Long-Term Benefits of Protecting Table Grape Vineyards against Trunk Diseases in the California Desert
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fungal Isolation and Identification
2.3. Statistical Analysis
2.4. Economic Analysis
2.4.1. Avoided Replanting Cost
2.4.2. Yield Effects
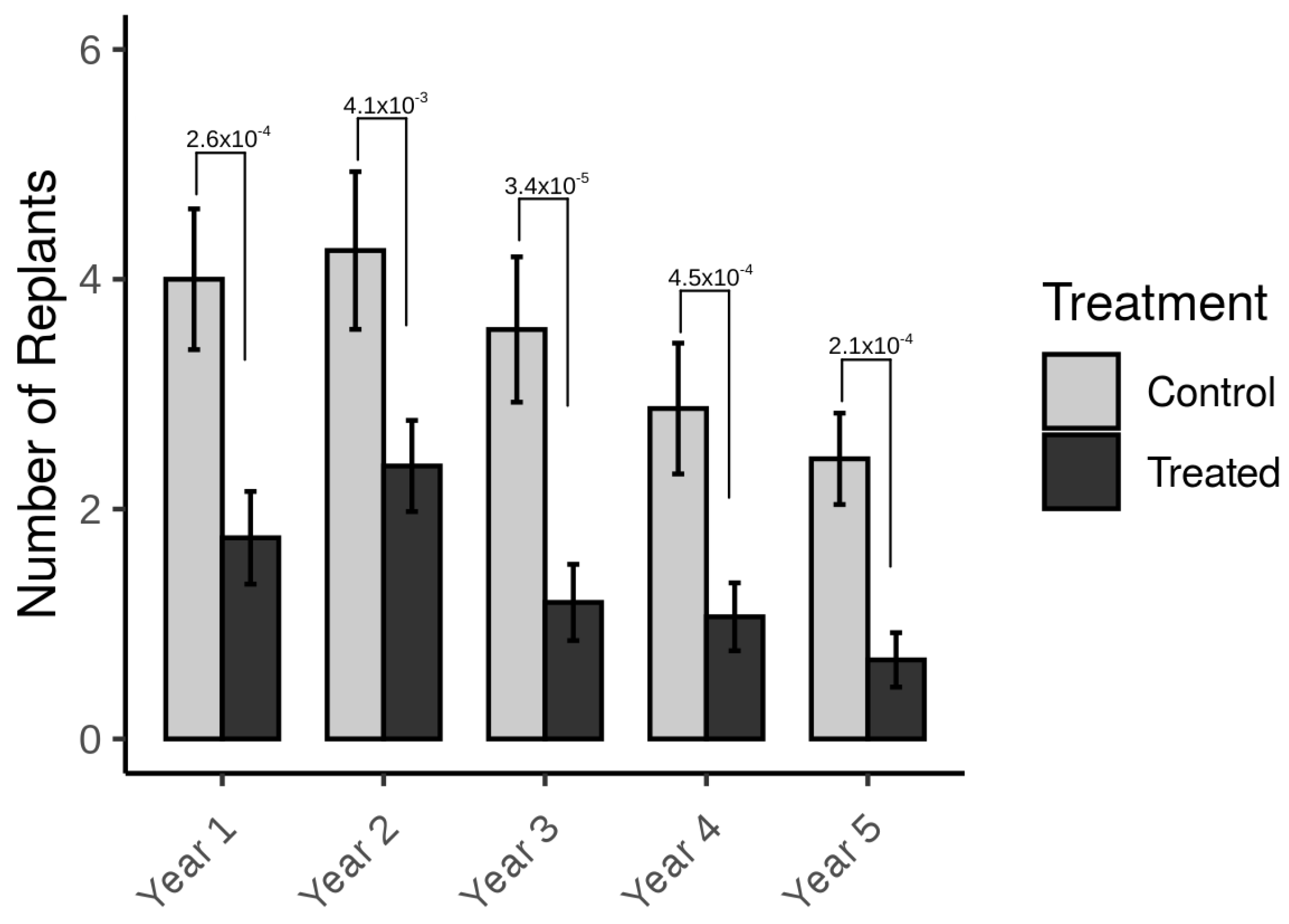
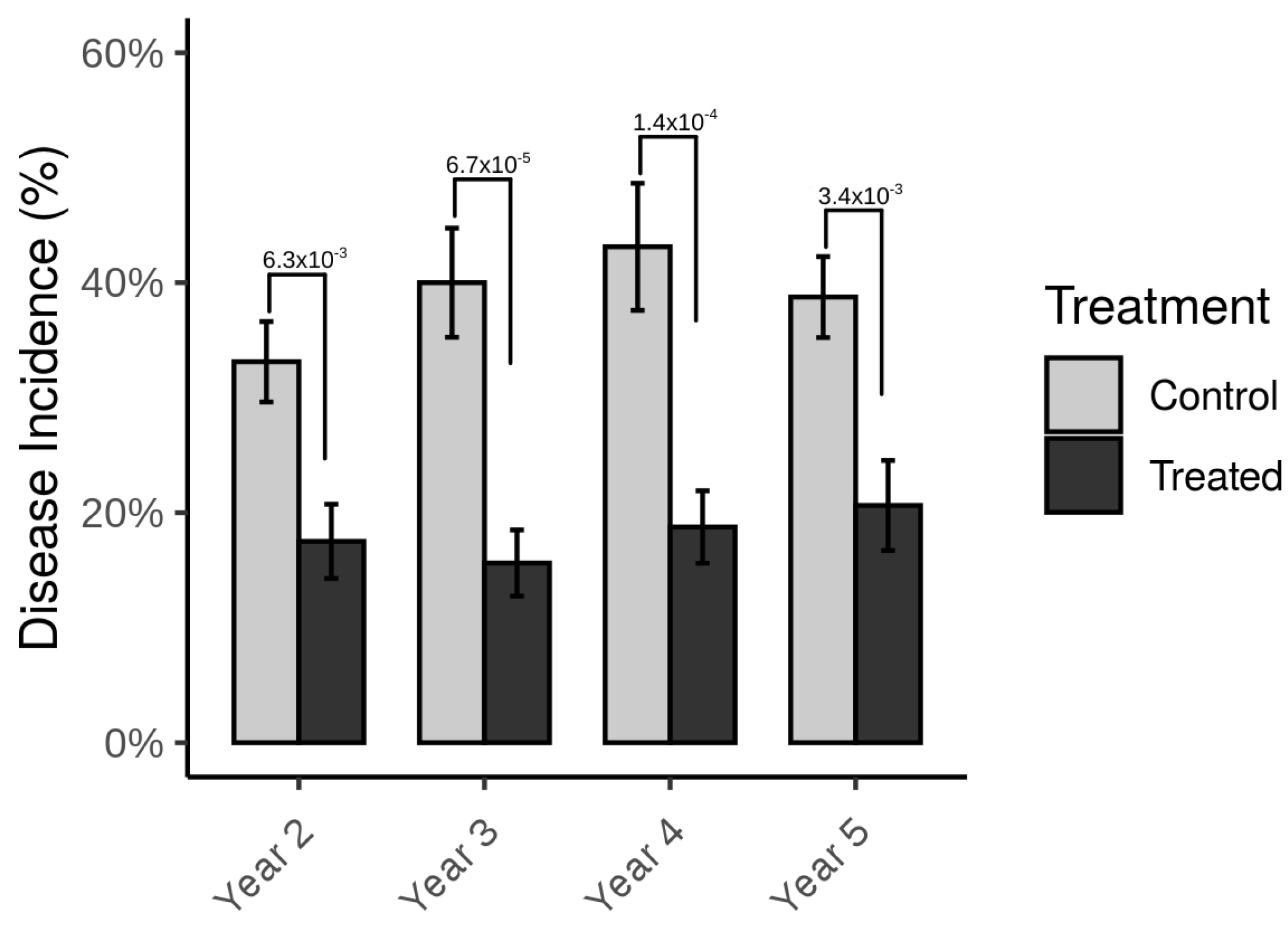
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OIV, International Organisation of Vine and Wine. Statistiical Report on World Vitiviniculture. 2019. Available online: http://oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 15 November 2020).
- USDA-NASS, United States Department of Agriculture National Agricultural Statistics Service. Statistics of Fruits, Tree Nuts, and Horticultural Specialties. 2019. Available online: https://www.nass.usda.gov/Publications/Ag_Statistics/ (accessed on 15 November 2020).
- CDFA, California Department of Food and Agriculture. California Agricultural Production Statistics. 2019. Available online: https://www.cdfa.ca.gov/statistics/ (accessed on 15 November 2020).
- Urbez-Torres, J.R.; Battany, M.; Bettiga, L.J.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R.J.; Verdegaal, P.; Gubler, W.D. Botryosphaeriaceae Species Spore-Trapping Studies in California Vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebert, J.B. Eutypa the economic toll on vineyards. Wines Vines 2001, April, 50–56. [Google Scholar]
- Kaplan, J.; Travadon, R.; Cooper, M.; Hillis, V.; Lubell, M.; Baumgartner, K. Identifying economic hurdles to early adoption of preventative practices: The case of trunk diseases in California winegrape vineyards. Wine Econ. Policy 2016, 5, 127–141. [Google Scholar] [CrossRef]
- Duthie, J.A.; Munkvold, G.P.; Marois, J.J. Relationship between age of vineyard and incidence of Eutypa dieback. Phytopathology 1991, 81, 1183. [Google Scholar]
- Munkvold, G.P.; Duthie, J.A.; Marois, J.J. Reductions in Yield and Vegetative Growth of Grapevines Due to Eutypa Dieback. Phytopathology 1994, 84, 186–192. [Google Scholar] [CrossRef]
- Petit, E.; Barriault, E.; Baumgartner, K.; Wilcox, W.F.; Rolshausen, P.E. Cylindrocarpon Species Associated with Black-Foot of Grapevine in Northeastern United States and Southeastern Canada. Am. J. Enol. Vitic. 2011, 62, 177–183. [Google Scholar] [CrossRef]
- Baumgartner, K.; Rizzo, D.M. Spread of Armillaria root disease in a California vineyard. Am. J. Enol. Vitic. 2002, 53, 197–203. [Google Scholar]
- van Niekerk, J.M.; Calitz, F.J.; Halleen, F.; Fourie, P.H. Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa. Eur. J. Plant Pathol. 2010, 127, 375–390. [Google Scholar] [CrossRef]
- Agusti-Brisach, C.; Gramaje, D.; Garcia-Jimenez, J.; Armengol, J. Detection of black-foot and Petri disease pathogens in soils of grapevine nurseries and vineyards using bait plants. Plant Soil 2013, 364, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Molnar, M.; Voegele, R.T.; Fischer, M. Grapevine trunk diseases in German viticulture IV. Spreading of spores of Phaeomoniella chlamydospora in Esca-affected vineyards. Vitis 2020, 59, 63–69. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Nouri, M.T.; Trouillas, F.P. Taxonomy and multi-locus phylogeny of cylindrocarpon-like species associated with diseased roots of grapevine and other fruit and nut crops in California. Fungal Syst. Evol. 2019, 4, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Urbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar]
- Rolshausen, P.E.; Baumgartner, K.; Travadon, R.; Fujiyoshi, P.; Pouzoulet, J.; Wilcox, W.F. Identification of Eutypa spp. Causing Eutypa Dieback of Grapevine in Eastern North America. Plant Dis. 2014, 98, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine Trunk Diseases in British Columbia: Incidence and Characterization of the Fungal Pathogens Associated with Black Foot Disease of Grapevine. Plant Dis. 2014, 98, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Pouzoulet, J.; Pivovaroff, A.L.; Santiago, L.S.; Rolshausen, P.E. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 5, 253. [Google Scholar] [CrossRef]
- Petzoldt, C.H.; Moller, W.J.; Sall, M.A. Eutypa Dieback of Grapevine—Seasonal Differences in Infection and Duration of Susceptibility of Pruning Wounds. Phytopathology 1981, 71, 540–543. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Urbez-Torres, J.R.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of Pruning Wound Susceptibility and Protection against Fungi Associated with Grapevine Trunk Diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar]
- Hillis, V.; Lubell, M.; Kaplan, J.; Baumgartner, K. Preventative Disease Management and Grower Decision Making: A Case Study of California Wine-Grape Growers. Phytopathology 2017, 107, 704–710. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 315–322. [Google Scholar] [CrossRef]
- Baumgartner, K.; Hillis, V.; Lubell, M.; Norton, M.; Kaplan, J. Managing Grapevine Trunk Diseases in California’s Southern San Joaquin Valley. Am. J. Enol. Vitic. 2019, 70, 267–276. [Google Scholar] [CrossRef]
- Fidelibus, M.; El-Kereamy, A.; Haviland, D.; Hembree, K.; Zhuang, G.; Stewart, D.; Sumner, D.A. Sample Costs to Establish and Produce Table Grapes: San Joaquin Valley South; University of California Cooperative Extension, Department of Agricultural and Resource Economics, UC Davis: Davis, CA, USA, 2018; Available online: http://coststudies.ucdavis.edu/ (accessed on 15 November 2020).
- Fuller, K.B.; Alston, J.M.; Golino, D.A. Economic Benefits from Virus Screening: A Case Study of Grapevine Leafroll in the North Coast of California. Am. J. Enol. Vitic. 2019, 70, 139–146. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Mundy, D.C. Pruning Wound Protection Strategies for Simultaneous Control of Eutypa and Botryosphaeria Dieback in New Zealand. Plant Dis. 2019, 103, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, G.A.; Latorre, B.A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop Prot. 2013, 46, 106–112. [Google Scholar] [CrossRef]
- Halleen, F.; Fourie, P.H.; Lombard, P.J. Protection of Grapevine Pruning Wounds against Eutypa lata by Biological and Chemical Methods. South Afr. J. Enol. Vitic. 2010, 31, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Hillis, V.; Lubell, M.; Kaplan, J.; Doll, D.; Baumgartner, K. The Role of Pest Control Advisers in Preventative Management of Grapevine Trunk Diseases. Phytopathology 2016, 106, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.A.; Trouillas, F.P.; Gubler, W.D. Double pruning of grapevines: A cultural practice to reduce infections by Eutypa lata. Am. J. Enol. Vitic. 2007, 58, 61–66. [Google Scholar]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases with Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Armengol, J. Fungal Trunk Pathogens in the Grapevine Propagation Process: Potential Inoculum Sources, Detection, Identification, and Management Strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Berlanas, C.; Ojeda, S.; Lopez-Manzanares, B.; Andres-Sodupe, M.; Bujanda, R.; Martinez-Diz, M.; Diaz-Losada, E.; Gramaje, D. Occurrence and Diversity of Black-Foot Disease Fungi in Symptomless Grapevine Nursery Stock in Spain. Plant Dis. 2020, 104, 94–104. [Google Scholar] [CrossRef]
- Urbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae Species Isolated from Grapevine Cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Rolshausen, P.E.; Akgul, D.S.; Perez, R.; Eskalen, A.; Gispert, C. First Report of Wood Canker Caused by Neoscytalidium dimidiatum on Grapevine in California. Plant Dis. 2013, 97, 1511. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (Black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorquin, J.S.; Wang, D.H.; Twizeyimana, M.; Eskalen, A. Identification, Distribution, and Pathogenicity of Diatrypaceae and Botryosphaeriaceae Associated with Citrus Branch Canker in the Southern California Desert. Plant Dis. 2016, 100, 2402–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, W.M.; Trouillas, F.P.; Gubler, W.D.; Savocchia, S.; Sosnowski, M.R. Pathogenicity of Diatrypaceous Fungi on Grapevines in Australia. Plant Dis. 2013, 97, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem Vessel Diameter Affects the Compartmentalization of the Vascular Pathogen Phaeomoniella chlamydospora in Grapevine. Front. Plant Sci. 2017, 8, 1442. [Google Scholar] [CrossRef] [Green Version]
- Spagnolo, A.; Magnin-Robert, M.; Alayi, T.D.; Cilindre, C.; Schaeffer-Reiss, C.; Van Dorsselaer, A.; Clement, C.; Larignon, P.; Ramirez-Suero, M.; Chong, J.; et al. Differential Responses of Three Grapevine Cultivars to Botryosphaeria Dieback. Phytopathology 2014, 104, 1021–1035. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gispert, C.; Kaplan, J.D.; Deyett, E.; Rolshausen, P.E. Long-Term Benefits of Protecting Table Grape Vineyards against Trunk Diseases in the California Desert. Agronomy 2020, 10, 1895. https://doi.org/10.3390/agronomy10121895
Gispert C, Kaplan JD, Deyett E, Rolshausen PE. Long-Term Benefits of Protecting Table Grape Vineyards against Trunk Diseases in the California Desert. Agronomy. 2020; 10(12):1895. https://doi.org/10.3390/agronomy10121895
Chicago/Turabian StyleGispert, Carmen, Jonathan D. Kaplan, Elizabeth Deyett, and Philippe E. Rolshausen. 2020. "Long-Term Benefits of Protecting Table Grape Vineyards against Trunk Diseases in the California Desert" Agronomy 10, no. 12: 1895. https://doi.org/10.3390/agronomy10121895



