Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. NIR Evaluations and Prediction Correcting of ZX-50 Methods
2.2. Validation and Application of ZX-50 Methods
3. Results and Discussion
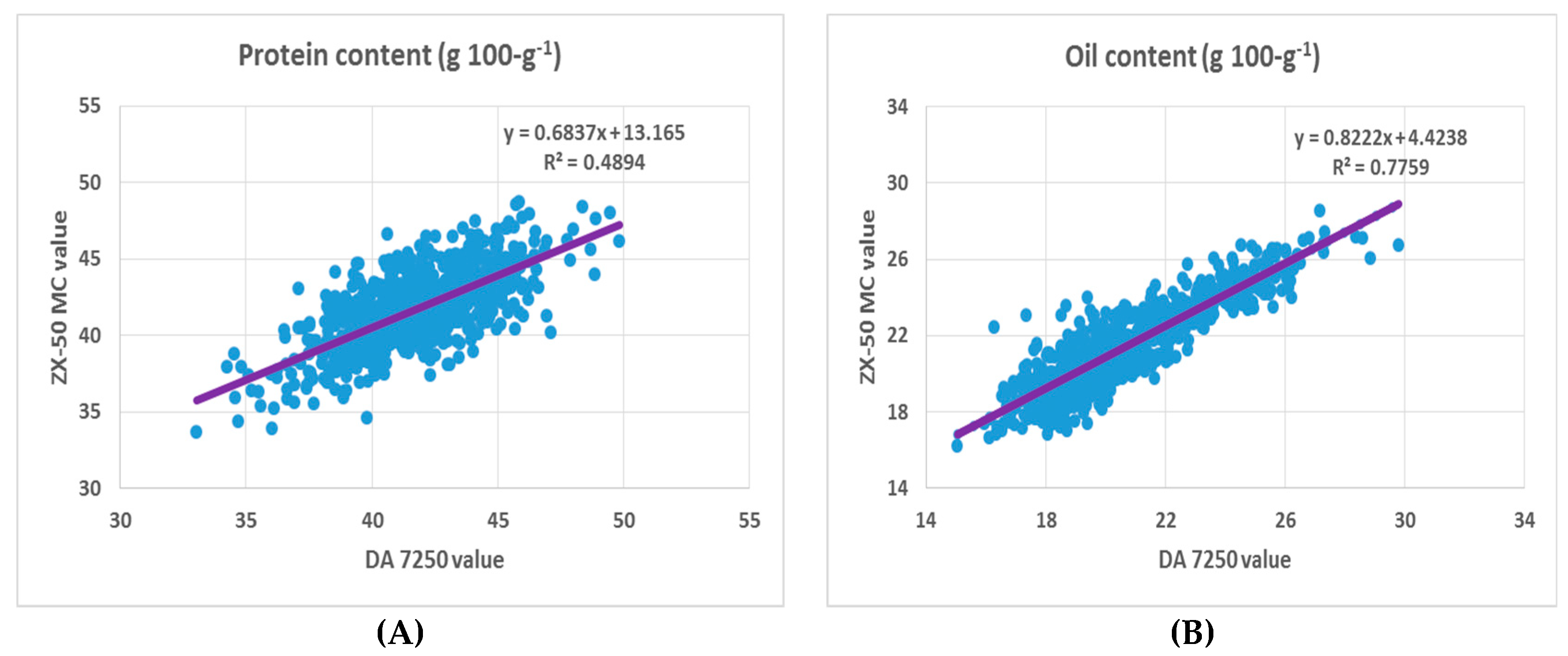
3.1. NIR Predictions and Comparisons of Protein and Oil Content
3.2. Seed Size and Correction of Predictions by ZX-50 and ZX-50 MC
- where y = the difference between the predictions of ZX-50 methods and DA 7250, and x = the 100-seed weight.
- where y = the calibrated value, OV = the original value, and x = the 100-seed weight.
3.3. Repeatability and Correlation
3.4. Validation and Application of ZX-50 MC with Data Correcting in Bulk-Seed Samples
3.5. Validation and Use of ZX-50 MC in Single Plant Evaluation
3.6. Other Issues
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Cho, B.K.; Kim, M.S.; Lee, W.H.; Tewari, J.; Bae, H.; Sohn, S.I.; Chi, H.Y. Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens. Actuators B Chem. 2013, 185, 694–700. [Google Scholar] [CrossRef]
- Liu, K. Soybeans: Chemistry, Technology, and Utilization; Aspen Publishers, Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Huang, H.; Yu, H.; Xu, H.; Ying, Y. Near infrared spectroscopy for on/in-line monitoring of quality in foods and beverages: A review. J. Food Eng. 2008, 87, 303–313. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists (AACC). Approved Methods of the AACC, Methods 46–30; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Font, R.; del Rio-Celestino, M.; de Haro-Bailon, A. The use of near-infrared spectroscopy (NIRS) in the study of seed quality components in plant breeding programs. Ind. Crop. Prod. 2006, 24, 307–313. [Google Scholar] [CrossRef]
- Weir, A.D.; Omielan, J.; Lee, E.A.; Rajcan, I. Use of NMR for predicting protein concentration in soybean seeds based on oil measurements. JAOCS 2005, 82, 87–91. [Google Scholar] [CrossRef]
- Santos, L.R.; Zangirolami, M.D.S.; Silva, N.O.; Valderrama, P.; Março, P.H. Rapid non-invasive assessment of quality parameters in ground soybean using near-infrared spectroscopy. Pesq. Agropec. Bras. 2018, 53, 97–104. [Google Scholar] [CrossRef]
- Singh, S.; Patel, S.; Litoria, N.; Gandhi, K.; Faldu, P.; Patel, K.G. Comparative efficiency of conventional and NIR based technique for proximate composition of pigeon pea, soybean and rice cultivars. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 773–782. [Google Scholar] [CrossRef]
- Grunvald, A.K.; Carvalho, C.G.P.; Leite, R.S.; Mandarino, J.M.G.; Andrade, C.A.B.; Scapim, C.A. Predicting the oil contents in sunflower genotype seeds using near-infrared reflectance (NIR) spectroscopy. Acta Scientiarum. Agron. 2014, 36, 233–237. [Google Scholar] [CrossRef]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Stuth, J.; Jama, A.; Tolleson, D. Direct and indirect means of predicting forage quality through near infrared reflectance spectroscopy. Field Crop. Res. 2003, 84, 45–56. [Google Scholar] [CrossRef]
- Osborne, B.G. Applications of near infrared spectroscopy in quality screening of early-generation material in cereal breeding programmes. J. Near Infrared Spectrosc. 2006, 14, 93–101. [Google Scholar] [CrossRef]
- Pathmell, C.A. Rapid NIR Measurement of Oil and Protein Content in Soybean. 2016. Available online: https://oceanoptics.com/rapid-soybean-oil-protein-nir-measurement/ (accessed on 15 January 2019).
- Patil, A.G.; Oak, M.D.; Taware, S.P.; Tamhankar, S.A.; Rao, V.S. Nondestructive estimation of fatty acid composition in soybean [Glycine max (L.) Merrill] seeds using near-infrared transmittance spectroscopy. Food Chem. 2010, 120, 1210–1217. [Google Scholar] [CrossRef]
- Pazdernik, D.; Killam, A.S.; Orf, J.H. Analysis of amino and fatty acid composition in soybean seed, using near infrared reflectance spectroscopy. Agron. J. 1997, 89, 679–685. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.L.; Green, M.; Scott, R.A.; Song, Q.; Hyten, D.L.; Cregan, P.B. Identification and validation of quantitative trait loci for seed yield, oil and protein contents in two recombinant inbred populations of soybean. Mol. Genet. Genom. 2014, 289, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, S.; Wu, X.; Xing, C.; Yuan, J. Determination of soybean routine quality parameters using near-infrared spectroscopy. Food Sci. Nutr. 2018, 6, 1109–1118. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhu, J.; Wei, L.M.; Dai, J.R.; Song, T.M.; Yan, Y.L.; Chen, S.J. Analysis of protein, starch and oil content of single intact kernels by near infrared reflectance spectroscopy (NIRS) in maize (Zea mays L.). Plant Breed. 2007, 126, 492–497. [Google Scholar] [CrossRef]
- Tallada, J.G.; Palacios-Rojas, N.; Armstrong, P.R. Prediction of maize seed attributes using a rapid single kernel near infrared instrument. J. Cereal Sci. 2009, 50, 381–387. [Google Scholar] [CrossRef]
- Long, D.S.; Engel, R.E.; Siemens, M.C. Measuring grain protein concentration with in-line near infrared reflectance spectroscopy. Agron. J. 2008, 100, 247–252. [Google Scholar] [CrossRef]
- AACC International. Near-infrared reflectance method for whole-grain analysis in soybeans. In AACC Approved Methods of Analysis, 11th ed.; Method 39-21.01; Cereals & Grains Association: St. Paul, MN, USA, 2010. [Google Scholar]
- Biancolillo, A.; Marini, F. Chapter Four—Chemometrics Applied to Plant Spectral Analysis. In Vibrational Spectroscopy for Plant Varieties and Cultivars Characterization, Comprehensive, Analytical Chemistry; Lopes, J., Sousa, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 80, pp. 69–104. [Google Scholar] [CrossRef]
- Agelet, L.E.; Ellis, D.D.; Duvick, S.; Goggi, A.S.; Hurburgh, C.R.; Gardner, A.C. Feasibility of near infrared spectroscopy for analyzing corn kernel damage and viability of soybean and corn kernels. J. Cereal Sci. 2012, 55, 160–165. [Google Scholar] [CrossRef]
- Armstrong, P.R. Rapid single-kernel NIR measurement of grain and oil-seed attributes. Appl. Eng. Agric. 2006, 22, 767–772. [Google Scholar] [CrossRef]
- Spielbauer, G.; Armstrong, P.; Baier, J.W.; Allen, W.B.; Richardson, K.; Shen, B.; Settles, A.M. High-throughput near-infrared reflectance spectroscopy for predicting quantitative and qualitative composition phenotypes of individual maize kernels. Cereal Chem. 2009, 86, 556–564. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R., Jr. Limitations and current applications of near infrared spectroscopy for single seed analysis. Talanta 2014, 121, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C.; Li, K.; Nelson, R.; Ulanov, A.; DeMuro, C.M.; Baxter, I. Canopy position has a profound effect on soybean seed composition. PeerJ 2016, 4, e2452. [Google Scholar] [CrossRef] [PubMed]
- Huskey, L.L.; Snyder, H.E.; Gbur, E.E. Analysis of single soybean seeds for oil and protein. JAOCS 1990, 67, 686–688. [Google Scholar] [CrossRef]
- Mosjidis, J.A.; Yermanos, D.M. Plant position effect on seed weight, oil content, and oil composition in sesame. Euphytica 1985, 34, 193–199. [Google Scholar] [CrossRef]
- Perten Instruments AB. DA 7250 Installation and Operation Manual; Perten Instruments AB: Hagersten, Sweden, 2016. [Google Scholar]
- Jiang, G.L.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic analysis of sugar composition and its relationship with protein, oil, and fiber in soybean. Crop Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Jiang, G.L.; Rutto, L.K.; Ren, S.; Bowen, R.A.; Berry, H.; Epps, K. Genetic analysis of edamame seed composition and trait relationships in soybean lines. Euphytica 2018, 214, 158. [Google Scholar] [CrossRef]
- Smallwood, C.J.; Gillman, J.D.; Saxton, A.M.; Bhandari, H.S.; Wadl, P.A.; Fallen, B.D.; Hyten, D.L.; Song, Q.; Pantalone, V.R. Identifying and exploring significant genomic regions associated with soybean yield, seed fatty acids, protein and oil. J. Crop Sci. Biotech. 2017, 20, 243–253. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Lu, Y.; Bhusal, S.; Song, Q.; Cregan, P.B.; Yen, Y.; Brown, M.; Jiang, G.L. Genome-wide scan for seed composition provides insights into soybean quality improvement and the impacts of domestication and breeding. Mol. Plant 2018, 11, 460–472. [Google Scholar] [CrossRef]
- Zeltex, Inc. ZX-50 Portable Grain Analyzer with AutoBIAS; User’s Manual Version 4.1.; Zeltex, Inc.: Hagerstown, MD, USA, 2007. [Google Scholar]
- Scheffé, H. The Analysis of Variance; Wiley: New York, NY, USA, 1959. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Filho, M.M.; Destro, D.; Miranda, L.A.; Spinosa, W.A.; Carrao-Panizzi, M.C.; Montalvan, R. Relationships among oil content, protein content and seed size in soybeans. Braz. Arch. Biol. Technol. 2001, 44, 23–32. [Google Scholar] [CrossRef]
- Maestri, D.M.; Labuckas, D.O.; Guzman, C.A.; Giorda, L.M. Correlation between seed size, protein and oil contents, and fatty acid composition in soybean genotypes. Grasas y Aceites 1998, 49, 450–453. [Google Scholar] [CrossRef]
- Poeta, F.; Borras, L.; Rotundo, J.L. Variation in seed protein concentration and seed size affects soybean crop growth and development. Crop Sci. 2016, 56, 3196–3208. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cregan, P.B.; Jiang, G.L. Genome-wide association study, genomic prediction and marker-assisted selection for seed weight in soybean (Glycine max). Theor. Appl. Genet. 2016, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.D.; Hashiguchi, M.; Ishigaki, G.; Muguerza, M.; Oba, C.; Abe, J.; Harada, K.; Akashi, R. Evaluation of seed components of wild soybean (Glycine soja) collected in Japan using near-infrared reflectance spectroscopy. Plant Genet. Resour. 2017, 16, 94–102. [Google Scholar] [CrossRef]
- Crochet, W.D. Uniform Soybean Tests Northern States 2012; USDA-ARS and Purdue University: West Lafayette, IN, USA, 2013. [Google Scholar]
- Gillen, A.M.; Shelton, G.W. Uniform Soybean Tests Southern States 2013; USDA-Agricultural Research Service: Stoneville, MS, USA, 2014. [Google Scholar]
- O’Brien, T.; Graef, G. Regional Quality Traits Test 2013: Group 0-V; University of Nebraska: Lincoln, NE, USA, 2014. [Google Scholar]
| Trait | Data | Mean | Range | FM a | FG a | FMG a |
|---|---|---|---|---|---|---|
| Protein | Original | 38.7 ± 4.4 | 29.8–48.0 | 2819.34 ** | 325.03 ** | 16.97 ** |
| Corrected ZX-50 and ZX-50 MC | 42.0 ± 3.5 | 31.7–48.1 | 2.14 | 485.37 ** | 8.81 ** | |
| Oil | Original | 20.6 ± 2.2 | 16.3–25.5 | 774.10 ** | 137.61 ** | 5.97 ** |
| Corrected ZX-50 and ZX-50 MC | 19.4 ± 1.8 | 16.3–23.4 | 0.50 | 184.20 ** | 3.77 ** |
| Method | Protein Content (g 100 g−1) | Oil Content (g 100 g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Repeatability (%) | Correlation with DA 7250 | Mean | Range | Repeatability (%) | Correlation with DA 7250 | |
| Wet-chemistry | 41.8 ± 3.4 | 33.4–47.7 | 99.02 | 0.977 ** | 19.5 ± 1.9 | 17.0–23.1 | 96.31 | 0.960** |
| DA 7250 | 42.0 ± 4.0 | 32.3–47.3 | 99.36 | 19.4 ± 2.0 | 16.6–23.1 | 99.25 | ||
| ZX-50 | 36.3 ± 2.5 | 30.0–39.7 | 99.72 | 0.942 ** | 20.8 ± 1.5 | 18.4–23.9 | 99.35 | 0.908 ** |
| ZX-50 MC | 34.8 ± 1.6 | 31.1–37.0 | 94.71 | 0.889 ** | 22.9 ± 1.0 | 21.3–24.8 | 89.13 | 0.894 ** |
| Corrected ZX-50 | 42.0 ± 3.5 | 33.7–47.0 | 99.86 | 0.970 ** | 19.5 ± 1.9 | 16.9–23.2 | 99.58 | 0.946 ** |
| Corrected ZX-50 MC | 42.0 ± 3.1 | 35.7–47.0 | 98.66 | 0.918 ** | 19.4 ± 1.6 | 17.1–22.1 | 95.50 | 0.937 ** |
| Genotype | 100-Seed Weight (g) | Protein Content (g 100 g−1) | Oil Content (g 100 g−1) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Range | CV (%) | Mean | Range | CV (%) | ||
| PI 549058 | 20.3 | 45.4 ± 0.5 | 44.7–46.2 | 1.01 | 16.9 ± 0.3 | 16.4–17.4 | 1.90 |
| AGS 346 | 29.8 | 43.3 ± 0.7 | 41.6–44.7 | 1.71 | 18.2 ± 0.5 | 17.0–19.2 | 2.81 |
| IA 1008LF | 14.0 | 40.2 ± 0.3 | 39.4–40.7 | 0.78 | 18.5 ± 0.3 | 18.0–19.1 | 1.84 |
| NC 346 | 21.4 | 42.6 ± 0.8 | 41.8–44.5 | 1.77 | 19.1 ± 0.3 | 18.5–19.8 | 1.64 |
| VS12-0203 | 13.9 | 37.9 ± 0.7 | 37.3–39.2 | 1.72 | 23.1 ± 0.5 | 22.1–24.2 | 2.23 |
| VS12-0205 | 12.0 | 32.3 ± 0.7 | 31.5–34.1 | 2.17 | 21.0 ± 0.4 | 20.4–21.7 | 1.98 |
| VS12-0069 | 21.9 | 47.1 ± 0.9 | 45.8–48.4 | 1.89 | 16.6 ± 0.6 | 15.4–17.4 | 3.50 |
| VS11-0022 | 30.7 | 46.5 ± 0.8 | 45.2–48.0 | 1.80 | 17.3 ± 0.4 | 16.8–18.1 | 2.05 |
| NC Green | 33.7 | 47.3 ± 0.8 | 45.6–48.6 | 1.78 | 17.8 ± 0.4 | 17.2–18.6 | 2.11 |
| Osage | 15.8 | 44.2 ± 0.8 | 43.0–45.5 | 1.80 | 19.7 ± 0.4 | 19.1–20.2 | 1.96 |
| NC Raleigh | 15.0 | 36.0 ± 0.6 | 35.3–37.2 | 1.79 | 22.9 ± 0.3 | 22.4–23.5 | 1.45 |
| N6202-8 | 23.9 | 46.8 ± 0.7 | 45.5–47.6 | 1.51 | 17.0 ± 0.5 | 16.1–17.7 | 3.02 |
| Asmara | 23.0 | 40.8 ± 0.9 | 39.6–42.3 | 2.33 | 17.7 ± 0.7 | 16.3–18.6 | 3.75 |
| Ellis | 12.7 | 38.1 ± 0.8 | 36.9–39.7 | 2.08 | 22.3 ± 0.4 | 21.7–23.3 | 1.76 |
| VS22-450 | 13.6 | 41.3 ± 0.6 | 40.6–43.1 | 1.56 | 21.7 ± 0.3 | 20.8–22.0 | 1.52 |
| VS12-0128 | 24.9 | 38.8 ± 1.0 | 37.5–40.5 | 2.51 | 19.9 ± 0.6 | 18.5–20.5 | 2.85 |
| NC 346_(a) | 22.0 | 43.1 ± 1.0 | 42.0–44.9 | 2.39 | 19.9 ± 0.3 | 19.3–20.3 | 1.34 |
| Osage_(a) | 14.3 | 42.8 ± 0.7 | 41.9–44.1 | 1.69 | 20.0 ± 0.3 | 19.4–20.5 | 1.66 |
| N6202-8_(a) | 24.1 | 45.4 ± 1.2 | 43.9–47.1 | 2.58 | 18.0 ± 0.6 | 16.6–18.6 | 3.41 |
| Ellis_(a) | 13.3 | 39.9 ± 0.6 | 39.1–41.5 | 1.39 | 20.3 ± 0.3 | 19.9–20.7 | 1.45 |
| LSD0.05 | 0.92 | 0.50 | |||||
| Genotype | ZX-50 | ZX-50 MC | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | CV (%) | Difference | Mean | Range | CV (%) | Difference | |
| PI 549058 | 43.4 ± 0.4 | 42.6–44.1 | 0.94 | 2.0 | 43.5 ± 0.5 | 43.1–44.5 | 1.25 | 1.8 |
| AGS 346 | 44.2 ± 1.2 | 40.3–45.4 | 2.68 | −0.9 | 45.5 ± 0.6 | 44.9–46.3 | 1.32 | −2.3 |
| IA 1008LF | 40.2 ± 0.3 | 39.9–41.0 | 0.72 | −0.1 | 40.4 ± 0.3 | 40.0–40.7 | 0.76 | −0.3 |
| NC 346 | 43.7 ± 0.3 | 43.1–44.1 | 0.67 | −1.0 | 43.1 ± 0.5 | 42.5–43.8 | 1.12 | −0.4 |
| VS12-0203 | 37.7 ± 0.2 | 37.2–38.1 | 0.65 | 0.3 | 38.3 ± 0.6 | 37.2–38.7 | 1.64 | −0.3 |
| VS12-0205 | 33.7 ± 0.4 | 32.9–34.6 | 1.13 | −1.4 | 35.6 ± 0.2 | 35.3–35.9 | 0.61 | −3.3 |
| VS12-0069 | 45.8 ± 0.4 | 45.0–46.4 | 0.97 | 1.3 | 44.4 ± 0.6 | 43.4–44.8 | 1.26 | 2.7 |
| VS11-0022 | 45.4 ± 0.7 | 43.8–46.3 | 1.47 | 1.1 | 46.1 ± 0.3 | 45.8–46.6 | 0.71 | 0.4 |
| NC Green | 47.0 ± 0.8 | 44.6–47.9 | 1.77 | 0.3 | 46.9 ± 0.8 | 46.1–48.1 | 1.72 | 0.4 |
| Osage | 42.8 ± 0.3 | 42.1–43.5 | 0.81 | 1.4 | 42.0 ± 0.6 | 41.2–42.7 | 1.38 | 2.1 |
| NC Raleigh | 37.1 ± 0.2 | 36.8–37.4 | 0.54 | −1.1 | 38.1 ± 0.7 | 37.1–38.7 | 1.73 | −2.1 |
| N6202-8 | 46.1 ± 0.6 | 44.3–46.7 | 1.21 | 0.7 | 45.4 ± 0.3 | 45.1–45.7 | 0.67 | 1.4 |
| Asmara | 41.2 ± 0.3 | 40.5–41.7 | 0.74 | −0.4 | 41.4 ± 0.9 | 40.0–42.2 | 2.11 | −0.6 |
| Ellis | 38.5 ± 0.2 | 38.0–38.9 | 0.63 | −0.3 | 38.5 ± 0.2 | 38.3–38.7 | 0.41 | −0.4 |
| VS22-450 | 41.2 ± 0.4 | 40.2–41.9 | 1.06 | 0.1 | 40.2 ± 0.7 | 39.6–41.3 | 1.69 | 1.1 |
| VS12-0128 | 41.0 ± 0.3 | 40.5–41.5 | 0.65 | −2.2 | 41.7 ± 0.8 | 40.8–42.5 | 1.81 | −3.0 |
| NC 346_(a) | 43.9 ± 0.3 | 43.1–44.4 | 0.68 | −0.8 | 44.0 ± 0.4 | 43.6–44.5 | 0.80 | −0.9 |
| Osage_(a) | 41.5 ± 0.4 | 40.7–42.1 | 0.86 | 1.3 | 40.8 ± 0.6 | 39.7–41.3 | 1.50 | 2.0 |
| N6202-8_(a) | 45.7 ± 0.4 | 45.0–46.1 | 0.94 | −0.3 | 44.8 ± 0.7 | 43.9–45.5 | 1.59 | 0.6 |
| Ellis_(a) | 39.8 ± 0.3 | 39.1–40.6 | 0.81 | 0.2 | 40.0 ± 0.4 | 39.4–40.5 | 1.07 | 0.0 |
| LSD0.05 | 0.37 | 1.03 | ||||||
| Genotype | ZX-50 | ZX-50 MC | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | CV (%) | Difference | Mean | Range | CV (%) | Difference | |
| PI 549058 | 18.1 ± 0.4 | 17.4–18.9 | 2.40 | 1.1 | 18.4 ± 0.2 | 18.1–18.7 | 1.25 | 1.5 |
| AGS 346 | 18.7 ± 0.9 | 16.0–19.4 | 4.96 | 0.5 | 18.4 ± 1.1 | 17.4–20.2 | 5.81 | 0.2 |
| IA 1008LF | 18.8 ± 0.3 | 18.2–19.2 | 1.41 | 0.2 | 19.4 ± 0.4 | 18.8–19.9 | 2.23 | 0.9 |
| NC 346 | 18.0 ± 0.3 | 17.5–18.7 | 1.94 | −1.1 | 18.8 ± 0.3 | 18.5–19.2 | 1.53 | −0.4 |
| VS12-0203 | 23.1 ± 0.4 | 22.5–24.2 | 1.78 | 0.0 | 22.2 ± 0.7 | 21.0–22.8 | 3.20 | −1.0 |
| VS12-0205 | 21.6 ± 0.2 | 21.3–22.0 | 0.91 | 0.6 | 21.9 ± 0.2 | 21.6–22.1 | 1.04 | 0.9 |
| VS12-0069 | 16.9 ± 0.5 | 15.9–18.0 | 2.93 | 0.3 | 17.5 ± 0.3 | 17.2–18.0 | 1.83 | 0.9 |
| VS11-0022 | 18.5 ± 0.7 | 17.3–19.5 | 3.69 | 1.1 | 17.6 ± 0.4 | 17.1–18.0 | 2.08 | 0.2 |
| NC Green | 17.3 ± 0.8 | 15.2–18.6 | 4.80 | −0.5 | 16.8 ± 0.4 | 16.1–17.1 | 2.43 | −0.9 |
| Osage | 20.1 ± 0.3 | 19.7–20.6 | 1.36 | 0.3 | 19.5 ± 0.8 | 18.4–20.5 | 4.31 | −0.2 |
| NC Raleigh | 22.5 ± 0.2 | 22.2–23.1 | 0.96 | −0.4 | 21.8 ± 0.3 | 21.3–22.2 | 1.54 | −1.1 |
| N6202-8 | 17.0 ± 0.8 | 15.8–19.5 | 4.63 | 0.0 | 17.6 ± 0.2 | 17.3–17.8 | 1.18 | 0.5 |
| Asmara | 18.9 ± 0.4 | 18.3–19.6 | 1.92 | 1.2 | 18.9 ± 0.7 | 18.4–20.1 | 3.67 | 1.2 |
| Ellis | 21.8 ± 0.4 | 21.3–22.9 | 1.82 | −0.5 | 21.7 ± 0.3 | 21.5–22.2 | 1.20 | −0.6 |
| VS22-450 | 21.3 ± 0.5 | 19.9–22.0 | 2.33 | −0.4 | 21.2 ± 0.4 | 20.5–21.4 | 1.81 | −0.5 |
| VS12-0128 | 19.8 ± 0.3 | 19.3–20.3 | 1.44 | −0.1 | 19.7 ± 0.6 | 18.9–20.5 | 2.90 | −0.2 |
| NC 346_(a) | 19.0 ± 0.3 | 18.4–19.6 | 1.67 | −0.9 | 18.9 ± 0.4 | 18.3–19.4 | 2.20 | −1.0 |
| Osage_(a) | 20.6 ± 0.3 | 20.1–21.4 | 1.48 | 0.6 | 20.4 ± 0.2 | 20.0–20.5 | 1.06 | 0.4 |
| N6202-8_(a) | 17.4 ± 0.5 | 16.4–18.3 | 2.71 | −0.6 | 17.8 ± 0.3 | 17.4–18.1 | 1.62 | −0.2 |
| Ellis_(a) | 20.5 ± 0.2 | 19.7–20.7 | 1.20 | 0.2 | 20.3 ± 0.4 | 19.7–20.7 | 2.00 | 0.0 |
| LSD0.05 | 0.35 | 0.99 | ||||||
| Sample | 100-Seed Weight (g) | Method | Protein Content (g 100 g−1) | Oil Content (g 100 g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Correlation with DA 7250 | Mean | Range | Correlation with DA 7250 | ||
| Mature seed 2015 (117 lines, n = 234) | 20.1 ± 3.6 | 9.5–27.5 | DA 7250 | 43.5 ± 1.8 | 38.6–48.9 | 19.3 ± 1.3 | 16.8–22.5 | ||
| Corrected ZX-50 MC | 43.2 ± 1.5 | 39.9–48.2 | 0.732 ** | 20.4 ± 1.3 | 18.2–24.3 | 0.846 ** | |||
| Mature seed 2016 (147 lines, n = 147) | 20.3 ± 4.2 | 10.7–30.3 | DA 7250 | 41.9 ± 2.2 | 33.0–48.7 | 19.1 ± 1.5 | 15.1–22.8 | ||
| Corrected ZX-50 MC | 41.6 ± 2.1 | 33.7–47.1 | 0.823 ** | 18.9 ± 1.2 | 16.2–21.8 | 0.873 ** | |||
| Edamame seed 2015 (88 lines, n = 138) | 12.8 ± 2.2 | 7.0–20.5 | DA 7250 | 41.1 ± 2.1 | 34.5–46.0 | 20.2 ± 1.5 | 17.4–24.5 | ||
| Corrected ZX-50 MC | 43.5 ± 1.9 | 38.0–48.7 | 0.830 ** | 21.6 ± 1.1 | 19.4–25.8 | 0.791 ** | |||
| Edamame seed 2016 (127 lines, n = 241) | 12.6 ± 2.4 | 7.5–23.5 | DA 7250 | 40.6 ± 2.3 | 34.6–47.1 | 23.2 ± 1.9 | 17.8–29.8 | ||
| Corrected ZX-50 MC | 39.5 ± 1.6 | 34.8–44.6 | 0.765 ** | 23.8 ± 1.4 | 18.5–26.7 | 0.870 ** | |||
| Combined samples (n = 760) | 16.4 ± 5.0 | 6.0–30.3 | DA 7250 | 41.8 ± 2.5 | 33.0–49.8 | 20.6 ± 2.5 | 15.1–29.8 | ||
| Corrected ZX-50 MC | 41.8 ± 2.5 | 33.7–48.7 | 0.700 ** | 21.4 ± 2.3 | 16.2–28.5 | 0.881 ** | |||
| Method | Protein Content (g 100 g−1) | Oil Content (g 100 g−1) | ||||
|---|---|---|---|---|---|---|
| Mean | Range | Correlation with DA 7250 | Mean | Range | Correlation with DA 7250 | |
| DA 7250 | 42.8 ± 2.3 | 37.3–50.4 | 20.3 ± 1.2 | 16.5–24.0 | ||
| ZX-50 MC | 35.2 ± 1.3 | 32.5–40.6 | 0.632 ** | 23.6 ± 0.9 | 20.4–26.0 | 0.635 ** |
| Corrected ZX-50 MC | 42.5 ± 2.2 | 37.6–50.2 | 0.777 ** | 20.1 ± 1.2 | 16.5–22.7 | 0.756 ** |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.-L. Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding. Agronomy 2020, 10, 77. https://doi.org/10.3390/agronomy10010077
Jiang G-L. Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding. Agronomy. 2020; 10(1):77. https://doi.org/10.3390/agronomy10010077
Chicago/Turabian StyleJiang, Guo-Liang. 2020. "Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding" Agronomy 10, no. 1: 77. https://doi.org/10.3390/agronomy10010077
APA StyleJiang, G.-L. (2020). Comparison and Application of Non-Destructive NIR Evaluations of Seed Protein and Oil Content in Soybean Breeding. Agronomy, 10(1), 77. https://doi.org/10.3390/agronomy10010077




