Aegilops Species for the Improvement of the Leaf and Stripe Rust Resistance in Cultivated Triticale (×Triticosecale Wittmack)
Abstract
:1. Introduction
2. Current Status of Triticale Resistance against Leaf and Stripe Rusts
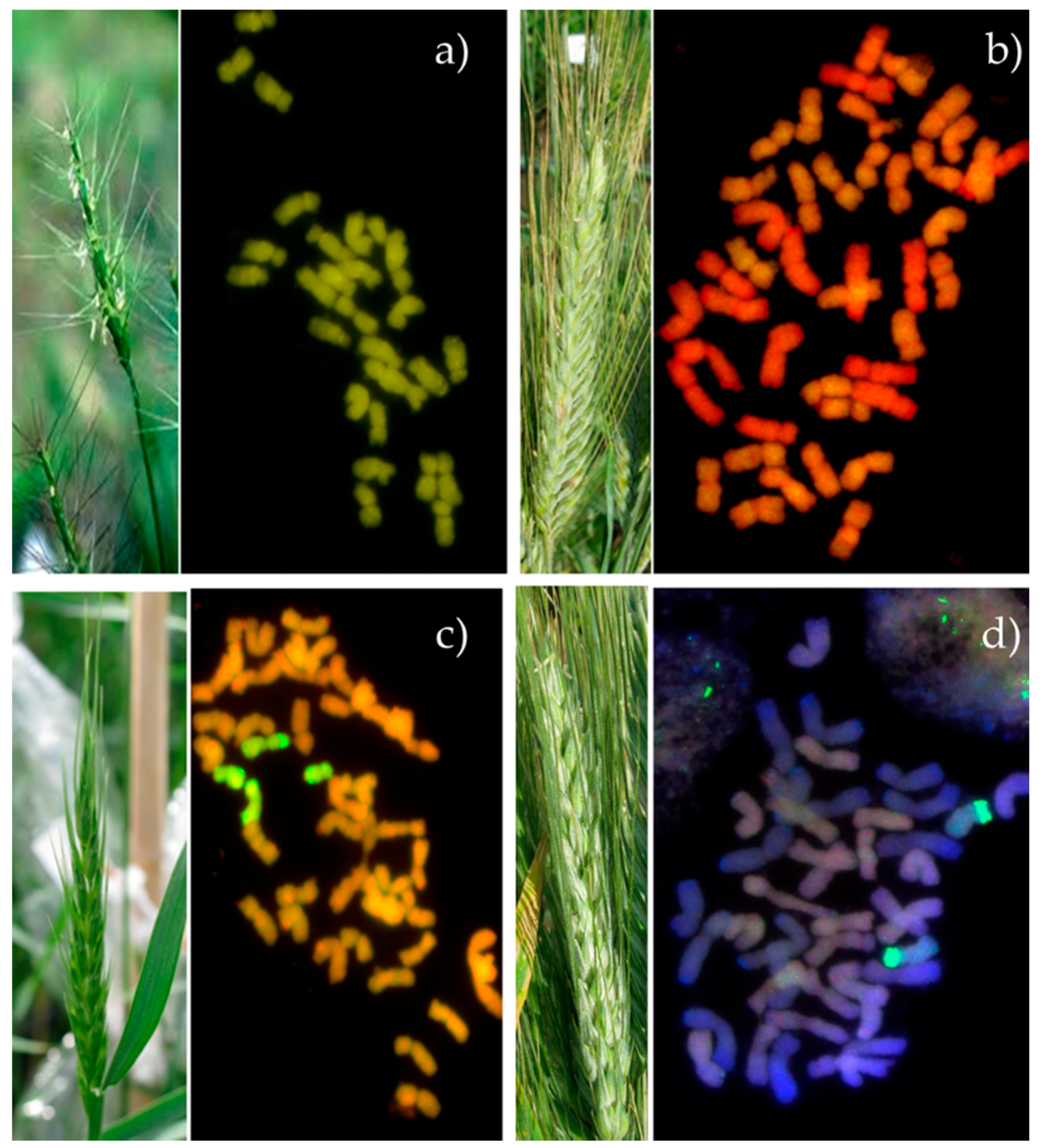
3. The Use of Aegilops Species for Improvement Leaf and Stripe Rust Resistance in Hexaploid Triticale
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2018–2027; OECD/FAO: Rome, Italy, 2018; ISBN 978-92-64-06203-0. [Google Scholar]
- Arseniuk, E. Triticale Abiotic Stresses—An Overview. In Triticale; Eudes, F., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 69–81. ISBN 978-3-319-22551-7. [Google Scholar]
- Ayalew, H.; Kumssa, T.T.; Butler, T.J.; Ma, X.-F. Triticale Improvement for Forage and Cover Crop Uses in the Southern Great Plains of the United States. Front. Plant Sci. 2018, 9, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woś, H.; Brzeziński, W. Triticale for Food—The Quality Driver. In Triticale; Eudes, F., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 213–232. ISBN 978-3-319-22551-7. [Google Scholar]
- Glatthar, J.; Heinisch, J.J.; Senn, T. The Use of Unmalted Triticale in Brewing and its Effect on Wort and Beer Quality. J. Am. Soc. Brew. Chem. 2003, 61, 182–190. [Google Scholar] [CrossRef]
- Klikocka, H.; Kasztelan, A.; Zakrzewska, A.; Wyłupek, T.; Szostak, B.; Skwaryło-Bednarz, B. The Energy Efficiency of the Production and Conversion of Spring Triticale Grain into Bioethanol. Agronomy 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Kwiatek, M.T.; Nawracała, J. Chromosome manipulations for progress of triticale (×Triticosecale) breeding. Plant Breed. 2018, 137, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Mergoum, M.; Gómez-Macpherson, H. Triticale improvement and production. In FAO Plant Production and Protection Papers; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; p. 179. [Google Scholar]
- Kiss, A. Kreuzungsversuche mit Triticale [Crossing experiments with Triticale]. Züchter Genet. Breed. Res. 1966, 36, 249–255. [Google Scholar] [CrossRef]
- Larter, E.N.; Shebeski, L.H.; McGinnis, R.C.; Evans, L.E.; Kultsikes, P.J. Rosner, a hexaploid triticale cultivar. Can. J. Plant Sci. 1970, 50, 122–124. [Google Scholar] [CrossRef] [Green Version]
- Niedziela, A.; Orłowska, R.; Machczyńska, J.; Bednarek, P.T. The genetic diversity of triticale genotypes involved in Polish breeding programs. Springerplus 2016, 5, 355. [Google Scholar] [CrossRef] [Green Version]
- Arseniuk, E. Triticale Diseases—A Review. In Triticale: Today and Tomorrow; Springer Science & Business Media: Cham, Switzerland, 1996; pp. 499–525. ISBN 978-94-010-6634-1. [Google Scholar]
- Kolmer, J.A. Wheat Rusts. In Encyclopedia of Plant and Crop Science; Goodman, R.M., Ed.; Routledge: Abingdon, UK, 2004. [Google Scholar]
- Lorrain, C.; dos Santos, K.C.G.; Germain, H.; Hecker, A.; Duplessis, S. Advances in understanding obligate biotrophy in rust fungi. New Phytol. 2019, 222, 1190–1206. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Savadi, S.; Bhardwaj, S.C.; Gupta, P.K. The progress of leaf rust research in wheat. Fungal Biol. 2020, 124, 537–550. [Google Scholar] [CrossRef]
- Schwessinger, B.; Chen, Y.J.; Tien, R.; Vogt, J.K.; Sperschneider, J.; Nagar, R.; McMullan, M.; Sicheritz-Ponten, T.; Sørensen, C.K.; Hovmøller, M.S.; et al. Distinct Life Histories Impact Dikaryotic Genome Evolution in the Rust Fungus Puccinia striiformis Causing Stripe Rust in Wheat. Genome Biol. Evol. 2020, 12, 597–617. [Google Scholar] [CrossRef]
- McIntosh, R.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Xia, X.C. Catalogue of Gene Symbols for Wheat. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp (accessed on 10 October 2020).
- Gill, B.S.; Friebe, B.R.; White, F.F. Alien introgressions represent a rich source of genes for crop improvement. Proc. Natl. Acad. Sci. USA 2011, 108, 7657–7658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiecicka, M.; Dmochowska-Boguta, M.; Orczyk, W.; Gradzielewska, A.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Changes in benzoxazinoid contents and the expression of the associated genes in rye (Secale cereale L.) due to brown rust and the inoculation procedure. PLoS ONE 2020, 15, e0233807. [Google Scholar] [CrossRef] [PubMed]
- Park, R.F.; Wellings, C.R. Somatic hybridization in the uredinales. Annu. Rev. Phytopathol. 2012, 50, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Knott, D. Suppression of rust resistance in bread wheat (Triticum aestivum L.) by D-genome chromosomes. Genome 2011, 35, 276–282. [Google Scholar] [CrossRef]
- Rodriguez-Algaba, J.; Sørensen, C.K.; Labouriau, R.; Justesen, A.F.; Hovmøller, M.S. Susceptibility of Winter Wheat and Triticale to Yellow Rust Influenced by Complex Interactions between Vernalisation, Temperature, Plant Growth Stage and Pathogen Race. Agronomy 2020, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Dundas, I.; Dong, C.; Li, G.; Trethowan, R.; Yang, Z.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef]
- Wellings, C. Global status of stripe rust. In Proceedings of the BGRI 2010 Technical Workshop Oral Presentations, St Petersburg, Russia, 30–31 May 2010; pp. 34–35. [Google Scholar]
- Tyryshkin, L.G.; Kurbanova, P.M.; Kurkiev, K.U.; Sarukhanov, I.G.; Kurkiev, U.K. Effective juvenile resistance to brown rust in hexaploid triticale. Zaščita Karantin Rastenij 2008, 10, 25. [Google Scholar]
- Mikhailova, L.A.; Merezhko, A.F.; Funtikova, E.Y. Triticale diversity in leaf rust resistance. Russ. Agric. Sci. 2009, 35, 320–323. [Google Scholar] [CrossRef]
- Manninger, K. Physiological Specialization of Puccinia triticina on Wheat and Triticale in Hungary in 2004. Acta Phytopathol. Entomol. Hung. 2006, 41, 93–100. [Google Scholar] [CrossRef]
- Czajkowski, G.; Karska, K.; Strzembicka, A. Characteristic of winter triticale breeding lines in term of the degree of infestation by Puccionia triticiana and Blumeria graminis. Biul. Inst. Hod. Aklim. Roślin 2013, 268, 59–67. [Google Scholar]
- Grzesik, H.; Strzembicka, A. Resistance of some winter triticale varieties to leaf rust (Puccinia recondita f. sp. tritici). Biul. Inst. Hod. Aklim. Roślin 2003, 230, 171–175. [Google Scholar]
- Grzesik, H.; Strzembicka, A. Studies on inheritance of the resistance to leaf rust (Puccinia recondita f.sp. tritici) of winter triticale varieties at the seedling and adult plant stages. Plant Breed. Seed Sci. 1999, 43, 85–89. [Google Scholar]
- Kwiatek, M.; Majka, M.; Wiśniewska, H.; Apolinarska, B.; Belter, J. Effective transfer of chromosomes carrying leaf rust resistance genes from Aegilops tauschii Coss. into hexaploid triticale (X Triticosecale Witt.) using Ae. tauschii × Secale cereale amphiploid forms. J. Appl. Genet. 2015, 56, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulaszewski, W.; Belter, J.; Wiśniewska, H.; Szymczak, J.; Skowrońska, R.; Phillips, D.; Kwiatek, M. Recovery of 2R.2Sk Triticale-Aegilops kotschyi Robertsonian Chromosome Translocations. Agronomy 2019, 9, 646. [Google Scholar] [CrossRef] [Green Version]
- Majka, M.; Serfling, A.; Czembor, P.; Ślusarkiewicz-Jarzina, A.; Kwiatek, M.T.; Ordon, F.; Wiśniewska, H. Resistance of (Aegilops tauschii × Secale cereale) × Triticosecale Hybrids to Leaf Rust (Puccinia triticina) Determined on the Macroscopic and Microscopic Level. Front. Plant Sci. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Kwiatek, M.T.; Wiśniewska, H.; Belter, J.; Ulaszewski, W.; Phillips, D. Development and cytomolecular identification of monosomic alien addition and substitution of 2Sk chromosome from Aegilops kotschyi to triticale (×Triticosecale Wittmack). Front. Plant Sci. 2020. [Google Scholar] [CrossRef]
- Kwiatek, M.T.; Kurasiak-Popowska, D.; Mikołajczyk, S.; Niemann, J.; Tomkowiak, A.; Weigt, D.; Nawracała, J. Cytological markers used for identification and transfer of Aegilops spp. chromatin carrying valuable genes into cultivated forms of Triticum. Comp. Cytogenet. 2019, 13, 41–59. [Google Scholar] [CrossRef]
- Rowland, G.G.; Kerber, E.R. Telocentric mapping in hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa. Can. J. Genet. Cytol. 1974, 16, 137–144. [Google Scholar] [CrossRef]
- Gill, B.S.; Raupp, W.J.; Browder, L.E.; Cox, T.S. Registration of KS86WGRC02 Leaf Rust Resistant Hard Red Winter Wheat Germplasm. Crop Sci. 1988, 28, 207. [Google Scholar] [CrossRef]
- Marais, G.F.; McCallum, B.; Marais, A.S. Leaf rust and stripe rust resistance genes Lr54 and Yr37 transferred to wheat from Aegilops kotschyi. Plant Breed. 2005, 124, 538–541. [Google Scholar] [CrossRef]
| Species | Resistance Genes (Chromosomal Location) | References |
|---|---|---|
| Ae. tauschii | Lr22a (2DS) Lr32 (3D) Lr39 (2DS) | [31,33] [31] [31,33] |
| Ae. kotschyi Boiss. | Lr54 (2SkL) | [32,34] |
| Yr37 (2SkL) | [32,34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulaszewski, W.; Kwiatek, M.T. Aegilops Species for the Improvement of the Leaf and Stripe Rust Resistance in Cultivated Triticale (×Triticosecale Wittmack). Agronomy 2020, 10, 1991. https://doi.org/10.3390/agronomy10121991
Ulaszewski W, Kwiatek MT. Aegilops Species for the Improvement of the Leaf and Stripe Rust Resistance in Cultivated Triticale (×Triticosecale Wittmack). Agronomy. 2020; 10(12):1991. https://doi.org/10.3390/agronomy10121991
Chicago/Turabian StyleUlaszewski, Waldemar, and Michał Tomasz Kwiatek. 2020. "Aegilops Species for the Improvement of the Leaf and Stripe Rust Resistance in Cultivated Triticale (×Triticosecale Wittmack)" Agronomy 10, no. 12: 1991. https://doi.org/10.3390/agronomy10121991






