Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Experimental Conditions
2.2. Irrigation Treatments
- Full irrigation (T1): irrigated to assure the crop needs. Irrigation was daily and irrigation scheduling was performed every week. Water needs were estimated with the crop evapotranspiration (ETc) approach according to Steduto et al. [23] using reduction coefficients (Kr) around 0.6. In addition, water status was evaluated using midday stem water potential and compared to the McCutchan and Shackel [24] baseline. When water status was more negative than expected, irrigation was increased by 150% ETc.
- Moderate RDI (T2): the water stress was imposed during the kernel-filling period; almond trees were irrigated when SWP was below −1.5 MPa, and for the rest of the time, trees were irrigated to keep an SWP as the baseline proposed by McCutchan and Shackel [24]. Equation (2) estimated optimum midday stem water potential in relation with vapor pressure deficit (VPD):where: SWP is optimum midday stem water potential (MPa) and VPD is vapor pressure deficit (KPa).
- Severe RDI (T3): the same as T2, except that trees were irrigated when SWP was below −2.0 MPa during kernel filling and maximum seasonal water was considered (120 mm, around 20% ETc). Therefore, after harvest, when total applied water was reached, irrigation stopped.
- SDI (T4): the same as T3, but tree water status was not considered. Irrigation was applied in a constant daily rate around 1–2 mm per day. The main differences between both strategies (T3 and T4) was that T4 limited postharvest irrigation more than T3.
2.3. Physical Parameters
2.3.1. Kernel Ratio
2.3.2. Dry Weight and Water Activity
2.3.3. Weight and Size
2.3.4. Instrumental Color
2.3.5. Instrumental Texture
2.4. Chemical and Functional Analysis/Parameters
2.4.1. Mineral Content Determination
2.4.2. Organic Acids and Sugars
2.4.3. Antioxidant Activity and Total Phenolic Content
2.4.4. Fatty Acids
2.5. Descriptive Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Agronomic Parameters
3.2. Morphological Parameters
3.3. Mineral, Organic Acids, and Sugars Content
3.4. Antioxidant Activity (AA) and Total Phenolic Compounds (TPC)
3.5. Fatty Acids
3.6. Descriptive Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vázquez-Araújo, L.; Verdú, A.; Navarro, P.; Martínez-Sánchez, F.; Carbonell-Barrachina, A.A. Changes in volatile compounds and sensory quality during toasting of Spanish almonds. Int. J. Food Sci. Technol. 2009, 44, 2225–2233. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Pesca y Alimentación MAPA. Informe del Consumo Alimentario en España. Available online: https://www.mapa.gob.esa (accessed on 15 May 2020).
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Grand View Research. Almond Milk Market Size, Share & Trends Analysis Report by Application (Beverages, Personal Care), by Distribution Channel (Hypermarkets & Supermarkets, Convenience Stores, Online), and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com (accessed on 21 September 2020).
- Lipan, L.; Rusu, B.; Lajos, E.S.; Sendra, E.; Hernández, F.; Vodnar, D.C.; Corell, M.; Carbonell-Barrachina, Á. Chemical and sensorial characterization of spray dried hydroSOStainable almond milk. J. Sci. Food Agric. 2020, in press. [Google Scholar] [CrossRef]
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Vodnar, D.C.; Carbonell-Barrachina, Á. Spray drying and storage of probiotic-enriched almond milk: Probiotic survival and physicochemical properties. J. Sci. Food Agric. 2020, 100, 3697–3708. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Gutiérrez Gordillo, S.; Souza, L.; Cuadros-Tavira, S.; Durán Zuazo, V.H. Fostering sustainable water use in almond (Prunus dulcis Mill.) orchards in a semiarid Mediterranean environment. Arch. Agron. Soil Sci. 2019, 65, 164–181. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations FAO. Producción de Almendra con Cáscara (FAOSTAT). Available online: http://www.fao.org/faostat/es/ (accessed on 12 May 2020).
- Ministerio de Agricultura Pesca y Alimentación MAPA. Datos Avances de Frutales no Cítricos y Frutales Secos Año. 2019. Available online: https://www.mapa.gob.es (accessed on 15 May 2020).
- Egea, G.; Nortes, P.A.; Domingo, R.; Baille, A.; Perez-Pastor, A.; Gonzalez-Real, M.M. Almond agronomic response to long-term deficit irrigation applied since orchard establishment. Irrig. Sci. 2013, 31, 445–454. [Google Scholar] [CrossRef]
- World Resource Institute WRI. 17 Countries, Home to One-Quarter of the World’s Population, Face Extremely High Water Stress. Available online: https://www.wri.org (accessed on 15 May 2020).
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Lazzarini, G.A.; Visschers, V.H.M.; Siegrist, M. How to improve consumers’ environmental sustainability judgements of foods. J Clean Prod. 2018, 198, 564–574. [Google Scholar] [CrossRef]
- European Environment Agency Report EEA. In Climate Change Adaptation In The Agriculture Sector In Europe; EEA Report 4/2019; EEA: Copenhagen, Denmark, 2019; p. 112. Available online: https://www.eea.europa.eu/publications/cc-adaptation-agriculture (accessed on 15 May 2020).
- De Ollas, C.; Morillón, R.; Fotopoulos, V.; Puértolas, J.; Ollitrault, P.; Gómez-Cadenas, A.; Arbona, V. Facing climate change: Biotechnology of iconic mediterranean woody crops. Front Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change IPCC. Global Warming of 1.5 °C. Available online: http://www.ipcc.ch/report/sr15/ (accessed on 15 May 2020).
- Du, T.; Kang, S.; Zhang, J.; Davies, W.J. Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M.; Salinas, M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2005, 24, 101–114. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Corell, M.; Sendra, E.; Hernández, F.; Stan, L.; Vodnar, D.C.; Vázquez-Araújo, L.; Carbonell-Barrachina, Á.A. Sensory profile and acceptability of hydrosostainable almonds. Foods 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Artiaga, L.; Lipan, L.; Vázquez-Araújo, L.; Barber, X.; Pérez-López, D.; Carbonell-barrachina, Á. Opinion of spanish consumers on hydrosustainable pistachios. J. Food Sci. 2016, 81, S2559–S2565. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Corell, M.; Hernández, F.; Sendra, E.; Moriana, A.; Carbonell-Barrachina, Á.A. Effect of Spanish-style processing on the quality attributes of HydroSOStainable green olives. J. Sci. Food Agric. 2019, 99, 1804–1811. [Google Scholar] [CrossRef]
- Myers, B.J. Water stress integral-a link between short-term stress and long-term growth. Tree Physiol. 1988, 4, 315–323. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop yield response to water. In FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 2012; p. 66. [Google Scholar]
- McCutchan, H.; A Shackel, K. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hort. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Lipan, L.; Martín-Palomo, M.J.; Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Sendra, E.; Hernández, F.; Burló, F.; Vázquez-Araújo, L.; Andreu, L.; Carbonell-Barrachina, Á.A. Almond fruit quality can be improved by means of deficit irrigation strategies. Agric Water Manag. 2019, 217, 236–242. [Google Scholar] [CrossRef]
- Lipan, L.; Moriana, A.; López Lluch, D.B.; Cano-Lamadrid, M.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Corell, M.; Carbonell-Barrachina, Á.A. Nutrition quality parameters of almonds as affected by deficit irrigation strategies. Molecules 2019, 24, 2646. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Huang, G. Almond Shelf Life Factors. Available at: Technical Summary, Almond Board of California, 2014, 1–4. Available online: https://www.almonds.com/sites/default/files/content/ (accessed on 20 May 2020).
- Lipan, L.; Garcia-Tejero, I.F.; Gutierrez-Gordillo, S.; Demirbas, N.; Sendra, E.; Hernandez, F.; Duran-Zuazo, V.H.; Carbonell-Barrachina, A.A. Enhancing nut quality parameters and sensory profiles in three almond cultivars by different irrigation regimes. J. Agric. Food Chem. 2020, 68, 2316–2328. [Google Scholar] [CrossRef]
- Nanos, G.; Kazantzis, I.; Kefalas, P.; Petrakis, C.; Stavroulakis, G. Irrigation and harvest time affect almond kernel quality and composition. Sci. Hort. 2002, 96, 249–256. [Google Scholar] [CrossRef]
- Carbonell-Barrachina, A.A.; Memmi, H.; Noguera-Artiaga, L.; Gijon-Lopez, M.D.; Ciapa, R.; Perez-Lopez, D. Quality attributes of pistachio nuts as affected by rootstock and deficit irrigation. J. Sci. Food Agric. 2015, 95, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lamadrid, M.; Girón, I.F.; Pleite, R.; Burló, F.; Corell, M.; Moriana, A.; Carbonell-Barrachina, A.A. Quality attributes of table olives as affected by regulated deficit irrigation. LWT-Food Sci. Technol. 2015, 62, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lu, M.; Tang, D.; Shi, Y. Composition of carotenoids and flavonoids in narcissus cultivars and their relationship with flower color. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Suleria, H.A.R.; et al. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. In Water Stress and Crop Plants: A Sustainable Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 2, pp. 649–668. [Google Scholar]
- Official Journal of the European Union EU. Regulation (EU) No 1169/2011 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu (accessed on 22 May 2020).
- Karim, M.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant. Nutr. Soil. Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Moradi, P.; MohseniFard, E.; Shekari, F.; Kompany-Zareh, M. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiol. Biochem. 2018, 132, 391–399. [Google Scholar] [CrossRef]
- Sanchez-Bel, P.; Egea, I.; Martinez-Madrid, M.C.; Flores, B.; Romojaro, F. Influence of irrigation and organic/inorganic fertilization on chemical quality of almond (Prunus amygdalus cv. Guara). J. Agric. Food Chem. 2008, 56, 10056–10062. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Gonzalez-Real, M.M.; Baille, A.; Nortes, P.A.; Sanchez-Bel, P.; Domingo, R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Peckmann, K.; Herppich, W.B. Effects of short-term drought and rewatering on the activity of mitochondrial enzymes and the oxidative capacity of leaf mitochondria from a CAM plant, Aptenia cordifolia. J. Plant Physiol. 1998, 152, 518–524. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, Z. Responses of ’Spring Bright’ and ’Summer Bright’ nectarines to deficit irrigation: Fruit growth and concentration of sugars and organic acids. Sci. Hortic. 2012, 135, 112–119. [Google Scholar] [CrossRef]
- Kashem, M.A.; Sultana, N.; Ikeda, T.; Hori, H.; Loboda, T.; Mitsui, T. Alteration of starch- sucrose transition in germinating wheat seed under sodium chloride salinity. J. Plant Biol. 2000, 43, 121–127. [Google Scholar] [CrossRef]
- Jahan, A.; Komatsu, K.; Wakida-Sekiya, M.; Hiraide, M.; Tanaka, K.; Ohtake, R.; Umezawa, T.; Toriyama, T.; Shinozawa, A.; Yotsui, I.; et al. Archetypal roles of an abscisic acid receptor in drought and sugar responses in liverworts. Plant Physiol. 2019, 179, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Carbonell-Barrachina, Á.A.; Wojdyło, A.; Sendra, E.; Hernández, F. Polyphenol profile in manzanilla table olives as affected by water deficit during specific phenological stages and spanish-style Processing. J. Agric. Food Chem. 2019, 67, 661–670. [Google Scholar] [CrossRef]
- Noguera-Artiaga, L.; Sánchez-Bravo, P.; Pérez-López, D.; Szumny, A.; Calin-Sánchez, Á.; Burgos-Hernández, A.; Carbonell-Barrachina, Á.A. Volatile, sensory and functional properties of hydrosos pistachios. Foods 2020, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Baek, K.-H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA. Food Data Central. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html (accessed on 15 May 2020).
- Zhu, Y.; Taylor, C.; Sommer, K.; Wilkinson, K.; Wirthensohn, M. Influence of deficit irrigation strategies on fatty acid and tocopherol concentration of almond (Prunus dulcis). Food Chem. 2015, 173, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M.; Anwar, F. Physico-chemical attributes of seed oil from drought stressed sunflower (Helianthus annuus L.) plants. Grasas y Aceites 2009, 60, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Artiaga, L.; Sánchez-Bravo, P.; Hernández, F.; Burgos-Hernández, A.; Pérez-López, D.; Carbonell-Barrachina, Á.A. Influence of regulated deficit irrigation and rootstock on the functional, nutritional and sensory quality of pistachio nuts. Sci. Hortic. 2020, 261. [Google Scholar] [CrossRef]
- Baldini, M.; Giovanardi, R.; Tahmasebi Enferadi, S.; Vannozzi, G. Effects of water regime on fatty acid accumulation and final fatty acid composition in the oil of standard and high oleic sunflower hybrids. Ital. J. Agron. 2002, 6, 119–126. [Google Scholar]
- Sánchez-Rodríguez, L.; Lipan, L.; Andreu, L.; Martín-Palomo, M.J.; Carbonell-Barrachina, Á.A.; Hernández, F.; Sendra, E. Effect of regulated deficit irrigation on the quality of raw and table olives. Agric. Water Manag. 2019, 221, 415–421. [Google Scholar] [CrossRef]
- Ros, E.; Mataix, J. Fatty acid composition of nuts-Implications for cardiovascular health. Br. J. Nutr. 2006, 96, S29–S35. [Google Scholar] [CrossRef] [Green Version]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Bio. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G.; et al. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration FDA. Qualified Health Claims: Letter of Enforcement Discretion-Nuts and Coronary Heart Disease (Docket No 02P-0505). Available online: https://www.fda.gov/food/food-labeling-nutrition/qualified-health-claims-letters-enforcement-discretion (accessed on 15 May 2020).
- Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Carbonell-Barrachina, Á.A.; Sendra, E.; Hernández, F. Volatile composition, sensory profile and consumer acceptability of hydrosostainable table olives. Foods 2019, 8, 470. [Google Scholar] [CrossRef] [Green Version]

 R = 1.00;
R = 1.00;  significant (p < 0.05) positive correlation;
significant (p < 0.05) positive correlation;  significant (p < 0.05) negative correlation;
significant (p < 0.05) negative correlation;  positive but not correlated;
positive but not correlated;  negative but not correlated. *, **, ***, significant at p < 0.05, 0.01, and 0.001, respectively. SWP = minimum stem water potential; SI = stress integral; AW = applied water; KR = kernel ratio; DW = dry weight; aw = water activity.
negative but not correlated. *, **, ***, significant at p < 0.05, 0.01, and 0.001, respectively. SWP = minimum stem water potential; SI = stress integral; AW = applied water; KR = kernel ratio; DW = dry weight; aw = water activity.
 R = 1.00;
R = 1.00;  significant (p < 0.05) positive correlation;
significant (p < 0.05) positive correlation;  significant (p < 0.05) negative correlation;
significant (p < 0.05) negative correlation;  positive but not correlated;
positive but not correlated;  negative but not correlated. *, **, ***, significant at p < 0.05, 0.01, and 0.001, respectively. SWP = minimum stem water potential; SI = stress integral; AW = applied water; KR = kernel ratio; DW = dry weight; aw = water activity.
negative but not correlated. *, **, ***, significant at p < 0.05, 0.01, and 0.001, respectively. SWP = minimum stem water potential; SI = stress integral; AW = applied water; KR = kernel ratio; DW = dry weight; aw = water activity.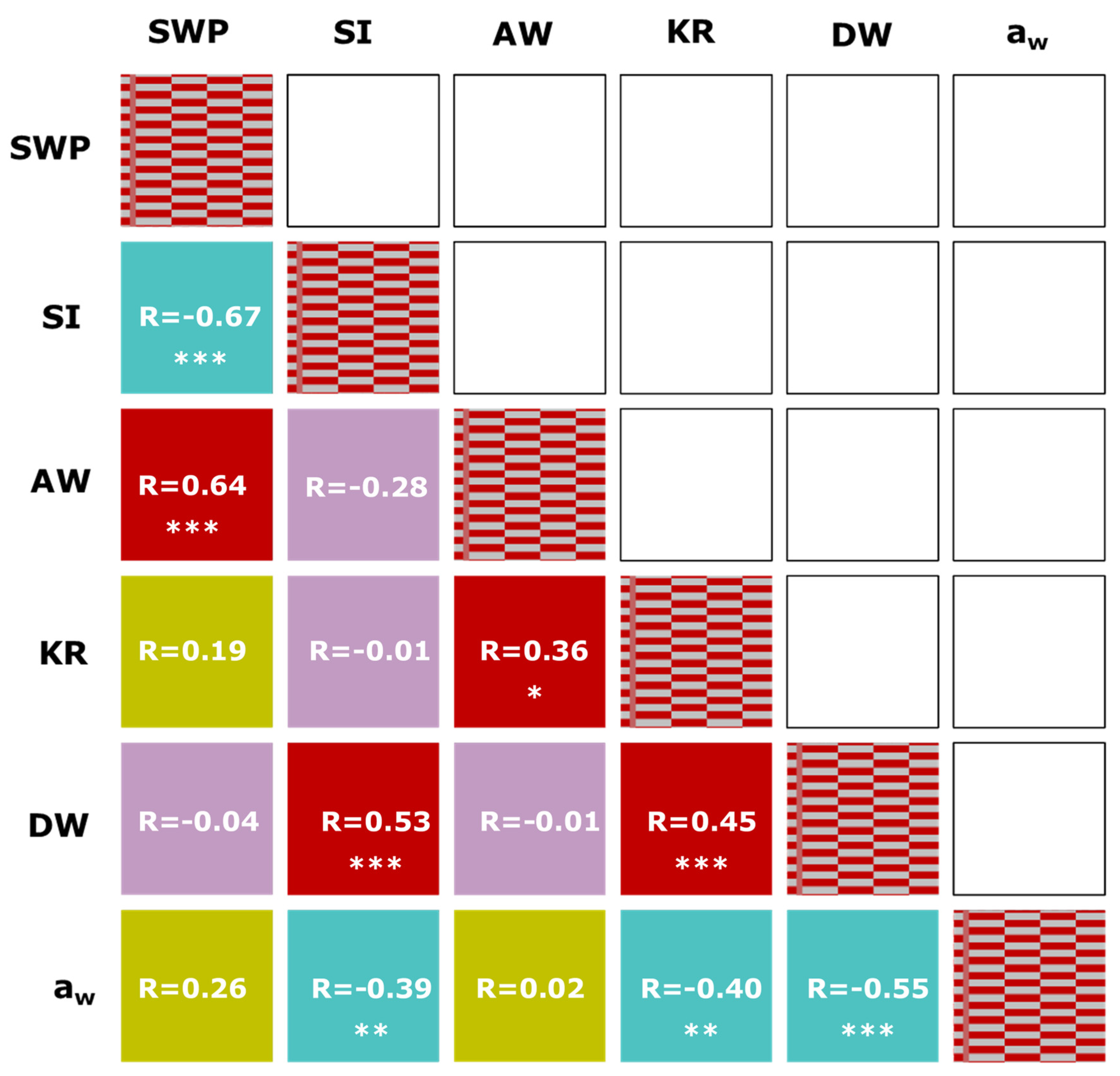
| SWP | SI | AWe | KWe | SHWe | AL | KL | AWi | KWi | ATi | KTi | L* | a* | b* | Hue | C | H | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWP | 1.00 | ||||||||||||||||
| SI | −0.67 *** | 1.00 | |||||||||||||||
| AWe | 0.17 | −0.39 ** | 1.00 | ||||||||||||||
| KWe | 0.35 * | −0.56 *** | 0.85 *** | 1.00 | |||||||||||||
| SHWe | 0.11 | −0.32 * | 0.99 *** | 0.75 *** | 1.00 | ||||||||||||
| AL | 0.05 | 0.08 | 0.75 *** | 0.43 ** | 0.80 *** | 1.00 | |||||||||||
| KL | 0.26 | −0.57 *** | 0.84 *** | 0.91 *** | 0.77 *** | 0.40 ** | 1.00 | ||||||||||
| AWi | 0.08 | −0.17 | 0.90 *** | 0.64 *** | 0.92 *** | 0.89 *** | 0.60 *** | 1.00 | |||||||||
| KWi | 0.18 | −0.51 *** | 0.92 *** | 0.88 *** | 0.87 *** | 0.55 *** | 0.88 *** | 0.79 *** | 1.00 | ||||||||
| ATi | 0.33 * | −0.41 ** | 0.80 *** | 0.63 *** | 0.80 *** | 0.68 *** | 0.61 *** | 0.81 *** | 0.70 *** | 1.00 | |||||||
| KTi | 0.32 * | 0.07 | −0.36 * | −0.04 | −0.44 ** | −0.30 * | −0.16 | −0.41 ** | −0.30 * | −0.15 | 1.00 | ||||||
| L* | −0.20 | 0.61 *** | −0.65 *** | −0.55 *** | −0.64 *** | −0.38 ** | −0.61 *** | −0.58 *** | −0.64 *** | −0.64 *** | 0.47 *** | 1.00 | |||||
| a* | −0.30 * | 0.80 *** | −0.41 ** | −0.57 *** | −0.34 * | 0.14 | −0.69 *** | −0.13 | −0.55 *** | −0.29 * | 0.19 | 0.65 *** | 1.00 | ||||
| b* | −0.21 | 0.72 *** | −0.53 *** | −0.55 *** | −0.50 *** | −0.10 | −0.67 *** | −0.36 * | −0.61 *** | −0.49 *** | 0.37 * | 0.87 *** | 0.90 *** | 1.00 | |||
| Hue | 0.07 | 0.02 | 0.32 *** | −0.04 | 0.40 ** | 0.59 *** | −0.06 | 0.55 *** | 0.16 | 0.48 *** | −0.50 *** | −0.42 ** | 0.27 | −0.05 | 1.00 | ||
| C | −0.09 | 0.10 | −0.40 * | −0.06 | −0.48 *** | −0.59 *** | −0.06 | −0.60 *** | −0.26 | −0.56 *** | 0.55 *** | 0.56 *** | −0.12 | 0.23 | −0.98 *** | 1.00 | |
| H | 0.06 | −0.40 | 0.61 *** | 0.60 *** | 0.58 *** | 0.31 * | 0.67 *** | 0.48 *** | 0.63 *** | 0.52 *** | −0.32 * | −0.56 *** | −0.56 *** | −0.63 *** | −0.04 | −0.08 | 1.00 |
| SWP | SI | Ca | Mg | K | Fe | Mn | Zn | Cit | Tar | Mal | ΣOA | Suc | Glu | Fru | ΣS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWP | 1.00 | |||||||||||||||
| SI | −0.67 *** | 1.00 | ||||||||||||||
| Ca | 0.14 | −0.60 *** | 1.00 | |||||||||||||
| Mg | 0.13 | −0.35 ** | −0.12 | 1.00 | ||||||||||||
| K | −0.06 | 0.60 *** | −0.79 *** | 0.39 ** | 1.00 | |||||||||||
| Fe | −0.18 | 0.64 *** | 0.79 *** | −0.20 | −0.65 *** | 1.00 | ||||||||||
| Mn | 0.13 | −0.05 | −0.51 *** | −0.46 *** | 0.44 ** | −0.40 ** | 1.00 | |||||||||
| Zn | −0.41 ** | 0.44 ** | −0.26 | −0.15 | 0.40 ** | 0.11 | 0.29 * | 1.00 | ||||||||
| Cit | −0.19 | 0.65 *** | −0.61 *** | 0.34 * | 0.58 *** | −0.58 *** | 0.10 | 0.02 | 1.00 | |||||||
| Tar | −0.07 | 0.35 | 0.07 | 0.75 *** | 0.04 | −0.05 | −0.80 | −0.24 | 0.41 ** | 1.00 | ||||||
| Mal | −0.01 | −0.17 | −0.23 | −0.56 *** | 0.07 | −0.01 | 0.68 *** | 0.22 | −0.22 | −0.74 *** | 1.00 | |||||
| ΣOA | −0.04 | −0.09 | −0.29* | −0.49 *** | 0.13 | −0.07 | 0.67 *** | 0.21 | −0.10 | −0.66 *** | 0.99 *** | 1.00 | ||||
| Suc | −0.42 ** | 0.71 *** | −0.62 *** | 0.06 | 0.52 *** | −0.43 ** | 0.17 *** | 0.19 | 0.71 *** | 0.20 | −0.05 | 0.02 | 1.00 | |||
| Glu | −0.02 | 0.26 | 0.03 | 0.57 *** | 0.12 | −0.07 | −0.51 *** | −0.02 | 0.13 | 0.63 *** | −0.53 *** | −0.50 *** | 0.03 | 1.00 | ||
| Fru | −0.36 * | 0.30 * | 0.21 | 0.28 * | −0.24 | 0.28 | −0.55 *** | −0.14 | 0.16 | 0.51 *** | −0.38 ** | −0.34 * | 0.19 | 0.24 | 1.00 | |
| ΣS | −0.39 ** | 0.70 *** | −0.36 * | 0.44 ** | 0.37 * | −0.27 | −0.31 * | 0.09 | 0.60 *** | 0.62 *** | −0.43 ** | −0.35 * | 0.75 *** | 0.64 *** | 0.50 *** | 1.00 |
| SWP | SI | ABTS•+K | DPPH•K | FRAP K | TPC K | ABTS•+S | DPPH•S | FRAP S | TPC S | |
|---|---|---|---|---|---|---|---|---|---|---|
| SWP | 1.00 | |||||||||
| SI | −0.67 *** | 1.00 | ||||||||
| ABTS•+K | −0.44 ** | 0.79 *** | 1.00 | |||||||
| DPPH•K | −0.09 | −0.25 | −0.46 *** | 1.00 | ||||||
| FRAP K | −0.07 | 0.34 * | 0.57 *** | −0.54 *** | 1.00 | |||||
| TPC K | 0.05 | −0.14 | 0.21 | −0.75 *** | 0.38 ** | 1.00 | ||||
| ABTS•+S | −0.30 * | 0.44 ** | 0.12 | 0.50 *** | −0.20 | −0.82 *** | 1.00 | |||
| DPPH•S | −0.07 | 0.10 | −0.20 | 0.80 *** | −0.46 *** | −0.95 *** | 0.82 *** | 1.00 | ||
| FRAP S | −0.19 | 0.41 ** | 0.14 | 0.24 | −0.17 | −0.63 *** | 0.87 *** | 0.62 *** | 1.00 | |
| TPC S | −0.11 | 0.16 | −0.15 | 0.72 *** | −0.43** | −0.92 *** | 0.88 *** | 0.94 *** | 0.73 *** | 1.00 |
| SWP | SI | C14:0 | C16:0 | C16:1 | C17:0 | C17:1 | C18:0 | C18:1n9 | C18:1n7 | C18:2 | O/L | SFA | MUFA | PUFA | PUFA/SFA | PUFA/MUFA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWP | 1.00 | ||||||||||||||||
| SI | −0.67 *** | 1.00 | |||||||||||||||
| C14:0 | −0.14 | 0.73 *** | 1.00 | ||||||||||||||
| C16:0 | −0.20 | 0.81 *** | 0.95 *** | 1.00 | |||||||||||||
| C16:1 | −0.13 | 0.75 *** | 0.92 *** | 0.96 *** | 1.00 | ||||||||||||
| C17:0 | −0.14 | 0.69 *** | 0.88 *** | 0.87 *** | 0.86 *** | 1.00 | |||||||||||
| C17:1 | −0.06 | 0.65 *** | 0.90 *** | 0.90 *** | 0.85 *** | 0.84 *** | 1.00 | ||||||||||
| C18:0 | −0.20 | 0.80 *** | 0.91 *** | 0.97 *** | 0.95 *** | 0.86 *** | 0.86 *** | 1.00 | |||||||||
| C18:1n9 | 0.22 | −0.82 *** | −0.94 *** | −0.99 *** | −0.95 *** | −0.86 *** | −0.90 *** | −0.98 *** | 1.00 | ||||||||
| C18:1n7 | −0.17 | 0.48 *** | 0.51 *** | 0.55 *** | 0.40** | 0.47 *** | 0.62 *** | 0.57 *** | −0.60 *** | 1.00 | |||||||
| C18:2 | −0.32 * | 0.82 *** | 0.86 *** | 0.91 *** | 0.86 *** | 0.76 *** | 0.75 *** | 0.88 *** | −0.93 *** | 0.46 *** | 1.00 | ||||||
| O/L | 0.24 | −0.82 *** | −0.93 *** | −0.97 *** | −0.93 *** | −0.84 *** | −0.84 *** | −0.95 *** | 0.98 *** | −0.52 *** | −0.98 *** | 1.00 | |||||
| SFA | −0.21 | 0.81 *** | 0.94 *** | 0.99 *** | 0.96 *** | 0.88 *** | 0.89 *** | 0.99 *** | −0.99 *** | 0.55 *** | 0.90 *** | −0.97 *** | 1.00 | ||||
| MUFA | 0.27 | −0.84 *** | −0.92 *** | −0.97 *** | −0.93 *** | −0.84 *** | −0.83 *** | −0.95 *** | 0.98 *** | −0.49 *** | −0.97 *** | 0.99 *** | −0.97 *** | 1.00 | |||
| PUFA | −0.32 * | 0.83 *** | 0.86 *** | 0.91 *** | 0.86 *** | 0.76 *** | 0.75 *** | 0.88 *** | −0.93 *** | 0.46 *** | 1.00 *** | −0.98 *** | 0.90 *** | −0.97 *** | 1.00 | ||
| PUFA/SFA | 0.05 | −0.65 *** | −0.84 *** | −0.89 *** | −0.90 *** | −0.83 *** | −0.86 *** | −0.91 *** | 0.86 *** | −0.46 *** | −0.64 *** | 0.78 *** | −0.90 *** | 0.79 *** | −0.64 *** | 1.00 | |
| PUFA/MUFA | −0.32 * | 0.85 *** | 0.89 *** | 0.94 *** | 0.88 *** | 0.80 *** | 0.79 *** | 0.91 *** | −0.96 *** | 0.50 *** | 0.99 *** | −0.99 *** | 0.94 *** | −0.99 ** | 0.99 *** | −0.70 *** | 1.00 |
| SWP | SI | Color | Size | Sweet | Bitter | Astr | Nutty | Al ID | Benz | Woody | Hardness | Crispiness | Aftertaste | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWP | 1.00 | |||||||||||||
| SI | −0.69 * | 1.00 | ||||||||||||
| Color | 0.24 | 0.04 | 1.00 | |||||||||||
| Size | 0.35 | −0.90 *** | −0.09 | 1.00 | ||||||||||
| Sweet | −0.43 | 0.35 | −0.68 * | −0.26 | 1.00 | |||||||||
| Bitter | 0.12 | −0.62 * | 0.27 | 0.78 ** | −0.42 | 1.00 | ||||||||
| Astr | 0.32 | −0.70 * | 0.12 | 0.77 ** | −0.29 | 0.51 | 1.00 | |||||||
| Nutty | 0.04 | −0.39 | −0.45 | 0.83 *** | 0.21 | 0.64 * | 0.59 * | 1.00 | ||||||
| Al ID | −0.09 | −0.34 | −0.84 * | 0.40 | 0.64 * | 0.03 | 0.27 | 0.78 ** | 1.00 | |||||
| Benz | 0.09 | −0.60 * | −0.68 * | 0.69 * | 0.38 | 0.26 | 0.43 | 0.83 *** | 0.83 *** | 1.00 | ||||
| Woody | 0.16 | −0.71* | −0.67 * | 0.76 ** | 0.20 | 0.39 | 0.48 | 0.89 *** | 0.82 *** | 0.91 *** | 1.00 | |||
| Hardness | −0.32 | 0.03 | −0.81 *** | 0.05 | 0.53 | −0.34 | 0.10 | 0.36 | 0.73 ** | 0.57 | 0.59 * | 1.00 | ||
| Crispiness | −0.17 | −0.12 | −0.95 *** | 0.17 | 0.64 * | −0.29 | 0.05 | 0.48 | 0.85 *** | 0.71 ** | 0.71 ** | 0.91 *** | 1.00 | |
| Aftertaste | −0.15 | −0.29 | −0.83 *** | 0.36 | 0.51 | −0.11 | 0.31 | 0.65 * | 0.91 *** | 0.82 *** | 0.81 *** | 0.86 *** | 0.89 *** | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipan, L.; Cano-Lamadrid, M.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, Á.A. Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds. Agronomy 2020, 10, 1470. https://doi.org/10.3390/agronomy10101470
Lipan L, Cano-Lamadrid M, Hernández F, Sendra E, Corell M, Vázquez-Araújo L, Moriana A, Carbonell-Barrachina ÁA. Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds. Agronomy. 2020; 10(10):1470. https://doi.org/10.3390/agronomy10101470
Chicago/Turabian StyleLipan, Leontina, Marina Cano-Lamadrid, Francisca Hernández, Esther Sendra, Mireia Corell, Laura Vázquez-Araújo, Alfonso Moriana, and Ángel A. Carbonell-Barrachina. 2020. "Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds" Agronomy 10, no. 10: 1470. https://doi.org/10.3390/agronomy10101470