Pyrolysis Improves the Effect of Straw Amendment on the Productivity of Perennial Ryegrass (Lolium perenne L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Plant Yields
2.3. Root Measurements
2.4. Statistics
3. Results
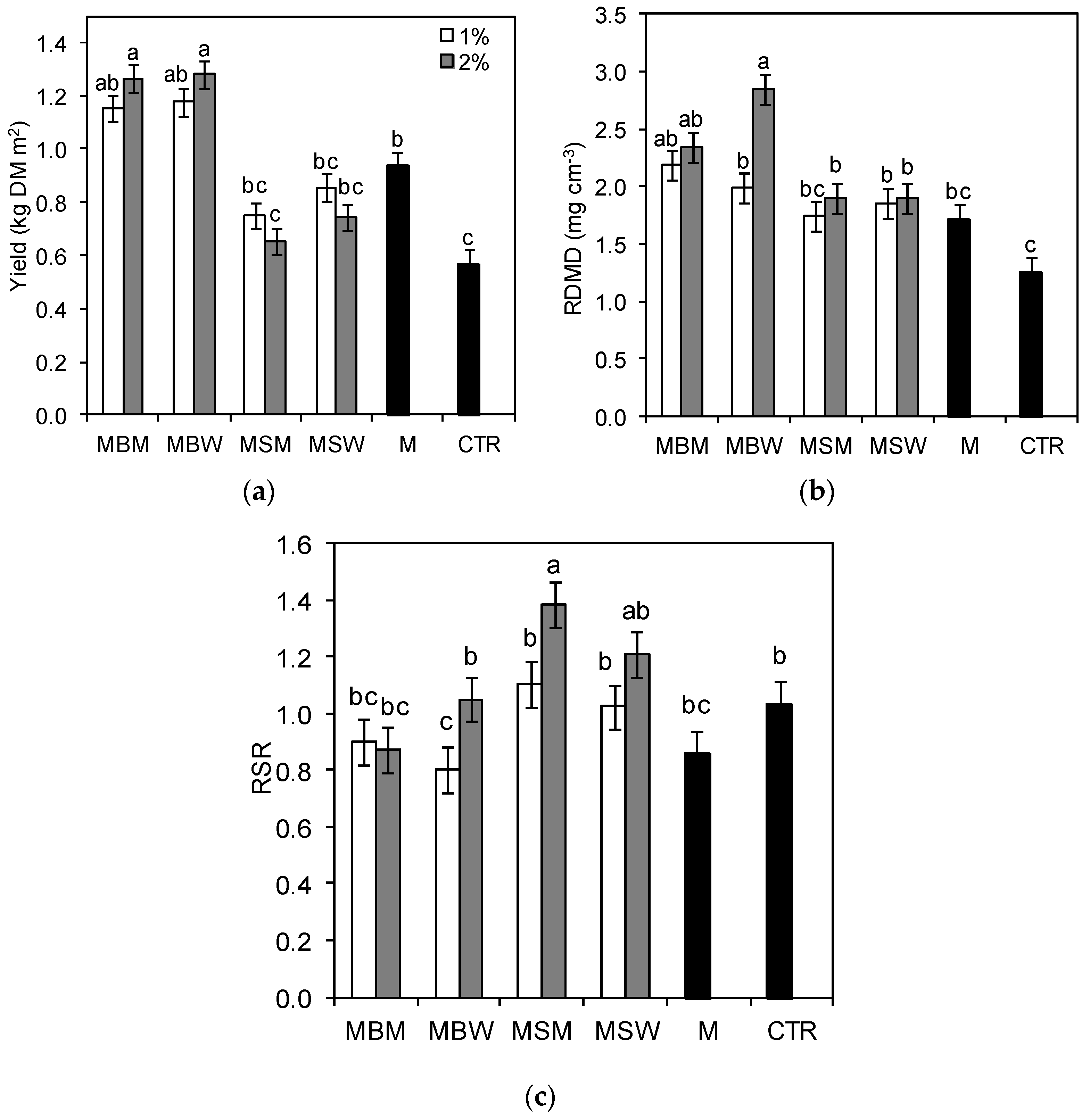
3.1. Biochar Effects on Perennial Ryegrass Yields
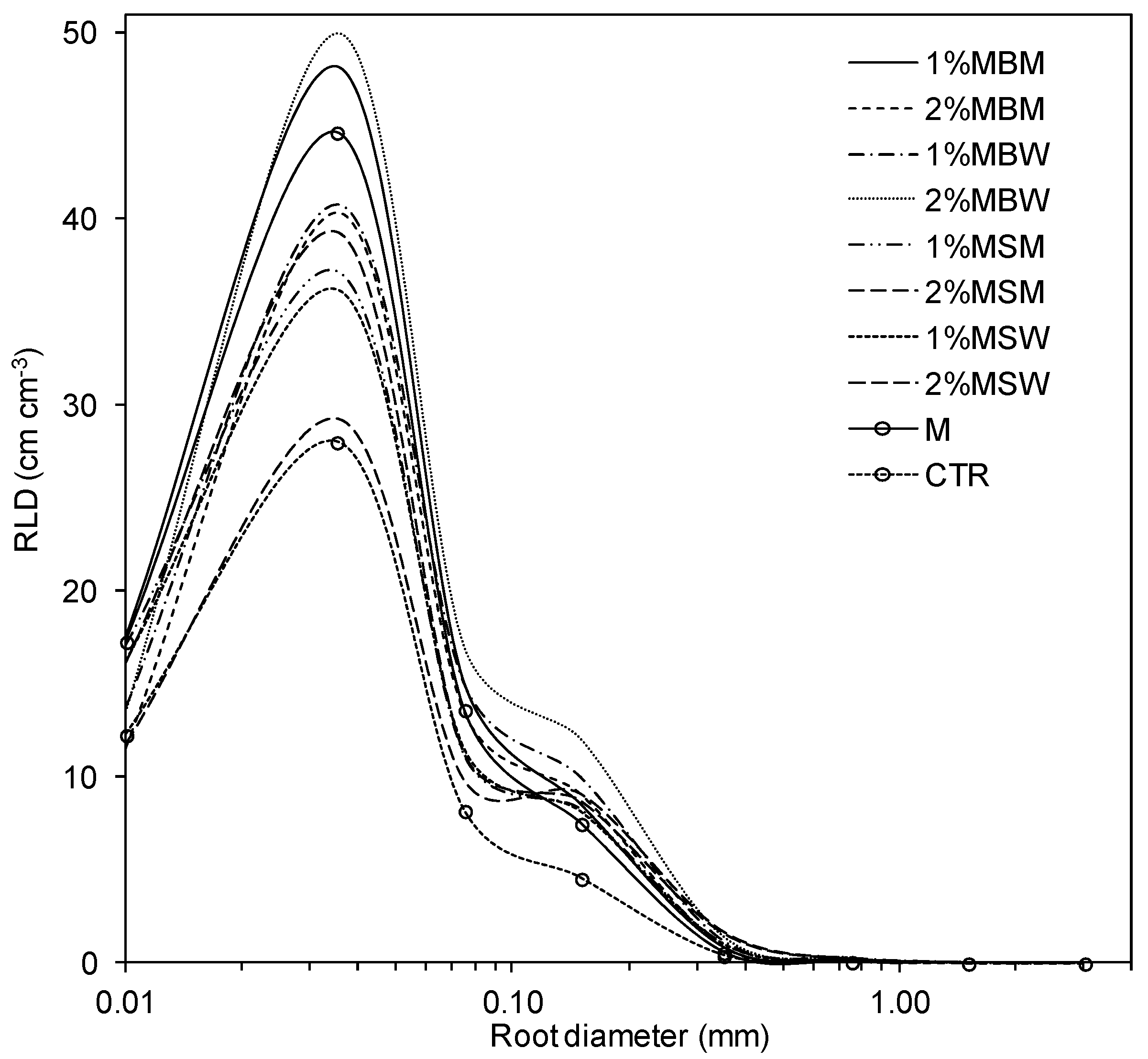
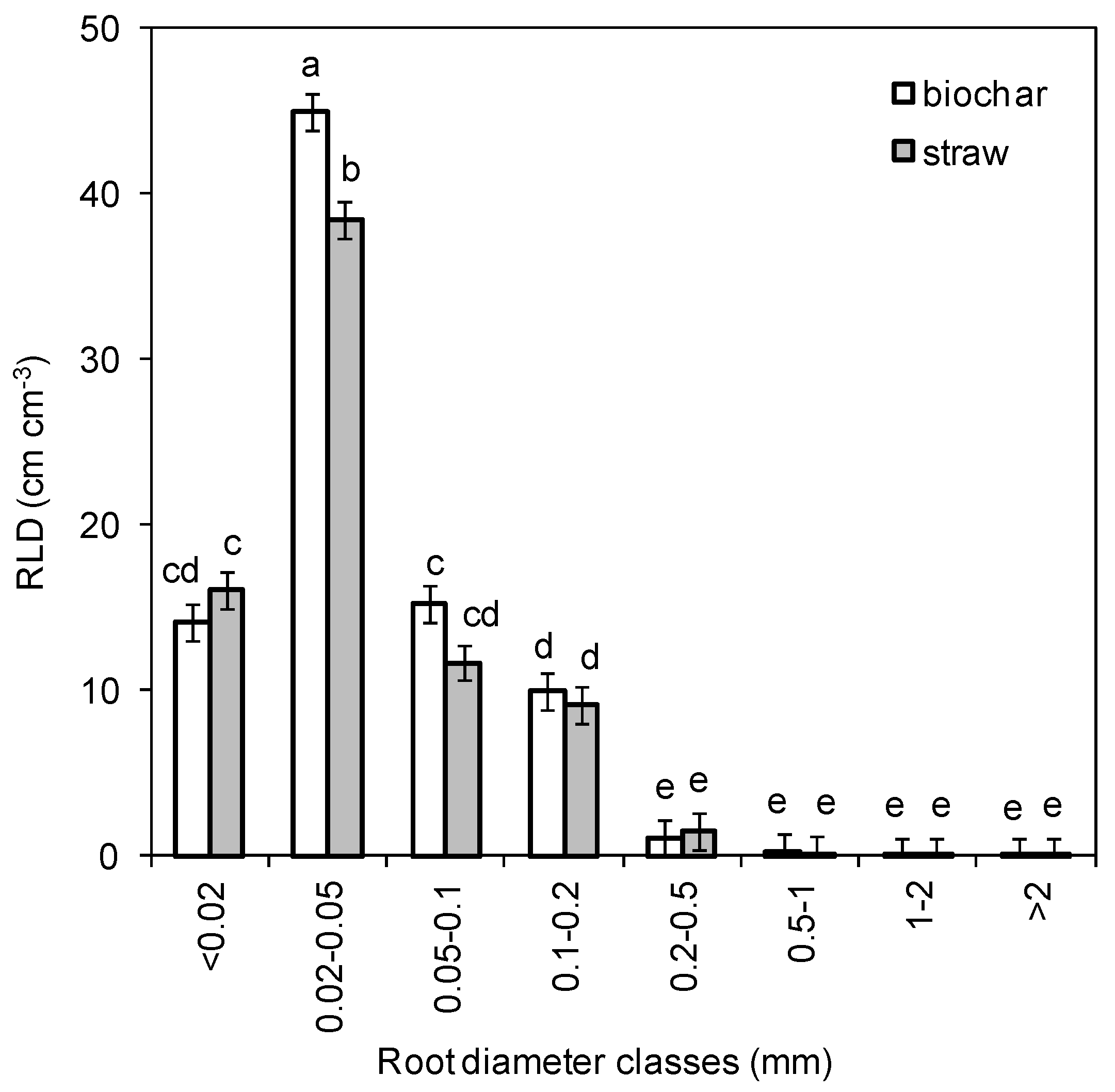
3.2. Root Morphometric Parameters
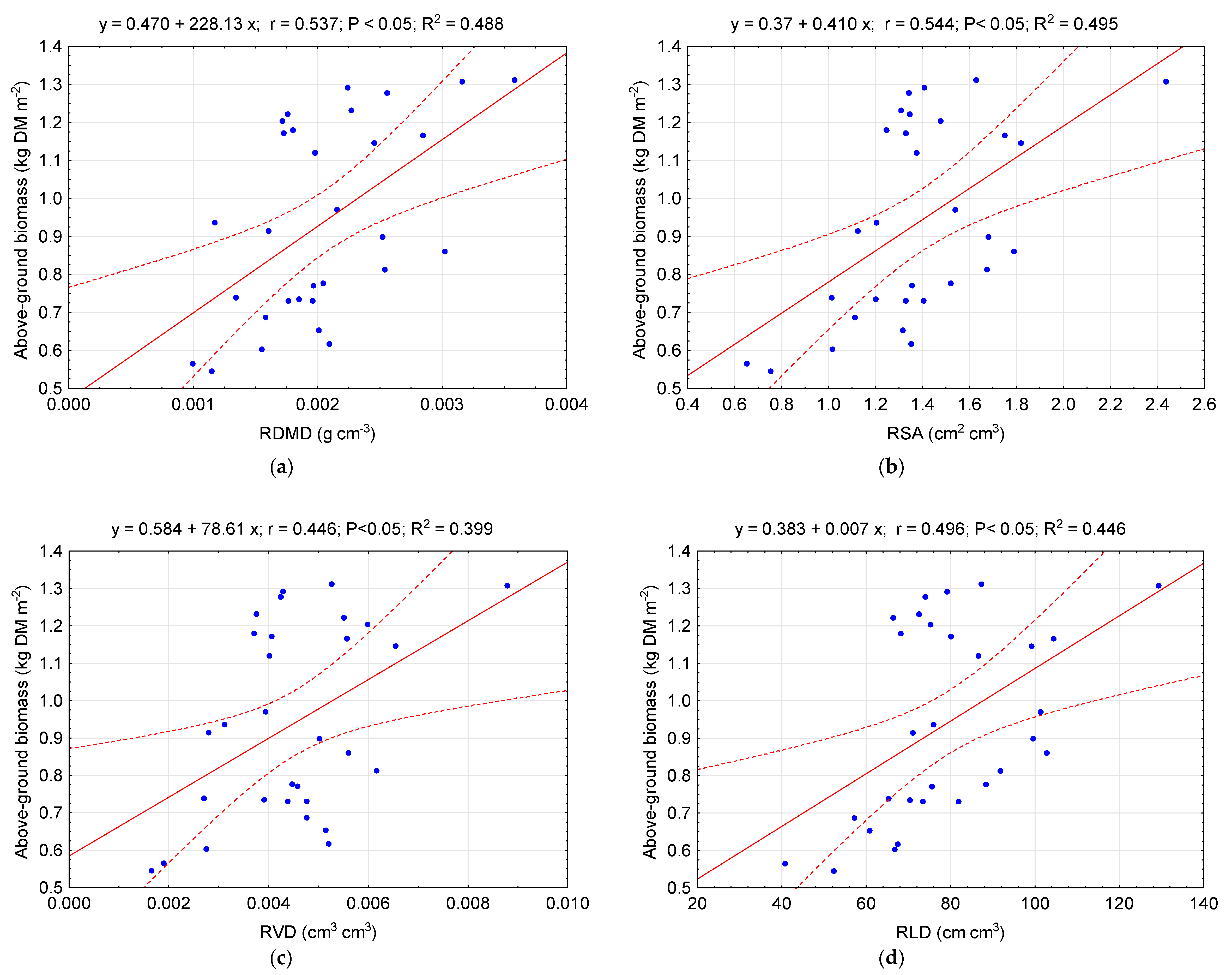
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Głąb, T.; Kulig, B. Effect of mulch and tillage system on soil porosity under wheat (Triticum aestivum). Soil Tillage Res. 2008, 99, 169–178. [Google Scholar] [CrossRef]
- Xu, J.; Han, H.; Ning, T.; Li, Z.; Lal, R. Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system. Field Crops Res. 2019, 233, 33–40. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Sun, Y.; Wu, D.; Meng, J.; Chen, W. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. Catena 2019, 173, 481–493. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Yu, Z.R.; Ma, Y.L. The effect of stubble return on agro-ecological system and crop growth. Chin. J. Soil Sci. 2001, 5, 209–213. [Google Scholar]
- Tan, D.S.; Jin, J.Y.; Huang, S.W.; Li, S.T.; He, P. Effect of long-term application of K fertilizer and wheat straw to soil on crop yield and soil K under different planting systems. Agric. Sci. China 2007, 40, 133–139. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Liu, X.; Zhao, X.; Lu, D.; Zhou, J.; Li, C. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Zhao, H.; Shar, A.G.; Li, S.; Chen, Y.; Shi, J.; Zhang, X.; Tian, X. Effect of straw return mode on soil aggregation and aggregate carbon content in an annual maize wheat double cropping system. Soil Tillage Res. 2018, 175, 178–186. [Google Scholar] [CrossRef]
- Wang, H.; Shen, M.; Hui, D.; Chen, J.; Sun, G.; Wang, X.; Lu, C.; Sheng, J.; Chen, L.; Luo, Y.; et al. Straw incorporation influences soil organic carbon sequestration, greenhouse gas emission, and crop yields in a Chinese rice (Oryza sativa L.)—wheat (Triticum aestivum L.) cropping system. Soil Tillage Res. 2019, 195, 104377. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Wei, T.; Yang, Z.; Jia, Z.; Yang, B.; Han, Q.; Ren, X. Effects of straw incorporation on the soil nutrient contents enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar]
- Zhang, F.; Zhang, W.; Li, M.; Yang, Y.; Li, F.M. Does long-term plastic film mulching really decrease sequestration of organic carbon in the Loess Plateau? Eur. J. Agron. 2017, 89, 53–60. [Google Scholar] [CrossRef]
- Kong, L. Maize residues, soil quality, and wheat growth in China. A review. Agron. Sustain. Dev. 2014, 34, 405–416. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Liang, L.; Zhao, Y.; Yang, B.; Ding, R.; Wang, J.; Nie, J. Changes in soil characteristics and maize yield under straw returning system in dryland farming. Field Crops Res. 2018, 218, 11–17. [Google Scholar] [CrossRef]
- He, L.; Zhong, Z.; Yang, H. Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Han, Y.; Yao, S.H.; Jiang, H.; Ge, X.; Zhang, Y.; Mao, J.; Dou, S.; Zhang, B. Effects of mixing maize straw with soil and placement depths on decomposition rates and products at two cold sites in the mollisol region of China. Soil Tillage Res. 2020, 197, 104519. [Google Scholar] [CrossRef]
- Xia, L.L.; Wang, S.W.; Yan, X.Y. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice-wheat cropping system in China. Agric. Ecosyst. Environ. 2014, 197, 118–127. [Google Scholar] [CrossRef]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560. [Google Scholar] [CrossRef]
- Peake, L.; Freddo, A.; Reid, B.J. Sustaining Soils and Mitigating Climate Change Using Biochar. In Sustainability Science and Technology; De Las Heras, A., Ed.; Taylor&Francis Group, CRC Press: Cleveland, OH, USA, 2014; pp. 109–126. [Google Scholar]
- Wang, X.; Zhou, W.; Liang, G.; Song, D.; Zhang, X. Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci. Total Environ. 2015, 538, 137–144. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci. Total Environ. 2014, 487, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Purakayastha, T.J.; Das, K.C.; Gaskin, J.; Harris, K.; Smith, J.L.; Kumari, S. Effect of pyrolysis temperatures on stability and priming effects of C3 and C4 biochars applied to two different soils. Soil Tillage Res. 2016, 155, 107–115. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For. Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar Impacts on Soil Physical Properties and Greenhouse Gas Emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Herath, H.M.S.K.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Pranagal, J.; Oleszczuk, P.; Tomaszewska-Krojańska, D.; Kraska, P.; Różyło, K. Effect of biochar application on the physical properties of Haplic podzol. Soil Tillage Res. 2017, 174, 92–103. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Bass, A.M.; Bird, M.I.; Kay, G.; Muirhead, B. Soil properties, greenhouse gas emissions and crop yield under compost, biochar and co-composted biochar in two tropical agronomic systems. Sci. Total Environ. 2016, 550, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Mróz, T. The influence of biochar enriched with magnesium and sulfur on the amount of perennial ryegrass biomass and selected chemical properties and biological of sandy soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1257–1265. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Well, R.; Shan, J.; Yan, X.; Bol, R.; Senbayram, M. Straw amendment with nitrate-N decreased N2O/(N2O+N2) ratio but increased soil N2O emission: A case study of direct soil-born N2 measurements. Soil Biol. Biochem. 2018, 127, 301–304. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Clay, S.A.; Krack, K.K.; Bruggeman, S.A.; Papiernik, S.; Schumacher, T.E. Maize, switchgrass, and ponderosa pine biochar added to soil increased herbicide sorption and decreased herbicide efficacy. J. Environ. Sci. Health 2016, 51, 497–507. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Głąb, T.; Żabiński, A.; Sadowska, U.; Gondek, K.; Kopeć, M.; Mierzwa-Hersztek, M.; Tabor, S. Effects of co-composted maize, sewage sludge, and biochar mixtures on hydrological and physical qualities of sandy soil. Geoderma 2018, 315, 27–35. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.J.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.J.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251, 47–54. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils: A Critical Scientific Review on Effects on Soil Properties, Processes and Functions; Joint Research Centre (JRC) Scientific and Technical Report; Office for the Official Publications of the European Communities: Luxemberg, 2010. [Google Scholar]
- Paz-Ferreiro, J.; Fu, S.L.; Mendez, A.; Gasco, G. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J. Soils Sediments 2017, 14, 483–494. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Jha, P.; Biswas, A.K.; Lakaria, B.L.; Rao, A.S. Biochar in agriculture-prospects and related implications. Curr. Sci. 2010, 99, 1218–1225. [Google Scholar]
- Abbruzzini, T.F.; Davies, C.A.; Toledo, F.H.; Cerri, C.A.P. Dynamic biochar effects on nitrogen use efficiency, crop yield and soil nitrous oxide emissions during a tropical wheat-growing season. J. Environ. Manag. 2019, 252, 109638. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Fan, L.; Jiang, L.; Yang, S.; Zou, Y.; Uphoff, N. Continuous applications of biochar to rice: Effects on grain yield and yield attributes. J. Integr. Agric. 2019, 18, 563–570. [Google Scholar] [CrossRef]
- Olmo, M.; Lozano, A.M.; Barrón, V.; Villar, R. Spatial heterogeneity of soil biochar content affects soil quality and wheat growth and yield. Sci. Total Environ. 2016, 562, 690–700. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Bonin, C.L.; Fidel, R.B.; Banik, C.; Laird, D.A.; Mitchell, R.; Heaton, E.A. Perennial biomass crop establishment, community characteristics, and productivity in the upper US Midwest: Effects of cropping systems seed mixtures and biochar applications. Eur. J. Agron. 2018, 101, 121–128. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237, 105–116. [Google Scholar] [CrossRef]
- Van de Voorde, T.F.J.; Bezemer, T.M.; Van Groenigen, J.W.; Jeffery, S.; Mommer, L. Soil biochar amendment in a nature restoration area: Effects on plant productivity and community composition. Ecol. Appl. 2014, 2, 1167–1177. [Google Scholar] [CrossRef]
- Adams, M.; Benjamin, T.; Emery, N.; Brouder, S.; Gibson, K.D. The effect of biochar on native and invasive prairie plant species. Invasive Plant Sci. Manag. 2013, 6, 197–202. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Głąb, T.; Szewczyk, W. The effect of traffic on turfgrass root morphological features. Sci. Hortic. 2015, 197, 542–554. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Głąb, T.; Strojny, J. The Key Role of Variety and Method of Sowing Selection in Pea Roots’ Parameters Development under Sustainable Practice. Sustainability 2019, 11, 1824. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That is Used in Soil. Final Report; Report No. IBISTD-2.0; Washington. 2014. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 2 September 2020).
- Gondek, K.; Mierzwa-Hersztek, M. Effect of low-temperature biochar derived from pig manure and poultry litter on mobile and organic matter-bound forms of Cu, Cd, Pb and Zn in sandy soil. Soil Use Manag. 2016, 32, 357–367. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Mendez, A.; Terradillos, M.; Gasco, G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Gondek, K.; Baran, A.; Kopeć, M. The effect of low-temperature transformation of mixtures of sewage sludge and plant material on content, leachability and toxicity of heavy metals. Chemosphere 2014, 117, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Domene, X.; Enders, A.; Hanley, K.; Lehmann, J. Ecotoxicological characterization of biochars: Role of feedstock and pyrolysis temperature. Sci. Total Environ. 2015, 512, 552–561. [Google Scholar] [CrossRef]
- Smucker, A.J.M.; McBurney, S.L.; Srivastava, A.K. Quantitative separation of roots from compacted soil profiles by the hydropneumatic elutriation system. Agron. J. 1982, 74, 500–503. [Google Scholar] [CrossRef]
- Bauhus, J.; Messier, C. Evaluation of fine root length and diameter measurements obtained using RHIZO image analysis. Agron. J. 1999, 91, 142–147. [Google Scholar] [CrossRef]
- Hopkins, A.; Murray, P.J.; Bowling, P.J.; Rook, A.J.; Johnson, J. Productivity and nitrogen uptake of ageing and newly sown swards of perennial ryegrass (Lolium perenne L.) at different sites and with different nitrogen fertilizer treatments. Eur. J. Agron. 1995, 4, 65–75. [Google Scholar] [CrossRef]
- Huang, S.; Zeng, Y.; Wu, J.; Shi, Q.; Pan, X. Effect of crop residue retention on rice yield in China: A meta-analysis. Field Crops Res. 2013, 154, 188–194. [Google Scholar] [CrossRef]
- Cui, S.; Liang, S.; Zhang, X.; Li, Y.; Liang, W.; Sun, L.; Wang, J.; Bezemer, M.T.; Li, Q. Long-term fertilization management affects the C utilization from crop residues by the soil micro-food web. Plant Soil. 2018, 429, 335–348. [Google Scholar]
- Yang, H.; Wu, G.; Mo, P.; Chen, S.; Wang, S.; Xiao, Y.; Ma, H.; Wen, T.; Guo, X.; Fan, G. The combined effects of maize straw mulch and no-tillage on grain yield and water and nitrogen use efficiency of dry-land winter wheat (Triticum aestivum L.). Soil Tillage Res. 2020, 197, 104485. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Tang, Y.; Wu, X.; Wu, C.; Huang, G.; Zeng, H. Stand establishment, root development and yield of winter wheat as affected by tillage and straw mulch in the water deficit hilly region of southwestern China. J. Integr. Agric. 2016, 15, 1480–1489. [Google Scholar] [CrossRef]
- Xu, X.; Pang, D.; Chen, J.; Luo, Y.; Zheng, M.; Yin, Y.; Li, Y.; Li, Y.; Wang, Z. Straw return accompany with low nitrogen moderately promoted deep root. Field Crops Res. 2018, 221, 71–80. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.H.; Park, J.H.; Seo, D.C.; Ok, Y.S.; Cho, J.S. Effect of biochar derived from barley straw on soil physicochemical properties, crop growth, and nitrous oxide emission in an upland field in South Korea. Environ. Sci. Pollut. Res. 2018, 25, 25813–25821. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; del Campillo, M.; Gallardo, A.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475–484. [Google Scholar] [CrossRef]
- Mclennon, E.; Solomon, J.K.Q.; Neupane, D.; Davison, J. Biochar and nitrogen application rates effect on phosphorus removal from a mixed grass sward irrigated with reclaimed wastewater. Sci. Total Environ. 2020, 715, 137012. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Sun, H.; Lü, F.; He, P. Three-year rice grain yield responses to coastal mudflat soil properties amended with straw biochar. J. Environ. Manag. 2019, 239, 23–29. [Google Scholar] [CrossRef]
- Madari, B.E.; Silva, M.A.S.; Carvalho, M.T.M.; Maia, A.H.N.; Petter, F.A.; Santos, J.L.S.; Tsai, S.M.; Leal, W.G.O.; Zeviani, W.M. Properties of a sandy clay loam Haplic Ferralsol and soybean grain yield in a five-year field trial as affected by biochar amendment. Geoderma 2017, 305, 100–112. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Enquist, B.J. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Bonifas, K.D.; Walters, D.T.; Cassman, K.G.; Lindquist, J.L. Nitrogen supply affects root:Shoot ratio in corn and velvetleaf (Abutilon theophrasti). Weed Sci. 2005, 53, 670–675. [Google Scholar] [CrossRef]
- Agren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]

| pH (H2O) | 5.67 ± 0.05 | |
| Soil organic carbon | g kg−1 | 6.43 ± 0.08 |
| Total N | g kg−1 | 0.54 ± 0.01 |
| P | mg kg−1 | 188 ± 11 |
| K | mg kg−1 | 305 ± 29 |
| Ca | mg kg−1 | 207 ± 17.9 |
| Mg | mg kg−1 | 236 ± 12.5 |
| Electrical conductivity | µS cm−1 | 32.2 ± 4.35 |
| Solid particle density | g cm−3 | 2.65 ± 0.06 |
| Bulk density | g cm−3 | 1.78 ± 0.04 |
| Sand | g kg−1 | 850 |
| Silt | g kg−1 | 90 |
| Clay | g kg−1 | 60 |
| Parameter | Unit | Triticum aestivum L | Miscanthus × giganteus | ||
|---|---|---|---|---|---|
| Straw | Biochar | Straw | Biochar | ||
| pH (H2O) | 5.84 ± 0.15 | 6.52 ± 0.60 | 6.18 ± 0.43 | 6.28 ± 0.42 | |
| EC | µS cm−1 | 4.48 ± 0.21 | 378 ± 21 | 3.23 ± 0.45 | 345 ± 18 |
| DM | g kg−1 | 952 ± 0.2 | 966 ± 2 | 947 ± 0.3 | 977 ± 1 |
| Ash | g·kg−1 | 59 ± 2 | 134 ± 5 | 54 ± 1 | 87 ± 3 |
| Total C | g·kg−1 | 441 ± 2 | 628 ± 2 | 456 ± 2 | 651 ± 6 |
| Total N | g·kg−1 | 7.16 ± 0.32 | 12.4 ± 0.36 | 3.97 ± 0.29 | 7.31 ± 0.09 |
| K | g·kg−1 | 4.95 ± 0.66 | 11.9 ± 0.29 | 1.33 ± 0.06 | 2.81 ± 0.17 |
| P | g·kg−1 | 1.04 ± 0.05 | 1.17 ± 0.04 | 0.73 ± 0.04 | 0.94 ± 0.06 |
| Treatment | 2017 | 2018 | Annual Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Cut | 2nd Cut | 3rd Cut | 1st Cut | 2nd Cut | 3rd Cut | 1st Cut | 2nd Cut | 3rd Cut | |
| 1% MBM | 0.396 | 0.319 | 0.200 | 1.075 b | 0.194 | 0.120 | 0.735 | 0.256 | 0.160 |
| 2% MBM | 0.367 | 0.410 | 0.210 | 1.197 ab | 0.218 | 0.129 | 0.782 | 0.314 | 0.169 |
| 1% MBW | 0.379 | 0.394 | 0.228 | 1.048 b | 0.148 | 0.154 | 0.713 | 0.271 | 0.191 |
| 2% MBW | 0.362 | 0.337 | 0.277 | 1.224 a | 0.230 | 0.129 | 0.793 | 0.283 | 0.203 |
| 1% MSM | 0.131 | 0.238 | 0.124 | 0.838 de | 0.065 | 0.103 | 0.484 | 0.151 | 0.113 |
| 2% MSM | 0.066 | 0.189 | 0.087 | 0.811 ef | 0.038 | 0.110 | 0.439 | 0.113 | 0.099 |
| 1% MSW | 0.149 | 0.166 | 0.257 | 0.921 c | 0.091 | 0.128 | 0.535 | 0.128 | 0.193 |
| 2% MSW | 0.055 | 0.058 | 0.283 | 0.864 d | 0.099 | 0.129 | 0.459 | 0.078 | 0.206 |
| M | 0.341 | 0.310 | 0.198 | 0.877 d | 0.070 | 0.083 | 0.609 | 0.190 | 0.140 |
| CTR | 0.050 | 0.112 | 0.069 | 0.794 f | 0.048 | 0.068 | 0.422 | 0.080 | 0.068 |
| Means for feedstock species × material interaction | |||||||||
| MBM | 0.381 | 0.364 a | 0.205 b | 1.136 a | 0.206 a | 0.124 | 0.759 a | 0.285 | 0.165 |
| MBW | 0.370 | 0.365 a | 0.252 a | 1.136 a | 0.189 a | 0.142 | 0.753 a | 0.277 | 0.197 |
| MSM | 0.098 | 0.213 b | 0.105 c | 0.825 c | 0.051 c | 0.107 | 0.461 c | 0.132 | 0.106 |
| MSW | 0.102 | 0.112 c | 0.270 a | 0.893 b | 0.095 b | 0.128 | 0.497 b | 0.103 | 0.199 |
| Means for amendments rate | |||||||||
| 1% | 0.263 a | 0.279 | 0.202 | 0.970 b | 0.124 b | 0.127 | 0.617 | 0.202 | 0.164 |
| 2% | 0.213 b | 0.249 | 0.214 | 1.024 a | 0.146 a | 0.124 | 0.618 | 0.197 | 0.169 |
| Means for feedstock species | |||||||||
| Miscanthus | 0.240 | 0.289 a | 0.155 b | 0.980 b | 0.129 | 0.116 b | 0.610 | 0.209 a | 0.135 b |
| Wheat | 0.236 | 0.239 b | 0.261 a | 1.014 a | 0.142 | 0.135 a | 0.625 | 0.190 b | 0.198 a |
| Means for amendment material | |||||||||
| Biochar | 0.376 a | 0.365 a | 0.229 a | 1.136 a | 0.197 a | 0.133 | 0.756 a | 0.281 a | 0.181 a |
| Straw | 0.100 b | 0.162 b | 0.188 b | 0.859 b | 0.073 b | 0.118 | 0.479 b | 0.118 b | 0.153 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głąb, T.; Gondek, K.; Mierzwa-Hersztek, M. Pyrolysis Improves the Effect of Straw Amendment on the Productivity of Perennial Ryegrass (Lolium perenne L.). Agronomy 2020, 10, 1455. https://doi.org/10.3390/agronomy10101455
Głąb T, Gondek K, Mierzwa-Hersztek M. Pyrolysis Improves the Effect of Straw Amendment on the Productivity of Perennial Ryegrass (Lolium perenne L.). Agronomy. 2020; 10(10):1455. https://doi.org/10.3390/agronomy10101455
Chicago/Turabian StyleGłąb, Tomasz, Krzysztof Gondek, and Monika Mierzwa-Hersztek. 2020. "Pyrolysis Improves the Effect of Straw Amendment on the Productivity of Perennial Ryegrass (Lolium perenne L.)" Agronomy 10, no. 10: 1455. https://doi.org/10.3390/agronomy10101455
APA StyleGłąb, T., Gondek, K., & Mierzwa-Hersztek, M. (2020). Pyrolysis Improves the Effect of Straw Amendment on the Productivity of Perennial Ryegrass (Lolium perenne L.). Agronomy, 10(10), 1455. https://doi.org/10.3390/agronomy10101455







