Mixed Cropping as Affected by Phosphorus and Water Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Analyses
2.3. Statistical Analyses
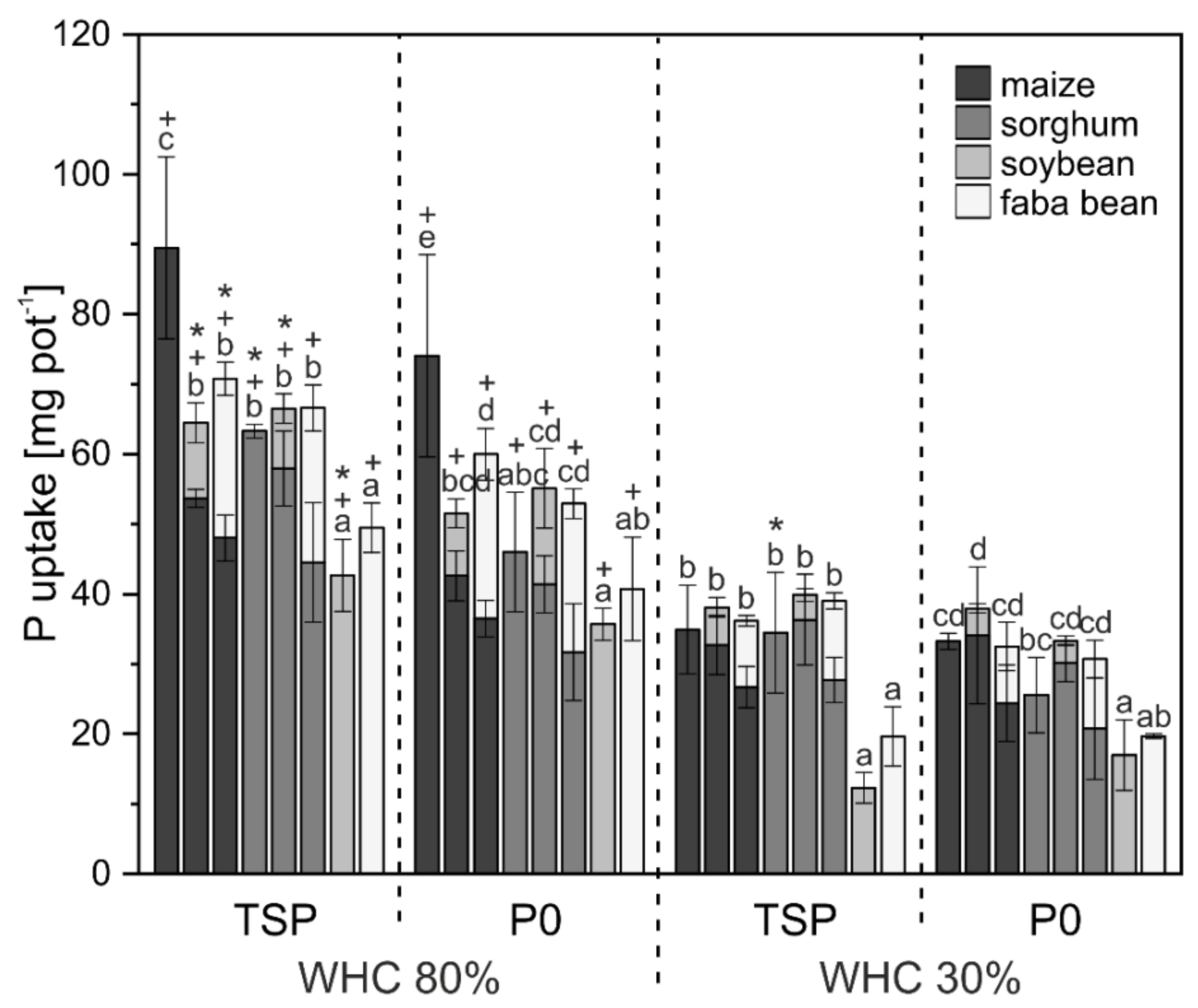
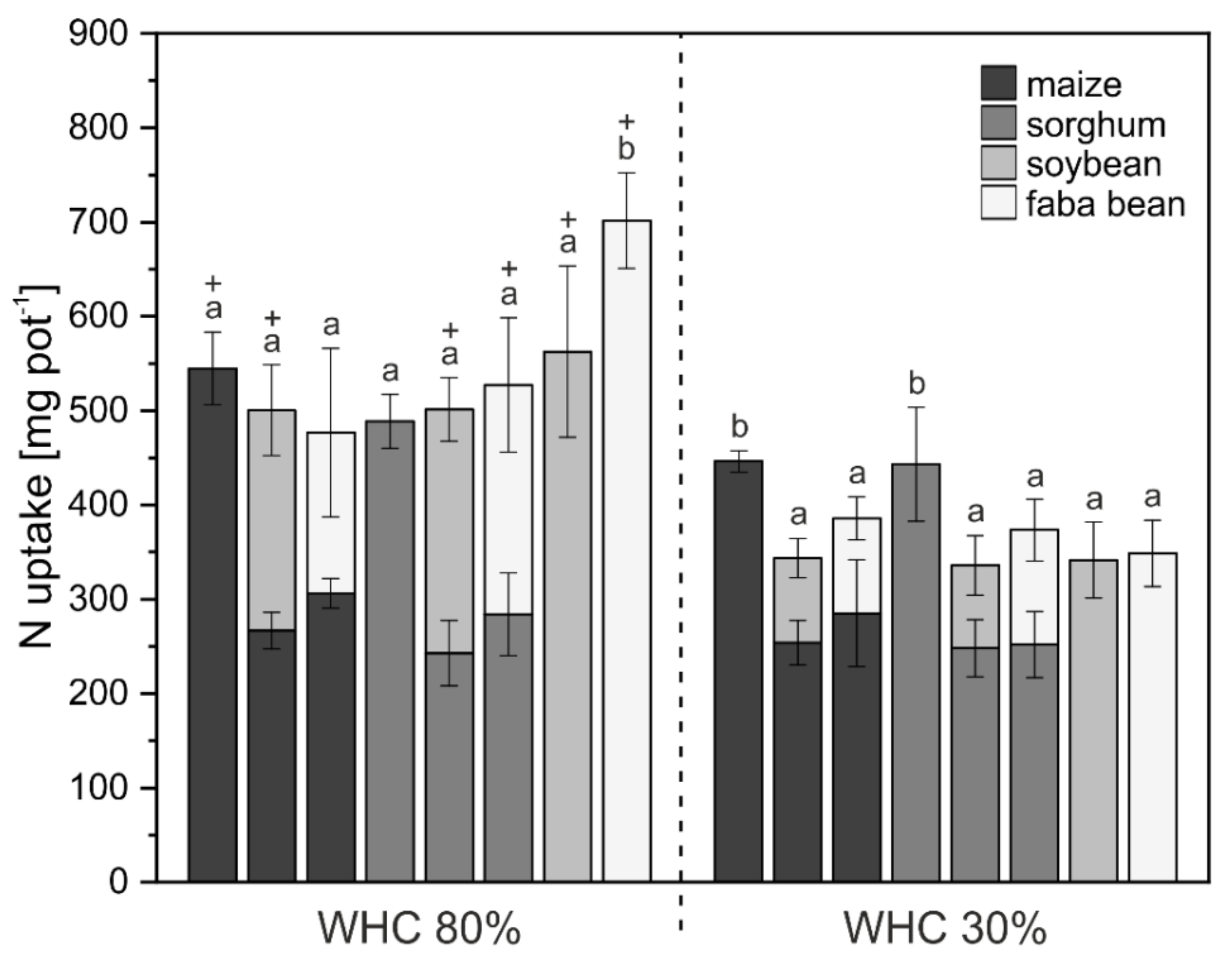
3. Results
3.1. Crop Biomass and Nutrient Uptake
3.2. Phosphorus Pools in Soil
3.3. Activity of Enzymes in Soil
4. Discussion
4.1. Biomass and Nutrient Uptake Mainly Affected by Water Supply
4.2. P mobilization in Mixed Cropping
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Water | Crop | Water × Crop | |
|---|---|---|---|
| shoot dry mass | 0.000 | 0.000 | 0.000 |
| (0.950) | (0.948) | (0.778) | |
| P uptake | 0.000 | 0.000 | 0.000 |
| (0.945) | (0.759) | (0.539) | |
| N uptake | 0.000 | 0.001 | 0.000 |
| (0.778) | (0.395) | (0.516) | |
| root length | 0.000 | 0.000 | 0.008 |
| (0.554) | (0.713) | (0.339) | |
| root-shoot-ratio | 0.000 | 0.000 | 0.000 |
| (0.607) | (0.731) | (0.494) | |
| pH | 0.397 | 0.147 | 0.604 |
| (0.016) | (0.204) | (0.109) | |
| Pw | 0.904 | 0.000 | 0.852 |
| (0.000) | (0.437) | (0.068) | |
| Pdl | 0.000 | 0.000 | 0.280 |
| (0.241) | (0.530) | (0.166) | |
| Pox | 0.040 | 0.644 | 0.259 |
| (0.090) | (0.103) | (0.171) |
| Water | P Supply | Crop | Crop × P Supply | Crop × Water | P Supply × Water | Crop × P Supply × Water | |
|---|---|---|---|---|---|---|---|
| shoot dry mass | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.761 |
| (0.902) | (0.492) | (0.920) | (0.205) | (0.621) | (0.216) | (0.043) | |
| P uptake | 0.000 | 0.000 | 0.000 | 0.174 | 0.000 | 0.000 | 0.954 |
| (0.877) | (0.349) | (0.765) | (0.102) | (0.373) | (0.160) | (0.022) | |
| N uptake | 0.000 | 0.000 | 0.000 | 0.322 | 0.000 | 0.028 | 0.953 |
| (0.806) | (0.209) | (0.809) | (0.082) | (0.389) | (0.051) | (0.022) | |
| pH | 0.005 | 0.075 | 0.000 | 0.502 | 0.000 | 0.569 | 0.020 |
| (0.081) | (0.034) | (0.613) | (0.064) | (0.430) | (0.003) | (0.160) | |
| Pw | 0.761 | 0.000 | 0.000 | 0.899 | 0.000 | 0.679 | 0.085 |
| (0.001) | (0.754) | (0.649) | (0.029) | (0.345) | (0.002) | (0.123) | |
| Pdl | 0.150 | 0.000 | 0.001 | 0.397 | 0.781 | 0.474 | 0.191 |
| (0.022) | (0.400) | (0.218) | (0.074) | (0.041) | (0.006) | (0.099) | |
| Pox | 0.506 | 0.000 | 0.000 | 0.870 | 0.199 | 0.687 | 0.852 |
| (0.005) | (0.234) | (0.405) | (0.033) | (0.098) | (0.002) | (0.034) | |
| acid Pase | 0.000 | 0.005 | 0.000 | 0.005 | 0.002 | 0.134 | 0.001 |
| (0.259) | (0.083) | (0.400) | (0.191) | (0.209) | (0.024) | (0.232) | |
| alk Pase | 0.400 | 0.011 | 0.000 | 0.068 | 0.000 | 0.001 | 0.845 |
| (0.008) | (0.067) | (0.513) | (0.129) | (0.285) | (0.110) | (0.035) | |
| dehydrogenase | 0.425 | 0.444 | 0.000 | 0.027 | 0.000 | 0.767 | 0.000 |
| (0.007) | (0.006) | (0.414) | (0.152) | (0.350) | (0.001) | (0.291) |
| MA | MA + SB | MA + FB | SO | SO + SB | SO + FB | SB | FB |
|---|---|---|---|---|---|---|---|
| Change in Shoot Biomass (DM) Due to Drought Stress (%) | |||||||
| −58.9 | −42.7 | −38.9 | −51.7 | −41.1 | −45.3 | −49.2 | −48.0 |
| Change in P Uptake Due to Drought Stress (%) | |||||||
| −68.1 | −61.9 | −50.0 | −57.4 | −51.1 | −52.4 | −49.7 | −62.8 |
| Change in N Uptake Due to Drought Stress (%) | |||||||
| −18.2 | −31.3 | −19.1 | −9.41 | −32.9 | −29.2 | −39.3 | −50.3 |
| WHC % | Fertil. | Ma | Ma + SB | Ma + FB | SO | SO + SB | SO + FB | SB | FB |
|---|---|---|---|---|---|---|---|---|---|
| Change in Shoot Biomass (DM) Due to Drought Stress (%) | |||||||||
| TSP | −59.4 | −41.0 | −46.6 | −46.6 | −36.0 | −33.8 | −66.3 | −59.2 | |
| P0 | −55.0 | −37.9 | −35.4 | −43.0 | −29.9 | −34.8 | −48.3 | −47.2 | |
| mean | −57.4 | −39.6 | −41.5 | −45.1 | −33.3 | −34.3 | −58.1 | −53.7 | |
| Change in Shoot Biomass (DM) Due to P Deficit (%) | |||||||||
| 80 | −19.9 | −19.4 | −18.8 | −28.6 | −23.9 | −13.3 | −17.5 | −16.7 | |
| 30 | −11.3 | −15.3 | −1.78 | −23.7 | −16.7 | −14.7 | +26.5 | +7.80 | |
| mean | −17.4 | −17.9 | −12.8 | −26.9 | −21.1 | −13.9 | +6.40 | −9.57 | |
| Change in P Uptake Due to Drought Stress (%) | |||||||||
| TSP | −61.0 | −40.9 | −48.9 | −45.5 | −40.0 | −41.4 | −71.2 | −60.4 | |
| P0 | −55.0 | −26.2 | −45.9 | −44.3 | −39.6 | −42.0 | −52.4 | −51.6 | |
| mean | −58.3 | −34.3 | −47.4 | −45.0 | −39.8 | −41.6 | −62.5 | −56.3 | |
| Change in P Uptake Due to P-Deficit (%) | |||||||||
| 80 | −17.3 | −20.2 | −15.3 | −27.3 | −17.1 | −20.6 | −16.4 | −17.8 | |
| 30 | −4.58 | −0.26 | −10.2 | −25.8 | −16.5 | −21.3 | +38.2 | +0.51 | |
| mean | −13.7 | −12.8 | −13.6 | −26.8 | −16.9 | −20.8 | −4.18 | −12.6 | |
| Change in N Uptake Due to Drought Stress (%) | |||||||||
| TSP | −50.4 | −23.2 | −39.7 | −34.8 | −23.9 | −30.1 | −45.8 | −56.1 | |
| P0 | −46.7 | −22.5 | −38.2 | −34.4 | −18.5 | −31.2 | −36.8 | −44.7 | |
| mean | −48.6 | −22.9 | −39.0 | −34.5 | −21.4 | −30.5 | −41.0 | −51.0 | |
| Change in N Uptake Due to P-Deficit (%) | |||||||||
| 80 | −11.6 | −7.49 | −9.61 | −17.0 | −15.9 | −12.6 | +1.4 | −19.9 | |
| 30 | −4.89 | −6.64 | −7.28 | −16.6 | −9.95 | −13.9 | +18.2 | +0.70 | |
| mean | −9.38 | −7.12 | −8.73 | −16.9 | −13.4 | −13.1 | +7.33 | −13.6 | |
| WHC 80% | WHC 30% | Change Due to Mixed Cropping | ||||
|---|---|---|---|---|---|---|
| Solo | Mix | Solo | Mix | WHC 80% | WHC 30% | |
| maize | 16.6 | 20.9 | 6.83 | 12.64 | +25.6 | +85.0 |
| sorghum | 12.3 | 14.3 | 5.96 | 8.64 | +16.3 | +45.1 |
| soybean | 5.96 | 4.93 | 3.17 | 1.98 | −17.2 | −37.7 |
| faba bean | 5.73 | 3.59 | 2.98 | 2.14 | −37.4 | −28.2 |
| WHC % | 80 | 30 | Mean | |||
|---|---|---|---|---|---|---|
| Root Length (m pot−1) | ||||||
| MA | 664 ± 94.2 | D * | 381 ± 87.2 | E | 522 | F |
| MA + SB | 469 ± 89.1 | C * | 221 ± 11.3 | ABC | 363 | D |
| MA + FB | 299 ± 16.4 | B * | 251 ± 14.6 | BCD | 275 | BC |
| SO | 457 ± 83.3 | C | 390 ± 11.4 | E | 428 | E |
| SO + SB | 485 ± 65.7 | C | 302 ± 28.4 | D | 378 | DE |
| SO + FB | 360 ± 72.4 | B | 281 ± 40.2 | CD | 321 | CD |
| SB | 286 ± 23.5 | B * | 184 ± 16.9 | AB | 235 | AB |
| FB | 188 ± 22.6 | A | 175 ± 34.4 | A | 181 | A |
| mean | 401 | b | 271 | a | ||
| Root: Shoot Ratio (m g−1) | ||||||
| MA | 10.0 ± 1.28 | B | 14.0 ± 3.47 | B | 12.0 | BC |
| MA + SB | 9.18 ± 1.39 | B | 7.79 ± 0.75 | A | 8.59 | A |
| MA + FB | 6.22 ± 0.16 | A | 8.71 ± 1.57 | A | 7.46 | A |
| SO | 9.23 ± 1.00 | B * | 15.3 ± 1.29 | B | 11.8 | BC |
| SO + SB | 13.1 ± 1.85 | C | 13.8 ± 1.33 | B | 13.5 | C |
| SO + FB | 9.32 ± 1.80 | B * | 13.4 ± 1.83 | B | 11.3 | B |
| SB | 12.0 ± 1.22 | C | 14.6 ± 1.87 | B | 13.3 | C |
| FB | 8.37 ± 1.90 | B * | 14.7 ± 1.66 | B | 11.5 | B |
| mean | 9.68 | 12.9 | ||||
References
- Fachagentur Nachwachsende Rohstoffe. Basisdaten Bioenergie Deutschland 2019; Fachagentur Nachwachsende Rohstoffe e.V.: Gilcov, Germany, 2019; Volume 17. [Google Scholar]
- Gründinger, W. The Renewable Energy Sources Act (EEG). In Energiepolitik und Klimaschutz. Energy Policy and Climate Protection; Gründinger, W., Ed.; Springer: Wiesbaden, Germany, 2017; pp. 257–419. [Google Scholar] [CrossRef]
- Huth, E.; Paltrinieri, S.; Thiele, J. Bioenergy and its effects on landscape aesthetics—A survey contrasting conventional and wild crop biomass production. Biomass Bioenergy 2019, 122, 313–321. [Google Scholar] [CrossRef]
- Das, A.; Lal, R.; Somireddy, U.; Bonin, C.; Verma, S.; Rimal, B.K. Changes in soil quality and carbon storage under biofuel crops in central Ohio. Soil Res. 2016, 54, 371–382. [Google Scholar] [CrossRef]
- Gevers, J.; Høye, T.T.; Topping, C.J.; Glemnitz, M.; Schröder, B. Biodiversity and the mitigation of climate change through bioenergy: Impacts of increased maize cultivation on farmland wildlife. GCB Bioenergy 2011, 3, 472–482. [Google Scholar] [CrossRef]
- Seifert, C.; Leuschner, C.; Culmsee, H. Arable plant diversity on conventional cropland—The role of crop species, management and environment. Agric. Ecosyst. Environ. 2015, 213, 151–163. [Google Scholar] [CrossRef]
- von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for A More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jensen, E.S. Facilitative root interactions in intercrops. Plant Soil 2005, 274, 237–250. [Google Scholar] [CrossRef]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; Zhang, L.; van der Werf, W. A meta-analysis of relative crop yields in cereal/legume mixtures suggests options for management. Field Crops Res. 2016, 198, 269–279. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef]
- Zhang, W.-P.; Liu, G.-C.; Sun, J.-H.; Zhang, L.-Z.; Weiner, J.; Li, L. Growth trajectories and interspecific competitive dynamics in wheat/maize and barley/maize intercropping. Plant Soil 2015, 397, 227–238. [Google Scholar] [CrossRef]
- Eisenhauer, N. Aboveground–belowground interactions as a source of complementarity effects in biodiversity experiments. Plant Soil 2012, 351, 1–22. [Google Scholar] [CrossRef]
- Pappa, V.A.; Rees, R.M.; Walker, R.L.; Baddeley, J.A.; Watson, C.A. Legumes intercropped with spring barley contribute to increased biomass production and carry-over effects. J. Agric. Sci. 2012, 150, 584–594. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.; Maruyama, H.; Masuda, G.; Wasaki, J. Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 2015, 390, 223–236. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Eichler, B.; Caus, M.; Schnug, E.; Köppen, D. Soil acid and alkaline phosphatase activities in regulation to crop species and fungal treatment, Landbauforsch. Volkenrode 2004, 54, 1–5. [Google Scholar]
- Garland, G.M. Phosphorus Cycling in Soil Aggregate Fractions in Maize-Pigeon Pea Intercropping Systems of Malawi. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2017. [Google Scholar] [CrossRef]
- He, Y.; Ding, N.; Shi, J.; Wu, M.; Liao, H.; Xu, J. Profiling of microbial PLFAs: Implications for interspecific interactions due to intercropping which increase phosphorus uptake in phosphorus limited acidic soils. Soil Biol. Biochem. 2013, 57, 625–634. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Turner, L.B. Changes in the Phosphorus Content of Capsicum annuum Leaves during Water Stress. J. Plant Physiol. 1985, 121, 429–439. [Google Scholar] [CrossRef]
- Waraich, E.; Ahmad, A.; Ashraf, R.; Saifullah, M.Y.; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Belnap, J. Biological phosphorus cycling in dryland regions. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2011; pp. 371–406. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Prieto, P.; Estiarte, M. Drought and warming induced changes in P and K concentration and accumulation in plant biomass and soil in a Mediterranean shrubland. Plant Soil 2008, 306, 261–271. [Google Scholar] [CrossRef]
- Betencourt, E.; Duputel, M.; Colomb, B.; Desclaux, D.; Hinsinger, P. Intercropping promotes the ability of durum wheat and chickpea to increase rhizosphere phosphorus availability in a low P soil. Soil Biol. Biochem. 2012, 46, 181–190. [Google Scholar] [CrossRef]
- Daleo, P.; Iribarne, O. Beyond competition: The stress-gradient hypothesis tested in plant–herbivore interactions. Ecology 2009, 90, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Jamia, S.; Umar, H. Alleviating Adverse Effects of Water Stress on Yield of Sorghum, Mustard and Groundnut by Potassium Application. Pak. J. Bot. Pak. 2006. Available online: https://agris.fao.org/agris-search/search.do?recordID=PK2008000016 (accessed on 17 June 2020).
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F.-S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, J.; Wang, Z.; Zhang, S. Planting density and sowing proportions of maize–soybean intercrops affected competitive interactions and water-use efficiencies on the Loess Plateau, China. Eur. J. Agron. 2016, 72, 70–79. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. In Dehydrogenases; Canuto, R.A., Ed.; InTech: London, UK, 2012; pp. 183–210. [Google Scholar] [CrossRef]
- Ehrmann, J.; Ritz, K. Plant: Soil interactions in temperate multi-cropping production systems. Plant Soil 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Hupfauf, S.; Bachmann, S.; Juárez, M.F.; Insam, H.; Eichler-Löbermann, B. Biogas digestates affect crop P uptake and soil microbial community composition. Sci. Total Environ. 2016, 542, 1144–1154. [Google Scholar] [CrossRef]
- Raseduzzaman, M.d.; Jensen, E.S. Does intercropping enhance yield stability in arable crop production? A meta-analysis. Eur. J. Agron. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Kiær, L.P.; Weisbach, A.N.; Weiner, J. Root and shoot competition: A meta-analysis. J. Ecol. 2013, 101, 1298–1312. [Google Scholar] [CrossRef]
- Zicker, T.; von Tucher, S.; Kavka, M.; Eichler-Löbermann, B. Soil test phosphorus as affected by phosphorus budgets in two long-term field experiments in Germany. Field Crops Res. 2018, 218, 158–170. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass ashes and their phosphorus fertilizing effect on different crops. Nutr. Cycl. Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. (Eds.) Bodenbiologische Arbeitsmethoden, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbial Properties; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- van der Paauw, F. An effective water extraction method for the determination of plantavailable soil phosphorus. Plant Soil 1971, 34, 467–481. [Google Scholar] [CrossRef]
- Riehm, H. Arbeitsvorschrift zur Bestimmung der Phosphorsäure und des Kaliums nach Lactatverfahren. Zeitschrift Pflanzenernährung Düngung Bodenkunde 1948, 40, 152–156. [Google Scholar]
- Schwertmann, U. Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z. Für Pflanzenernähr. Düng. Bodenkd. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for the assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Thalmann, H. Zur Methodik der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 243–258. [Google Scholar]
- Rusinamhodzi, L.; Corbeels, M.; Nyamangara, J.; Giller, K.E. Maize–grain legume intercropping is an attractive option for ecological intensification that reduces climatic risk for smallholder farmers in central Mozambique. Field Crops Res. 2012, 136, 12–22. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Andersen, M.K.; Jørnsgaard, B.; Jensen, E.S. Density and relative frequency effects on competitive interactions and resource use in pea–barley intercrops. Field Crops Res. 2006, 95, 256–267. [Google Scholar] [CrossRef]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models. A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Garg, B.K.; Burman, U.; Kathju, S. The influence of phosphorus nutrition on the physiological response of moth bean genotypes to drought. J. Plant Nutr. Soil Sci. 2004, 167, 503–508. [Google Scholar] [CrossRef]
- Burman, U.; Garg, B.K.; Kathju, S. Effect of Phosphorus Application on Clusterbean under Different Intensities of Water Stress. J. Plant Nutr. 2009, 32, 668–680. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Baddeley, J.A.; Watson, C.A. Models of biological nitrogen fixation of legumes. A review. Agron. Sustain. Dev. 2011, 31, 155–172. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. The efficiency of a durum wheat-winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil 2010, 330, 19–35. [Google Scholar] [CrossRef]
- Morgado, L.B.; Willey, R.W. Effects of plant population and nitrogen fertilizer on yield and efficiency of maize-bean intercropping. Pesqui. Agropecuária Bras. 2003, 38, 1257–1264. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Liu, Y.; He, C.; Zhang, X.; Zhang, J. Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth. Planta 2011, 233, 1129–1143. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.-S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, J.; Zhang, F.; Marschner, P.; Cawthray, G.; Rengel, Z. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fertil. Soils 2010, 46, 79–91. [Google Scholar] [CrossRef]
- Latati, M.; Blavet, D.; Alkama, N.; Laoufi, H.; Drevon, J.J.; Gérard, F.; Pansu, M.; Ounane, S.M. The intercropping cowpea-maize improves soil phosphorus availability and maize yields in an alkaline soil. Plant Soil 2014, 385, 181–191. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Rengel, Z.; Zhang, F. Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 2003, 248, 297–303. [Google Scholar] [CrossRef]
- Ohm, M.; Paulsen, H.M.; Moos, J.H.; Eichler-Löbermann, B. Long-term negative phosphorus budgets in organic crop rotations deplete plant-available phosphorus from soil. Agron. Sustain. Dev. 2017, 37, 17. [Google Scholar] [CrossRef]
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresour. Technol. 2000, 75, 43–48. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Li, X.; Christie, P.; Sun, J.; Yang, S.; Tang, C. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosyst. 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Zhang, F.; Clairotte, M.; Drevon, J.J.; le Cadre, E.; Hinsinger, P. Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant Soil 2008, 312, 139–150. [Google Scholar] [CrossRef]
- Devau, N.; Hinsinger, P.; le Cadre, E.; Gerard, F. Root-induced processes controlling phosphate availability in soils with contrasted P-fertilized treatments. Plant Soil 2011, 348, 203–218. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Seasonal patterns of root-surface phosphatase activities in a Mediterranean shrubland. Responses to experimental warming and drought. Biol. Fertil. Soils 2007, 43, 779–786. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichler-Löbermann, B.; Busch, S.; Jablonowski, N.D.; Kavka, M.; Brandt, C. Mixed Cropping as Affected by Phosphorus and Water Supply. Agronomy 2020, 10, 1506. https://doi.org/10.3390/agronomy10101506
Eichler-Löbermann B, Busch S, Jablonowski ND, Kavka M, Brandt C. Mixed Cropping as Affected by Phosphorus and Water Supply. Agronomy. 2020; 10(10):1506. https://doi.org/10.3390/agronomy10101506
Chicago/Turabian StyleEichler-Löbermann, Bettina, Stefanie Busch, Nicolai David Jablonowski, Mareike Kavka, and Christine Brandt. 2020. "Mixed Cropping as Affected by Phosphorus and Water Supply" Agronomy 10, no. 10: 1506. https://doi.org/10.3390/agronomy10101506
APA StyleEichler-Löbermann, B., Busch, S., Jablonowski, N. D., Kavka, M., & Brandt, C. (2020). Mixed Cropping as Affected by Phosphorus and Water Supply. Agronomy, 10(10), 1506. https://doi.org/10.3390/agronomy10101506







