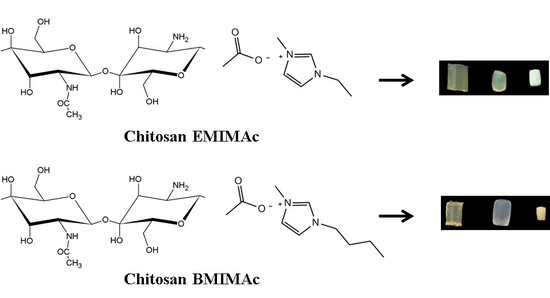
2.2. Solutions of Chitosan in Ionic Liquids
2.3. Formation of Ionogels
2.4. Formation of Aerogels
2.5. Characterization of Aerogels
2.5.1. Chemical Identity
2.5.2. Structural Analysis
2.5.3. Degree of Swelling
3. Results and Discussion
3.1. Characterization of Chitosan Aerogels
3.2. Diffusion Properties of Aerogels
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valentin, R.; Bonelli, B.; Garrone, E.; Di Renzo, F.; Quignard, F. Accessibility of the Functional Groups of Chitosan Aerogel Probed by FT-IR-Monitored Deuteration. Biomacromolecules 2007, 8, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Pohako-Esko, K.; Bahlmann, M.; Schulz, P.S.; Wasserscheid, P. Chitosan Containing Supported Ionic Liquid Phase Materials for CO2 Absorption. Ind. Eng. Chem. Res. 2016, 55, 7052–7059. [Google Scholar] [CrossRef]
- Guyomard-Lack, A.; Buchtová, N.; Humbert, B.; Bideau, J.L. Ion segregation in an ionic liquid confined within chitosan based chemical ionogels. Phys. Chem. Chem. Phys. 2015, 17, 23947–23951. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose-chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol-gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef]
- Ma, B.; Li, X.; Qin, A.; He, C. A comparative study on the chitosan membranes prepared from glycine hydrochloride and acetic acid. Carbohydr. Polym. 2013, 91, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, O.; Heinze, T.; Wawro, D. Blending of Cellulose and Chitosan in Alkyl Imidazolium Ionic Liquids. ISRN Polym. Sci. 2012, 2012, 251950. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, H.; Wu, C.; Wang, R. Preparation and characterization of conductive chitosan–ionic liquid composite membranes. Polym. Adv. Technol. 2012, 23, 1429–1434. [Google Scholar] [CrossRef]
- Silva, S.S.; Santos, T.C.; Cerqueira, M.T.; Marques, A.P.; Reys, L.L.; Silva, T.H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. The use of ionic liquids in the processing of chitosan/silk hydrogels for biomedical applications. Green Chem. 2012, 14, 1463–1470. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Liu, C.; Jiang, Y.; Yu, G.; Mu, X.; Wang, X. Magnetic cellulose–chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions. Chem. Commun. 2012, 48, 7350–7352. [Google Scholar] [CrossRef] [PubMed]
- Naseeruteen, F.; Hamid, N.S.A.; Suah, F.B.M.; Ngah, W.S.W.; Mehamod, F.S. Adsorption of malachite green from aqueous solution by using novel chitosan ionic liquid beads. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Biomedical Exploitation of Chitin and Chitosan via Mechano-Chemical Disassembly, Electrospinning, Dissolution in Imidazolium Ionic Liquids, and Supercritical Drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Silva, S.S.; Duarte, A.R.C.; Carvalho, A.P.; Mano, J.F.; Reis, R.L. Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 2011, 7, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Various Methods for Determination of the Degree of N-Acetylation of Chitin and Chitosan: A Review. J. Agric. Food Chem. 2009, 57, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Ottøy, M.H.; Vårum, K.M.; Smidsrød, O. Compositional heterogeneity of heterogeneously deacetylated chitosans. Carbohydr. Polym. 1996, 29, 17–24. [Google Scholar] [CrossRef]
- Scherließ, R.; Buske, S.; Young, K.; Weber, B.; Rades, T.; Hook, S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine 2013, 31, 4812–4819. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Solubilization of Chitosan in Strong Acid Medium. Int. J. Polym. Anal. Charact. 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in aqueous solution without cross-linking agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.; Argüelles-Monal, W.; Goycoolea, F.M.; Higuera-Ciapara, I.; Peniche, C. Diffusion through Membranes of the Polyelectrolyte Complex of Chitosan and Alginate. Macromol. Biosci. 2003, 3, 535–539. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Yan, C.; Cao, Y.; Mu, T. The Dynamic Process of Atmospheric Water Sorption in [EMIM][Ac] and Mixtures of [EMIM][Ac] with Biopolymers and CO2 Capture in These Systems. J. Phys. Chem. B 2014, 118, 11523–11536. [Google Scholar] [CrossRef] [PubMed]
- Vachoud, L.; Domard, A. Physicochemical properties of physical chitin hydrogels: Modeling and relation with the mechanical properties. Biomacromolecules 2001, 2, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Quignard, F.; Valentin, R.; Renzo, F.D. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Chichester, NY, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, German, 2008; ISBN 978-3-527-61004-4. [Google Scholar]
- Chang, X.; Chen, D.; Jiao, X. Chitosan-Based Aerogels with High Adsorption Performance. J. Phys. Chem. B 2008, 112, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Stefopoulos, A.; Kokkinomalis, I.; Papadopoulou, L.; Panayiotou, C. Development of micro- and nano-porous composite materials by processing cellulose with ionic liquids and supercritical CO2. Green Chem. 2008, 10, 965–971. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Fernández-Valle, M.E.; Aranaz, I.; Heras, Á. pH- and Temperature-Sensitive Chitosan Hydrogels: Swelling and MRI Studies. Macromol. Chem. Phys. 2011, 212, 887–895. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1979; ISBN 0-19-853411-6. [Google Scholar]
- Vázquez, B.; San Roman, J.; Peniche, C.; Cohen, M.E. Polymeric Hydrophilic Hydrogels with Flexible Hydrophobic Chains. Control of the Hydration and Interactions with Water Molecules. Macromolecules 1997, 30, 8440–8446. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Wynne-Jones, W.F.K.; Eyring, H. The Absolute Rate of Reactions in Condensed Phases. J. Chem. Phys. 1935, 3, 492–502. [Google Scholar] [CrossRef]
- Eyring, H. Viscosity, Plasticity, and Diffusion as Examples of Absolute Reaction Rates. J. Chem. Phys. 1936, 4, 283–291. [Google Scholar] [CrossRef]
- Kincaid, J.F.; Eyring, H.; Stearn, A.E. The Theory of Absolute Reaction Rates and its Application to Viscosity and Diffusion in the Liquid State. Chem. Rev. 1941, 28, 301–365. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry; Cornell University Press: Ithaca, NY, USA, 1960; ISBN 978-0-8014-0333-0. [Google Scholar]
- Prabhumirashi, L.S.; Jose, C.I. Infra-red studies and thermodynamics of hydrogen bonding in ethylene glycol monoalkyl ethers. Evidence for a ten membered ring dimer. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1975, 71, 1545–1554. [Google Scholar] [CrossRef]
- Ishizuka, T.; Ohzu, S.; Kotani, H.; Shiota, Y.; Yoshizawa, K.; Kojima, T. Hydrogen atom abstraction reactions independent of C–H bond dissociation energies of organic substrates in water: Significance of oxidant–substrate adduct formation. Chem. Sci. 2014, 5, 1429–1436. [Google Scholar] [CrossRef]








| Cs-IL Solution | Non-Solvent Agent | |
|---|---|---|
| Water | Ethanol | |
| CsEMIM | Ionogel (soft) | Ionogel (CsE) |
| CsBMIM | dilution | Ionogel (CsB) |
| Parameters | CsE | CsB |
|---|---|---|
| SBET (m2/g) | 358 ± 79 | 478 ± 264 |
| Vp (cm3/g) | 0.0733 ± 0.016 | 0.236 ± 0.083 |
| Dp (nm) | 31.0 ± 1.4 | 46.0 ± 3.6 |
| T (°C) | CsE | CsB | ||||||
|---|---|---|---|---|---|---|---|---|
| W∞ (g H2O/g gel) | D × 1010 (m2/s) a | k × 104 (s−1) b | n c | W∞ (g H2O/g gel) | D × 1010 (m2/s) a | k × 104 (s−1) b | n c | |
| 20 | 4.64 | 3.99 | 3.011 | 0.16 | 6.56 | 3.36 | 1.111 | 0.26 |
| 25 | 3.96 | 3.55 | 3.426 | 0.20 | 5.22 | 3.32 | 1.367 | 0.31 |
| 30 | 3.79 | 3.01 | 3.464 | 0.23 | 6.43 | 3.09 | 1.703 | 0.35 |
| 40 | 3.59 | 2.54 | 4.481 | 0.29 | 5.15 | 2.68 | 2.738 | 0.37 |
| Aerogel | Ea (kJ/mol) | ΔH‡ (kJ/mol) | ΔS‡ (J/mol·K) | −TΔS‡ a (kJ/mol) | ΔG‡ a (kJ/mol) |
|---|---|---|---|---|---|
| CsE | −17.5 | 12.1 | −271 | 80.7 | 92.9 |
| CsB | −9.2 | 32.1 | −211 | 63.0 | 95.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-López, G.; Argüelles-Monal, W.; Carvajal-Millan, E.; López-Franco, Y.L.; Recillas-Mota, M.T.; Lizardi-Mendoza, J. Aerogels from Chitosan Solutions in Ionic Liquids. Polymers 2017, 9, 722. https://doi.org/10.3390/polym9120722
Santos-López G, Argüelles-Monal W, Carvajal-Millan E, López-Franco YL, Recillas-Mota MT, Lizardi-Mendoza J. Aerogels from Chitosan Solutions in Ionic Liquids. Polymers. 2017; 9(12):722. https://doi.org/10.3390/polym9120722
Chicago/Turabian StyleSantos-López, Gonzalo, Waldo Argüelles-Monal, Elizabeth Carvajal-Millan, Yolanda L. López-Franco, Maricarmen T. Recillas-Mota, and Jaime Lizardi-Mendoza. 2017. "Aerogels from Chitosan Solutions in Ionic Liquids" Polymers 9, no. 12: 722. https://doi.org/10.3390/polym9120722
APA StyleSantos-López, G., Argüelles-Monal, W., Carvajal-Millan, E., López-Franco, Y. L., Recillas-Mota, M. T., & Lizardi-Mendoza, J. (2017). Aerogels from Chitosan Solutions in Ionic Liquids. Polymers, 9(12), 722. https://doi.org/10.3390/polym9120722