Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review
Abstract
:1. Introduction
1.1. Stomatal Development
1.2. Stomatal Opening
2. Light Regulation of Stomatal Development and Opening
2.1. Stomatal Development and Light
2.2. Stomatal Opening and Light
3. Carbon Dioxide Regulation of Stomatal Development and Opening
3.1. Stomatal Development and Carbon Dioxide
3.2. Stomatal Opening and Carbon Dioxide
4. Effect of Temperature on Stomatal Development and Opening
4.1. Stomatal Development and Temperature
4.2. Stomatal Opening and Temperature
5. Effect of Relative Humidity on Stomatal Development and Opening
5.1. Stomatal Development and Humidity
5.2. Stomatal Opening and Humidity
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zoulias, N.; Harrison, E.L.; Casson, S.A.; Gray, J.E. Molecular control of stomatal development. Biochem. J. 2018, 475, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, A.; Anderegg, W.R.L.; Pacala, S.W. Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proc. Natl. Acad. Sci. USA 2016, 113, E7222–E7230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waisel, Y.; Eshel, A.; Beeckman, T.; Kafkafi, U. Plant Roots: The Hidden Half. Ann. Bot. 2002, 90, 775–776. [Google Scholar]
- Richardson, F.; Brodribb, T.J.; Jordan, G.J. Amphistomatic leaf surfaces independently regulate gas exchange in response to variations in evaporative demand. Tree Physiol. 2017, 37, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of Stomatal Development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef] [Green Version]
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Stomatal control as a driver of plant evolution. J. Exp. Bot. 2011, 62, 2419–2423. [Google Scholar] [CrossRef] [Green Version]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. Phytochrome B and PIF4 Regulate Stomatal Development in Response to Light Quantity. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.; Hamdani, S.; Bunce, J.A. The physiology and genetics of stomatal adjustment under fluctuating and stressed environments. Appl. Photosynth. New Prog. 2016. [Google Scholar] [CrossRef] [Green Version]
- KBecklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining plant physiological responses to climate change through an evolutionary lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar]
- Lee, L.R.; Bergmann, D.C. The plant stomatal lineage at a glance. J. Cell Sci. 2019, 132, jcs228551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, J.; Zou, J.; Yang, K.; Wang, M. Signaling to stomatal initiation and cell division. Front. Plant Sci. 2014, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lau, O.S.; Bergmann, D.C. Stomatal development: A plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 2012, 139, 3683–3692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, D.C.; Sack, F.D. Stomatal Development. Annu. Rev. Plant Biol. 2007, 58, 163–181. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Z.; Hou, S. SPEECHLESS Speaks Loudly in Stomatal Development. Front. Plant Sci. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Sloan, D.B.; Bogenschutz, N.L.; Torii, K.U. Termination of asymmetric cell division and differentiation of stomata. Nature 2006, 445, 501–505. [Google Scholar] [CrossRef]
- Lampard, G.R.; MacAlister, C.A.; Bergmann, D.C. Arabidopsis Stomatal Initiation Is Controlled by MAPK-Mediated Regulation of the bHLH SPEECHLESS. Science 2008, 322, 1113–1116. [Google Scholar] [CrossRef] [Green Version]
- LaRue, H.; Zhang, S. SCREAM in the making of stomata. Nat. Plants 2019, 5, 648–649. [Google Scholar] [CrossRef]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.-K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 Specify Three Cell-State Transitional Steps Leading to Arabidopsis Stomatal Differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Q.; Chen, T.; Niu, G.; Zhang, H.; Zhou, C.; Hong, Z. N-glycosylation is involved in stomatal development by modulating the release of active abscisic acid and auxin in Arabidopsis. J. Exp. Bot. 2020, 71, 5865–5879. [Google Scholar] [CrossRef]
- Han, Y.; Watanabe, S.; Shimada, H.; Sakamoto, A. Dynamics of the leaf endoplasmic reticulum modulate β-glucosidase-mediated stress-activated ABA production from its glucosyl ester. J. Exp. Bot. 2020, 71, 2058–2071. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, Y.; Von Schaewen, A.; Koiwa, H. Function of N-glycosylation in plants. Plant Sci. 2018, 274, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Guo, K.; Zhang, D.; Ince, M.; Jammes, F. ABA-glucose ester hydrolyzing enzyme ATBG1 and PHYB antagonistically regulate stomatal development. PLoS ONE 2019, 14, e0218605. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, M.; Hoecker, U. Auxin—A novel regulator of stomata differentiation. Trends Plant Sci. 2014, 19, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or Close the Gate—Stomata Action under the Control of Phytohormones in Drought Stress Conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flütsch, S.; Wang, Y.; Takemiya, A.; Vialet-Chabrand, S.; Klejchová, M.; Nigro, A.; Hills, A.; Lawson, T.; Blatt, M.R.; Santelia, D. Guard Cell Starch Degradation Yields Glucose for Rapid Stomatal Opening in Arabidopsis. Plant Cell 2020, 32, 2325–2344. [Google Scholar] [CrossRef] [PubMed]
- Robaina-Estévez, S.; Daloso, D.M.; Zhang, Y.; Fernie, A.R.; Nikoloski, Z. Resolving the central metabolism of Arabidopsis guard cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Choi, Y.; Burla, B.; Kim, Y.-Y.; Jeon, B.; Maeshima, M.; Yoo, J.-Y.; Martinoia, E.; Lee, Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008, 10, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.-I.; Kinoshita, T. Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.-M.; Kuchitsu, K. Early ABA Signaling Events in Guard Cells. J. Plant Growth Regul. 2005, 24, 296–307. [Google Scholar] [CrossRef]
- Arve, L.E.; Kruse, O.M.O.; Tanino, K.K.; Olsen, J.E.; Futsaether, C.M.; Torre, S. Growth in continuous high air humidity increases the expression of CYP707A-genes and inhibits stomatal closure. Environ. Exp. Bot. 2015, 115, 11–19. [Google Scholar] [CrossRef]
- Merilo, E.; Yarmolinsky, D.; Jalakas, P.; Parik, H.; Tulva, I.; Rasulov, B.; Kilk, K.; Kollist, H. Stomatal VPD Response: There Is More to the Story Than ABA. Plant Physiol. 2018, 176, 851–864. [Google Scholar] [CrossRef] [Green Version]
- Bögre, L.; Beemster, G.T. Plant Growth Signaling; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Sager, J.C.; Farlane, J.C.M. Chapter 1. Radiation, Growth Chamber Handbook; Iowa State University of Science and Technology: Ames, IA, USA, 2003. [Google Scholar] [CrossRef]
- Lake, J.A.; Quick, W.P.; Beerling, D.J.; Woodward, F.I. Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Endo, H.; Torii, K.U. Stomatal Development and Perspectives toward Agricultural Improvement. Cold Spring Harb. Perspect. Biol. 2019, 11, a034660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casson, S.; Hetherington, A.M. Phytochrome B Is Required for Light-Mediated Systemic Control of Stomatal Development. Curr. Biol. 2014, 24, 1216–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Woodward, F.I.; Quick, W.P. Systemic irradiance signalling in tobacco. New Phytol. 2003, 161, 193–198. [Google Scholar] [CrossRef]
- Coupe, S.A.; Palmer, B.G.; Lake, J.A.; Overy, S.A.; Oxborough, K.; Woodward, F.I.; Gray, J.E.; Quick, W.P. Systemic signalling of environmental cues in Arabidopsis leaves. J. Exp. Bot. 2006, 57, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Devlin, P.F.; Yanovsky, M.J.; Kay, S.A. A Genomic Analysis of the Shade Avoidance Response in Arabidopsis. Plant Physiol. 2003, 133, 1617–1629. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, D.C. Integrating signals in stomatal development. Curr. Opin. Plant Biol. 2004, 7, 26–32. [Google Scholar] [CrossRef] [PubMed]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.-Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.-H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Assmann, S.M.; Shimazaki, K.-I. The Multisensory Guard Cell. Stomatal Responses to Blue Light and Abscisic Acid. Plant Physiol. 1999, 119, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelfsema, M.R.G.; Hanstein, S.; Felle, H.H.; Hedrich, R. CO2 provides an intermediate link in the red light response of guard cells. Plant J. 2002, 32, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.-I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Geng, S.; Chakravorty, D.; Guan, Q.; Chen, S.; Assmann, S.M. Metabolomics of red-light-induced stomatal opening in Arabidopsis thaliana: Coupling with abscisic acid and jasmonic acid metabolism. Plant J. 2019, 101, 1331–1348. [Google Scholar] [CrossRef]
- Ando, E.; Kinoshita, T. Red Light-Induced Phosphorylation of Plasma Membrane H+-ATPase in Stomatal Guard Cells. Plant Physiol. 2018, 178, 838–849. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of blue and red light in stomatal dynamic behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Kotilainen, T.; Aphalo, P.; Brelsford, C.; Böök, H.; Devraj, S.; Heikkilä, A.; Hernández, R.; Kylling, A.; Lindfors, A.; Robson, T.M. Patterns in the spectral composition of sunlight and biologically meaningful spectral photon ratios as affected by atmospheric factors. Agric. For. Meteorol. 2020, 291, 108041. [Google Scholar] [CrossRef]
- Brelsford, C.; Nybakken, L.; Kotilainen, T.; Robson, T.M. The influence of spectral composition on spring and autumn phenology in trees. Tree Physiol. 2019, 39, 925–950. [Google Scholar] [CrossRef]
- Assman, S.H. Enhancement of the Stomatal Response to Blue Light by Red Light, Reduced Intercellular Concentrations of CO2, and Low Vapor Pressure Differences. Plant. Physiol. 1988, 87, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, T.C.; Allaway, W.G.; Evans, L.T. Action Spectra for Guard Cell Rb+ Uptake and Stomatal Opening in Vivia faba. Plant Physiol. 1973, 51, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iino, M.; Ogawa, T.; Zeiger, E. Kinetic properties of the blue-light response of stomata. Proc. Natl. Acad. Sci. USA 1985, 82, 8019–8023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiyama, A.; Takemiya, A.; Munemasa, S.; Okuma, E.; Sugiyama, N.; Tada, Y.; Murata, Y.; Shimazaki, K.-I. Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K.-I. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin Blue-Light Receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Xiao, Y.-G.; Li, X.; Ni, M. Light-Regulated Stomatal Aperture in Arabidopsis. Mol. Plant 2012, 5, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Takemiya, A.; Shimazaki, K.-I. Phosphatidic Acid Inhibits Blue Light-Induced Stomatal Opening via Inhibition of Protein Phosphatase 1. Plant Physiol. 2010, 153, 1555–1562. [Google Scholar] [CrossRef]
- Shen, L.; Tian, Q.; Yang, L.; Zhang, H.; Shi, Y.; Shen, Y.; Zhou, Z.; Wu, Q.; Zhang, Q.; Zhang, W. Phosphatidic acid directly binds with rice potassium channel OsAKT2 to inhibit its activity. Plant J. 2020, 102, 649–665. [Google Scholar] [CrossRef]
- Schwartz, A.; Zeiger, E. Metabolic energy for stomatal opening. Roles of photophosphorylation and oxidative phosphorylation. Planta 1984, 161, 129–136. [Google Scholar] [CrossRef]
- Tominaga, M.; Kinoshita, T.; Shimazaki, K.-I. Guard-Cell Chloroplasts Provide ATP Required for H+ Pumping in the Plasma Membrane and Stomatal Opening. Plant Cell Physiol. 2001, 42, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, E.; Kinoshita, T. Fluence rate dependence of red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells. Plant Signal. Behav. 2019, 14, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroli, I.; Price, G.D.; Badger, M.R.; Von Caemmerer, S. The Contribution of Photosynthesis to the Red Light Response of Stomatal Conductance. Plant Physiol. 2007, 146, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Talbott, L.D.; Hammad, J.W.; Harn, L.C.; Nguyen, V.H.; Patel, J.; Zeiger, E. Reversal by Green Light of Blue Light-stimulated Stomatal Opening in Intact, Attached Leaves of Arabidopsis Operates Only in the Potassium-dependent, Morning Phase of Movement. Plant Cell Physiol. 2006, 47, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Talbott, L.D.; Nikolova, G.; Ortiz, A.; Shmayevich, I.; Zeiger, E. Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. Am. J. Bot. 2002, 89, 366–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouly, J.-P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; et al. Cryptochrome Blue Light Photoreceptors Are Activated through Interconversion of Flavin Redox States. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar]
- Huche-Thelier, L.; Crespel, L.; Gourrierec, J.G.-L.; Morel, P.; Sakr, S.; LeDuc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Eisinger, W.; Swartz, T.E.; Bogomolni, R.A.; Taiz, L. The Ultraviolet Action Spectrum for Stomatal Opening in Broad Bean. Plant Physiol. 2000, 122, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Teramura, A.H.; Tevini, M.; Iwanzik, W. Effects of ultraviolet-B irradiation on plants during mild water stress. I. Effects on diurnal stomatal resistance. Physiol. Plant. 1983, 57, 175–180. [Google Scholar] [CrossRef]
- Zhang, J.; De-Oliveira-Ceciliato, P.; Takahashi, Y.; Schulze, S.; Dubeaux, G.; Hauser, F.; Azoulay-Shemer, T.; Tõldsepp, K.; Kollist, H.; Rappel, W.-J.; et al. Insights into the Molecular Mechanisms of CO2-Mediated Regulation of Stomatal Movements. Curr. Biol. 2018, 28, R1356–R1363. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.E.; Holroyd, G.H.; Van Der Lee, F.M.; Bahrami, A.R.; Sijmons, P.C.; Woodward, F.I.; Schuch, W.; Hetherington, A.M. The HIC signalling pathway links CO2 perception to stomatal development. Nature 2000, 408, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Negi, J.; Young, J.; Israelsson, M.; Schroeder, J.I.; Iba, K. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 2006, 8, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Scripps. The Keeling Curve. 2019. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 23 October 2019).
- Ciais, P.; Denning, A.S.; Tans, P.P.; Berry, J.A.; Randall, D.; Collatz, G.J.; Sellers, P.J.; White, J.W.C.; Trolier, M.; Meijer, H.A.J.; et al. A three-dimensional synthesis study of δ18O in atmospheric CO2: 1. Surface fluxes. J. Geophys. Res. Space Phys. 1997, 102, 5857–5872. [Google Scholar] [CrossRef]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Beerling, D.J.; Chaloner, W.G. Evolutionary responses of stomatal density to global CO2 change. Biol. J. Linn. Soc. 1993, 48, 343–353. [Google Scholar] [CrossRef]
- Engineer, C.B.; Ghassemian, M.; Anderson, J.C.; Peck, S.C.; Hu, H.; Schroeder, J.I. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 2014, 513, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.-J.; Iba, K.; Schroeder, J.I. CO 2 Sensing and CO 2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Yokoo, T.; Kajita, R.; Onishi, T.; Yahata, S.; Peterson, K.M.; Torii, K.U.; Kakimoto, T. Epidermal Cell Density is Autoregulated via a Secretory Peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis Leaves. Plant Cell Physiol. 2009, 50, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Hunt, L.; Gray, J.E. The Signaling Peptide EPF2 Controls Asymmetric Cell Divisions during Stomatal Development. Curr. Biol. 2009, 19, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Higaki, T.; Akita, K.; Hasezawa, S. Elevated CO2 promotes satellite stomata production in young cotyledons of Arabidopsis thaliana. Genes Cells 2020, 25, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse Stomatal Signaling and the Signal Integration Mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Merilo, E.; Laanemets, K.; Waadt, R.; Pater, D.; Kollist, H.; Schroeder, J.I. Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc. Natl. Acad. Sci. USA 2018, 115, E9971–E9980. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, N.; Miao, Y.; Hauser, F.; McCammon, J.A.; Rappel, W.-J.; Schroeder, J.I. Identification of SLAC1 anion channel residues required for CO2/bicarbonate sensing and regulation of stomatal movements. Proc. Natl. Acad. Sci. USA 2018, 115, 11129–11137. [Google Scholar] [CrossRef] [Green Version]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.-K.; McLachlan, D.H.; Casson, S.A.; Isner, J.C.; Wilson, I.D.; et al. Elevated CO2 -Induced Responses in Stomata Require ABA and ABA Signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef] [Green Version]
- Leymarie, J.; Vavasseur, A.; Lasceve, G. CO2 sensing in stomata of abi1-1 and abi2-1 mutants of Arabidopsis thaliana. Plant Physiol. Biochem. 1998, 36, 539–543. [Google Scholar] [CrossRef]
- Webb, A.A.R.; Hetherington, A.M. Convergence of the Abscisic Acid, CO2, and Extracellular Calcium Signal Transduction Pathways in Stomatal Guard Cells. Plant Physiol. 1997, 114, 1557–1560. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Hu, H.; Ries, A.; Merilo, E.; Kollist, H.; Schroeder, J.I. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011, 30, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Merilo, E.; Laanemets, K.; Hu, H.; Xue, S.; Jakobson, L.; Tulva, I.; Gonzalez-Guzman, M.; Rodriguez, P.L.; Schroeder, J.I.; Broschè, M.; et al. PYR/RCAR Receptors Contribute to Ozone-, Reduced Air Humidity-, Darkness-, and CO2-Induced Stomatal Regulation. Plant Physiol. 2013, 162, 1652–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The Regulatory Domain of SRK2E/OST1/SnRK2.6 Interacts with ABI1 and Integrates Abscisic Acid (ABA) and Osmotic Stress Signals Controlling Stomatal Closure inArabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, U. Stomatal opening at elevated temperature: An underestimated regulatory mechanism. Gen. Appl. Plant Physiol. Spec. Issue 2006, 19–31. [Google Scholar] [CrossRef]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar] [CrossRef]
- Lau, O.S.; Song, Z.; Zhou, Z.; Davies, K.A.; Chang, J.; Yang, X.; Wang, S.; Lucyshyn, D.; Tay, I.H.Z.; Wigge, P.A.; et al. Direct Control of SPEECHLESS by PIF4 in the High-Temperature Response of Stomatal Development. Curr. Biol. 2018, 28, 1273–1280.e3. [Google Scholar] [CrossRef] [Green Version]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef] [Green Version]
- Koini, M.A.; Alvey, L.; Allen, T.; Tilley, C.A.; Harberd, N.P.; Whitelam, G.C.; Franklin, K.A. High Temperature-Mediated Adaptations in Plant Architecture Require the bHLH Transcription Factor PIF4. Curr. Biol. 2009, 19, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Lucyshyn, D.; Wigge, P.A. Plant development: PIF4 integrates diverse environmental signals. Curr. Biol. 2009, 19, R265–R266. [Google Scholar] [CrossRef] [Green Version]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Kostaki, K.-I.; Coupel-Ledru, A.; Bonnell, V.C.; Gustavsson, M.; Sun, P.; McLaughlin, F.J.; Fraser, D.P.; McLachlan, D.H.; Hetherington, A.M.; Dodd, A.N.; et al. Guard Cells Integrate Light and Temperature Signals to Control Stomatal Aperture. Plant Physiol. 2020, 182, 1404–1419. [Google Scholar] [CrossRef] [Green Version]
- Devireddy, A.R.; Arbogast, J.; Mittler, R. Coordinated and rapid whole-plant systemic stomatal responses. New Phytol. 2020, 225, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto-Sugimoto, M.; Higaki, T.; Yaeno, T.; Nagami, A.; Irie, M.; Fujimi, M.; Miyamoto, M.; Akita, K.; Negi, J.; Shirasu, K.; et al. A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat. Commun. 2013, 4, 2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanourakis, D.; Bouranis, D.; Giday, H.; Carvalho, D.R.; Nejad, A.R.; Ottosen, C.-O. Improving stomatal functioning at elevated growth air humidity: A review. J. Plant Physiol. 2016, 207, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Zheng, Y.; Piao, S.; Ciais, P.; Lombardozzi, D.L.; Wang, Y.; Ryu, Y.; Chen, G.; Dong, W.; Hu, Z.; et al. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci. Adv. 2019, 5, eaax1396. [Google Scholar] [CrossRef] [Green Version]
- McAdam, S.A.; Brodribb, T.J. The Evolution of Mechanisms Driving the Stomatal Response to Vapor Pressure Deficit. Plant Physiol. 2015, 167, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Fanourakis, D.; Heuvelink, E.; Carvalho, S.M. A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. J. Plant Physiol. 2013, 170, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.R.; Van Meeteren, U. Stomatal response characteristics of Tradescantia virginiana grown at high relative air humidity. Physiol. Plant. 2005, 125, 324–332. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Matamoros, P.M.; Van Meeteren, U. Stomatal malfunctioning under low VPD conditions: Induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling? Physiol. Plant. 2014, 152, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Van Meeteren, U. Stomatal characteristics and desiccation response of leaves of cut chrysanthemum (Chrysanthemum morifolium) flowers grown at high air humidity. Sci. Hortic. 2016, 205, 84–89. [Google Scholar] [CrossRef]
- Bakker, J. Effects of humidity on stomatal density and its relation to leaf conductance. Sci. Hortic. 1991, 48, 205–212. [Google Scholar] [CrossRef]
- McKown, A.D.; Guy, R.D.; Quamme, L.; Klápště, J.; La Mantia, J.; Constabel, C.P.; El-Kassaby, Y.A.; Hamelin, R.C.; Zifkin, M.; Azam, M.S. Association genetics, geography and ecophysiology link stomatal patterning inPopulus trichocarpawith carbon gain and disease resistance trade-offs. Mol. Ecol. 2014, 23, 5771–5790. [Google Scholar] [CrossRef] [PubMed]
- Pantin, F.; Blatt, M.R. Stomatal Response to Humidity: Blurring the Boundary between Active and Passive Movement. Plant Physiol. 2018, 176, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Chen, G.; Wang, Y.; Huang, Y.; Marchant, B.; Wang, Y.; Yang, Q.; Dai, F.; Hills, A.; Franks, P.J.; et al. Evolutionary Conservation of ABA Signaling for Stomatal Closure. Plant Physiol. 2017, 174, 732–747. [Google Scholar] [CrossRef] [PubMed]
- Kübarsepp, L.; Laanisto, L.; Niinemets, Ü.; Talts, E.; Tosens, T. Are stomata in ferns and allies sluggish? Stomatal responses to CO2, humidity and light and their scaling with size and density. New Phytol. 2019, 225, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.; Kamisugi, Y.; Movahedi, M.; Fleming, A.; Cuming, A.C.; Gray, J.E.; Beerling, D.J. Regulatory Mechanism Controlling Stomatal Behavior Conserved across 400 Million Years of Land Plant Evolution. Curr. Biol. 2011, 21, 1025–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdam, S.A.; Sussmilch, F.C.; Brodribb, T.J. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016, 39, 485–491. [Google Scholar] [CrossRef]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.-O. Foliar abscisic acid content underlies genotypic variation in stomatal responsiveness after growth at high relative air humidity. Ann. Bot. 2013, 112, 1857–1867. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzõrjova, I.; Brosché, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.-S.; Lindgren, O.; Salojärvi, J.; et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef]
- Xie, X.D.; Wang, Y.B.; Williamson, L.; Holroyd, G.H.; Tagliavia, C.; Murchie, E.; Theobald, J.C.; Knight, M.R.; Davies, W.J.; Leyser, H.M.O.; et al. The Identification of Genes Involved in the Stomatal Response to Reduced Atmospheric Relative Humidity. Curr. Biol. 2006, 16, 882–887. [Google Scholar] [CrossRef]






| Stomatal Development | ||
| Environmental Factor | Stomatal Response | Molecular Components Involved in Stomatal Response |
| Light | ↑ light intensity: ↑stomatal index | phyB, PIF4 |
| CO2 | ↑ CO2-concentration: downregulation of stomatal development: ↓ stomatal index | CA enzymes, HIC gene, ERECTA receptor kinase, EPF1, EPF2, LRR, TMM, ER, SDD1, CDC6 |
| Temperature | ↑ ambient temperature: ↓stomatal development | PIF4, SPCH |
| Relative humidity | ↑ RH: ↑ stomatal density and ↑ stomatal length | |
| Stomatal Opening | ||
| Environmental Factor | Stomatal Response | Molecular Components Involved in Stomatal Response |
| Light | Light induces stomatal opening Blue and red light induces stomatal opening by two distinct pathways | phot1, phot2 PM H+-ATPase K+ inward channels Malate2+, Cl−, glucose, sucrose PP1, 14-3-3 |
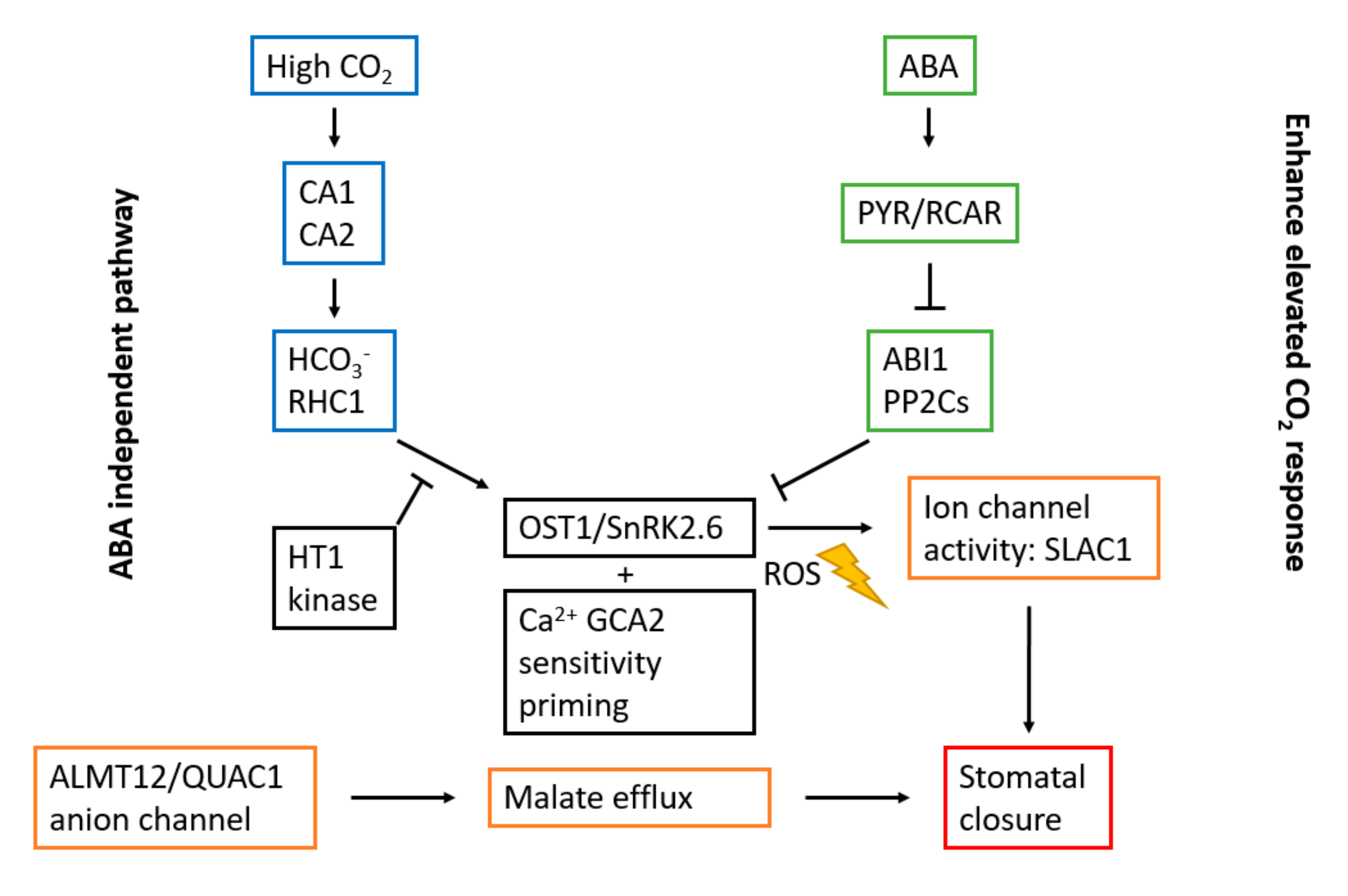
| CO2 | ↑ CO2-concentration induces stomatal closure | βCA1, βCA2, HT1, OST1/SnRK2.6, SLAC1, ALMT12/QUAC1, RHC1, GCA2, MPK12, GHR1, AHA1 |
| Temperature | ↑ temperature induces stomatal opening | Phototropin-dependent pathway: BLUS1, 14-3-3 proteins, AHA1, PATROL1 Phototropin-independent pathway: RBOHD-mediated ROS production |
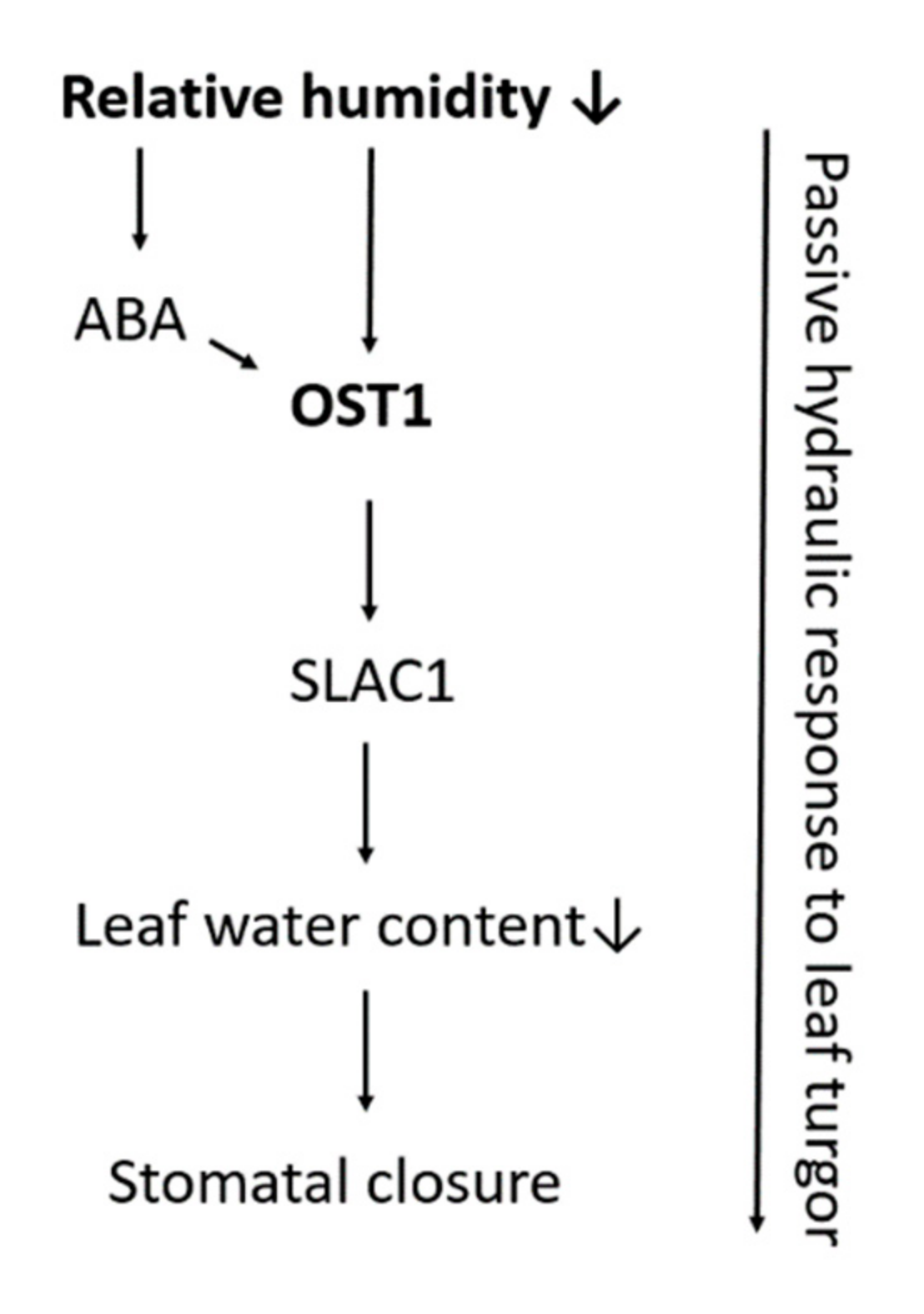
| Relative humidity | ↓ relative humidity induces stomatal closure | Passive hydraulic response Active/ABA driven response: NCED genes, OST1, SLAC1, ABA2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. https://doi.org/10.3390/agronomy10121975
Driesen E, Van den Ende W, De Proft M, Saeys W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy. 2020; 10(12):1975. https://doi.org/10.3390/agronomy10121975
Chicago/Turabian StyleDriesen, Elisa, Wim Van den Ende, Maurice De Proft, and Wouter Saeys. 2020. "Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review" Agronomy 10, no. 12: 1975. https://doi.org/10.3390/agronomy10121975
APA StyleDriesen, E., Van den Ende, W., De Proft, M., & Saeys, W. (2020). Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy, 10(12), 1975. https://doi.org/10.3390/agronomy10121975








