Recent Achievements and New Research Opportunities for Optimizing Macronutrient Availability, Acquisition, and Distribution for Perennial Fruit Crops
Abstract
:1. Introduction
2. Mineral Nutrient Cycling in Perennial Fruit Crops
3. Soil Nutrient Cycling
4. Soil Carbon
5. Soil Fertility
5.1. Nitrogen (N)
5.2. Phosphorus (P)
5.3. Potassium (K), Calcium (Ca), Magnesium (Mg), and Sulfur (S)
5.4. Effects of Organic Soil Amendments on Soil Nutrient Dynamics
5.5. Effects of Irrigation Management on Soil Nutrient Dynamics
6. Nutrient Interactions in the Rhizosphere
7. Rootstocks and Mineral Nutrition
8. Root Morphology and Competition with Other Root Systems
9. Root Macronutrient Uptake
9.1. Nitrogen (N)
9.2. Phosphorus (P)
9.3. Potassium (K)
9.4. Calcium (Ca)
9.5. Magnesium (Mg)
9.6. Sulfur (S)
10. Calcium Distribution among Roots, Leaves, and Fruit
11. Foliar Spray Supplements
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuchi, S.; Cesco, S.; Varanini, Z.; Pinton, R.; Astolfi, S. Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta 2009, 230, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, W.S.; Vyvyan, M.C. Root Studies V Rootstock and soil effects on apple root systems. J. Hortic. Sci. 1934, 12, 110–150. [Google Scholar] [CrossRef]
- Atkinson, D.; Wilson, S.A. The growth and distribution of fruit tree roots: Some consequences for nutrient uptake. Acta Hortic. 1979, 92, 137–150. [Google Scholar]
- Atkinson, D. Crop attributes facilitating the use of soil resources. In The Sci.ence Beneath Organic Production; Atkinson, D., Watson, C.A., Eds.; John Willey: Hoboken, NJ, USA, 2020; pp. 169–212. [Google Scholar]
- Welch, R.M.; Shuman, L. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [Green Version]
- Swietlik, D.; Faust, M. Foliar nutrition of fruit crops. Hortic. Rev. 1984, 6, 287–355. [Google Scholar]
- Ferguson, I.B.; Watkins, C.B. Bitter pit in apple fruit. Hortic. Rev. 1989, 11, 289–355. [Google Scholar] [CrossRef]
- Marcelle, R. Mineral nutrition and fruit quality. Acta Hortic. 1995, 383, 219–226. [Google Scholar] [CrossRef]
- Tagliavini, M.; Failla, O.; Xyloyannis, C. Mineral Nutrition. In Principles of Modern Fruit Science; Sansavini, S., Costa, G., Gucci, R., Inglese, P., Ramina, A., Xiloyannis, C., Desjardins, Y., Eds.; International Society for Horticultural Sciences: Leuven, Belgium, 2019; pp. 341–348. [Google Scholar]
- Proietti, P. Gas exchange in senescing leaves of Olea europaea L. Photosynthetica 1998, 35, 579–587. [Google Scholar] [CrossRef]
- Millard, P. Ecophysiology of the internal cycling of nitrogen for tree growth. J. Plant Nutri. Soil Sci. 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Niederholzer, F.J.A.; DeJong, T.M.; Saenz, J.L.; Muraoka, T.T.; Weinbaum, S.A. Effectiveness of fall versus spring soil fertilization of field-grown peach trees. J. Am. Soc. Hortic. Sci. 2001, 126, 644–648. [Google Scholar] [CrossRef]
- Neilsen, D.; Millard, P.; Neilsen, G.H.; Hogue, E.J. Sources of N used for leaf growth in a high density apple (Malus domestica) orchard irrigated with ammonium nitrate solution. Tree Physiol. 1997, 17, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Millard, P.; Wendler, R.; Minotta, G.; Tagliavini, M. Measurements of xylem sap amino acid concentrations in conjunction with whole tree transpiration estimates spring N remobilization by cherry trees. Plant Cell Environ. 2002, 25, 1689–1699. [Google Scholar] [CrossRef]
- Ferrara, G.; Malerba, A.D.; Matarrese, A.M.S.; Mondelli, D.; Mazzeo, A. Nitrogen distribution in annual growth of ‘Italia table grape vines. Front. Plant Sci. 2018, 9, a1374. [Google Scholar] [CrossRef] [Green Version]
- Granatstein, D.; Sánchez, E. Research knowledge and needs for orchard floor management in organic tree fruit systems. Int. J. Fruit Sci. 2009, 9, 257–281. [Google Scholar] [CrossRef]
- Ventura, M.; Scandellari, F.; Bonora, E.; Tagliavini, M. Nutrient release during decomposition of leaf litter in a peach (Prunus persica L.) orchard. Nutr. Cycl. Agroecosyst. 2010, 87, 115–123. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.-L.; García-Ruiz, R.; Francaviglia, R.; Aguilera, E.; Smith, P. Carbon sequestration rates under Mediterranean woody crops using recommended practices—A meta-analysis. Agr. Ecosyst. Environ. 2016, 235, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Midwood, A.J.; Hannam, K.D.; Forge, T.A.; Neilsen, D.; Emde, D.; Jones, M.D. Importance of drive-row vegetation for soil carbon storage in woody perennial crops: A regional study. Geoderma 2020, 377, 114591. [Google Scholar] [CrossRef]
- Regni, L.; Nasini, L.; Ilarioni, L.; Brunori, A.; Massaccesi, L.; Agnelli, A.; Proietti, P. Long term amendment with fresh and composted solid olive mill waste on olive grove affects carbon sequestration by prunings, fruits and soil. Front. Plant Sci. 2016, 7, a2042. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Mezzapesa, G.N.; Stellacci, A.M.; Ferrara, G.; Occhiogrosso, G.; Petrelli, G.; Castellini, M.; Spagnuolo, M. Cover crop for a sustainable viticulture: Effects on soil properties and table grape production. Agronomy 2020, 10, 1334. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Lowery, T.; Forge, T.; Neilsen, D. Organic fruit production in British Columbia. Can. J. Plant Sci. 2009, 89, 677–692. [Google Scholar] [CrossRef]
- Massaccesi, L.; De Feudis, M.; Agnelli, A.E.; Nasini, L.; Regni, L.; D’scoli, R.; Castaldi, S.; Proietti, P.; Agnelli, A. Organic carbon pools and storage in the soil of olive groves of different age. Eur. J. Soil Sci. 2018, 69, 843–855. [Google Scholar] [CrossRef]
- Forge, T.; Neilsen, G.H.; Neilsen, D. Organically acceptable practices to improve replant success of temperate tree-fruit crops. Sci. Hortic. 2016, 200, 205–214. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, G.W.B.D.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Fentabil, M.M.; Nichol, C.F.; Jones, M.D.; Neilsen, G.H.; Neilsen, D.; Hannam, K.D. Effect of drip irrigation frequency. Nitrogen rate and mulching on nitrous oxide emissions in a semi-arid climate: An assessment across two years in an apple orchard. Agric. Ecosyst. Environ. 2016, 235, 242–252. [Google Scholar] [CrossRef]
- Hannam, K.D.; Neilsen, G.H.; Forge, T.A.; Neilsen, D.; Losso, I.; Jones, M.D.; Nichol, C.; Fentabil, M.M. Irrigation practices, nutrient applications, and mulches affect soil nutrient dynamics in a young Merlot (Vitis vinifera L.) vineyard. Can. J. Soil Sci. 2016, 96, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, D.; Neilsen, G.H.; Forge, T. Building resilience: Future directions in mineral nutrition of woody perennial crops. Acta Hortic. 2018, 1217. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosys. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Ren, W.; Coyne, M.; Jacinthe, P.-A.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate smart agricultural practices: A meta-analysis. Glob. Chang. Biol. 2019, 25, 2591–2606. [Google Scholar] [CrossRef]
- Tagliavini, M.; Tonon, G.; Scandarelli, F.; Quinones, A.; Palmieri, S.; Menarbin, G.; Gioacchini, P.; Masia, A. Nutrient cycling during the decomposition of apple leaves (Malus domestica) and mow grasses in an orchard. Agric. Ecosyst. Environ. 2007, 118, 191–200. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Neilsen, D.; Parchomchuk, P.; Neilsen, G.H.; Hogue, E. Use of soil solution monitoring to determine the effects of irrigation management and fertigation on nitrogen availability in high-density apple orchards. J. Am. Soc. Hortic. Sci. 1998, 123, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, G.H.; Neilsen, D. Precision Nutrient Management. In Automation in Tree Fruit Production; Zhang, Q., Ed.; CAB International: Boston, MA, USA, 2018; pp. 134–160. [Google Scholar]
- Baram, S.; Couvreur, V.; Harter, T.; Read, M.; Brown, P.H.; Hopmans, J.W.; Smart, D.R. Assessment of orchard N losses to groundwater with a vadose zone monitoring networks. Agric. Water Manag. 2016, 172, 83–95. [Google Scholar] [CrossRef]
- Atucha, A.; Merwin, I.A.; Purohit, C.K.; Brown, M.G. Nitrogen dynamics and nutrient budgets in four orchard groundcover management systems. HortScience 2011, 46, 1184–1193. [Google Scholar] [CrossRef] [Green Version]
- Hungate, B.A.; Dukes, J.S.; Shaw, M.R.; Luo, Y.; Field, C.B. Nitrogen and climate change. Science 2003, 302, 1512–1513. [Google Scholar] [CrossRef] [Green Version]
- Alsina, M.; Fanton Borges, A.C.; Smart, D.R. Spatiotemporal variation of event related N2O and CH4 emissions during fertigation in a California almond orchard. Ecosphere 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Forge, T. Environmental limiting factors for cherry production. In Cherries: Botany Production and Uses; Quero-Garcia, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CAB International: Boston, MA, USA, 2017; pp. 189–222. [Google Scholar]
- Nelson, N.O.; Janke, R.R. Phosphorus sources and management in organic production systems. HortTechnology 2007, 17, 442–454. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, A.; Mazzeo, A.; Lopriore, G.; Disciglio, G.; Gagliardi, A.; Nuzzo, V.; Ferrara, G. Nutrients in clusters and leaves of Italian table grapes are affected by the use of cover crops in the vineyard. J. Berry Res. 2020, 10, 157–173. [Google Scholar] [CrossRef]
- Neilsen, G.; Forge, T.; Angers, D.; Neilsen, D.; Hogue, E. Suitable orchard floor management strategies in organic apple orchards that augment soil organic matter and maintain tree performance. Plant Soil 2014, 378, 325–335. [Google Scholar] [CrossRef]
- Neilsen, D.; Neilsen, G.H.; Gregory, D.; Forge, T.; Zebarth, B. Drainage losses of water, N and P from micro-irrigation systems in a young high-density apple planting. Acta Hortic. 2008, 792, 483–490. [Google Scholar] [CrossRef]
- Forge, T.; Kempler, C. Use of organic mulches as primary N sources for red raspberry: Influences on root growth and nematode communities. Can. J. Plant Pathol. 2009, 31, 241–249. [Google Scholar] [CrossRef]
- Gransee, A.; Fuhrs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Neilsen, G.H.; Neilsen, D.; Forge, T.; Hannam, K. Advances in soil and nutrient management in apple cultivation. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; p. 38. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D. Response of high-density apple orchards on coarse-textured soil to form of potassium applied by fertigation. Can. J. Soil Sci. 2006, 86, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Hogue, E.J.; Cline, J.A.; Neilsen, G.; Neilsen, D. Growth and yield responses to mulches and cover crops under low potassium conditions in drip-irrigated apple orchards on coarse soils. HortScience 2010, 45, 1866–1871. [Google Scholar] [CrossRef] [Green Version]
- Kowalenko, C.G.; Bittman, S.; Neilsen, G.H.; Kenney, E.; Hunt, D.E.; Neilsen, D. Potential for improving sulfur tests on agricultural soils in contrasting ecoregions in British Columbia, Canada. Geoderma Reg. 2014, 1, 10–20. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zhao, S.; Han, Q.; Pan, X.; He, F.; Chen, C. Assessment of the responses of soil pore properties to combined soil structure amendments using X-ray computed tomography. Nature Sci. Rep. 2018, 8, 695. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.A.; Hopmans, J.W.; Filipovic, V.; Van der Ploeg, M.; Lebron, I.; Jones, S.B.; Reinsch, S.; Jarvis, N.; Tuller, M. Global environmental changes impact soil hydraulic functions through biophysical feedbacks. Glob. Chang. Biol. 2019, 25, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasny, B.; McBratney, A.B. Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 2018, 69, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Karl, A.D.; Merwin, I.A.; Brown, M.G.; Hervieux, R.A.; Vanden Heuvel, J.E. Under-vine management impacts soil properties and leachate composition in a New York State vineyard. HortScience 2016, 51, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Mays, N.; Rom, C.R.; Brye, K.R.; Savin, M.C.; Garcia, M.E. Groundcover Management System and Nutrient Source Impacts on Soil Quality Indicators in an Organically Managed Apple (Malus domestica Borkh.) orchard in the Ozark Highlands. HortScience 2015, 50, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Demir, Z.; Tursun, N.; Işık, D. Effects of different cover crops on soil quality parameters and yield in an apricot orchard. Int. J. Agric. Biol. 2019. [Google Scholar] [CrossRef]
- Lepsch, H.C.; Brown, P.H.; Peterson, C.A.; Gaudin, A.C.M.; Khalsa, S.D.S. Impact of organic matter amendments on soil and tree water status in a California orchard. Agric. Water Manag. 2019, 222, 204–212. [Google Scholar] [CrossRef]
- Cline, J.; Neilsen, G.; Hogue, E.; Kuchta, S.; Neilsen, D. Spray-on-mulch technology for intensively grown irrigated apple orchards: Influence on tree establishment, early yields, and soil physical properties. HortTechnology 2011, 21, 398–411. [Google Scholar] [CrossRef] [Green Version]
- Torres, R.; Ferrara, G.; Soto, F.; López, J.A.; Sanchez, F.; Mazzeo, A.; Pérez-Pastor, A.; Domingo, R. Effects of soil and climate in a table grape vineyard with cover crops. Irrigation management using sensors networks. Cienc. Tec. Vitivvinic 2017, 32, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.T.; Nelson, L.M.; Neilsen, D.; Neilsen, G.H.; Forge, T.A. Low-volume irrigation systems influence Pratylenchus penetrans populations, root colonization by arbuscular mycorrhizal fungi, and replant establishment of sweet cherry. Sci. Hortic. 2018, 239, 50–56. [Google Scholar] [CrossRef]
- Lordan, J.; Pascual, M.; Villar, J.M.; Fonseca, F.; Papió, J.; Montilla, V.; Rufat, J. Use of organic mulch to enhance water-use efficiency and peach production under limiting soil conditions in a three-year-old orchard. Span. J. Agric. Res. 2015, 13, e0904. [Google Scholar] [CrossRef]
- Sarkar, B.; Singh, M.; Mandal, S.; Churchman, G.J.; Bolan, N.S. Clay minerals—Organic matter interactions in relation to carbon stabilization in soils. In The future of Soil Carbon; Academic Press: Cambridge, MA, USA, 2018; pp. 71–86. [Google Scholar]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Innangi, M.; Niro, E.; D’Ascoli, R.; Danise, T.; Proietti, P.; Nasini, L.; Regni, L.; Castaldi, S.; Fioretto, A. Effects of olive pomace amendment on soil enzyme activities. Appl. Soil Ecol. 2017, 119, 242–249. [Google Scholar] [CrossRef]
- Federici, E.; Massaccesi, L.; Pezzolla, D.; Fidati, L.; Montalbani, E.; Proietti, P.; Nasini, L.; Regni, L.; Scargetta, S.; Gigliotti, G. Short-term modifications of soil microbial community structure and soluble organic matter chemical composition following amendment with different solid olive mill waste and their derived composts. Appl. Soil Ecol. 2017, 119, 234–241. [Google Scholar] [CrossRef]
- Proietti, P.; Federici, E.; Fidati, L.; Scargetta, S.; Massaccesi, L.; Nasini, L.; Regni, L.; Ricci, A.; Cenci, G.; Gigliotti, G. Effects of amendment with oil mill waste and its derived-compost on soil chemical and microbiological characteristics and olive (Olea europaea L.) productivity. Agric. Ecosyst. Environ. 2015, 207, 51–60. [Google Scholar] [CrossRef]
- Nasini, L.; Gigliotti, G.; Balduccini, M.A.; Federici, E.; Cenci, G.; Proietti, P. Effect of solid olive-mill waste amendment on soil fertility and olive (Olea europaea L.) tree activity. Agric. Ecosyst. Environ. 2013, 164, 292–297. [Google Scholar] [CrossRef]
- Neilsen, D.; Neilsen, G.H. Optimizing precision in irrigation and nutrient management. In Achieving Sustainable Cultivation of Temperate Zone Tree Fruits and Berries; Lang, G.A., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019. [Google Scholar]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hinsinger, P.; Gobran, G.R.; Gregory, P.J.; Wenzel, W.W. Rhizosphere geometry and heterogeneity arising from rootmediated physical and chemical processes. New Phytol. 2005, 168, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef]
- Terzano, R.; Cesco, S.; Mimmo, T. Dynamics, thermodynamics and kinetics of exudates: Crucial issues in understanding rhizosphere processes. Plant Soil 2015, 386, 399–406. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, P.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- el Zahar Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Astolfi, S.; Lehto, N.; Robinson, B.; Brunetto, G.; Terzano, R.; Cesco, S. Nutrient availability in the rhizosphere: A review. Acta Hortic. 2018, 1217, 13–27. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Ann. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borruso, L.; Bacci, G.; Mengoni, A.; De Philippis, R.; Brusetti, L. Rhizosphere effect and salinity competing to shape microbial communities in Phragmites australis (Cav.) Trin. ex-Steud. FEMS Microbiol. Lett. 2014, 359, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Mommer, L.; Hinsinger, P.; Prigent-Combaret, C.; Visser, E.J.W. Advances in the rhizosphere: Stretching the interface of life. Plant Soil 2016, 407, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 135–191. [Google Scholar]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.A.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.O.; Lastovetsky, O.A.; et al. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef] [Green Version]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Hines, D.E.; Ray, S.; Borrett, S.R. Uncertainty analyses for Ecological Network Analysis enable stronger inferences. Environ. Model Softw. 2018, 101, 117–127. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Constructing and Analyzing Microbiome Networks in R; Humana Press: New York, NY, USA, 2018; pp. 243–266. [Google Scholar] [CrossRef]
- Esposito, A.; Colantuono, C.; Ruggieri, V.; Chiusano, M.L. Bioinformatics for agriculture in the next-generation sequencing era. Chem. Biol. Technol Agric. 2016, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Fazio, G.; Chang, L.; Grusak, M.A.; Robinson, T.L. Apple rootstocks influence mineral nutrient concentration of leaves and fruit. N. Y. Fruit Q. 2015, 23, 11–15. [Google Scholar]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yang, H. Effect of Soil Type on Root Architecture and Nutrient Uptake by Roots of Young Apple Rootstocks. Acta Hortic. 2011, 903, 885–890. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sharma, R.M. Effect of rootstocks on tree growth, yield, quality and leaf mineral composition of lemon (Citrus limon (L.) Burm.). Sci. Hortic. 2016, 200, 131–136. [Google Scholar] [CrossRef]
- Glass, A.D.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.A.; Guy, R.D. Variation in fluxes estimated from nitrogen isotope discrimination corresponds with independent measures of nitrogen flux in Populus balsamifera L. Plant Cell Environ. 2016, 39, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Da Ros, L.M.; Soolanayakanahally, R.Y.; Guy, R.D.; Mansfield, S.D. Phosphorus storage and resorption in riparian tree species: Environmental applications of poplar and willow. Environ. Exp. Bot. 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Pii, Y.; Alessandrini, M.; Guardini, K.; Zamboni, A.; Varanini, Z. Induction of high-affinity NO3–uptake in grapevine roots is an active process correlated to the expression of specific members of the NRT2 and plasma membrane H+-ATPase gene families. Funct. Plant Biol. 2014, 41, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Livigni, S.; Lucini, L.; Sega, D.; Navacchi, O.; Pandolfini, T.; Zamboni, A.; Varanini, Z. The different tolerance to magnesium deficiency of two grapevine rootstocks relies on the ability to cope with oxidative stress. BMC Plant Biol. 2019, 19, 148. [Google Scholar] [CrossRef]
- Cesco, S.; Rombolà, A.D.; Tagliavini, M.; Varanini, Z.; Pinton, R. Phytosiderophores released by graminaceous species promote 59 Fe-uptake in citrus. Plant Soil 2006, 287, 223–233. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Forde, B.G. Quantitative Analysis of Lateral Root Development: Pitfalls and How to Avoid Them: Quantitative Analysis of Lateral Roots. Plant Cell 2012, 24, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; Von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Lobet, G.; Couvreur, V.; Meunier, F.; Javaux, M.; Draye, X. Plant water uptake in drying soils. Plant Physiol. 2014, 164, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, L.; Fabrice, M.R.; Tian, Y.; Qi, K.; Chen, Q.; Cao, P.; Wang, P.; Zhang, S.; Wu, J.; et al. Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 2018, 871, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management, and breeding. Hortic. Rev. 2018, 45, 197–312. [Google Scholar] [CrossRef]
- Atkinson, D. Apple root systems for organic orchards: What might be the contribution of the rootstock? Acta Hortic. 2018, 1217, 285–292. [Google Scholar] [CrossRef]
- Mullinix, K.; Granatstein, D. Potential nitrogen contributions from legumes in pacific Northwest apple orchards. Int. J. Fruit Sci. 2011, 11, 74–87. [Google Scholar] [CrossRef]
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. Phytopathology 2015, 105, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Tahir, I.I.; Svensson, S.E.; Hansson, D. Floor Management Systems in an Organic Apple Orchard Affect Fruit Quality and Storage Life. HortScience 2015, 50, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Filleur, S.; Dorbe, M.F.; Cerezo, M.; Orsel, M.; Granier, F.; Gojon, A.; Daniel-Vedele, F. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 2001, 489, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, M.Y.; Glass, A.D.; Ruth, T.J.; Fernando, M. Studies of the regulation of nitrate influx by barley seedlings using 13NO3−. Plant Physiol. 1989, 90, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Touraine, B.; Glass, A.D. NO3− and ClO3− fluxes in the chl1-5 mutant of Arabidopsis thaliana. Does the CHL1-5 gene encode a low-affinity NO3− transporter? Plant Physiol. 1997, 114, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pii, Y.; Aldrighetti, A.; Valentinuzzi, F.; Mimmo, T.; Cesco, S. Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 2019, 70, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Tomasi, N.; Wirdnam, C.; Meier, S.; Komarova, N.Y.; Mimmo, T.; Cesco, S.; Rentsch, D.; Pinton, R. Isolation and functional characterization of a high affinity urea transporter from roots of Zea mays. BMC Plant Biol. 2014, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pinton, R.; Tomasi, N.; Zanin, L. Molecular and physiological interactions of urea and nitrate uptake in plants. Plant Signal. Behav. 2016, 11, e1076603. [Google Scholar] [CrossRef]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Mata, C.; Van Vemde, N.; Clarkson, D.T.; Martins-Loução, M.A.; Lambers, H. Influx, efflux and net uptake of nitrate in Quercus suber seedlings. Plant Soil 2000, 221, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Sorgonà, A.; Abenavoli, M.R.; Cacco, G. A comparative study between two citrus rootstocks: Effect of nitrate on the root morpho-topology and net nitrate uptake. Plant Soil 2005, 270, 257–267. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Dong, Q.; Huang, D.; Li, C.; Li, P.; Ma, F. Genome-wide identification and analysis of apple nitrate transporter 1/peptide transporter family (NPF) genes reveals MdNPF6. 5 confers high capacity for nitrogen uptake under low-nitrogen conditions. Int. J. Mol. Sci. 2018, 19, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasi, N.; Monte, R.; Varanini, Z.; Cesco, S.; Pinton, R. Induction of nitrate uptake in Sauvignon Blanc and Chardonnay grapevines depends on the scion and is affected by the rootstock. Aust. J. Grape Wine Res. 2015, 21, 331–338. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Poirier, Y.; Jung, J.Y. Phosphate Transporters. In Annual Plant Reviews Volume 48: Phosphorus Metabolism in Plants; Plaxton, W.C., Lambers, H., Eds.; John Wiley & Sons: West Sussex, UK, 2015; pp. 125–158. [Google Scholar] [CrossRef]
- Sun, S.; Gu, M.; Cao, Y.; Huang, X.; Zhang, X.; Ai, P.; Fan, X.; Xu, G. A constitutive expressed phosphate transporter, OsPht1; 1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol. 2012, 159, 1571–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Lebaudy, A.; Véry, A.A.; Sentenac, H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. Cellular mechanisms of potassium transport in plants. Physiol. Plant 2008, 133, 637–650. [Google Scholar] [CrossRef]
- Fazio, G.E.; Kviklys, D.A.; Grusak, M.A.; Robinson, T.L. Phenotypic diversity and QTL mapping of absorption and translocation of nutrients by apple rootstocks. Asp. Appl. Biol. 2013, 119, 37–50. [Google Scholar]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- González-Fontes, A.; Navarro-Gochicoa, M.T.; Ceacero, C.J.; Herrera-Rodríguez, M.B.; Camacho-Cristóbal, J.J.; Rexach, J. Understanding calcium transport and signaling, and its use efficiency in vascular plants. In Plant Macronutrient Use Efficiency; Hossein, M.A., Kamiya, T., Burritt, D., Phan Tran, L.S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 165–180. [Google Scholar]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Miedema, H.; Demidchik, V.; Véry, A.A.; Bothwell, J.H.; Brownlee, C.; Davies, J.M. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytol. 2008, 179, 378–385. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. (Eds.) Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Knoop, V.; Groth-Malonek, M.; Gebert, M.; Eifler, K.; Weyand, K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Gen. Genom. 2005, 274, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Kuhn, A.J.; Schröder, W.H.; Bauch, J. The kinetics of calcium and magnesium entry into mycorrhizal spruce roots. Planta 2000, 210, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, M.; Tester, M. Characterization of a voltage dependent Ca2+-selective channel from wheat roots. Planta 1995, 195, 478–488. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tutone, A.F.; Drummond, R.S.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef] [Green Version]
- Pisat, N.P.; Pandey, A.; MacDiarmid, C.W. MNR2 regulates intracellular magnesium storage in Saccharomyces cerevisiae. Genetics 2009, 183, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Waters, B.M. Moving magnesium in plant cells. New Phytol. 2011, 190, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Mao, D.D.; Tian, L.F.; Li, L.G.; Chen, J.; Deng, P.Y.; Li, D.P.; Luan, S. AtMGT7: An Arabidopsis gene encoding a low affinity magnesium transporter. J. Integr. Plant Biol. 2008, 50, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Schock, I.; Gregan, J.; Steinhauser, S.; Schweyen, R.; Brennicke, A.; Knoop, V.A. A member of a novel Arabidopsis thaliana gene family of candidate Mg ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J. 2000, 24, 489–501. [Google Scholar] [CrossRef]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Qi, Y.P.; Lee, J.; Yang, L.T.; Guo, P.; Jiang, H.X.; Chen, L.S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015, 16, 253. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Davidian, J.C.; Grignon, C. Sulphate/proton cotransport in plasma-membrane vesicles isolated from roots of Brassica napus L.: Increased transport in membranes isolated from sulphur-starved plants. Planta 1993, 190, 297–304. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3; 5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H. Sulfate transport systems in plants: Functional diversity and molecular mechanisms underlying regulatory coordination. J. Exp. Bot. 2019, 70, 4075–4087. [Google Scholar] [CrossRef]
- Torres, E.; Recasens, I.; Lordan, J.; Alegre, S. Combination of strategies to supply calcium and reduce bitter pit in ‘Golden Delicious’ apples. Sci. Hortic. 2017, 217, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal accumulation of mineral nutrients by kiwifruit 2. Fruit. New Phytol. 1988, 108, 399–409. [Google Scholar] [CrossRef]
- Stiegler, J.C.; Richardson, M.D.; Karcher, D.E. Foliar nitrogen uptake following urea application to putting green turfgrass species. Crop. Sci. 2011, 51, 1253–1260. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Will, S.; Eichert, T.; Fernandez, V.; Möhring, J.; Römheld, V. Absorption and mobility of foliar-applied boron in soybean as affected by plant boron status and application as a polyol complex. Plant Soil 2011, 344, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Nuzzo, V.; Clearwater, M.J.; Xiloyannis, C. Internal versus external control of calcium nutrition in kiwifruit. J. Plant Nut. Soil Sci. 2014, 177, 819–830. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2015, 181, 67–74. [Google Scholar] [CrossRef]
- Song, W.; Yi, J.; Kurniadinata, O.F.; Wang, H.; Huang, X. Linking fruit Ca uptake capacity to fruit growth and pedicel anatomy, a cross-species study. Front. Plant Sci. 2018, 9, 575. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A. Quantifying the Uptake as Well as the Allocation/Distribution of Ca to Different Plant Organs After a Ca Soil Application During the Second White Root Flush in Young Potted Apple Trees. Master’s Thesis, Faculty of AgriScience, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Cheng, L.; Sazo, M.M. Why is ‘Honeycrisp’ so susceptible to bitter pit? Fruit Q. 2018, 26, 19–23. [Google Scholar]
- Wang, Y.; Zhang, X.; Wang, Y.; Yang, S.; Qu, H. The changes of intracellular calcium concentration and distribution in the hard end pear (Pyrus pyrifolia cv. ‘Whangkeumbae’) fruit. Cell Calcium 2018, 71, 15–23. [Google Scholar] [CrossRef]
- Levin, A.G.; Yermiyahu, U.; Doron, I.; Shtienberg, D. The role of calcium concentration in the endocarp wall of apple fruit in the development of core rot. Crop. Prot. 2019, 120, 67–74. [Google Scholar] [CrossRef]
- Reig, G.; Lordan, J.; Fazio, G.; Grusak, M.A.; Hoying, S.; Cheng, L.; Francescatto, P.; Robinson, T. Horticultural performance and elemental nutrient concentrations on ‘Fuji’ grafted on apple rootstocks under New York State climatic conditions. Sci. Hortic. 2018, 227, 22–37. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Cheng, L.; Kalcsits, L. Apple scion and rootstock contribute to nutrient uptake and partitioning under different belowground environments. Agronomy 2019, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Gomez, R.; Kalcsits, L. Physiological factors affecting nutrient uptake and distribution and fruit quality in ‘Honeycrisp’ and ‘WA 38’ apple (Malus × domestica Borkh.). HortScience 2020, 55, 1327–1336. [Google Scholar] [CrossRef]
- Kalcsits, L.; Mattheis, J.P.; Giordani, L.; Reid, M.; Mullin, K. Fruit canopy positioning affects fruit calcium and potassium concentrations, disorder incidence, and fruit quality for ‘Honeycrisp’ apple. Can. J. Plant Sci. 2019, 99, 761–771. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Fallahi, E.; Fallahi, B.; Neilsen, G.H.; Neilsen, D.; Peryea, F.J. Effect of mineral nutrition on fruit quality and nutritional disorders in apples. Acta Hortic. 2010, 868, 49–59. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, J.; Francescatto, P.; Cheng, L.; Wallis, A.; Grusak, M.A.; Robinson, T.L. ‘Honeycrisp’ apple fruit nutrient concentration affected by apple rootstocks. Acta Hortic. 2018, 1228, 223–228. [Google Scholar] [CrossRef]
- Le Roux, E. Investigating the Effect of Metalosate Ca on Fruit Quality of Apples and Citrus. Master’s Thesis, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Lötze, E.; Frazenburg, M.; Turketti, S.S.; Dreyer, L. Calcium dynamics of reproductive apple buds during the dormant season in the Western Cape, South Africa. Sci. Hortic. 2019, 256, 108533. [Google Scholar] [CrossRef]
- Wilsdorf, R.E.; Theron, K.I.; Lötze, E. Evaluating the effectiveness of different strategies for calcium application on the accumulation of calcium in apple (Malus × domestica Borkh. ‘Braeburn’) fruit. J. Hortic. Sci. Biotechnol. 2012, 87, 565–570. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Miqueloto, A.; Steffens, C.A.; Dos Santos, A.; Argenta, L.C. Changes in xylem functionality during apple fruit development: Implications on calcium concentration and incidence of bitter pit. Acta Hortic. 2013, 1012, 135–140. [Google Scholar] [CrossRef]
- Lötze, E.; Joubert, J.; Theron, K.I. Assessment of pre-harvest physiological infiltration methods for predicting commercial bitter pit in ‘Braeburn’ and ‘Golden Delicious’. Acta Hortic. 2010, 868, 347–351. [Google Scholar] [CrossRef]
- Baugher, T.A.; Marini, R.; Schupp, J.R.; Watkins, C.B. Prediction of bitter pit in ‘Honeycrisp’ apples and best management implications. HortScience 2017, 52, 1368–1374. [Google Scholar] [CrossRef]
- Eichert, T.; Goldbach, H.E.; Burkhardt, J. Evidence for the uptake of large anions through stomatal pores. Bot. Acta 1998, 111, 461–466. [Google Scholar] [CrossRef]
- Nikolic, M.; Cesco, S.; Monte, R.; Tomasi, N.; Gottardi, S.; Zamboni, A.; Pinton, R.; Varanini, Z. Nitrate transport in cucumber leaves is an inducible process involving an increase in plasma membrane H+-ATPase activity and abundance. BMC Plant Biol. 2012, 12, 66. [Google Scholar] [CrossRef] [Green Version]
- Mwije, A.; Hoffman, E.W.; Lötze, E. Apple peel biochemical changes after foliar application of combined boron and calcium I. Phenolics and physico-chemical attributes. Am. J. Plant Sci. 2020, 11, 965–986. [Google Scholar] [CrossRef]
- Mwije, A.; Hoffman, E.W.; Lötze, E. Apple peel biochemical changes after foliar application of combined boron and calcium II. Photosynthetic pigments, total peroxides and photochemical efficiency. Am. J. Plant Sci. 2020, 11, 939–964. [Google Scholar] [CrossRef]
- Knoche, M.; Winkler, A. Rain-induced cracking of sweet cherries. In Cherries, Botany, Production and Uses; QueroGarcia, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Wallingford, UK, 2017; pp. 140–165. [Google Scholar]
- Ouzounis, T.; Lang, G.A. Foliar applications of urea affect nitrogen reserves and cold acclimation of sweet cherries (Prunus avium L.) on dwarfing rootstocks. HortScience 2011, 46, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Lötze, E.; Daiber, S.H.; Midgley, S.J.E. Evaluating the efficacy of a pre-harvest combination of calcium and boron as foliar application to reduce sunburn on ‘Cripps Pink’ apples. Acta Hortic. 2018, 1217, 61–68. [Google Scholar] [CrossRef]
- Kalcsits, L.; Van der Heijden, G.; Reid, M.; Mullin, K. Calcium absorption during fruit development in ‘Honeycrisp’ apple measured using 44Ca as a stable isotope tracer. HortScience 2017, 52, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Peryea, F.J.; Neilsen, G.H.; Faubion, D. Start-timing for calcium chloride spray programs influences fruit calcium and bitter pit in ‘Braeburn’ and ‘Honeycrisp’ apples. J. Plant Nutr. 2007, 30, 1213–1227. [Google Scholar] [CrossRef]
- Biggs, A.R.; Peck, G.M. Managing bitter pit in ‘Honeycrisp’ apples grown in the Mid-Atlantic United States with foliar-applied calcium chloride and some alternatives. HortTechnology 2015, 25, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.A. Non-destructive measurement of calcium and potassium in apple and pear using handheld X-ray fluorescence. Front. Plant Sci. 2016, 7, 442. [Google Scholar] [CrossRef] [Green Version]
- Lötze, E.; Wilsdorf, R.E.; Turketti, S.S.; Przybylowics, W.J.; Mesjasz-Przybylowicz, J. Revisiting calcium concentration and distribution in apple fruit (Malus domestica Borkh.). J. Plant Nutr. 2015, 38, 1469–1477. [Google Scholar] [CrossRef]
- Donner, E.; De Jonge, M.D.; Kopittke, P.M.; Lombi, E. Mapping element distributions in plant tissues using synchrotron X-ray fluorescence techniques. In Plant Mineral Nutrients; Maathuis, F.J.M., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 143–159. [Google Scholar] [CrossRef]
- Wilsdorf, R.E.; Lötze, E.; Mesjasz-Przybylowicz, J.; Przybylowicz, W.J. Mapping the distribution of calcium on apple tissue with proton-induced x-ray emission-after application of additional pre-harvest foliar or soil calcium. Acta Hortic. 2013, 984, 347–355. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Do Amarante, C.V.T.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Lötze, E.; Turketti, S. Efficacy of foliar application of calcium products on tomatoes as defined by penetration depth of and concentration within fruit tissues. J. Plant Nutr. 2015, 38, 2112–2125. [Google Scholar] [CrossRef]
- Amiri, M.E.; Fallahi, E.; Safi-Songhorabad, M. Influence of rootstock on mineral uptake and scion growth of ‘Golden Delicious’ and ‘Royal Gala’ apples. J. Plant Nutr. 2014, 37, 16–29. [Google Scholar] [CrossRef]
- McLaren, T.I.; Guppy, C.N.; Tighe, M.K. A rapid and nondestructive plant nutrient analysis using portable X-ray fluorescence. Soil Sci. Soc. Am. J. 2012, 76, 1446–1453. [Google Scholar] [CrossRef]
- Tanino, K.; Willick, I.R.; Hamilton, K.; Vijayan, P.; Jiang, Y.; Brar, G.S.; Yu, P.; Kalcsits, L.; Lahlali, R.; Smith, B.; et al. Chemotyping using synchrotron mid-infrared and X-ray spectroscopy to improve agricultural production. Can. J. Plant Sci. 2017, 97, 982–996. [Google Scholar] [CrossRef] [Green Version]
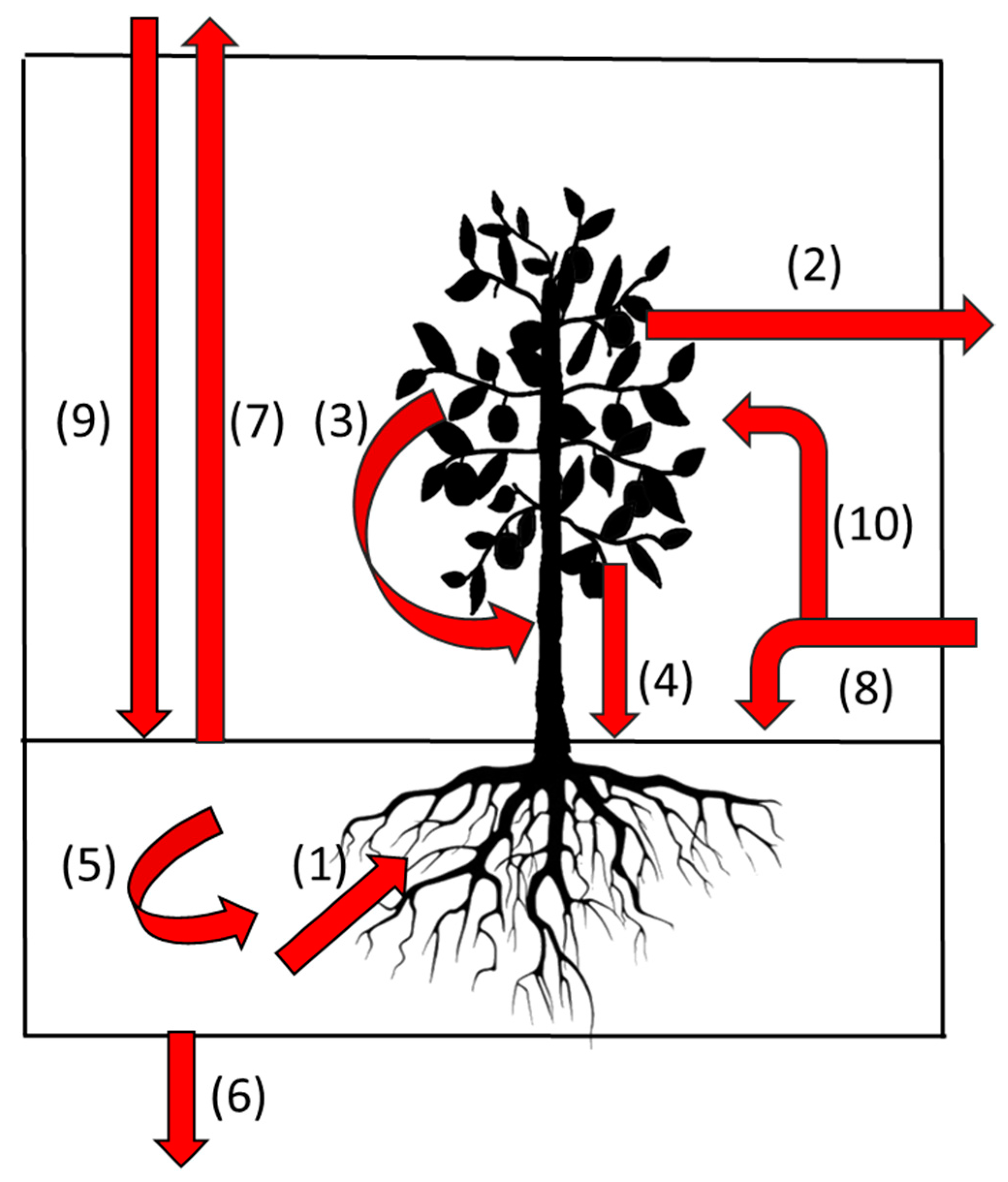

| Initial Allocation | Allocation after Primary Stage | Amount/Proportion |
|---|---|---|
| New white root | Brown root | All below-ground primary production |
| Root cortex | Tissue is external to the stele and is shed as part of the aging process and representing a significant C input to the soil | |
| Root exudate | Largely unquantified but this is the source of most carbon for soil bacterial populations | |
| Arbuscular Mycorrhizal Fungi (AMF) | Not well quantified but may be substantial. Infection influences white root survival. | |
| Brown root | Surviving brown root | Can survive as an isolated stele for significant periods |
| Woody root | A major recipient of root carbon commonly the fate of 20–25% of root length | |
| Soil organic matter | A major fate of root carbon but with time lag from initial allocation to becoming soil carbon varying with root type | |
| AMF | Soil organic matter | Potentially a major source especially for small soil pores < 8 um |
| Woody root | Survival until tree death then soil organic matter | A major storage compartment for C during the life of a tree |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalcsits, L.; Lotze, E.; Tagliavini, M.; Hannam, K.D.; Mimmo, T.; Neilsen, D.; Neilsen, G.; Atkinson, D.; Casagrande Biasuz, E.; Borruso, L.; et al. Recent Achievements and New Research Opportunities for Optimizing Macronutrient Availability, Acquisition, and Distribution for Perennial Fruit Crops. Agronomy 2020, 10, 1738. https://doi.org/10.3390/agronomy10111738
Kalcsits L, Lotze E, Tagliavini M, Hannam KD, Mimmo T, Neilsen D, Neilsen G, Atkinson D, Casagrande Biasuz E, Borruso L, et al. Recent Achievements and New Research Opportunities for Optimizing Macronutrient Availability, Acquisition, and Distribution for Perennial Fruit Crops. Agronomy. 2020; 10(11):1738. https://doi.org/10.3390/agronomy10111738
Chicago/Turabian StyleKalcsits, Lee, Elmi Lotze, Massimo Tagliavini, Kirsten D. Hannam, Tanja Mimmo, Denise Neilsen, Gerry Neilsen, David Atkinson, Erica Casagrande Biasuz, Luigimaria Borruso, and et al. 2020. "Recent Achievements and New Research Opportunities for Optimizing Macronutrient Availability, Acquisition, and Distribution for Perennial Fruit Crops" Agronomy 10, no. 11: 1738. https://doi.org/10.3390/agronomy10111738
APA StyleKalcsits, L., Lotze, E., Tagliavini, M., Hannam, K. D., Mimmo, T., Neilsen, D., Neilsen, G., Atkinson, D., Casagrande Biasuz, E., Borruso, L., Cesco, S., Fallahi, E., Pii, Y., & Valverdi, N. A. (2020). Recent Achievements and New Research Opportunities for Optimizing Macronutrient Availability, Acquisition, and Distribution for Perennial Fruit Crops. Agronomy, 10(11), 1738. https://doi.org/10.3390/agronomy10111738








