Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review
Abstract
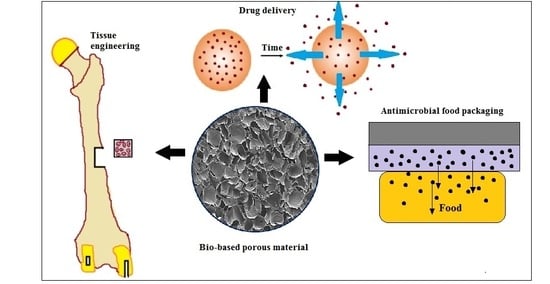
:1. Introduction
2. Biomedical Applications
2.1. Porous Scaffold for Tissue Engineering
2.1.1. Porous Scaffold by Thermally Induced Phase Separation
2.1.2. Porous Scaffold by Freeze-Drying/Lyophilization
2.1.3. Porous Scaffold by Supercritical Fluid Processing
2.2. Porous Carriers for Drug Delivery
Microporous and Superporous Matrices for Drug Delivery
2.3. Wound Healing Material
3. Microbiological Applications
3.1. Encapsulation of Microorganisms in Food Industry
3.2. Encapsulation of Probiotic Bacteria
3.3. Antimicrobial Food Packaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rubentheren, V.; Thomas, A.W.; Ching, Y.C.; Praveena, N.; Erfan, S.; Christopher, F. Effects of heat treatment on chitosan nanocomposite film reinforced with nanocrystalline cellulose and tannic acid. Carbohydr. Polym. 2016, 140, 202. [Google Scholar] [CrossRef] [PubMed]
- Kucińska-Lipka, J.; Gubańska, I.; Janik, H. Polyurethanes modified with natural polymers for medical application Part II: Polyurethane/gelatin, polyurethane/starch, polyurethane/cellulose. Polimery 2014, 3, 197. [Google Scholar] [CrossRef]
- Pan, P.; Wu, S.; Hu, X.; Liu, G.; Li, B. Effect of material composition and environmental condition on thermal characteristics of conductive asphalt concrete. Materials 2017, 10, 218. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Synthesis and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar]
- Mohd, A.C.M.; Ching, Y.C.; Luqman, C.A.; Poh, S.C.; Chuah, C.H. Review of bionanocomposite coating films and their applications. Polymers 2016, 8, 246. [Google Scholar]
- Ching, Y.C.; Ershad, A.; Luqman, C.A.; Choo, K.W.; Yong, C.K.; Sabariah, J.J.; Chuah, C.H.; Liou, N.S. Rheological properties of cellulose nanocrystal-embedded polymer composites: A review. Cellulose 2016, 23, 1011–1030. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.W.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.; Liou, N.S. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. Bio. Resources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Ramesh, S. Poly(vinyl alcohol)-α-chitin composites reinforced by oil palm empty fruit bunch fiber-derived nanocellulose. Int. J. Polym. Anal. Charact. 2017, 128, 339–345. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. JCDR 2015, 9, ZE21. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.O.; Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Effects of encapsulation of microorganisms on product formation during microbial fermentations. Appl. Microbiol. Biotechnol. 2012, 96, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, O.A.; Darwish, S.M. Fabrication of tissue engineering scaffolds using rapid prototyping techniques. World Acad. Sci. Eng. Technol. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2011, 5, 2317–2325. [Google Scholar]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599. [Google Scholar] [CrossRef] [PubMed]
- Cam, C.; Zhu, S.; Truong, N.F.; Scumpia, P.O.; Segura, T. Systematic evaluation of natural scaffolds in cutaneous wound healing. J. Mater. Chem. B 2015, 3, 7986–7992. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in alginate and chitosan microgels to enhance viability of bifidobacterium longum for oral delivery. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Baillargeon, A.L.; Mequanint, K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. BioMed Res. Int. 2014, 2014, 761373. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 38, 364–371. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-gag scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.; Lee, E.; Orgill, D.; Skrabut, E.; Murphy, G. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Stevens, R.; Pearson, R.; Davies, M.; Tendler, S.; Roberts, C.; Williams, P.; Shakesheff, K. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J. Biomed. Mater. Res. 2002, 61, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Ma, H.; Hu, J.; Ma, P.X. Polymer scaffolds for small-diameter vascular tissue engineering. Adv. Funct. Mater. 2010, 20, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Pavia, F.C.; La Carrubba, V.; Piccarolo, S.; Brucato, V. Polymeric scaffolds prepared via thermally induced phase separation: Tuning of structure and morphology. J. Biomed. Mater. Res. A 2008, 86, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Martel-Estrada, S.A.; Martínez-Pérez, C.A.; Chacón-Nava, J.G.; García-Casillas, P.E.; Olivas-Armendariz, I. Synthesis and thermo-physical properties of chitosan/poly (dl-lactide-co-glycolide) composites prepared by thermally induced phase separation. Carbohydr. Polym. 2010, 81, 775–783. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, L.; Guo, X.; Yu, Y. Culture of primary rat hepatocytes within porous chitosan scaffolds. J. Biomed. Mater. Res. A 2003, 67, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh Narbat, M.; Orang, F.; Solati Hashtjin, M.; Goudarzi, A. Fabrication of porous hydroxyapatite-gelatin composite scaffolds for bone tissue engineering. Iran. Biomed. J. 2006, 10, 215–223. [Google Scholar]
- Hilmi, A.; Halim, A.S.; Hassan, A.; Lim, C.K.; Noorsal, K.; Zainol, I. In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. Springerplus 2013, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.-L.; Zhao, L.-J.; Zhang, Q.-A.; Zhang, J.; Zhang, Z.-Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- Teng, X.; Ren, J.; Gu, S. Preparation and characterization of porous PDLLA/HA composite foams by supercritical carbon dioxide technology. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bozsak, F.; Gonzalez-Rodriguez, D.; Sternberger, Z.; Belitz, P.; Bewley, T.; Chomaz, J.-M.; Barakat, A.I. Optimization of drug delivery by drug-eluting stents. PLoS ONE 2015, 10, e0130182. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Charoenteeraboon, J. Antibacterial activity and drug release of chitosan sponge containing doxycycline hyclate. AAPS PharmSciTech 2008, 9, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, B.E.; Ramazani, S.A.A.; Shafiee, M.; Danaei, M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Dorj, B.; Won, J.-E.; Purevdorj, O.; Patel, K.D.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. A novel therapeutic design of microporous-structured biopolymer scaffolds for drug loading and delivery. Acta Biomater. 2014, 10, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Pichatborvonkul, P.; Kaewsrichan, J.; Kaewsichan, L. Development of hydroxyapatite-chitosan beads as a sustained drug delivery system to bone. Tissue Eng. Regen. Med. Soc. Malays. 2012, 1, 66–73. [Google Scholar]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. Biomater. Sci. Polym. Ed. 2004, 15, 607–619. [Google Scholar] [CrossRef]
- Chavda, H.; Modhia, I.; Mehta, A.; Patel, R.; Patel, C. Development of bioadhesive chitosan superporous hydrogel composite particles based intestinal drug delivery system. BioMed Res. Int. 2013, 2013, 563651. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.V.; Shivakumar, H. Preparation and characterization of superporous hydrogels as gastroretentive drug delivery system for rosiglitazone maleate. Daru 2010, 18, 200–210. [Google Scholar]
- Chavda, H.; Patel, C. Preparation and in vitro evaluation of a stomach specific drug delivery system based on superporous hydrogel composite. Indian J. Pharm. Sci. 2011, 73, 30. [Google Scholar] [CrossRef] [PubMed]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef]
- Ghosh, S.; Jassal, M. Use of polysaccharide fibres for modern wound dressings. Indian J. Fibre Text. Res. 2002, 27, 434–452. [Google Scholar]
- Piraino, F.; Selimović, Š. A current view of functional biomaterials for wound care, molecular and cellular therapies. BioMed Res. Int. 2015, 2015, 403801. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Chen, Y.; Zhang, S. Study on chitosan-lactate sponges with oriented pores as potential wound dressing. Mater. Sci. Appl. 2013, 4, 458–470. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arotzena, O.; Meier, J.G.; Del Amo, C.; Garcia-Aznar, J.M. Characterization of fibrin and collagen gels for engineering wound healing models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S. Development of polyvinyl alcohol-sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef] [PubMed]
- El Salmawi, K.M. Gamma radiation-induced crosslinked PVA/chitosan blends for wound dressing. J. Macromol. Sci. A 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Stolarzewicz, I.; Białecka-Florjańczyk, E.; Majewska, E.; Krzyczkowska, J. Immobilization of yeast on polymeric supports. Chem. Biochem. Eng. Q. 2011, 25, 135–144. [Google Scholar]
- Verbelen, P.J.; De Schutter, D.P.; Delvaux, F.; Verstrepen, K.J.; Delvaux, F.R. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol. Lett. 2006, 28, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.A.; Obradovic, B.; Leskosek-Cukalovic, I.; Vunjak-Novakovic, G. Immobilized yeast bioreactor systems for brewing—Recent achievements. In Engineering and Manufacturing for Biotechnology; Springer: Berlin, Germany, 2001; pp. 277–292. [Google Scholar]
- Willaert, R.; Nedovic, V.A. Primary beer fermentation by immobilised yeast—A review on flavour formation and control strategies. J. Chem. Technol. Biotechnol. 2006, 81, 1353–1367. [Google Scholar] [CrossRef]
- Jovanović-Malinovska, R.; Cvetkovska, M.; Kuzmanova, S.; Tsvetanov, C.; Winkelhausen, E. Immobilization of saccharomyces cerevisiae in novel hydrogels based on hybrid networks of poly(ethylene oxide), alginate and chitosan for ethanol production. Maced. J. Chem. Chem. Eng. 2010, 29, 169–179. [Google Scholar]
- Wang, C. Immobilization of Gluconobacter oxydans by entrapment in porous chitosan sponge. J. Bioprocess. Biotech. 2013, 3, 132. [Google Scholar] [CrossRef]
- Saeki, A. Studies on acetic acid fermentation, 2: Vinegar production using immobilized Acetobacter aceti cells entrapped in calcium alginate gel beads. J. Jpn. Soc. Food Sci. Technol. 1990, 37, 191–198. [Google Scholar] [CrossRef]
- Fumi, M.D.; Silva, A.; Battistotti, G.; Colagrande, O. Living immobilized acetobacter in CA-alginate in vinegar production: Perliminary study on optimum conditions for immobilization. Biotechnol. Lett. 1992, 14, 605–608. [Google Scholar] [CrossRef]
- Sun, Y.; Furusaki, S. Continuous production of acetic acid using immobilized Acetobacter aceti in a three-phase fluidized bed bioreactor. J. Ferment. Bioeng. 1990, 69, 102–110. [Google Scholar] [CrossRef]
- Krisch, J.; Szajáni, B. Effects of immobilization on biomass production and acetic acid fermentation of Acetobacter aceti as a function of temperature and ph. Biotechnol. Lett. 1996, 18, 393–396. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr. Issues Intest. Microbiol. 2002, 3, 39–48. [Google Scholar] [PubMed]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization technologies in probiotic food production. J. Nutr. Metab. 2013, 2013, 716861. [Google Scholar] [CrossRef] [PubMed]
- Sathyabama, S.; Vijayabharathi, R. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Tarzi, B.G.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014, 13, 843. [Google Scholar]
- Rao, A.; Shiwnarain, N.; Maharaj, I. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can. Inst. Food Sci. Technol. J. 1989, 22, 345–349. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of cellulose acetate based antimicrobial food packaging materials for controlled release of lysozyme. J. Food Eng. 2009, 90, 453–462. [Google Scholar] [CrossRef]
- Mangalassary, S. Antimicrobial food packaging to enhance food safety: Current developments and future challenges. J. Food Process. Technol. 2012, 3, 2157–2164. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Production of antimicrobial membranes loaded with potassium sorbate using a supercritical phase separation process. Innov. Food Sci. Emerg. Technol. 2016, 34, 77–85. [Google Scholar] [CrossRef]
- Sampath, U.G.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef]
- Chen, M.C.; Yeh, G.H.C.; Chiang, B.H. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Process. Preserv. 1996, 20, 379–390. [Google Scholar] [CrossRef]
- Saini, S.; Sillard, C.; Belgacem, M.N.; Bras, J. Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv. 2016, 6, 12422–12430. [Google Scholar] [CrossRef]



| Cell types cultured | Optimal pore size (µm) | Reference |
|---|---|---|
| Hepatocytes | 20 µm | [26] |
| Osteogenic cells | 100–150 µm | [27] |
| Fibroblast | 5–15 µm | [26] |
| Adult mammalian skin cells | 20–125 µm | [28] |
| Smooth muscle cells | 60–150 µm | [29] |
| Endothelial cells | <80 µm | [30] |
| Cell/tissue type | Biopolymer | Method of fabrication | Pore characteristics | Reference |
|---|---|---|---|---|
| Small-diameter blood vessels | Poly(l-lactic acid) (PLLA) | Thermally induced phase-separation | Porosity decreased from 95% to 90% with increasing the polymer concentration from 2.5% to 10%. Pore size decreased from 115–140 µm to 20–40 µm with decreasing the phase-separation temperature from −20 to −196 °C | [32] |
| Hepatocytes | Chitosan | Lyophilization | Porosity of 90% and mean pore size between 50–200 µm | [35] |
| Bone tissue | Hydroxyapatite and gelatin | Solvent-casting method combined with freeze drying | Open, interconnected porous structure with a pore size of 80–400 µm and porosity 70% | [36] |
| Human dermal fibroblasts | Chitosan | Freeze drying | Pore size between 40–140 µm, and average porosity about 93% ± 12.57% | [37] |
| Osteoblast | Poly(lactic-co-glycolic acid)/Hyaluronic acid/collagen | Supercritical CO2 | Porosity of 88.9% and pore size of 205.7 µm | [38] |
| Type of drug | Biopolymer | Method of fabrication | Pore characteristics | Reference |
|---|---|---|---|---|
| Doxycycline hyclate | Chitosan | Freeze drying | Well interconnected pores with diameter about 80–130 µm | [43] |
| Ampicillin and cytochrome C | Poly(lactic acid) | Robotic dispensing technique and room temperature ionic liquid | Pore size of 2.43 µm and microporosity of ~70% | [45] |
| Metoprolol succinate | Hydroxypropyl-methylcellulose and chitosan | Gas blowing | Pore size between 100–1000 µm and porosity of 47.11% ± 1.80% | [48] |
| Amoxicillin trihydrate | Chitosan | Freeze drying | Pore sizes were obtained from100 to 500 µm with increasing the crosslinking agent from 1:0.068 to 1:0.30 (molar ratio-chitosan: crosslinker) | [44] |
| Tetracycline hydrochloride | Hydroxyapatite/chitosan | Freeze drying | Pore diameter 45 ± 17 µm. | [46] |
| Rosiglitazone maleate | Chitosan/poly(vinyl alcohol) | Gas foaming | Superporous hydrogel with capillary porous structures. Porosity increased from 38.3 ± 2.2 to 88.2 ± 2.1 with increasing the amount of glyoxal (crosslinker) | [49] |
| Ranitidine | Carboxymethylcellulose hydrogel | Gas foaming | Porosity decreased from 69.30 ± 4.36 to 42.38 ± 2.68 with the addition of sodium carboxymethyl cellulose | [50] |
| Curcumin | Nanocellulose reinforced chitosan hydrogel | Gas foaming | Highly interconnected pores with pore sizes >100 µm | [51] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udenni Gunathilake, T.M.S.; Ching, Y.C.; Ching, K.Y.; Chuah, C.H.; Abdullah, L.C. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. https://doi.org/10.3390/polym9050160
Udenni Gunathilake TMS, Ching YC, Ching KY, Chuah CH, Abdullah LC. Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers. 2017; 9(5):160. https://doi.org/10.3390/polym9050160
Chicago/Turabian StyleUdenni Gunathilake, T. M. S., Yern Chee Ching, Kuan Yong Ching, Cheng Hock Chuah, and Luqman Chuah Abdullah. 2017. "Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review" Polymers 9, no. 5: 160. https://doi.org/10.3390/polym9050160
APA StyleUdenni Gunathilake, T. M. S., Ching, Y. C., Ching, K. Y., Chuah, C. H., & Abdullah, L. C. (2017). Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers, 9(5), 160. https://doi.org/10.3390/polym9050160