Effect of Biostimulants and Storage on Discoloration Potential of Carrot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Storage Conditions
2.3. Sample Preparation
Preparation of Extracts for Measurement of Polyphenolic Compounds and Antioxidant Capacity FRAP
2.4. Isolation and Determination of Pectin Fractions
2.5. Determination of Total Polyphenolic Compounds
2.6. Determination of Chlorogenic Acid
2.7. Determination of Flavonoids
2.8. Determination of Total Anthocyanins
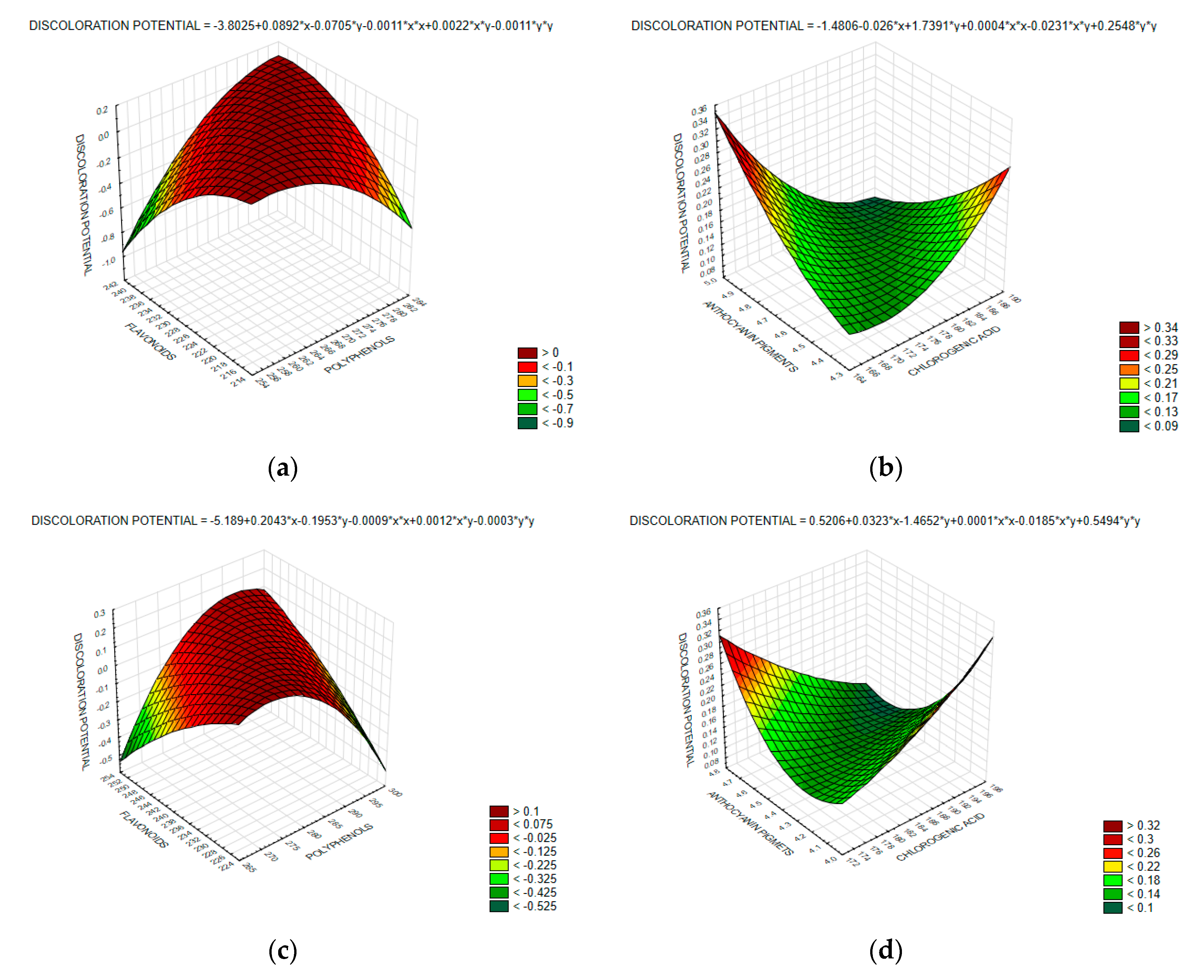
2.9. Determination of Discoloration Potential
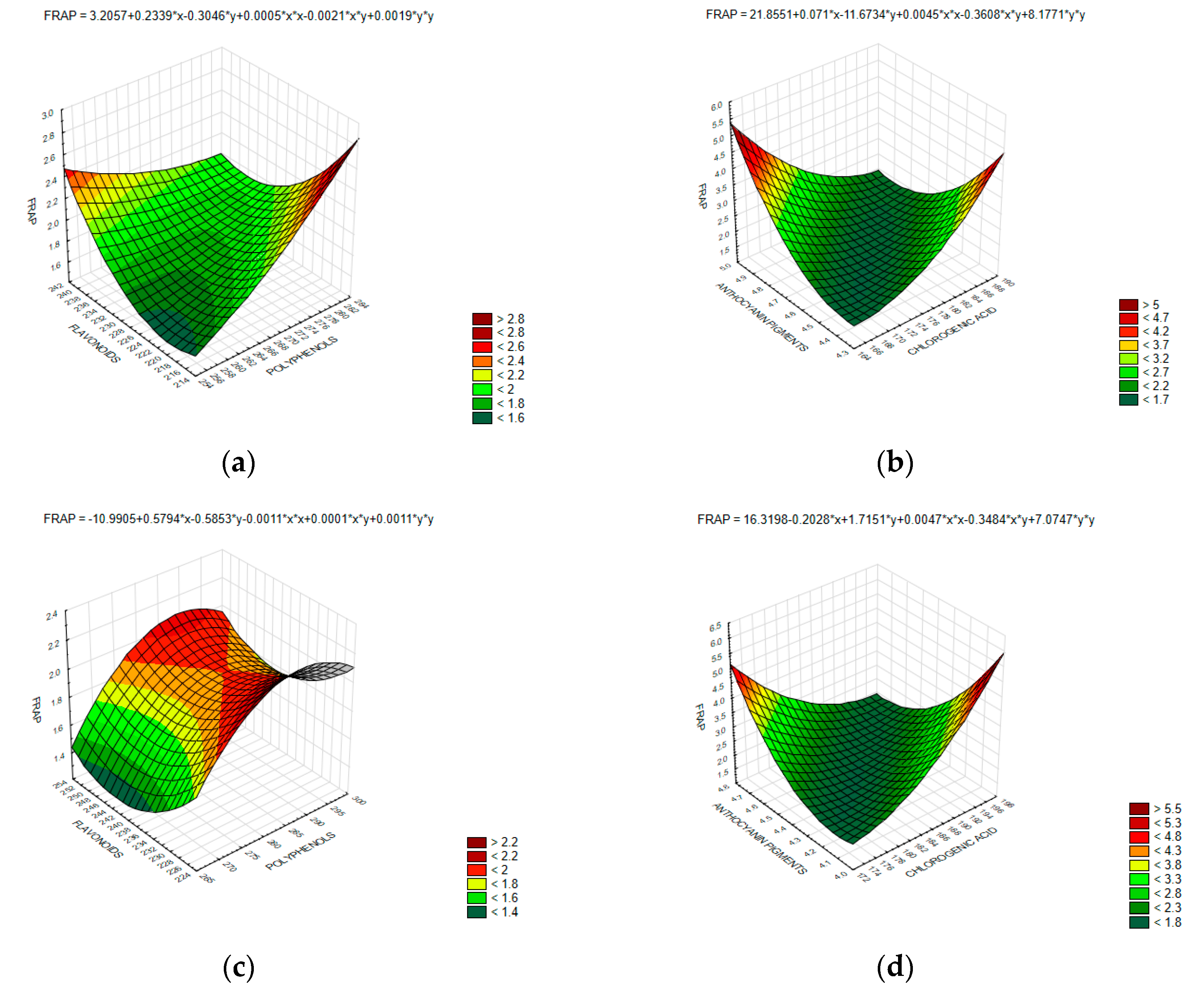
2.10. Assessment of the Antioxidant Capacity FRAP
2.11. Determination of the Antioxidant Capacity by the ABTS+• Cation Radical Method
2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karklelienė, R.; Radzevičius, A.; Dambrauskienė, E.; Duchovskienė, L.; Bobinas, Č.; Kavaliauskaitė, D. Reproduction features of organically grown edible carrot cultivars (Daucus sativus Röhl.) in Lithuania. Agron. Res. 2009, 7, 305–310. [Google Scholar]
- Keutgen, A.J.; Wszelaczyńska, E.; Pobereżny, J. Influence of cultivar and UGmax on antioxidative properties of carrot roots (Daucus carota L.) and their stability during freezing process. Environ. Prot. Nat. Resour. 2014, 25, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Keutgen, A.J.; Wszelaczyńska, E.; Pobereżny, J.; Kozera, W.; Knapowski, T.; Mozolewski, W. Właściwości prozdrowotne marchwi (Daucus carota L.) jako funkcja odmiany oraz stosowania użyźniacza UGmax. Ekol. Technol. 2015, 23, 206–210. (In Polish) [Google Scholar]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jihong, W.; Haiyan, G.; Lei, Z.; Xiaojun, L.; Fang, C.; Zhnefu, W.; Xiaosong, H. Chemical com-positional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Perera, C.O. Selected quality attributes of dried foods. Dry. Technol. 2005, 23, 717–730. [Google Scholar] [CrossRef]
- Pellegrini, N.; Simonetti, P.; Gardana, C.; Brenna, O.; Brighenti, F.; Pietta, P. Polyphenol content and total antioxidant activity of vini novelli (young red wines). J. Agric. Food Chem. 2000, 48, 732–735. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Szczepanek, M.; Siwik-Ziomek, A. P and K accumulation by rapeseed as affected by biostimulant under different NPK and S fertilization doses. Agronomy 2019, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J.; Ochmian, I. Response of onion (Allium cepa L.) to the method of seaweed biostimulant application. Acta Sci. Pol. Hortorum Cultus 2017, 16, 113–122. [Google Scholar]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Szczepanek, M.; Wilczewski, E.; Pobereżny, J.; Wszelaczyńska, E.; Ochmian, I. Carrot root size distribution in response to biostimulant application. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 334–339. [Google Scholar] [CrossRef]
- Wszelaczyńska, E.; Szczepanek, M.; Pobereżny, J.; Kazula, M. Effect of biostimulant application and long-term storage on the nutritional value of carrot. Hortic. Bras. 2019, 37, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Pobereżny, J.; Szczepanek, M.; Wszelaczyńska, E.; Prus, P. The quality of carrot after field biostimulant application and after storage. Sustainability 2020, 12, 1386. [Google Scholar] [CrossRef] [Green Version]
- Plant Growth in Harmony with Nature. Available online: https://www.kelpak.com/ (accessed on 6 November 2020).
- Anyszka, Z. Metodyka integrowanej produkcji marchwi. Na podstawie art. 57 ust. 2 pkt 2 ustawy z dnia 8 marca 2013 r. o środkach ochrony roślin. In (Dz.U. z 2018 r. poz. 1310) Zatwierdzona przez Głównego Inspektora Ochrony Roślin i Nasiennictwa; Instytut Ogrodnictwa: Warszawa, Poland, 2018. (In Polish) [Google Scholar]
- Keutgen, A.J.; Pawelzik, E. Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001, 73, 393–396. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Bain, H.; Dale, M.F.B. Development of rapid colorimetric method for the determination of chlorogenic acid in freezedried potato tubers. J. Sci. Food Agric. 1992, 58, 41–48. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Dean, B.B.; Jakowiak, N.; Nagle, M.; Pavek, J.; Corsini, D. Blackspot pigment development of resistant and susceptible Solanum tuberosum L. genotypes at harvest and during storage measured by three methods of evaluation. Am. Potato J. 1993, 70, 201–217. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Keutgen, A.; Pobereżny, J.; Wszelaczyńska, E.; Murawska, B.; Spychaj-Fabisiak, E. Wpływ przechowywania na procesy ciemnienia bulw ziemniaka (Solanum tuberosum L.) i ich właściwości prozdrowotne. Inż. Ap. Chem. 2014, 53, 86–88. (In Polish) [Google Scholar]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their efects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Wszelaczyńska, E. Wpływ nawożenia magnezem na zawartość kwasów organicznych i ciemnienie miąższu bulw ziemniaka odmiany Mila. Acta Sci. Pol. Agric. 2004, 3, 175–186. (In Polish) [Google Scholar]
- Koley, T.K.; Singh, S.; Khemariya, P.; Sarkar, A.; Kaur, C.; Chaurasia, S.N.S.; Naik, P.S. Evaluation of bioactive properties of Indian carrot (Daucus carota L.): A chemometric approach. Food Res. Inter. 2014, 60, 76–85. [Google Scholar] [CrossRef]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef]
- Singh, B.K.; Koley, T.K.; Maurya, A.; Singh, P.M.; Singh, B. Phytochemical and antioxidative potential of orange, red, yellow, rainbow and black coloured tropical carrots (Daucus carota subsp. sativus Schubl. & Martens). Physiol. Mol. Biol. Plants 2018, 24, 899–907. [Google Scholar] [CrossRef]
- Yangilar, F. The Application of Dietary Fibre in Food Industry: Structural Features, Effects on Health and Definition, Obtaining and Analysis of Dietary Fibre: A Review. J. Food Nutr. Res. 2013, 1, 13–23. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of diferentially expressed genes in Solanum Lycopersicon, L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sra, S.K.; Sandhu, K.S.; Ahluwalia, P. Effect of treatments and packaging on the quality of dried carrot slices during storage. J. Food Sci. Technol. 2014, 51, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Wang-Pruski, G.; Nowak, J. Potato after-cooking darkening. Am. J. Potato Res. 2004, 81, 7–16. [Google Scholar] [CrossRef]
- Singh, D.P.; Beloy, J.; McInerney, J.K.; Day, L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrots (Daucus carota). Food Chem. 2012, 132, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kreutzmann, S.; Christensen, L.P.; Edelenbos, M. Investigation of bitterness in carrots (Daucus carota L.) based on quantitative chemical and sensory analyses. LWT Food Sci. Technol. 2008, 41, 193–205. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.; Boukhari, M.; Barakate, M.; Youness Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R. Processing effects on carrot phytonutrients. Hortic. Sci. 2006, 41, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Bartley, G.E.; Avena-Bustillos, R.J.; Du, W.-X.; Hidalgo, M.; Cain, B.; Breksa, A.P. Transcriptional regulation of chlorogenic acid biosynthesis in carrot root slices exposed to UV-B light. Plant Gene 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Augšpole, I.; Kince, T.; Cinkmanis, I. Changes of polyphenol compound concentrations in hybrids of nante type carrots during storage. Proceedings of the Latvian Academy of Sciences. Sect. B. Nat. Exact Appl. Sci. 2017, 71, 492–495. [Google Scholar] [CrossRef] [Green Version]
- Søltoft, M.; Nielsen, J.; Holst Laursen, K.; Husted, S.; Halekoh, U.; Knuthsen, P. Effects of organic and conventional growth systems on the content of flavonoids in onions and phenolic acids in carrots and potatoes. J. Agric. Food Chem. 2010, 58, 10323–10329. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruom, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Ranjitha, K.; Rao, D.S.; Shivashankara, K.S.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 2017, 42, 91–100. [Google Scholar] [CrossRef]
- Lazcano, C.A.; Yoo, K.S.; Pike, L.M. A method for measuring anthocyanins after removing carotenes in purple colored carrots. Sci. Hortic. Amst. 2001, 90, 321–324. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Detection of peonidin and pelargonidin glycosides in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Compos. Anal. 2016, 51, 76–81. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kidoń, M.; Czapski, J. Ocena zmian zawartości składników bioaktywnych oraz zdolności antyoksydacyjnej soków z marchwi purpurowej podczas przechowywania (Assessment of the changes in the bioactive components and anti-oxidizing properties of black carrot juice during storage). Bromat. Chem. Toksykol. 2009, 3, 848–853. (In Polish) [Google Scholar]
- Koley, T.K.; Kaur, C.; Nagal, S.; Walia, S.; Jaggi, S.; Sarika, J. Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk). Arab. J. Chem. 2011, 9, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
| Biostimulant | Rates (dm3 ha−1) | Date of Application |
|---|---|---|
| Control | - | - |
| Kelpak 1 | 3 | in 4-leaf stage (BBCH 14) |
| Kelpak 2 | 2 | in 4-leaf stage (BBCH 14) |
| Kelpak 3 | 3 + 2 | in 4-leaf stage (BBCH 14) + 14 days after 1st treatment |
| Kelpak 4 | 2 + 2 | |
| Kelpak 5 | 3 + 2 + 2 | in 4-leaf stage (BBCH 14) + 14 days after 1st treatment + 28 days after 1st treatment |
| Kelpak 6 | 2 + 2 + 2 | |
| Kelpak 7 | 3 + 2 | in 4-leaf stage (BBCH 14) + 28 days after 1st treatment |
| Kelpak 8 | 2 + 2 | |
| Asahi | 0.5 + 0.5 | in 4-leaf stage (BBCH 14) + 14 days after 1st treatment |
| Biostimulant | Discoloration Potential [AU475] | FRAP [mM Fe2 kg−1 FW] | ABTS [mM Trolox kg−1] | Pectins [g kg−1 FW] |
|---|---|---|---|---|
| After harvest | ||||
| Control | 0.143 ± 0.023 c | 1.66 ± 0.05 a | 0.655 ± 0.0124 a | 10.37 ± 0.39 a |
| Kelpak 1 | 0.099 ± 0.007 a | 1.84 ± 0.08 de | 0.822 ± 0.0212 b | 11.57 ± 0.47 cd |
| Kelpak 2 | 0.095 ± 0.008 a | 1.87 ± 0.07 e | 0.921 ± 0.0242 c | 11.67 ± 0.45 d |
| Kelpak 3 | 0.131 ± 0.020 b | 1.72 ± 0.08 a–d | 0.666 ± 0.0341 a | 10.75 ± 0.41 ab |
| Kelpak 4 | 0.135 ± 0.025 b | 1.69 ± 0.06 a–d | 0.651 ± 0.0164 a | 10.65 ± 0.38 ab |
| Kelpak 5 | 0.134 ± 0.021 b | 1.74 ± 0.08 ab | 0.659 ± 0.0235 a | 10.89 ± 0.37 a–c |
| Kelpak 6 | 0.135 ± 0.019 b | 1.76 ± 0.08 a–e | 0.802 ± 0.03144 b | 11.27 ± 0.36 b–d |
| Kelpak 7 | 0.135 ± 0.022 b | 1.78 ± 0.09 a–e | 0.801 ± 0.04121 b | 11.06 ± 0.37 a–d |
| Kelpak 8 | 0.126 ± 0.023 ab | 1.81 ± 0.07 b–e | 0.831 ± 0.0333 b | 11.47 ± 0.36 cd |
| Asahi | 0.110 ± 0.002 a | 1.84 ± 0.07 c–e | 0.829 ± 0.0123 b | 10.45 ± 0.49 a |
| Mean Kelpak Mean total | 0.124 0.124 | 1.78 1.77 | 0.769 0.764 | 11.17 11.01 |
| After storage | ||||
| Control | 0.148 ± 0.029 c | 1.73 ± 0.07 a | 0.666 ± 0.02852 a | 10.03 ± 0.51 a |
| Kelpak 1 | 0.109 ± 0.003 a | 1.92 ± 0.08 bc | 0.934 ± 0.0324 d | 11.35 ± 0.72 b |
| Kelpak 2 | 0.106 ± 0.093 a | 1.94 ± 0.06 c | 0.930 +± 0.0124 d | 11.45 ± 0.74 b |
| Kelpak 3 | 0.133 ± 0.024 b | 1.79 ± 0.08 ab | 0.721 ± 0.0341 bc | 10.52 ± 0.66 ab |
| Kelpak 4 | 0.140 ± 0.030 b | 1.76 ± 0.08 ab | 0.674 ± 0.01658 ab | 10.24 ± 0.47 ab |
| Kelpak 5 | 0.139 ± 0.026 b | 1.81 ± 0.10 a–c | 0.704 ± 0.0328 ab | 10.69 ± 0.59 ab |
| Kelpak 6 | 0.139 ± 0.019 b | 1.84 ± 0.09 a–c | 0.744 ± 0.0328 c | 11.13 ± 0.75 ab |
| Kelpak 7 | 0.138 ± 0.023 b | 1.85 ± 0.10 a–c | 0.738 ± 0.01899 c | 10.87 ± 0.67 ab |
| Kelpak 8 | 0.130 ± 0.026 ab | 1.88 ± 0.09 bc | 0.754 ± 0.0234 c | 11.15 ± 0.76 ab |
| Asahi | 0.118 ± 0.055 a | 1.91 ± 0.07 bc | 0.928 ± 0.03777d | 10.17 ± 0.62 a |
| Mean Kelpak Mean total | 0.129 0.130 | 1.85 1.84 | 0.775 0.779 | 10.93 10.76 |
| Susceptibility Classes | Colorimetric Method [AU475] |
|---|---|
| Resistant to darkening processes | 0–0.20 |
| Moderately resistant to darkening processes | 0.21–0.40 |
| Moderately susceptible to darkening processes | 0.41–0.60 |
| Susceptible to darkening processes | 0.61–0.80 |
| Very susceptible to darkening processes | >0.80 |
| Biostimulant | Discoloration Potential | FRAP †† | ABTS †† | Pectin | Polyphenols | Chlorogenic Acid | Flavonoids | Anthocyanins |
|---|---|---|---|---|---|---|---|---|
| Control | +3.5 * | +4.2 | +1.7 | −3.3 | +4.5 | +4.8 | +5.5 | −3.3 |
| Asahi | +7.3 | +3.8 | +11.9 | −2.7 | +4.9 | +4.1 | +5.4 | −4.0 |
| Kelpak † | +4.6 | +3.9 | −2.0 | −2.3 | +4.8 | +3.3 | +5.2 | −3.4 |
| Biostimulant | Polyphenols [mg kg−1 FW] | Chlorogenic Acid [mg kg−1 FW] | Flavonoids [mg kg−1 FW] | Anthocyanin Pigments [mg kg−1 FW] |
|---|---|---|---|---|
| After harvest | ||||
| Control | 260.8 ± 6.8 a | 169.9 ± 4.0 a | 21.9 ± 4.0 a | 4.45 ± 0.08 a |
| Kelpak 1 | 276.9 ± 6.1 cd | 182.6 ± 1.0 c–f | 23.2 ± 2.9 ef | 4.67 ± 0.03 c–e |
| Kelpak 2 | 277.2 ± 6.1 d | 188.0 ± 1.3 f | 23.6 ± 3.9 f | 4.87 ± 0.04 g |
| Kelpak 3 | 268.3 ± 2.8 b | 178.2 ± 1.7 b–d | 22.7 ± 1.9 b–d | 4.62 ± 0.04 cd |
| Kelpak 4 | 268.2 ± 2.8 b | 175.4 ± 4.9 bc | 22.5 ± 1.6 bc | 4.53 ± 0.04 bc |
| Kelpak 5 | 268.6 ± 2.8 ab | 179.8 ± 2.7 b–e | 22.8 ± 2.2 b–e | 4.68 ± 0.07 c–f |
| Kelpak 6 | 270.0 ± 2.4 bc | 184.0 ± 4.7 d–f | 23.0 ± 1.1 c–e | 4.74 ± 0.04 ef |
| Kelpak 7 | 269.3 ± 1.4 b | 182.4 ± 3.6 c–f | 22.9 ± 1.7 c–e | 4.72 ± 0.05 d–f |
| Kelpak 8 | 270.2 ± 7.1 bc | 185.4 ± 3.1 f | 23.1 ± 1.1 de | 4.77 ± 0.04 fg |
| Asahi | 267.5 ± 6.3 ab | 172.5 ± 5.1 ab | 22.4 ± 1.3 ab | 4.49 ± 0.06 ab |
| Mean Kelpak Mean total | 271.1 269.7 | 182.0 179.8 | 23.0 22.8 | 4.70 4.65 |
| After storage | ||||
| Control | 272.6 ± 7.1 a | 178.1 ± 3.2 a | 23.1 ± 4.9 a | 4.25 ± 0.10 a |
| Kelpak 1 | 286.4 ± 6.3 cd | 186.6 ± 1.0 c–e | 24.6 ± 3.1 de | 4.56 ± 0.02 c–f |
| Kelpak 2 | 287.7 ± 6.4 d | 194.7 ± 1.7 g | 24.8 ± 3.8 e | 4.70 ± 0.03 f |
| Kelpak 3 | 282.5 ± 3.0 b | 184.8 ± 1.8 cd | 23.9 ± 2.1 bc | 4.45 ± 0.04 b–d |
| Kelpak 4 | 281.4 ± 2.9 b | 181.3 ± 1.8 bc | 23.7 ± 3.4 bc | 4.39 ± 0.12 bc |
| Kelpak 5 | 283.8 ± 2.9 ab | 187.2 ± 2.8 c–f | 24.0 ± 2.7 bc | 4.50 ± 0.07 b–e |
| Kelpak 6 | 285.2 ± 2.5 b | 189.7 ± 1.6 ef | 24.3 ± 2.8 cd | 4.60 ± 0.12 def |
| Kelpak 7 | 284.5 ± 2.9 b | 188.7 ± 1.9 d–f | 24.2 ± 2.9 cd | 4.56 ± 0.09 c–f |
| Kelpak 8 | 286.5 ± 2.5 bc | 190.8 ± 1.4 fg | 24.3 ± 2.0 c–e | 4.64 ± 0.08 ef |
| Asahi | 280.6 ± 2.5 ab | 179.5 ± 2.4 ab | 23.6 ± 3.1 ab | 4.31 ± 0.13 ab |
| Mean Kelpak Mean total | 282.4 280.9 | 188.0 186.2 | 24.2 24.0 | 4.55 4.50 |
| Characteristics | DP † | FRAP † | ABTS † | Polyphenols | Flavonoids | Chlorogenic Acid | Anthocyanin Pigments |
|---|---|---|---|---|---|---|---|
| After harvest | |||||||
| Pectins | −0.503 | 0.569 | 0.702 | 0.749 | 0.910 | 0.926 | 0.811 |
| DP | n.s | n.s | n.s | n.s | −0.474 | −0.477 | |
| FRAP | 0.858 | 0.836 | 0.671 | 0.592 | n.s | ||
| ABTS | 0.719 | 0.729 | 0.711 | 0.603 | |||
| Polyphenols | 0.848 | 0.727 | 0.543 | ||||
| Flavonoids | 0.929 | 0.855 | |||||
| Chlorogenic acid | 0.936 | ||||||
| After storage | |||||||
| Pectins | n.s | n.s | 0.728 | 0.811 | 0.915 | 0.694 | 0.864 |
| DP | n.s | n.s | n.s | n.s | n.s | n.s | |
| FRAP | 0.695 | 0.697 | 0.522 | n.s | n.s | ||
| ABTS | 0.634 | 0.674 | 0.730 | 0.588 | |||
| Polyphenols | 0.892 | 0.540 | 0.734 | ||||
| Flavonoids | 0.803 | 0.938 | |||||
| Chlorogenic acid | 0.929 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanek, M.; Pobereżny, J.; Wszelaczyńska, E.; Gościnna, K. Effect of Biostimulants and Storage on Discoloration Potential of Carrot. Agronomy 2020, 10, 1894. https://doi.org/10.3390/agronomy10121894
Szczepanek M, Pobereżny J, Wszelaczyńska E, Gościnna K. Effect of Biostimulants and Storage on Discoloration Potential of Carrot. Agronomy. 2020; 10(12):1894. https://doi.org/10.3390/agronomy10121894
Chicago/Turabian StyleSzczepanek, Małgorzata, Jarosław Pobereżny, Elżbieta Wszelaczyńska, and Katarzyna Gościnna. 2020. "Effect of Biostimulants and Storage on Discoloration Potential of Carrot" Agronomy 10, no. 12: 1894. https://doi.org/10.3390/agronomy10121894
APA StyleSzczepanek, M., Pobereżny, J., Wszelaczyńska, E., & Gościnna, K. (2020). Effect of Biostimulants and Storage on Discoloration Potential of Carrot. Agronomy, 10(12), 1894. https://doi.org/10.3390/agronomy10121894






