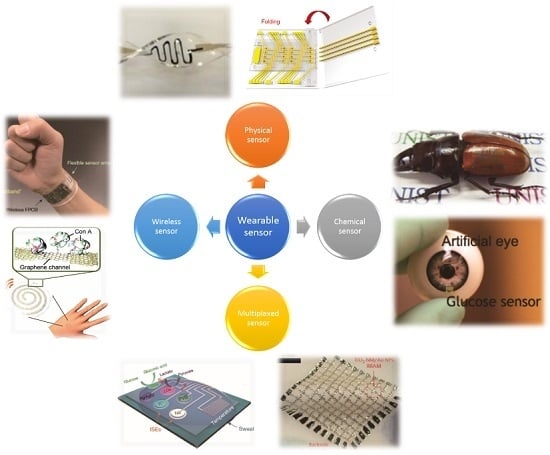
Smart Sensor Systems for Wearable Electronic Devices
Abstract
:1. Introduction
2. Individual Sensors
2.1. Physical Sensors
2.1.1. Temperature Sensors
2.1.2. Pressure Sensors
2.1.3. Strain Sensors
2.2. Chemical Sensors
2.2.1. Gas Sensors
2.2.2. Ion Sensors
2.2.3. Biosensors
3. Multiplexed Sensors
4. Wireless Sensors
4.1. Resonance Antenna Integrated Sensors
4.2. Bluetooth Integrated Sensors
4.3. Near Field Communication (NFC) Integrated Sensors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonato, P. Wearable sensors/systems and their impact on biomedical engineering. IEEE Eng. Med. Biol. Mag. 2003, 22, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Electronic Skin: Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals (Adv. Mater. 9/2014). Adv. Mater. 2014, 26, 1309. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.-G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.-H.; Bao, Z. Highly skin-conformal microhairy sensor for pulse signal amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Lee, C.; Suh, K.-Y. Recent advances in flexible sensors for wearable and implantable devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Honda, W.; Harada, S.; Arie, T.; Akita, S. Toward Flexible and Wearable Human-Interactive Health-Monitoring Devices. Adv. Healthc. Mater. 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Di, C.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horiz. 2015, 2, 140–156. [Google Scholar] [CrossRef]
- Chortos, A.; Bao, Z. Skin-inspired electronic devices. Mater. Today 2014, 17, 321–331. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Joe, P.; Tuzman, O.L.; Park, K.-I.; Lee, K.J.; Shi, Y.; Huang, Y.; Rogers, J.A. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extrem. Mech. Lett. 2016, 9, 269–281. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Li, Z.; Wang, Z.L. Energy harvesting from the animal/human body for self-powered electronics. Annu. Rev. Biomed. Eng. 2017, 19, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.-S.; Jeon, S.; Kim, M.; Kim, S.; Kim, K.; Bien, F.; Hong, S.Y.; Park, J.-U. Highly Transparent and Stretchable Field-Effect Transistor Sensors Using Graphene—Nanowire Hybrid Nanostructures. Adv. Mater. 2015, 27, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- An, B.W.; Hyun, B.G.; Kim, S.-Y.; Kim, M.; Lee, M.-S.; Lee, K.; Koo, J.B.; Chu, H.Y.; Bae, B.-S.; Park, J.-U. Stretchable and Transparent Electrodes using Hybrid Structures of Graphene—Metal Nanotrough Networks with High Performances and Ultimate Uniformity. Nano Lett. 2014, 14, 6322–6328. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Lee, K.; Kim, S.-Y.; Lee, H.; Park, J.; Choi, K.-H.; Kim, H.-K.; Kim, D.-G.; Lee, D.-Y.; Nam, S.; et al. High-Performance, Transparent, and Stretchable Electrodes Using Graphene—Metal Nanowire Hybrid Structures. Nano Lett. 2013, 13, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Jang, J.; Cho, E.; Kim, S.-H.; Kang, E.-S.; Kim, J.; Kim, H.-K.; Kong, H.; Kim, S.-K.; Kim, J.-Y.; et al. High Dielectric Performances of Flexible and Transparent Cellulose Hybrid Films Controlled by Multidimensional Metal Nanostructures. Adv. Mater. 2017, 29, 1700538. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, J.Y.; Kim, S.-Y.; Seo, Y.-H.; Jang, K.-S.; Lee, S.Y.; Jung, S.; Ryu, B.-H.; Kim, H.-S.; Park, J.-U.; et al. 3D-printable, highly conductive hybrid composites employing chemically-reinforced, complex dimensional fillers and thermoplastic triblock copolymers. Nanoscale 2017, 9, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Hyun, B.G.; Kim, K.; Lee, S.Y.; Kim, S.-H.; Kim, J.-Y.; Song, M.H.; Park, J.-U. Photo-patternable and transparent films using cellulose nanofibers for stretchable origami electronics. NPG Asia Mater. 2016, 8, e299. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, K.; Kim, S.-Y.; Hyung Cheong, W.; Park, K.; Hyeb Song, J.; Namgoong, G.; Joon Kim, J.; Heo, J.; et al. Wearable, wireless gas sensors using highly stretchable and transparent structures of nanowires and graphene. Nanoscale 2016, 8, 10591–10597. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.D.; Nam, Y.S.; Seo, H.; Lee, B.R.; Yu, J.C.; Lee, S.Y.; Kim, J.-Y.; Park, J.-U.; Song, M.H. Highly efficient flexible optoelectronic devices using metal nanowire-conducting polymer composite transparent electrode. Electron. Mater. Lett. 2015, 11, 906–914. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, J.; Park, J.; Park, J.-U. Studies on the mechanical stretchability of transparent conductive film based on graphene-metal nanowire structures. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, J.; Lee, M.-S.; Kim, J.; Hyun, B.G.; Kang, D.J.; Na, K.; Lee, C.Y.; Bien, F.; Park, J.-U. In-situ Synthesis of Carbon Nanotube—Graphite Electronic Devices and Their Integrations onto Surfaces of Live Plants and Insects. Nano Lett. 2014, 14, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.J.; Park, G.U.; Lee, H.H.; Park, H.Y.; Park, J.-U. Photopatternable and refractive-index-tunable sol—Gel-derived silica—Titania nanohybrid materials. Curr. Appl. Lett. 2013, 13, 1732–1737. [Google Scholar] [CrossRef]
- Hyun, B.G.; Son, H.J.; Ji, S.; Jang, J.; Hur, S.-H.; Park, J.-U. Multi-dimensional carbon nanofibers for supercapacitor electrodes. J. Electroceram. 2017, 38, 43–50. [Google Scholar] [CrossRef]
- Kim, K.; Hyun, B.G.; Jang, J.; Cho, E.; Park, Y.-G.; Park, J.-U. Nanomaterial-based stretchable and transparent electrodes. J. Inf. Disp. 2016, 17, 131–141. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, K.; Hwang, Y.H.; Park, J.; Jang, J.; Nam, Y.; Kang, Y.; Kim, M.; Park, H.J.; Lee, Z.; et al. High-resolution electrohydrodynamic inkjet printing of stretchable metal oxide semiconductor transistors with high performance. Nanoscale 2016, 8, 17113–17121. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, G.; Lee, B.R.; Ji, S.; Kim, S.-Y.; An, B.W.; Song, M.H.; Park, J.-U. High-resolution electrohydrodynamic jet printing of small-molecule organic light-emitting diodes. Nanoscale 2015, 7, 13410–13415. [Google Scholar] [CrossRef] [PubMed]
- An, B.W.; Kim, K.; Lee, H.; Kim, S.-Y.; Shim, Y.; Lee, D.-Y.; Song, J.Y.; Park, J.-U. High-Resolution Printing of 3D Structures Using an Electrohydrodynamic Inkjet with Multiple Functional Inks. Adv. Mater. 2015, 27, 4322–4328. [Google Scholar] [CrossRef] [PubMed]
- An, B.W.; Kim, K.; Kim, M.; Kim, S.-Y.; Hur, S.-H.; Park, J.-U. Direct Printing of Reduced Graphene Oxide on Planar or Highly Curved Surfaces with High Resolutions Using Electrohydrodynamics. Small 2015, 11, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.; Koncar, V.; Lewandowski, M.; Dufour, C. Design and Development of a Flexible Strain Sensor for Textile Structures Based on a Conductive Polymer Composite. Sensors (Basel) 2007, 7, 473–492. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Lee, P.S. Stretchable Graphene Thermistor with Tunable Thermal Index. ACS Nano 2015, 9, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Ji, S.; Choi, S.; Pyo, K.-H.; An, B.W.; Park, J.; Kim, J.; Kim, J.-Y.; Lee, K.-S.; Kwon, S.-Y.; et al. Integrated arrays of air-dielectric graphene transistors as transparent active-matrix pressure sensors for wide pressure ranges. Nat. Commun. 2017, 8, 14950. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Hwang, B.-U.; Kim, D.; Kim, B.-Y.; Lee, N.-E. Stretchable, Transparent, Ultrasensitive, and Patchable Strain Sensor for Human—Machine Interfaces Comprising a Nanohybrid of Carbon Nanotubes and Conductive Elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Rodger, D.C.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Scalable high lead-count parylene package for retinal prostheses. Sens. Actuators B Chem. 2006, 117, 107–114. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, J.S.; Kim, J.-S.; Choe, J.-H.; Chung, K.H.; Shin, J.-W.; Kim, J.T.; Youn, D.-H.; Kim, K.-C.; Lee, J.-I.; et al. Flexible and transparent gas molecule sensor integrated with sensing and heating graphene layers. Small 2014, 10, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.-H. Reverse-Micelle-Induced Porous Pressure-Sensitive Rubber for Wearable Human-Machine Interfaces. Adv. Mater. 2014, 26, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.S.; Bae, S.-H.; Chen, H.; Yang, J.L.; Kim, J.; Andrews, A.M.; Weiss, P.S.; Yang, Y.; Tseng, H.-R. Printable Ultrathin Metal Oxide Semiconductor-Based Conformal Biosensors. ACS Nano 2015, 9, 12174–12181. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Ma, H.; Park, J.; Yeo, J.; Park, J.-U.; Bien, F. Graphene-Based Wireless Environmental Gas Sensor on PET Substrate. IEEE Sens. J. 2016, 16, 5003–5009. [Google Scholar] [CrossRef]
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.-H.; Kim, Y.-S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional Epidermal Electronics Printed Directly Onto the Skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-Direction-Sensitive and Stretchable Electronic Skins Based on Human-Skin-Inspired Interlocked Microstructures. ACS Nano 2014, 8, 12020–12029. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, Y.; Jung, E.S.; Kwon, S. Implantable hybrid chrome silicide temperature sensor for power MEMS devices. IET Micro Nano Lett. 2011, 6, 895–899. [Google Scholar] [CrossRef]
- Brenci, M.; Contorti, G.; Falciai, R.; Mignani, A.G.; Scheggi, A.M. Thermochromic Transducer Optical Fiber Temperature Sensor. In Proceedings of the 2nd International Conference on Optical Fiber Sensors, Stuttgart, Germany, 5–7 September 1984; Volume 0514, pp. 155–160. [Google Scholar]
- Gao, L.; Zhang, Y.; Malyarchuk, V.; Jia, L.; Jang, K.-I.; Webb, R.C.; Fu, H.; Shi, Y.; Zhou, G.; Shi, L.; et al. Epidermal photonic devices for quantitative imaging of temperature and thermal transport characteristics of the skin. Nat. Commun. 2014, 5, 4938. [Google Scholar] [CrossRef] [PubMed]
- Ploss, B.; Domig, A. Static and dynamic pyroelectric properties of PVDF. Ferroelectrics 1994, 159, 263–268. [Google Scholar] [CrossRef]
- Broadhurst, M.G.; Davis, G.T.; McKinney, J.E.; Collins, R.E. Piezoelectricity and pyroelectricity in polyvinylidene fluoride—A model. J. Appl. Phys. 1978, 49, 4992–4997. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Lee, Y.; Lee, H.S.; Ko, H. Fingertip skin—Inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 2015, 1, e1500661. [Google Scholar] [CrossRef] [PubMed]
- Cosseddu, P.; Viola, F.; Lai, S.; Raffo, L.; Bonfiglio, A. A Temperature Transducer Based on a Low-Voltage Organic Thin-Film Transistor Detecting Pyroelectric Effect. IEEE Electron. Dev. Lett. 2014, 35, 1296–1298. [Google Scholar] [CrossRef]
- Tien, N.T.; Jeon, S.; Kim, D.-I.; Trung, T.Q.; Jang, M.; Hwang, B.-U.; Byun, K.-E.; Bae, J.; Lee, E.; Tok, J.B.-H.; et al. A Flexible Bimodal Sensor Array for Simultaneous Sensing of Pressure and Temperature. Adv. Mater. 2014, 26, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Wang, S.; Keum, H.; Ghaffari, R.; Kim, Y.-S.; Tao, H.; Panilaitis, B.; Li, M.; Kang, Z.; Omenetto, F.; et al. Thin, Flexible Sensors and Actuators as “Instrumented” Surgical Sutures for Targeted Wound Monitoring and Therapy. Small 2012, 8, 3263–3268. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, H.-B.-R.; Bao, Z. Flexible Wireless Temperature Sensors Based on Ni Microparticle-Filled Binary Polymer Composites. Adv. Mater. 2013, 25, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Sim, J.K.; Cho, Y.-H. A Flexible and Wearable Human Stress Monitoring Patch. Sci. Rep. 2016, 6, 23468. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Honda, W.; Arie, T.; Akita, S.; Takei, K. Fully Printed, Highly Sensitive Multifunctional Artificial Electronic Whisker Arrays Integrated with Strain and Temperature Sensors. ACS Nano 2014, 8, 3921–3927. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Yu, S.U.; Kim, S.H.; Yoon, Y.S.; Kim, D.Y.; Kwon, A.H.; Meyyappan, M.; Kim, K.S. Highly selective CO2 capture on N-doped carbon produced by chemical activation of polypyrrole functionalized graphene sheets. Chem. Commun. 2012, 48, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed]
- Bendi, R.; Bhavanasi, V.; Parida, K.; Nguyen, V.C.; Sumboja, A.; Tsukagoshi, K.; Lee, P.S. Self-powered graphene thermistor. Nano Energ. 2016, 26, 586–594. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Z.; Yu, H.; Jiang, H. A stretchable temperature sensor based on elastically buckled thin film devices on elastomeric substrates. Appl. Phys. Lett. 2009, 95, 141912. [Google Scholar] [CrossRef]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, Human-Interactive, Health-Monitoring, Wireless Devices Fabricated by Macroscale Printing Techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Yang, J.; Wei, D.; Tang, L.; Song, X.; Luo, W.; Chu, J.; Gao, T.; Shi, H.; Du, C. Wearable temperature sensor based on graphene nanowalls. RSC Adv. 2015, 5, 25609–25615. [Google Scholar] [CrossRef]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Shi, Y.; Joe, P.; Ghaffari, R.; Balooch, G.; Usgaonkar, K.; Gur, O.; Tran, P.L.; Crosby, J.R.; Meyer, M.; et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 2015, 14, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dagdeviren, C.; Shi, Y.; Ma, Y.; Feng, X.; Rogers, J.A.; Huang, Y. Computational models for the determination of depth-dependent mechanical properties of skin with a soft, flexible measurement device. Proc. R. Soc. A 2016, 472, 20160225. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.-K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zirkl, M.; Sawatdee, A.; Helbig, U.; Krause, M.; Scheipl, G.; Kraker, E.; Ersman, P.A.; Nilsson, D.; Platt, D.; Bodö, P.; Bauer, S.; Domann, G.; Stadlober, B. An All-Printed Ferroelectric Active Matrix Sensor Network Based on Only Five Functional Materials Forming a Touchless Control Interface. Adv. Mater. 2011, 23, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, K.-Y.; Cheong, O.J.; Kim, J.H.; Jang, J. Highly Sensitive and Multifunctional Tactile Sensor Using Free-standing ZnO/PVDF Thin Film with Graphene Electrodes for Pressure and Temperature Monitoring. Sci. Rep. 2015, 5, 7887. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.-D.; Lim, H.; Kim, S.Y.; Ko, H. Giant Tunneling Piezoresistance of Composite Elastomers with Interlocked Microdome Arrays for Ultrasensitive and Multimodal Electronic Skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.-Y.; Kim, T.; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Huang, D.; Gao, X.; Di, C.; Zhu, D. Flexible suspended gate organic thin-film transistors for ultra-sensitive pressure detection. Nat. Commun. 2015, 6, 6269. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.-E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Smith, C.S. Piezoresistance Effect in Germanium and Silicon. Phys. Rev. 1954, 94, 42–49. [Google Scholar] [CrossRef]
- Lu, N.; Lu, C.; Yang, S.; Rogers, J. Highly Sensitive Skin-Mountable Strain Gauges Based Entirely on Elastomers. Adv. Funct. Mater. 2012, 22, 4044–4050. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene–Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, W.; Kim, S.-W.; Kim, J.J.; Kim, B.-S. Flexible Textile Strain Wireless Sensor Functionalized with Hybrid Carbon Nanomaterials Supported ZnO Nanowires with Controlled Aspect Ratio. Adv. Funct. Mater. 2016, 26, 6206–6214. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, L.; Zhong, J.; Ding, T.; Liu, Y.; Cai, Z.; Rong, Y.; Han, H.; Zhou, J.; Wang, Z.L. High-Strain Sensors Based on ZnO Nanowire/Polystyrene Hybridized Flexible Films. Adv. Mater. 2011, 23, 5440–5444. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.S.; Khan, U.; Backes, C.; O’Neill, A.; McCauley, J.; Duane, S.; Shanker, R.; Liu, Y.; Jurewicz, I.; Dalton, A.B.; Coleman, J.N. Sensitive, High-Strain, High-Rate Bodily Motion Sensors Based on Graphene–Rubber Composites. ACS Nano 2014, 8, 8819–8830. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and Highly Sensitive Graphene Strain Sensors for Human Motion Monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Hwang, B.-U.; Lee, J.-H.; Trung, T.Q.; Roh, E.; Kim, D.-I.; Kim, S.-W.; Lee, N.-E. Transparent Stretchable Self-Powered Patchable Sensor Platform with Ultrasensitive Recognition of Human Activities. ACS Nano 2015, 9, 8801–8810. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Lai, D.T.H.; Su, B.; Si, K.J.; Ma, Z.; Yap, L.W.; Guo, P.; Cheng, W. Highly Stretchy Black Gold E-Skin Nanopatches as Highly Sensitive Wearable Biomedical Sensors. Adv. Electron. Mater. 2015, 1, 1400063. [Google Scholar] [CrossRef]
- Saha, B.; Baek, S.; Lee, J. Highly Sensitive Bendable and Foldable Paper Sensors Based on Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Mitra, D.; Peterson, K.; Maharbiz, M.M. A Highly Elastic, Capacitive Strain Gauge Based on Percolating Nanotube Networks. Nano Lett. 2012, 12, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F.; et al. Super-stretchable, Transparent Carbon Nanotube-Based Capacitive Strain Sensors for Human Motion Detection. Sci. Rep. 2013, 3, 3048. [Google Scholar] [CrossRef] [PubMed]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Mengüç, Y.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive Soft Strain Sensors via Multicore–Shell Fiber Printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Kim, M.H.; Oh, Y.S.; Jung, S.-H.; Jung, J.H.; Sung, H.J.; Lee, H.W.; Lee, H.M. Highly Stretchable, Hysteresis-Free Ionic Liquid-Based Strain Sensor for Precise Human Motion Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.B.; Arutselvan, K.; Liu, Y.; Armstrong, D.; Lin, Y.; Khan, M.R.; Genzer, J.; Dickey, M.D. Stretchable Capacitive Sensors of Torsion, Strain, and Touch Using Double Helix Liquid Metal Fibers. Adv. Funct. Mater. 2017, 27, 1605630. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, C.-Y.; Zhang, Y.; Chen, K.-H.; Yang, Y.; Hu, Y.; He, J.-H.; Wang, Z.L. Ultrahigh Sensitive Piezotronic Strain Sensors Based on a ZnSnO3 Nanowire/Microwire. ACS Nano 2012, 6, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bae, S.-H.; Lin, L.; Yang, Y.; Park, C.; Kim, S.-W.; Cha, S.N.; Kim, H.; Park, Y.J.; Wang, Z.L. Super-Flexible Nanogenerator for Energy Harvesting from Gentle Wind and as an Active Deformation Sensor. Adv. Funct. Mater. 2013, 23, 2445–2449. [Google Scholar] [CrossRef]
- Lee, S.; Hinchet, R.; Lee, Y.; Yang, Y.; Lin, Z.-H.; Ardila, G.; Montès, L.; Mouis, M.; Wang, Z.L. Ultrathin Nanogenerators as Self-Powered/Active Skin Sensors for Tracking Eye Ball Motion. Adv. Funct. Mater. 2014, 24, 1163–1168. [Google Scholar] [CrossRef]
- Zhong, J.; Zhong, Q.; Hu, Q.; Wu, N.; Li, W.; Wang, B.; Hu, B.; Zhou, J. Stretchable Self-Powered Fiber-Based Strain Sensor. Adv. Funct. Mater. 2015, 25, 1798–1803. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12, 12–20. [Google Scholar] [CrossRef]
- Roberts, M.E.; Sokolov, A.N.; Bao, Z. Material and device considerations for organic thin-film transistor sensors. J. Mater. Chem. 2009, 19, 3351–3363. [Google Scholar] [CrossRef]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: a review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors (Basel) 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yu, L.; Garcia, D.; Ren, R.X.; Zeng, X. Ionic Liquid High-Temperature Gas Sensor Array. Anal. Chem. 2006, 78, 6980–6989. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-S.; Gwo, S. Quantitative surface acoustic wave detection based on colloidal gold nanoparticles and their bioconjugates. Anal. Chem. 2008, 80, 3318–3326. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Materials for Electrochemical Gas Sensors with Liquid and Polymer Electrolytes. In Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013; pp. 353–364. [Google Scholar]
- Wang, T.; Guo, Y.; Wan, P.; Sun, X.; Zhang, H.; Yu, Z.; Chen, X. A flexible transparent colorimetric wrist strap sensor. Nanoscale 2017, 9, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, G.; Zhu, L.; Li, G. Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem. Commun. 2011, 13, 31–33. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Shur, M.S.; Potyrailo, R.A.; Balandin, A.A. Selective gas sensing with a single pristine graphene transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, M.; Voznyy, O.; Hu, L.; Fu, Q.; Zhou, D.; Xia, Z.; Sargent, E.H.; Tang, J. Physically flexible, rapid-response gas sensor based on colloidal quantum dot solids. Adv. Mater. 2014, 26, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Forleo, A.; Francioso, L.; Capone, S.; Siciliano, P.; Lommens, P.; Hens, Z. Synthesis and gas sensing properties of ZnO quantum dots. Sens. Actuators B Chem. 2010, 146, 111–115. [Google Scholar] [CrossRef]
- Levine, P.M.; Gong, P.; Levicky, R.; Shepard, K.L. Active CMOS Sensor Array for Electrochemical Biomolecular Detection. IEEE J. Solid State Circ. 2008, 43, 1859–1871. [Google Scholar] [CrossRef]
- Mu, X.; Wang, Z.; Zeng, X.; Mason, A.J. A Robust Flexible Electrochemical Gas Sensor Using Room Temperature Ionic Liquid. IEEE Sens. J. 2013, 13, 3976–3981. [Google Scholar] [CrossRef]
- Lehnhardt, A.; Kemper, M.J. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr. Nephrol. 2011, 26, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Fay, C.; Coyle, S.; Byrne, R.; O’Toole, C.; Barry, C.; Hughes, S.; Moyna, N.; Diamond, D.; Benito-Lopez, F. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B Chem. 2012, 171–172, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, M.D.; Kassal, P.; Steinberg, I.M. System Architectures in Wearable Electrochemical Sensors. Electroanalysis 2016, 28, 1149–1169. [Google Scholar] [CrossRef]
- Parrilla, M.; Cánovas, R.; Jeerapan, I.; Andrade, F.J.; Wang, J. A Textile-Based Stretchable Multi-Ion Potentiometric Sensor. Adv. Healthc. Mater. 2016, 5, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.Y.; Hong, Y.K.; Oh, B.K.; Cha, G.S.; Nam, H.; Lee, S.B.; Jin, J.-I. Potentiometric Properties of Ion-Selective Electrode Membranes Based on Segmented Polyether Urethane Matrices. Anal. Chem. 1997, 69, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Cosofret, V.V.; Erdosy, M.; Raleigh, J.S.; Johnson, T.A.; Neuman, M.R.; Buck, R.P. Aliphatic polyurethane as a matrix for pH sensors: Effects of native sites and added proton carrier on electrical and potentiometric properties. Talanta 1996, 43, 143–151. [Google Scholar] [CrossRef]
- Kwan, R.C.H.; Leung, H.F.; Hon, P.Y.T.; Cheung, H.C.F.; Hirota, K.; Renneberg, R. Amperometric biosensor for determining human salivary phosphate. Anal. Biochem. 2005, 343, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-U.; Nam, S.; Lee, M.-S.; Lieber, C.M. Synthesis of monolithic graphene–graphite integrated electronics. Nat. Mater. 2012, 11, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.G.; Marshall, R.W.; Bowers, G.N. Ionized Calcium in Body Fluids. CRC Crit. Rev. Clin. Lab. Sci. 1981, 15, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.Y.; Kim, J.S.; Choi, B.R.; Lee, K.; Lee, H. Hydrogel Based Biosensors for In Vitro Diagnostics of Biochemicals, Proteins, and Genes. Adv. Healthc. Mater. 2017, 6, 1601475. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Panneer Selvam, A.; Muthukumar, S.; Kamakoti, V.; Prasad, S. A wearable biochemical sensor for monitoring alcohol consumption lifestyle through Ethyl glucuronide (EtG) detection in human sweat. Sci. Rep. 2016, 6, 23111. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Bandodkar, A.J.; Parkhomovsky, S.; Wang, J. Stamp transfer electrodes for electrochemical sensing on non-planar and oversized surfaces. Analyst 2012, 137, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Screen-printed biosensors in microbiology; a review. Talanta 2010, 82, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Miyahara, Y. Current and emerging challenges of field effect transistor based bio-sensing. Nanoscale 2013, 5, 10702–10718. [Google Scholar] [CrossRef] [PubMed]
- Karthick Kannan, P.; Late, D.J.; Morgan, H.; Sekhar Rout, C. Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 2015, 7, 13293–13312. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Ophthalmic glucose sensing: a novel monosaccharide sensing disposable and colorless contact lens. Analyst 2004, 129, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. A glucose-sensing contact lens: from bench top to patient. Curr. Opin. Biotechnol. 2005, 16, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A Gelated Colloidal Crystal Attached Lens for Noninvasive Continuous Monitoring of Tear Glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef]
- Speedy, D.B.; Noakes, T.D.; Schneider, C. Exercise-associated hyponatremia: a review. Emerg. Med. 2001, 13, 17–27. [Google Scholar] [CrossRef]
- Talary, M.S.; Dewarrat, F.; Huber, D.; Caduff, A. In vivo life sign application of dielectric spectroscopy and non-invasive glucose monitoring. J. Non-Cryst. Solids 2007, 353, 4515–4517. [Google Scholar] [CrossRef]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P. Lactate in human sweat: a critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Sprigle, S.; Linden, M.; McKenna, D.; Davis, K.; Riordan, B. Clinical skin temperature measurement to predict incipient pressure ulcers. Adv. Skin Wound Care 2001, 14, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Jang, K.-I.; Ma, Y.; Koh, A.; Chen, H.; Jung, H.N.; Kim, Y.; Kwak, J.W.; Wang, L.; Xue, Y.; et al. Chemical Sensing Systems that Utilize Soft Electronics on Thin Elastomeric Substrates with Open Cellular Designs. Adv. Funct. Mater. 2017, 27, 1605476. [Google Scholar] [CrossRef]
- Ho, J.S.; Yeh, A.J.; Neofytou, E.; Kim, S.; Tanabe, Y.; Patlolla, B.; Beygui, R.E.; Poon, A.S.Y. Wireless power transfer to deep-tissue microimplants. Proc. Natl. Acad. Sci. USA 2014, 111, 7974–7979. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Rodger, D.C.; Saati, S.; Humayun, M.S.; Tai, Y.-C. Microfabricated implantable parylene-based wireless passive intraocular pressure sensors. J. Microelectromech. Syst. 2008, 17, 1342–1351. [Google Scholar] [CrossRef]
- Mei, H.; Irazoqui, P.P. Miniaturizing wireless implants. Nat. Biotechnol. 2014, 32, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Brenner, D.S.; Shin, G.; Morgan, C.D.; Copits, B.A.; Chung, H.U.; Pullen, M.Y.; Noh, K.N.; Davidson, S.; Oh, S.J.; et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015, 33, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, J.; Hu, F.; Hao, Q. Bluetooth low energy for wearable sensor-based healthcare systems. In Proceedings of the 2014 IEEE Healthcare Innovation Conference (HIC), Seattle, WA, USA, 8–10 October 2014; pp. 251–254. [Google Scholar]
- Malhi, K.; Mukhopadhyay, S.C.; Schnepper, J.; Haefke, M.; Ewald, H. A Zigbee-Based Wearable Physiological Parameters Monitoring System. IEEE Sens. J. 2012, 12, 423–430. [Google Scholar] [CrossRef]
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.-I.; et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2016, 2, e1600418. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-Based Conformal, Adhesive, Edible Food Sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Cheng, H.; Shin, W.-J.; Fan, J.A.; Liu, Z.; Lu, C.-J.; Kong, G.-W.; Chen, K.; Patnaik, D.; et al. Materials and Designs for Wireless Epidermal Sensors of Hydration and Strain. Adv. Funct. Mater. 2014, 24, 3846–3854. [Google Scholar] [CrossRef]
- Kim, J.; Banks, A.; Xie, Z.; Heo, S.Y.; Gutruf, P.; Lee, J.W.; Xu, S.; Jang, K.-I.; Liu, F.; Brown, G.; et al. Miniaturized Flexible Electronic Systems with Wireless Power and Near-Field Communication Capabilities. Adv. Funct. Mater. 2015, 25, 4761–4767. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.-J.; Lu, C.-J.; Kong, G.-W.; Patnaik, D.; Lee, S.-H.; Cortes, J.F.; Rogers, J.A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 2014, 10, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Oh, J.; Shin, D.H.; Kim, S.G.; Lee, J.S.; Kim, W.; Jang, J. Wireless, Room Temperature Volatile Organic Compound Sensor Based on Polypyrrole Nanoparticle Immobilized Ultrahigh Frequency Radio Frequency Identification Tag. ACS Appl. Mater. Interfaces 2016, 8, 33139–33147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, Z. A Microfluidic, Reversibly Stretchable, Large-Area Wireless Strain Sensor. Adv. Funct. Mater. 2011, 21, 2282–2290. [Google Scholar] [CrossRef]
- Tang, J.; Guo, H.; Zhao, M.; Yang, J.; Tsoukalas, D.; Zhang, B.; Liu, J.; Xue, C.; Zhang, W. Highly Stretchable Electrodes on Wrinkled Polydimethylsiloxane Substrates. Sci. Rep. 2015, 5, 16527. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Cho, C.; Cooper, J.; Wang, Y.; Tentzeris, M.M.; Leon, R.T. Passive wireless antenna sensor for strain and crack sensing—Electromagnetic modeling, simulation, and testing. Smart Mater. Struct. 2013, 22, 085009. [Google Scholar] [CrossRef]
- Govil, J. 4G Mobile Communication Systems: Turns, Trends and Transition. In Proceedings of the 2007 International Conference on Convergence Information Technology (ICCIT 2007), Gyeongju, Korea, 21–23 November 2007; pp. 13–18. [Google Scholar]
- Tröster, G. The Agenda of Wearable Healthcare. Yearb. Med. Inform. 2005, 125–138. [Google Scholar]
- Laine, T.H.; Lee, C.; Suk, H. Mobile Gateway for Ubiquitous Health Care System Using ZigBee and Bluetooth. In Proceedings of the 2014 Eighth International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing, Birmingham, UK, 2–4 July 2014; pp. 139–145. [Google Scholar]
- Dementyev, A.; Hodges, S.; Taylor, S.; Smith, J. Power Consumption Analysis of Bluetooth Low Energy, ZigBee, and ANT Sensor Nodes in a Cyclic Sleep Scenario. In Proceedings of the 2013 IEEE International Wireless Symposium (IWS), Beijing, China, 14–18 April 2013. [Google Scholar]
- Barnes, S.J. Under the skin: short-range embedded wireless technology. Int. J. Inf. Manag. 2002, 22, 165–179. [Google Scholar] [CrossRef]
- Coskun, V.; Ozdenizci, B.; Ok, K. A Survey on Near Field Communication (NFC) Technology. Wirel. Pers. Commun. 2013, 71, 2259–2294. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed]
- Touati, F.; Tabish, R. U-Healthcare System: State-of-the-Art Review and Challenges. J. Med. Syst. 2013, 37, 9949. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Kim, S.-J.; Kim, I.-D. Ultrafast optical reduction of graphene oxide sheets on colorless polyimide film for wearable chemical sensors. NPG Asia Mater. 2016, 8, e315. [Google Scholar] [CrossRef]
- Lee, J.W.; Xu, R.; Lee, S.; Jang, K.-I.; Yang, Y.; Banks, A.; Yu, K.J.; Kim, J.; Xu, S.; Ma, S.; et al. Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. USA 2016, 113, 6131–6136. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Trans. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Kim, J.; Zhang, S.; Banks, A.; Crawford, K.E.; Sheng, X.; Gutruf, P.; Shi, Y.; Pielak, R.M.; Rogers, J.A. Materials and Device Designs for an Epidermal UV Colorimetric Dosimeter with Near Field Communication Capabilities. Adv. Funct. Mater. 2017, 27, 1604465. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Ad. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Lipson, H.; Kurman, M. Fabricated: The new World of 3D Printing; John Wiley & Sons: Weinheim, Germany, 2013. [Google Scholar]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M.; Fearing, R.S. Synthetic gecko foot-hair micro/nano-structures as dry adhesives. J. Adhes. Sci. Technol. 2003, 17, 1055–1073. [Google Scholar] [CrossRef]
- Kim, S.; Sitti, M. Biologically inspired polymer microfibers with spatulate tips as repeatable fibrillar adhesives. Appl. Phys. Lett. 2006, 89, 261911. [Google Scholar] [CrossRef]
- Murphy, M.P.; Aksak, B.; Sitti, M. Gecko-Inspired Directional and Controllable Adhesion. Small 2009, 5, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Glass, P.; Chung, H.; Washburn, N.R.; Sitti, M. Enhanced reversible adhesion of dopamine methacrylamide-coated elastomer microfibrillar structures under wet conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, J.; Hyeon, T.; Lee, M.; Kim, D.-H. Fabric-Based Integrated Energy Devices for Wearable Activity Monitors. Adv. Mater. 2014, 26, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Fan, F.R.; Zhou, T.; Tang, W.; Xue, F.; Wang, Z.L. Ultrasensitive self-powered pressure sensing system. Extrem. Mech. Lett. 2015, 2, 28–36. [Google Scholar] [CrossRef]
- Yi, F.; Wang, X.; Niu, S.; Li, S.; Yin, Y.; Dai, K.; Zhang, G.; Lin, L.; Wen, Z.; Guo, H.; et al. A highly shape-adaptive, stretchable design based on conductive liquid for energy harvesting and self-powered biomechanical monitoring. Sci. Adv. 2016, 2, e1501624. [Google Scholar] [CrossRef] [PubMed]







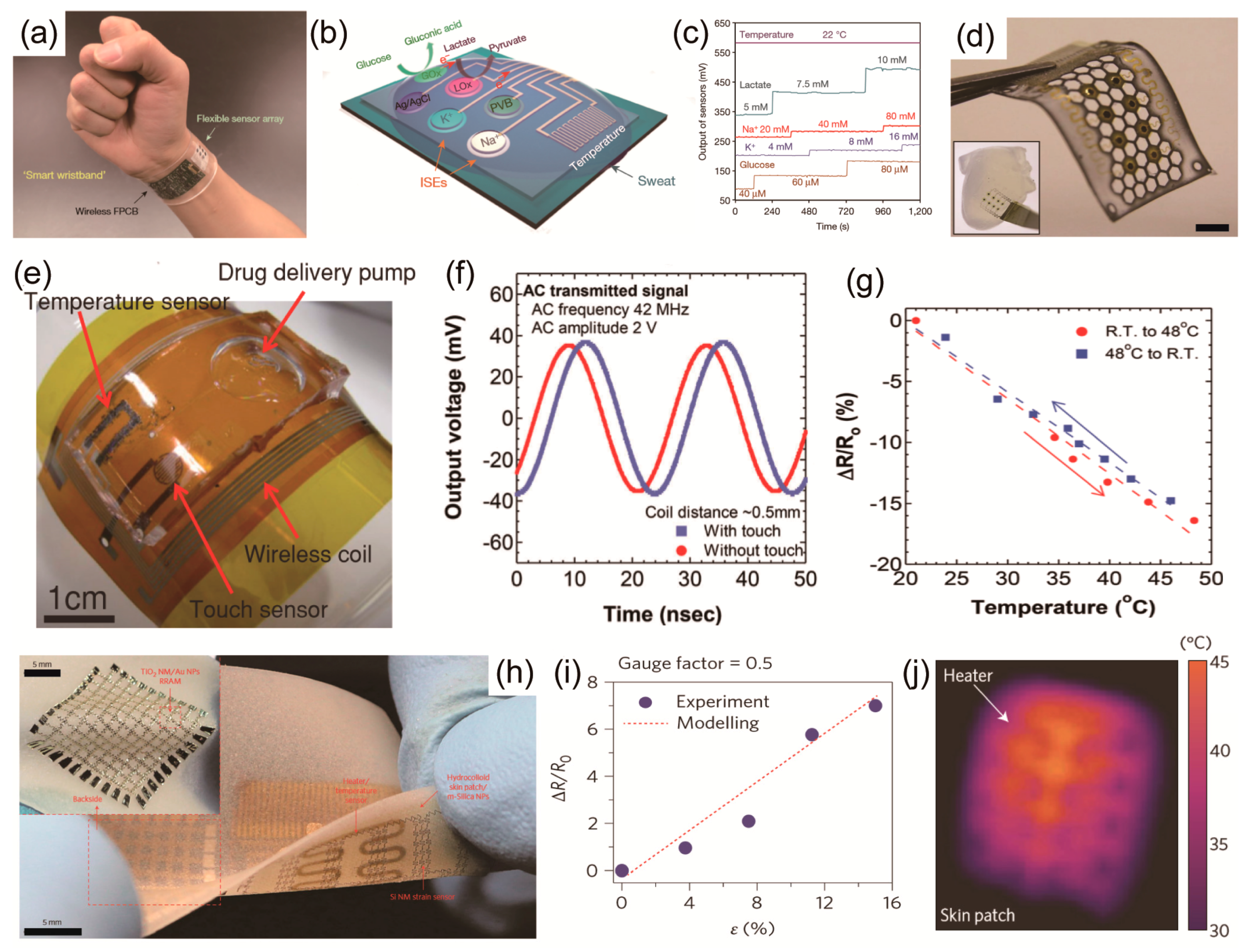



| Sensor Type | Sensing Element | Substrate | Electrode | Active Material | Working Voltage | Sensing Range | Sensitivity | Flexibility | Stretchability | Reliability | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | Human hand temperature | PDMS or thin PVA | Au | Au | 0.6 V | 20~150 °C | 1.9 Ohm K−1 | - | 100% | - | [42] |
| Temperature | Temperature | PDMS | AgNW | Graphene | 10 V | 30~100 °C | Nonlinear resistance response | - | 50% | 100 | [31] |
| Temperature | Endothelial layer | Polyester fabric strip coated with PDMS | Au | Platinum (Pt) | 0.8 V | 0~120 °C | 2.7 Ohm K−1 | 0.01% | - | - | [54] |
| pressure | Intraocular pressure (IOP) | Parylene | graphene-AgNW | ecoflex (capacitive-type) | - | 5~150 mmHg | - | Bending radius: 3.1 μm | 25% | 10,000 cycles with 25% strain | [32] |
| Pressure | Cutaneous pressure | Ecoflex | Au | Lead zirconate titanate (PbZr0.52Ti0.48O3, PZT) | VG: 3.5 V VD: 0.1 V | 0.005~10 Pa | 1.36 μA Pa−1 | 14.8 mm bending radius | 30% | 1000 | [3] |
| Pressure | Touch | PET or Polyurethane | Conductive carbon fabric | CNT/PDMS porous | 0.1 V | 0.25~100 kPa | - | 30 mm bending radius | 30% | 10 | [39] |
| Pressure | Artery wrist pulse or acoustic vibrations | PDMS | Au | Au NWs | 1.5 V | 13 Pa~50 kPa | 1.14 kPa−1 | 30 mm bending radius | 25% | 50,000 | [36] |
| Pressure | Pressure | PDMS and Epoxy (SU8) | Au or AgNW/graphene | Graphene | VG: 25 V VD: 0.1 V | 250 Pa~3 Mpa | 2.05 × 10−4 kPa−1 (below 500 kPa), 9.43 × 10−6 kPa−1 (above 500 kPa) | - | - | 1000 | [33] |
| Strain | Facial expressions | PDMS | Polyurethane-PEDOT:PSS | Single-walled carbon nanotubes | 1 V | 1.6~3.6%, 10~100% | 62 (ΔR/R)/ε | 3.60% | 100% | Minimum 50 cycles for bending, 1000 cycles for stretching | [34] |
| Strain | Heartbeats | PDMS | Graphene woven fabrics | Graphene woven fabrics | 1 V | under 0.2~30% | 1000 (ΔR/R)/ε | - | 30% | - | [86] |
| Strain | Stretch and pressure | PDMS | Eutectic Gallium Indium | Single-walled carbon nanotubes-Ecoflex | - | ~150% | 0.004 (ΔC/C)/ε | - | 150% | - | [69] |
| Strain | Joint movement (bending) | Silicon elastomer (Dragonskin 10 + Thi-Vex silicon thickener + Slo-jo platinum silicon cure retarder) | Silver wire | Ionic fluid - silicon elastomer | AC 5 V, 50~200 Hz | ~700% | 0.348 (ΔC/C)/ε | - | 700% | minimum 20 cycles for stretching | [92] |
| Strain | Bending | PDMS coated Polystyrene | Silver paste | ZnSnO3 nanowires | 1.2 V | ~0.33% | 3740 (ΔI/I)/ε | 0.33% | No stretchability | - | [95] |
| Strain | Lymphedema | Silicon rubber (Solaris) | Cu | Cu-PI (capacitive-type) | - | 0~30% | - | - | 30% | - | [160] |
| Strain | Strain | PET textile | Carbon nanotube/reduced Graphene oxide | ZnO Nanowire | - | Sensor limits of bacterium/μL | - | 3~5% | - | 100 bending cycles | [83] |
| Gas | O2 (oxygen) | Porous PTFE (porous polytetrafluoroethylene) | Au | High-purity 1-butyl-1-methylpyrrolidinium bis (trifluoro-methylsulfonyl)imide | −1.4V | 0~21% | 0.48 uA/% | Can be bent either convex or concave | - | - | [117] |
| Gas | NO2 (nitrogen dioxide) | PES (polyethersulfone) | Cr/Au | Graphene | 30 V | 0.5 to 40 ppm | ∆R/R0 = −40% (at 40 ppm N2) | ∆R/R0 = 5% under a bending strain of 1.4% | - | - | [37] |
| Gas | NO2 (nitrogen dioxide) | Paper | Au | NaNO2 treated PbS CQD | 4.1 V | 0.5 to 50 ppm | 0.41/ppm | Bending angle of 70° | - | 7% decrease in response under 5000 cyclic bending tests (Bending angle of 50°) | [114] |
| Gas | Volatile compouds (ammonia, acetic acid) | Plastic substrate | Cr/Au | Polypyrrole (Ppy) (functionalized by carboxyl group) | −0.1~0.1 V | 0.1~100 ppm | - | Bending angle: 15° | - | - | [163] |
| Gas | Dimethyl methylphosphonate (DMMP) | PI or PDMS or parylene | Graphene-AgNW | Graphene (functionalized by polypyrrole (Ppy)) | 0.1 V | 5~25 ppm | - | - | 20% | 1000 cycles with 5% strain | [18] |
| Gas | H2S, C2H5OH, H2 | PI | Ti/Au | Graphene oxide | - | Graphene limits of bacterium/μL | Toluene, Acetone, CO Ethanol at 20 PPM, Acetone at 20 ppm, H2S, H2 at 5–20 ppm | A 30° bending angle | - | 104 bending cycles | [175] |
| Bio | Protein (Concanavalin A, Con A) | PET or PDMS or parylene | Graphene-AgNW | Graphene (functionalized by mannosyl-pyrene) | 0.1 V | 1 mg/mL | - | Bending radius: 27 μm (bending strain: 2.59%) | 20% | - | [12] |
| Bio | Bacteria (E. coli, S. aureus, H. pylori) | Silk fibroin film | Cr/Au | graphene (functionalized by antimicrobial peptides (AMPs)) | - | Detection limits of bacterium/μL | 100~108 CFU/mL | - | - | - | [159] |
| Bio | Lactate | Temporary transfer tatto paper, GORETEX | Ag/AgCl, conductive carbon | lactate oxidase(LOx) | 0.05 V | 1 mM to 25 mM | 644.2 nA/mM at RT, 0.916 μA/mM at 37 °C | Bending angle of 90° | stretched at ~10% | The bending/stretching test: 10 times | [136] |
| Bio | D-glucose | Polyimide film (2 μm) | Cr/Au | In2O3, glucose oxidase | 0.2~0.8 V | 100 μM to 4 mM | - | Bending radius of 837 (0.078~0.082%) | - | - | [40] |
| Bio | Glucose | PDMS, Polyimide, Parylene | Graphene/AgNW | Graphene, glucose oxidase | 0.1 V | 1 μM~10 mM | - | - | ∆R < 6% at 25% tensile strain (5000 cycles) | ∆R ~20% at 10,000 cycles of stretching | [32] |
| Bio | Glucose, Lactate | PDMS | - | Enzyme and chromogenic reagent | - | Glucose: 0~25 mM Lactage: 0~100 mM | - | 5 cm bending radius | Strain 30% | - | [177] |
| Bio | Salivary Uric acid | PET | Ag/AgCl | Prussian-blue-graphite | - | Mouthguard limits of bacterium/μL | 2.45 μA/mM | - | - | - | [29] |
| Ion | Chloride, H+ | PDMS | - | Enzyme and chromogenic reagent | - | Chloride: 0~625 μM pH: 5.0~8.5 | - | 5 cm bending radius | Strain 30% | - | [177] |
| Ion | Na+, K+ | Textile | Ecoflex-containing Ag/AgCl ink, MWCNT | Multi-walled carbon nanotubes (functionalized by carboxylic acid) | - | Physiological range of human sweat (10~110 × 10−3 M NaCl, 1~8 × 10−3 M KCl) | 54.1 ± 1.5 mV/log [Na+], 56.9 ± 1.6 mV/log [K+] | Bending angle of 180° | 100 % (uniaxial stretching) | No sensitivity degradation at linear strain of 75% during 60 min at a speed of 1 mm/s | [122] |
| Ion | Na+, K+ | PET (polyethylene terephthalate) | Cr/Au, Ag/AgCl | Multi-walled carbon nanotubes | 3.7 V | 1 mM KCl and 10 mM NaCl (working condition) | 62.5 mV/log [Na+], 59.5 mV/log [K+] | Radius of curvature 1.5 cm | - | No sensitivity degradation by bending radius of 1.5 cm over 60 cycles | [38] |
| Ion | H+ | Poly (methylmethacrylate) | Graphite | Graphene | −0.4~0.4 V | pH 5~8 | 17 mV/pH | Radius of curvature: 1.2 cm | 0.04 | No significant change in the electrical response (mobility values remained constant) as a result of bending to radii of curvature as small as 0.7 cm (estimated bending-induced strain: 0.6%) | [126] |
| Ion | Ca2+, H+ | PET (polyethylene terephthalate) | Ag/AgCl reference electrode | Polyaniline (PANI) | - | Typical physiological [Ca2+] variations (e.g., 1 mM to 0.5 mM), pH 4~7 | 32.7 mV/log [Ca2+], 62.5 mV/pH | - | - | - | [127] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.W.; Shin, J.H.; Kim, S.-Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.-G.; Cho, E.; et al. Smart Sensor Systems for Wearable Electronic Devices. Polymers 2017, 9, 303. https://doi.org/10.3390/polym9080303
An BW, Shin JH, Kim S-Y, Kim J, Ji S, Park J, Lee Y, Jang J, Park Y-G, Cho E, et al. Smart Sensor Systems for Wearable Electronic Devices. Polymers. 2017; 9(8):303. https://doi.org/10.3390/polym9080303
Chicago/Turabian StyleAn, Byeong Wan, Jung Hwal Shin, So-Yun Kim, Joohee Kim, Sangyoon Ji, Jihun Park, Youngjin Lee, Jiuk Jang, Young-Geun Park, Eunjin Cho, and et al. 2017. "Smart Sensor Systems for Wearable Electronic Devices" Polymers 9, no. 8: 303. https://doi.org/10.3390/polym9080303
APA StyleAn, B. W., Shin, J. H., Kim, S.-Y., Kim, J., Ji, S., Park, J., Lee, Y., Jang, J., Park, Y.-G., Cho, E., Jo, S., & Park, J.-U. (2017). Smart Sensor Systems for Wearable Electronic Devices. Polymers, 9(8), 303. https://doi.org/10.3390/polym9080303