Potential of Biochar to Alternate Soil Properties and Crop Yields 3 and 4 Years after the Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Description of the Field Experiment
2.2. Soil Sampling and Analysis
2.3. Plant Sampling
2.4. Statistical Analysis
3. Results and Discussion
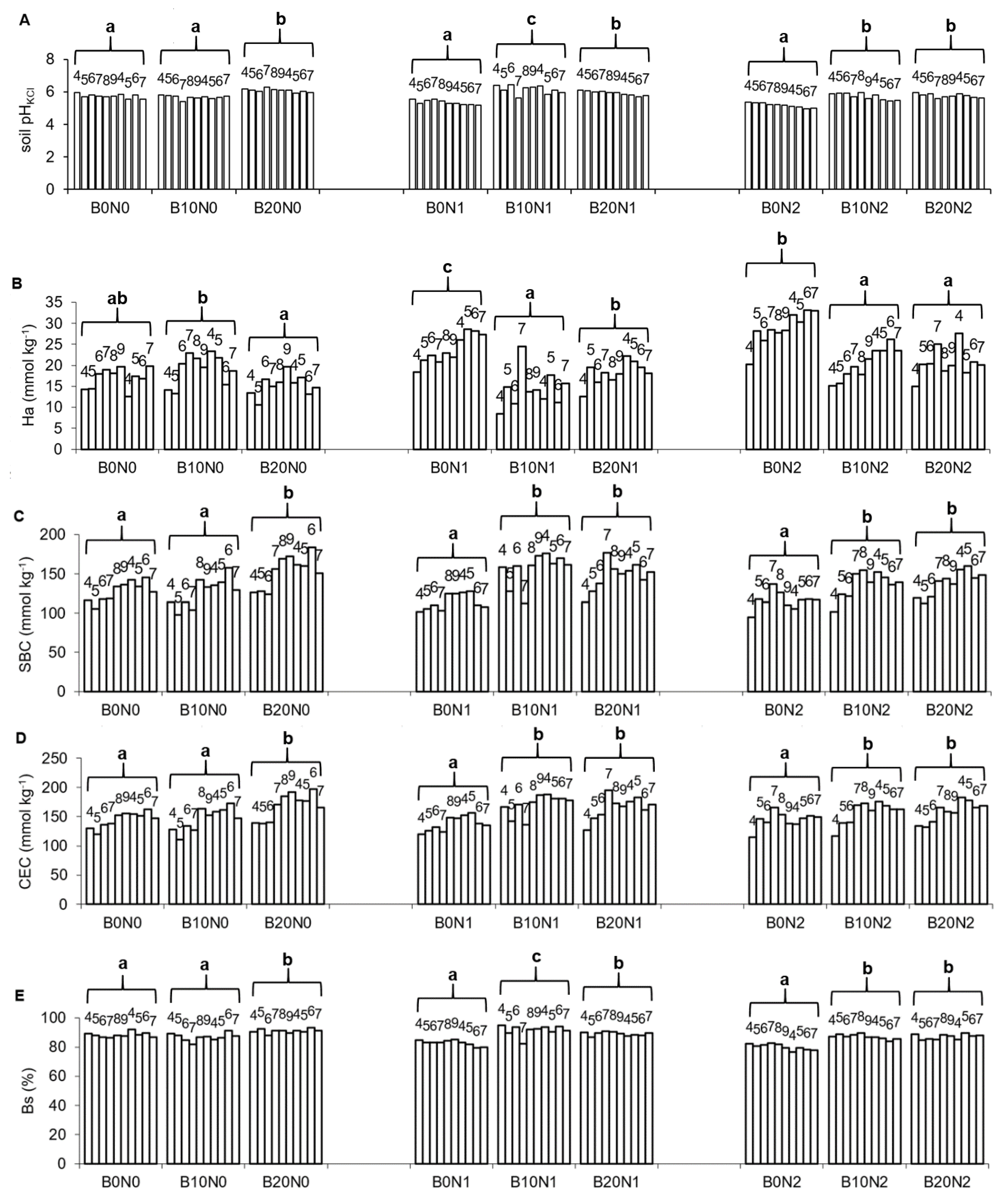
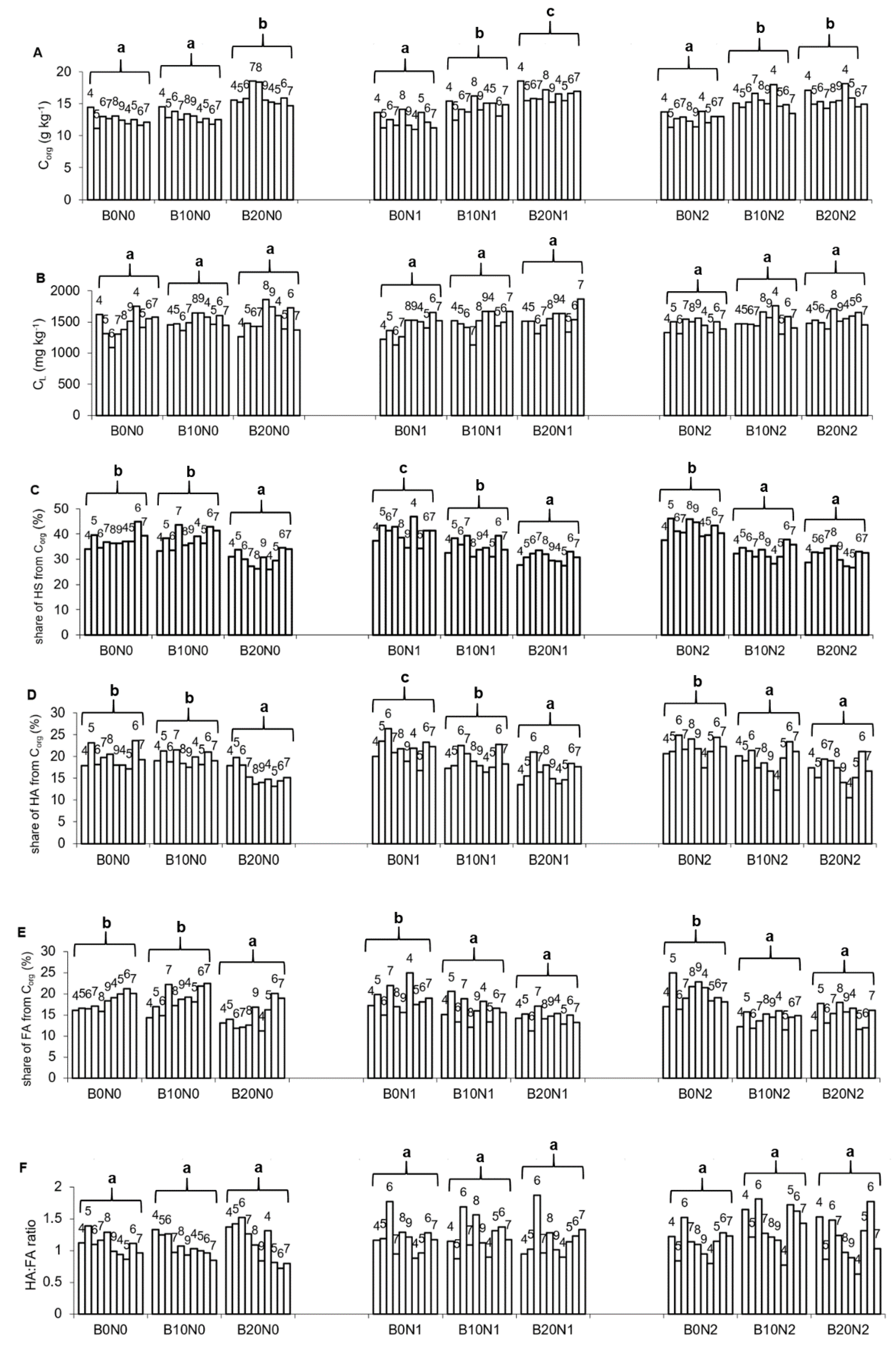
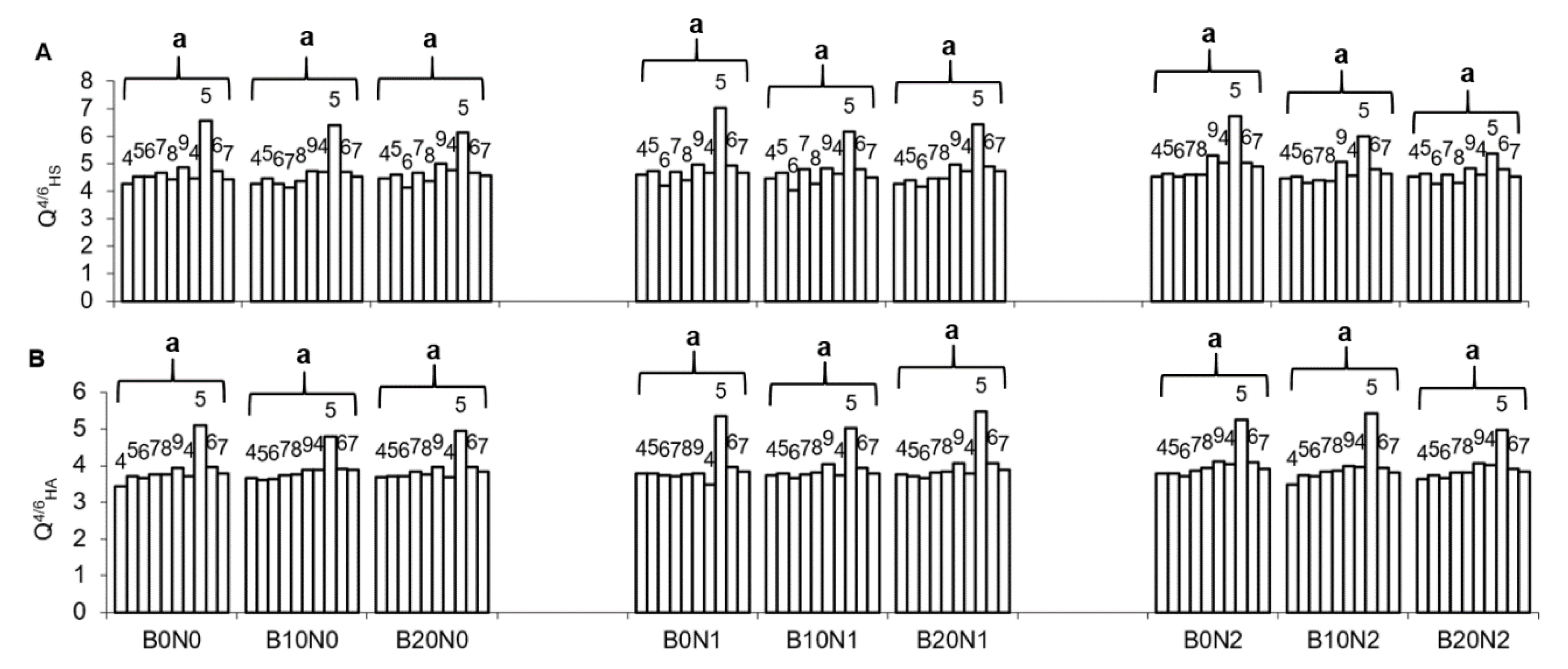
3.1. Soil Properties
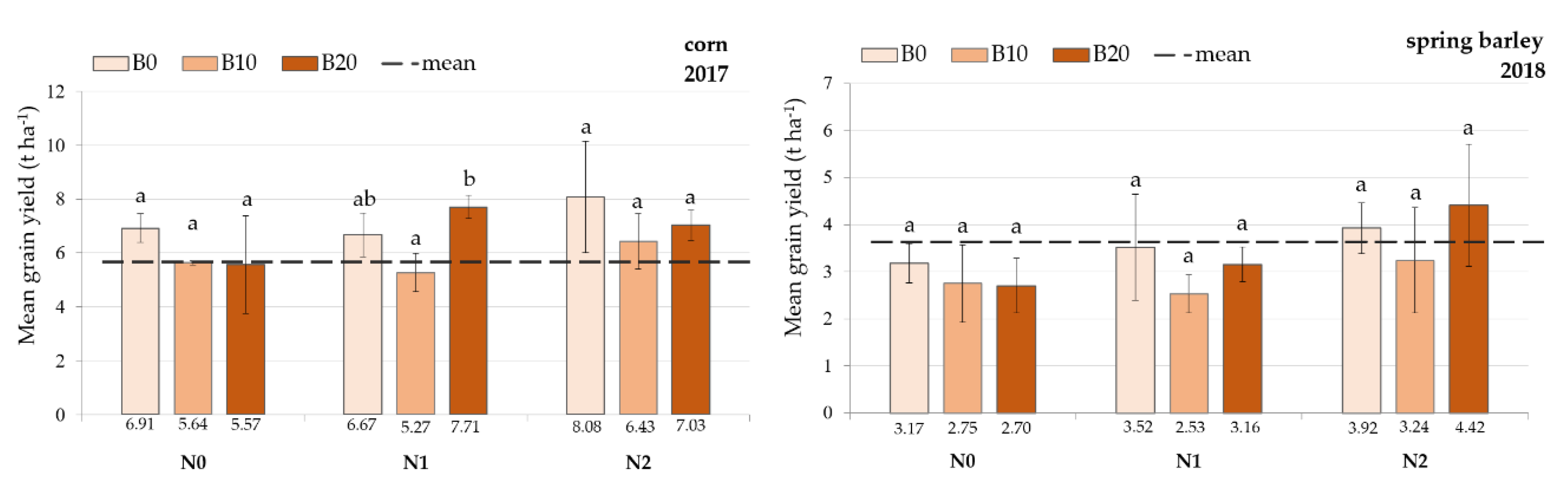
3.2. Crop Yields
3.3. Relation of Crop Yield of Maize and Spring Barley to Changes of Soil Properties
3.4. The Effect of Soil Amendments on Crop Yield of Maize and Spring Barley
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scherr, S.J.; Yadav, S. Land Degradation in the Developming World: Implications for Food, Agriculture, and the Environment to 2020. In Food, Agriculture, And The Environment, Discussion Paper 14; IFPRI: Washington, DC, USA, 1996; pp. 1–3. [Google Scholar]
- Wani, S.P.; Chander, G.; Pardhasaradhi, G. Soil Amendments for Sustainable Intensification. In Soil Amendments for Sustainability, Challenges and Perspectives; Taylor and Francis Group: Boca Raton, FL, USA, 2019; pp. 3–17. [Google Scholar]
- Curaqueo, G.; Meier, S.; Khan, N.; Cea, M.; Navia, R. Use of biochar on two volcanic soils: Effects on soil properties and barley yield. J. Soil Sci. Plant Nutr. 2014, 14, 911–924. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de ’ındio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Merino, A.; Pereira, P. Ash and fire, char, and biochar in the environment. Land Degrad. Dev. 2018, 29, 2040–2044. [Google Scholar] [CrossRef]
- De Jesus Duarte, S.; Glaser, B.; Pellegrino Cerri, C. Effect of biochar particle size on physical, hydrological and chemical properties of loamy and sandy tropical soils. Agronomy 2019, 9, 165. [Google Scholar] [CrossRef]
- Tammeorg, P.; Bastos, A.C.; Jeffery, S.; Rees, F.; Kern, J.; Graber, E.R.; Ventura, M.; Kibblewhite, M.; Amaro, A.; Budai, A.; et al. Biochars in soils: Towards the required level of scientific understanding. J. Environ. Eng. Landsc. Manag. 2017, 25, 192–207. [Google Scholar] [CrossRef]
- Horák, J.; Kondrlová, E.; Igaz, D.; Šimanský, V.; Felber, R.; Lukac, M.; Balashov, E.; Buchkina, N.; Rizhiya, E.Y.; Jankowski, M. Biochar and biochar with N–fertilizer affect soil N2O emission in Haplic Luvisol. Biologia 2017, 72, 9. [Google Scholar] [CrossRef]
- Igaz, D.; Šimanský, V.; Horák, J.; Kondrlová, E.; Domanová, J.; Rodný, M.; Buchkina, N.P. Can a single dose of biochar affect selected soil physical and chemical characteristics? J. Hydrol. Hydromech. 2018, 66, 421–428. [Google Scholar] [CrossRef]
- Hardy, B.; Cornelis, J.T.; Houben, D.; Leifeld, J.; Lambert, R.; Dufey, J.E. Evaluation of the long-term effect of biochar on properties of temperate agricultural soil at pre-industrial charcoal kiln sites in Wallonia, Belgium: Effect of pre-industrial charcoal kilns on agricultural soil properties. Eur. J. Soil Sci. 2017, 68, 80–89. [Google Scholar] [CrossRef]
- Lone, A.H.; Najar, G.R.; Ganie, M.A.; Sofi, J.A.; Ali, T. Biochar for sustainable soil health: A review of prospects and concerns. Pedosphere 2015, 25, 639–653. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinkelebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Horák, J.; Šimanský, V.; Igaz, D.; Juriga, M.; Aydin, E.; Lukac, M. Biochar: An important component ameliorating the productivity of intensively used soils—Review. Pol. J. Environ. Stud. 2020, 29, 2995–3001. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.M.S.K.; Xi, Y.G.; Xiao, X.J. Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag. 2014, 30, 119–128. [Google Scholar] [CrossRef]
- Šimanský, V.; Horák, J.; Igaz, D.; Jonczak, J.; Markiewicz, M.; Felber, R.; Rizhiya, E.Y.; Lukac, M. How dose of biochar and biochar with nitrogen can improve the parameters of soil organic matter and soil structure? Biologia 2016, 71, 989–995. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Baronti, S.; Alberti, G.; Vedove, G.D.; Gennaro, F.D.; Fellet, G.; Genesio, L.; Miglietta, F.; Peressotti, A.; Vaccari, F.P. The biochar option to improve plant yields: First results from some field and pot experiments in Italy. Ital. J. Agron. 2010, 5, 3–11. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Subedi, R.; Bertora, C.; Zavattaro, L.; Grignani, C. Crop response to soils amended with biochar: Expected benefits and unintended risks. Ital. J. Agron. 2017, 12, 161–173. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of biochar properties on soil conditions and agricultural sustainability: A review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties – New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef] [PubMed]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Bai, N.; Zhang, H.; Li, S.; Zheng, X.; Zhang, J.; Zhang, H.; Zhou, S.; Sun, H.; Lv, W. Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice—Wheat rotation system. PeerJ 2019, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, G.; Tewolde, H. Field Application of Biochar: Long Term Effect on the Physical and Hydraulic Properties of a Silty Loam Soil. In Proceedings of the ASA-CSSA-SSSA International Annual Meetings, San Antonio, TX, USA, 10–13 November 2019; Available online: https://scisoc.confex.com/scisoc/2019am/meetingapp.cgi/Paper/119406 (accessed on 29 April 2020).
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Qi, X.; Li, Z.; Nan, J.; Deng, J. The effects of biochar and hoggery biogas slurry on fluvo-aquic soil physical and hydraulic properties: A field study of four consecutive wheat–maize rotations. J. Soils Sediments 2016, 16, 2050–2058. [Google Scholar] [CrossRef]
- Altdorff, D.; Galagedara, L.; Abedin, J.; Unc, A. Effect of biochar application rates on the hydraulic properties of an agricultural-use boreal podzol. Soil Syst. 2019, 3, 53. [Google Scholar] [CrossRef]
- Karer, J.; Wimmer, B.; Zehetner, F.; Kloss, S.; Soja, G. Biochar application to temperate soils: Effects on nutrient uptake and crop yield under field conditions. Agric. Food Sci. 2013, 22, 390–403. [Google Scholar] [CrossRef]
- Šiška, B.; Špánik, F.; Repa, Š.; Gálik, M. Praktická Biometeorológia [Practical Biometeorology]; Slovenská Poľnohospodárska Univerzita: Nitra, Slovakia, 2005. (In Slovak) [Google Scholar]
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Kondrlova, E.; Horak, J.; Igaz, D. Effect of biochar and nutrient amendment on vegetative growth of spring barley (Hordeum vulgare L. var. Malz). Aust. J. Crop Sci. 2018, 12, 178–184. [Google Scholar] [CrossRef]
- Horák, J. Testing biochar as a possible way to ameliorate slightly acidic soil at the research field located in the danubian lowland. Acta Hortic. Reg. 2015, 18, 20–24. [Google Scholar] [CrossRef]
- Hrivňáková, K.; Makovníková, J.; Barančíková, G.; Bezák, P.; Bezáková, Z.; Dodok, R.; Grečo, V.; Chlpík, J.; Kobza, J.; Lištjak, M.; et al. Uniform Methods Of Soil Analyses; VÚPOP: Bratislava, Slovakia, 2011. [Google Scholar]
- Dziadowiec, H.; Gonet, S. Przewodnik Metodyczny do Badań Materii Organicznej Gleb [Methodological Guidebook For The Organic Matter Researches]; Prace Komisji Naukowych Polskiego Towarzystwa Naukowego 120: Warszawa, Poland, 1999; pp. 31–34. (In Polish) [Google Scholar]
- Walkley, A. An examination of methods for determining organic carbon and nitrogen in soils. With one text-figure. J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Loginow, W.; Wisniewski, W.; Gonet, S.S.; Ciescinska, B. Fractionation of organic carbon based on susceptibility to oxidation. Pol. J. Soil Sci. 1987, 20, 47–52. [Google Scholar]
- AHDB. Barley Growth Guide. Available online: https://projectblue.blob.core.windows.net/media/Default/Imported%20Publication%20Docs/Barley%20growth%20guide%20130718.pdf (accessed on 27 April 2020).
- Ghosh, S.; Ow, L.F.; Yusof, M.L. Biochar Application for Sustainable Soil Quality. A Case sTudy in Tropical Urban Environment. In Soil Amendments For Sustainability, Challenges And Perspectives; Taylor and Francis Group: Boca Raton, FL, USA, 2019; pp. 313–319. [Google Scholar]
- Kováčik, P.; Ryant, P. Agrochémia, Princípy A Prax [Agrochemistry, Principles And Practice]; SPU: Nitra, Slovakia, 2019. (In Slovak) [Google Scholar]
- Roy, R.N.; Finck, A.; Blair, G.J.; Tandon, H.L.S. Plant Nutrition for Food Security. A Guide for Integrated Nutrient Management. Available online: http://www.fao.org/3/a-a0443e.pdf (accessed on 18 June 2020).
- Chodak, M.; Pietrzykowski, M.; Sroka, K. Physiological profiles of microbial communities in mine soils afforested with different tree species. Ecol. Eng. 2015, 81, 462–470. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Kim, H.J.; Kim, K.H.; Yang, J.E.; Ok, Y.S.; Owens, G. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1–11. [Google Scholar] [CrossRef]
- Šimanský, V.; Horák, J.; Igaz, D.; Balashov, E.; Jonczak, J. Biochar and biochar with N fertilizer as a potential tool for improving soil sorption of nutrients. J. Soils Sediments 2018, 18, 1432–1440. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma 2015, 245, 56–64. [Google Scholar]
- Fischer, D.; Glaser, B. Synergisms between Compost and Biochar for Sustainable Soil Amelioration. In Management of Organic Waste; Kumar, S., Bharti, A., Eds.; Tech Europe: Rijeka, Croatia, 2012; pp. 167–198. [Google Scholar]
- Whitman, T.; Singh, B.P.; Zimmerman, A.R. Priming Effects in Biochar-Amended Soils: Implications of Biochar-Soil Organic Matter Interactions for Carbon Storage. In Biochar for Environmental Management, Science, Technology and Implementation; Taylor and Francis Group: London, UK; New York, NY, USA, 2015; pp. 455–487. [Google Scholar]
- Singh, B.P.; Cowie, A.L. Long-term influence of biochar on native organic carbon mineralisation in carbon clayey soil. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Conteh, A.; Blair, G.J.; Lefroy, R.D.B.; Whitbread, A. Labile organic carbon determined by permangante oxidation and its relationships to other measurements of soil organic carbon. Humic Subst. Environ. 1999, 1, 3–15. [Google Scholar]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition – an important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campell, C.A.; Ellert, B.H.; Bremer, E. Soil Organic Matter Dynamics and Their Relationship to Soil Quality. In Soil Quality For Crop Production And Ecosystem Health; Elsevier: Amsterdam, The Netherlands, 1997; pp. 277–291. [Google Scholar]
- Spaccini, R.; Piccolo, A.; Conte, P.; Haberhauder, G.; Gerzabek, M.H. Increased soil organic carbon sequestration through hydrophobic production by humic substances. Soil Biol. Biochem. 2002, 34, 1839–1851. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Capel, E.; Zwart, K.; Shackley, S.; Postma, R.; Stenstrom, J.; Rasse, D.P.; Budai, A.; Glaser, B. Biochar Properties. In Biochar in European Soils And Agriculture; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Routledge: London, UK; New York, NY, USA, 2016; pp. 41–72. [Google Scholar]
- Li, H.; Yutong, W.; Tianpei, W.; Hungrui, M. Effect of biochar on organic matter conservation and metabolic quotient of soil. Environ. Prog. Sustain. Energy 2015, 34, 1467–1472. [Google Scholar] [CrossRef]
- Szombathová, N. The comparison of soil carbon susceptibility to oxidation by KMnO4 solutions in different farming systems. Humic Subst. Environ. 1999, 1, 35–39. [Google Scholar]
- Zhao, S.; Ta, N.; Li, Z.; Yang, Y.; Zhang, X.; Liu, D.; Zhang, A.; Wang, X. Varying pyrolysis temperature impacts application effects of biochar on soil labile organic carbon and humic substances. Appl. Soil Ecol. 2017, 116, 399–409. [Google Scholar]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Adani, F.; Genevini, P.; Ricca, G.; Tambone, F.; Montoneri, E. Modyfication of soil humic matter after 4 years of compost application. Waste Manag. 2007, 27, 319–324. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Kopeć, M.; Ukalska-Jaruga, A. Biochar changes in soil based on quantitative and qualitative humus compounds parameters. Soil Sci. Ann. 2018, 69, 234–242. [Google Scholar] [CrossRef]
- STATdat. Branch Statistics > Agriculture, Forestry, Fisheries > Yields of Selected Agricultural Crops > Select Data > Hectare Yields (Tons). Available online: http://statdat.statistics.sk (accessed on 12 April 2020).
- NPCC; Skalský, R.; Slivková, M.; Kusý, D.; Sviček, M. Odhad Úrody a Produkcie Kukurice na Zrno, Cukrovej Repy Technickej, Slnečnice Ročnej a Zemiakov [Estimation of Harvest and Production of Maize, Sugar Beet, Sunflower and Potatoes]. 2018. Available online: http://www.podnemapy.sk/portal/verejnost/akt_poln_sezona/_vystupy/Odhad_200818.pdf (accessed on 12 April 2020).
- NPPC; Hašana, R.; Bušo, R.; Polakovič, J.; Zeleňáková, K.; Brezinová, M.; Vráblová, E.; Urbanová, E.; Palkovičová, D. Prognóza Úrody Pšenice Letnej f. Ozimnej a Jačmeňa Siateho Jarného v Roku 2019 [Yield Forecast of Winter Wheat and Spring Barley in 2019]. 2019. Available online: http://www.nppc.sk/pdf/SummerwheatcropPrognoza_urody_psenice_ozimnej_v_roku_2019.pdf (accessed on 12 April 2020).
- Kožnarová, V.; Klabzuba, J. Recommendation of world meteorological organization to describing meteorological or climatological conditions—Information. Plant Soil Environ. 2011, 48, 190–192. [Google Scholar] [CrossRef]
- Bárek, V.; Halaj, P.; Igaz, D. The Influence of Climate Change on Water Demands for Irrigation of Special Plants and Vegetables in Slovakia. In Bioclimatology and Natural Hazards; Springer: Dordrecht, NL, USA, 2009; pp. 271–282. [Google Scholar]
- Igaz, D.; Bárek, V.; Halaj, P.; Takáč, J.; Čimo, J. A comparison of measured soil moisture with simulated results obtained by selected models for Danubian lowland. Cereal Res. Commun. 2008, 36, 1619–1622. [Google Scholar]
- Cimo, J.; Malencikova, T.; Szomorova, L. Agroclimatic Indicators Review in Danubian Lowland for Period 1961–2000. In Proceedings of the 15th International Multidisciplinary Scientific GeoConference SGEM 2015, Albena, Bulgaria, 18–24 June 2015; Volume 2, pp. 433–440. [Google Scholar]
- Nleya, T.; Kleinja, J. Corn Growth and Development. Available online: https://extension.sdstate.edu/sites/default/files/2019-09/S-0003-05-Corn.pdf (accessed on 2 May 2020).
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Science 2018, 320, 629. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Singh, B.; Singh, B.P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef]
- Whitman, T.; Pal Singh, B.; Zimmerman, A.R. Priming Effects in Biochar-Amended Soils: Implications of Biochar-Soil Organic Matter Interactions for Carbon Storage. In Biochar For Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 455–488. [Google Scholar]
- Monostori, T. Crop Production. Available online: http://publicatio.bibl.u-szeged.hu/5833/1/CROP%20PRODUCTION%20-%20Tam%C3%A1s%20Monostori.pdf (accessed on 17 June 2020).
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci. Total Environ. 2016, 569, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Horák, J.; Šimanský, V.; Aydin, E. Benefits of biochar and its combination with nitrogen fertilization for soil quality and grain yields of barley, wheat and corn. J. Elem. 2020, 25, 16. [Google Scholar] [CrossRef]
- Gathorne-Hardy, A.E. The Role of Biochar in English Agriculture—Agronomy, Biodiversity, Economics and Climate Change. Ph.D. Thesis, Centre for Environmental Policy, Imperial College, London, UK, 2012. [Google Scholar]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
| Soil Properties | Values |
|---|---|
| Clay (g kg−1) | 249 |
| Silt (g kg−1) | 599 |
| Sand (g kg−1) | 152 |
| Soil organic carbon (g kg−1) | 9.13 |
| CEC (mmol kg−1) | 85 |
| Base saturation (%) | 142 |
| pH (in 1 mol dm−3 KCl) | 5.71 |
| Treatment | Description |
|---|---|
| B0N0 | no biochar, no N fertilization |
| B10N0 | biochar at rate of 10 t ha−1, no N fertilization |
| B20N0 | biochar at rate of 20 t ha−1, no N fertilization |
| B0N1 | no biochar combined with first level of N fertilization: doses of N were 160 and 40 kg N ha−1 in 2017 and 2018, respectively |
| B10N1 | biochar at rate of 10 t ha−1 with N: doses of N were 160 and 40 kg N ha−1 in 2017 and 2018, respectively |
| B20N1 | biochar at rate of 20 t ha−1 with N: doses of N were 160 and 40 kg N ha−1 in 2017 and 2018, respectively |
| B0N2 | no biochar combined with second level of N fertilization: doses of N were 240 and 80 kg N ha−1 in 2017 and 2018, respectively |
| B10N2 | biochar at rate of 10 t ha−1 with N: doses of N were 240 and 80 kg N ha−1 in 2017 and 2018, respectively |
| B20N2 | biochar at rate of 20 t ha−1 with N: doses of N were 240 and 80 kg N ha−1 in 2017 and 2018, respectively |
| Treatments | pHKCl | Ha | SBC | CEC | Bs |
|---|---|---|---|---|---|
| Mann–Kendall Trends | |||||
| B0N0 | Stable/No Trend | Stable/No Trend | Increasing | Increasing | Stable/No Trend |
| B10N0 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B20N0 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend g | Stable/No Trend |
| B0N1 | Decreasing | Increasing | Stable/No Trend | Stable/No Trend | Decreasing |
| B10N1 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B20N1 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B0N2 | Decreasing | Increasing | Stable/No Trend | Stable/No Trend | Decreasing |
| B10N2 | Stable/No Trend | Increasing | Stable/No Trend | Stable/No Trend | Decreasing |
| B20N2 | Increasing | Stable/No Trend | Increasing | Increasing | Stable/No Trend |
| Treatments | Corg | CL | HS | HA | FA | HA:FA | Q4/6HS | Q4/6HA |
|---|---|---|---|---|---|---|---|---|
| Mann–Kendall Trends | ||||||||
| B0N0 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Increasing | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B10N0 | Decreasing | Stable/No Trend | Stable/No Trend | Stable/No Trend | Increasing | Decreasing | Stable/No Trend | Increasing |
| B20N0 | Decreasing | Increasing | Stable/No Trend | Stable/No Trend | Stable/No Trend | Decreasing | Stable/No Trend | Increasing |
| B0N1 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B10N1 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B20N1 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Increasing |
| B0N2 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B10N2 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend |
| B20N2 | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Stable/No Trend | Increasing |
| Treatments | Aboveground Biomass (t ha−1) | No. of Plants (m2) | Ears per Plant | Grains per Ear | Weight of 1000 Grains (g) |
|---|---|---|---|---|---|
| No fertilization: N0 | Year 2017, crop: maize | ||||
| B0N0 | 12.9 ± 0.8 a | 10.5 ± 0.9 a | 1.0 ± 0.0 ab | 325 ± 26.6 a | 216.3 ± 24.3 a |
| B10N0 | 11.2 ± 0.2 a | 7.5 ± 0.9 a | 1.2 ± 0.1 b | 294 ± 13.4 a | 227.3 ± 6.7 a |
| B20N0 | 10.9 ± 3.4 a | 10.0 ± 1.0 a | 0.9 ± 0.1 a | 307 ± 72.9 a | 190.7 ± 8.1 a |
| Fertilization: N1 | |||||
| B0N1 | 12.7 ± 1.6 a | 9.0 ± 0.0 ab | 1.8 ± 0.8 a | 261 ± 78.8 a | 211.0 ± 14.7 a |
| B10N1 | 9.8 ± 1.4 a | 7.5 ± 0.0 a | 1.0 ± 0.0 a | 323 ± 32.9 a | 216.0 ± 7.1 a |
| B20N1 | 14.1 ± 0.8 a | 10.5 ± 1.5 b | 1.0 ± 0.0 a | 369 ± 25.0 a | 204.9 ± 12.7 a |
| Fertilization: N2 | |||||
| B0N2 | 15.3 ± 3.8 a | 9.0 ± 0.9a | 1.3 ± 0.2 b | 255 ± 31.9 a | 266.1 ± 9.4 b |
| B10N2 | 11.5 ± 1.8 a | 9.0 ± 1.5 a | 1.0 ± 0.0 ab | 362 ± 41.7 b | 200.9 ± 16.2 a |
| B20N2 | 13.1 ± 1.1 a | 9.5 ± 0.5 a | 0.9 ± 0.1 a | 393 ± 8.1 b | 199.6 ± 9.4 a |
| No fertilization: N0 | Year 2018, crop: spring barley | ||||
| B0N0 | 8.6 ± 0.8 a | 250.7 ± 46.3 a | 2.6 ± 0.2 a | 13 ± 0.5 a | 38.9 ± 1.0 a |
| B10N0 | 7.5 ± 2.0 a | 298.7 ± 32.4 a | 2.0 ± 0.2 a | 12 ± 1.0 a | 38.5 ± 1.7 a |
| B20N0 | 7.9 ± 1.0 a | 357.3 ± 17.5 a | 1.7 ± 0.3 a | 13 ± 2.3 a | 34.5 ± 2.6 a |
| Fertilization: N1 | |||||
| B0N1 | 9.3 ± 2.4 a | 296.0 ± 32.3 a | 2.0 ± 0.4 a | 14 ± 0.7 a | 40.3 ± 1.2 a |
| B10N1 | 8.2 ± 0.1 a | 312.0 ± 21.2 a | 1.7 ± 0.0 a | 12 ± 1.3 a | 40.6 ± 1.9 a |
| B20N1 | 9.2 ± 0.3 a | 237.3 ± 18.7 a | 2.2 ± 0.5 a | 15 ± 0.7 a | 43.2 ± 1.5 a |
| Fertilization: N2 | |||||
| B0N2 | 11.3 ± 1.3 a | 258.7 ± 53.1 a | 2.5 ± 0.2 a | 15 ± 0.8 a | 42.0 ± 0.8 a |
| B10N2 | 9.7 ± 2.1 a | 248.0 ± 37.8 a | 2.4 ± 0.4 a | 14 ± 4.6 a | 40.5 ± 2.5 a |
| B20N2 | 12.8 ± 3.6 a | 413.3 ± 131.4 a | 2.1 ± 0.1 a | 14 ± 1.4 a | 38.9 ± 2.3 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, E.; Šimanský, V.; Horák, J.; Igaz, D. Potential of Biochar to Alternate Soil Properties and Crop Yields 3 and 4 Years after the Application. Agronomy 2020, 10, 889. https://doi.org/10.3390/agronomy10060889
Aydin E, Šimanský V, Horák J, Igaz D. Potential of Biochar to Alternate Soil Properties and Crop Yields 3 and 4 Years after the Application. Agronomy. 2020; 10(6):889. https://doi.org/10.3390/agronomy10060889
Chicago/Turabian StyleAydin, Elena, Vladimír Šimanský, Ján Horák, and Dušan Igaz. 2020. "Potential of Biochar to Alternate Soil Properties and Crop Yields 3 and 4 Years after the Application" Agronomy 10, no. 6: 889. https://doi.org/10.3390/agronomy10060889
APA StyleAydin, E., Šimanský, V., Horák, J., & Igaz, D. (2020). Potential of Biochar to Alternate Soil Properties and Crop Yields 3 and 4 Years after the Application. Agronomy, 10(6), 889. https://doi.org/10.3390/agronomy10060889





