Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Experimental Setup
2.2. Experimental Sampling and Measurements
2.3. Comprehensive Evaluation Formula of The Saline-Alkaline-Tolerance Coefficient of Each Index
2.4. Statistical Analysis
3. Results
3.1. Genetic Variation in Plant Growth and Physiological Indices under SA Treatment vs. NSA Treatment
3.2. Classification of the SA-Tolerance of Alfalfa Based on the Different Methods
3.3. Identifying the Most Important Indices for Evaluating SA Tolerance in Alfalfa Based on Different Methods.
4. Discussion
4.1. Assessing the Appropriateness of SA-Tolerance Prediction and the Identification of Suitable Cultivars for saline–alkaline (SA)-Tolerance
4.2. Identification of the Best Markers for Predicting the saline–alkaline (SA)-Tolerance of Alfalfa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pandey, V.C.; Singh, K.; Singh, B.; Singh, R.P. New approaches to enhance eco restoration efficiency of degraded sodic lands: critical research needs and future prospects. Ecol. Restor. 2011, 29, 322–325. [Google Scholar] [CrossRef]
- Wong, V.; Greene, R.; Dalal, R.; Murphy, B.W. Soil carbon dynamics in saline and sodic soils: A review. Soil Use Manage. 2009, 26, 2–11. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D.; Schubert, S.; Noble, A.D.; Sahrawat, K.L. Phytoremediation of sodic and saline-sodic soils. Adv. Agron. 2007, 96, 197–247. [Google Scholar]
- Chi, C.M.; Wang, Z.C. Characterizing salt-affected soils of songnen plain using saturated paste and 1:5 soil-to-water extraction methods. Arid Land Res. Manag. 2010, 24, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.C.; Li, Q.S.; Li, X.J.; Song, C.C.; Zhang, G.X. Sustainable agriculture development in saline-alkali soil area of Songnen Plain, Northeast China. Chinese Geogr. Sci. 2003, 13, 171–174. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S. Degradation processes and nutrient constraints in sodic soils. Land Degrad Dev. 2002, 13, 275–294. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 37,613–620. [Google Scholar] [CrossRef]
- Naidu, R.; Rengasamy, P. Ion interactions and constraints to plant nutrition in Australian sodic soils. Aust. J. Soil Res. 1993, 31, 801–819. [Google Scholar] [CrossRef]
- Chi, C.M.; Zhao, C.W.; Sun, X.J.; Wang, Z.C. Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma 2012, 187, 24–30. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Shi, S.H.; Xu, S.Q.; Yang, F.; Li, X.J.; Wang, Z.C.; Tian, C.J. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 2018, 329, 108–117. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S.; Badia, D.; Sharma, B.R.; Qureshi, A.S.; Murtaza, G. Amelioration and nutrient management strategies for sodic and alkali soils. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Natural Resour. 2007, 21, 1–13. [Google Scholar] [CrossRef]
- Wang, M.M.; Yang, F.; Ma, H.Y.; Wei, L.X.; Huang, L.H.; Liu, M.; Yang, H.Y.; Li, J.P.; Li, X.W.; Liu, X.L.; et al. Cooperative effects of sand application and flushing during the sensitive stages of rice on its yield in a hard saline-sodic soil. Plant Prod. Sci. 2016, 19, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.M.; Rengasamy, P.; Wang, Z.C.; Yang, F.; Ma, H.Y.; Huang, L.H.; Liu, M.; Yang, H.Y.; Li, J.P.; An, F.H.; et al. Identification of the most limiting factor for rice yield using soil data collected before planting and during the reproductive stage. Land Degrad Dev. 2018, 29, 2310–2320. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Cui, Z.; Fang, Y.; He, H.H.; Liu, R.B.; Wu, G.L. Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crops Res. 2018, 221, 1–6. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Abiala, M.A.; Abdelrahman, M.; Burritt, D.J.; Tran, L.-S.P. Salt stress tolerance mechanisms and potential applications of legumes for sustainable reclamation of salt-degraded soils. Land Degrad Dev. 2018, 29, 3812–3822. [Google Scholar] [CrossRef]
- Peng, Y.L.; Gao, Z.W.; Gao, Y.; Liu, G.F.; Sheng, L.X.; Wang, D.L. Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J. Integr. Plant Biol. 2008, 50, 29–39. [Google Scholar] [CrossRef]
- Adem, G.D.; Roy, S.J.; Zhou, M.X.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.T.; Xu, Y.X.; Hettenhausen, C.; Lu, C.K.; Shen, G.J.; Zhang, C.P.; Li, J.; Song, J.; Lin, H.H.; Wu, J.Q. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar]
- Luo, D.; Zhou, Q.; Wu, Y.G.; Chai, X.T.; Liu, W.X.; Wang, Y.R.; Yang, Q.C.; Wang, Z.Y.; Liu, Z.P. Full-length transcript sequencing and comparative transcriptomic analysis to evaluate the contribution of osmotic and ionic stress components towards salinity tolerance in the roots of cultivated alfalfa (Medicago sativa L.). BMC Plant Biol. 2019, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell and Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.L.; Shi, F.C.; Fukuda, K.J.; Yang, Y.L. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Wang, X.S.; Ren, H.L.; Wei, Z.W.; Wang, Y.W.; Ren, W.B. Effects of neutral salt and alkali on ion distributions in the roots, shoots, and leaves of two alfalfa cultivars with differing degrees of salt tolerance. J. Integr. Agr. 2017, 16, 1800–1807. [Google Scholar] [CrossRef]
- An, Y.M.; Song, L.L.; Liu, Y.R.; Shu, Y.J.; Guo, C.H. Denovo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, R.C.; Sun, H.; Cao, C.Y.; Zhang, T.J.; Kang, J.M.; Wang, Z.; Li, M.N.; Gao, Y.L.; Li, X.; Yang, Q.C. Identification of alkali-responsive proteins from early seedling stage of two contrasting Medicago species by iTRAQ-based quantitative proteomic analysis. Environ. Exp. Bot. 2019, 157, 26–34. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting alfalfa cultivars for salt tolerance based on some physiochemical traits. Agron. J. 2014, 106, 1758–1764. [Google Scholar] [CrossRef]
- Hu, G.F.; Liu, Y.M.; Zhang, X.Z.; Yao, F.J.; Huang, Y.; Ervin, E.H.; Zhao, B.Y. Physiological evaluation of alkali-salt tolerance of thirty switchgrass (Panicum virgatum) lines. PLoS ONE 2015, 10, e0125305. [Google Scholar] [CrossRef] [Green Version]
- Tavakkoli, E.; Fatehi, F.; Rengasamy, P.; McDonald, G.K. A comparison of hydroponic and soil-based screening methods to identify salt tolerance in the field in barley. J. Exp. Bot. 2012, 63, 3853–3867. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Liu, M.; Liu, X.L.; Cheng, X.G.; Liang, Z.W. Silicon priming created an enhanced tolerance in alfalfa (Medicago sativa L.) seedlings in response to high alkaline stress. Front. Plant Sci. 2018, 9, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.P.; Chen, W.C.; Zhou, Y.; Han, J.Y.; Zhao, J.; Shi, D.; Yang, C. Comparison of adaptive strategies of alfalfa (Medicago sativa L.) to salt and alkali stresses. Aust. J. Crop Sci. 2012, 6, 309–315. [Google Scholar]
- Anower, M.R.; Mott, I.W.; Peel, M.D.; Wu, Y.J. Characterization of physiological responses of two alfalfa half-sib families with improved salt tolerance. Plant Physiol. Biochem. 2013, 71, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.P.; Zhang, H.; Zhao, Q.; Yoo, M.J.; Zhu, N.; Yu, J.L.; Yu, J.J.; Guo, S.Y.; Miao, Y.C.; Chen, S.X.; et al. Physiological and comparative proteomic analyses of saline-alkali NaHCO3—responses in leaves of halophyte Puccinellia tenuiflora. Plant Soil. 2019, 437, 137–158. [Google Scholar] [CrossRef]
- Genc, Y.; Tester, M.; McDonald, G.K. Calcium requirement of wheat in saline and non-saline conditions. Plant Soil. 2010, 327, 331–345. [Google Scholar] [CrossRef]
- Wang, X.P.; Geng, S.J.; Ma, Y.Q.; Shi, D.C.; Yang, C.W.; Wang, H. Growth, photosynthesis, solute accumulation, and ion balance of tomato plant under sodium-or potassium-salt stress and alkali stress. Agron. J. 2015, 107, 651–661. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M. Effect of salt stress on growth and ion accumulation of alfalfa (Medicago sativa L.) cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Yang, C.W.; Chong, J.N.; Li, C.Y.; Kim, C.M.; Shi, D.C.; Wang, D.L. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil. 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Khorshidi, M.B.; Yarnia, M.; Hassanpanah, D. Salinity effect on nutrients accumulation in alfalfa shoots in hydroponic condition. J. Food Agric. Environ. 2009, 7, 787–790. Available online: https://www.researchgate.net/publication/255613590_Salinity_effect_on_nutrients_accumulation_in_alfalfa_shoots_in_hydroponic_condition (accessed on 2 January 2020).
- Liu, X.L.; Zhang, H.; Jin, Y.Y.; Wang, M.M.; Yang, H.Y.; Ma, H.Y.; Jiang, C.J.; Liang, Z.W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes. Plant Soil. 2019, 438, 39–55. [Google Scholar] [CrossRef]
- Anower, M.R.; Peel, M.D.; Mott, I.W.; Wu, Y. Physiological processes associated with salinity tolerance in an alfalfa half-sib family. J. Agron. Crop. Sci. 2017, 203, 506–518. [Google Scholar] [CrossRef]
- Tavakoli, M.; Poustini, K.; Besharati, H.; Ali, S. Variable salinity responses of 25 alfalfa genotypes and comparative salt-response ion distribution. Russ. J. Plant Physiol. 2019, 66, 231–239. [Google Scholar] [CrossRef]
- Bao, A.K.; Guo, Z.G.; Zhang, H.F.; Wang, S.M. A procedure for assessing the salt tolerance of lucerne (Medicago sativa L.) cultivar seedlings by combining agronomic and physiological indicators. N. Z. J. Agr. Res. 2009, 52, 435–442. [Google Scholar]
- Majid, M.; Ali, A.; Essia, B. Effect of salinity on sodium and chloride uptake, proline and soluble carbohydrate contents in three alfalfa varieties. Iosr-Javs. 2012, 1, 01–06. [Google Scholar] [CrossRef]
- Monirifar, H.; Barghi, M. Identification and selection for salt tolerance in alfalfa (Medicago sativa L.) ecotypes via physiological traits. Not. Sci. Biol. 2009, 1, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.Q.; Wang, S.M. Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 2012, 58, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.Z.; Shen, Q.F.; Cai, S.G.; Chen, Z.H.; Dai, F.; Zhang, G.P. Ionomic responses and correlations between elements and metabolites under salt stress in wild and cultivated barley. Plant Cell Physiol. 2013, 54, 1976–1988. [Google Scholar] [CrossRef] [Green Version]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.T.; Mu, C.S. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci. Plant Nutr. 2009, 55, 685–697. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.Z.; Li, F.; Yan, C.R.; Zhong, X.L.; Liu, Q.; Xia, X.; Li, H.R.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Shi, L.X.; Yan, C.R.; Zhong, X.L.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H.R. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar]
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Bioch. 2015, 90, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.X.; Lv, B.S.; Li, X.W.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Yang, R.F.; Piao, Z.Z.; Wang, Z.H.; Lou, J.H.; et al. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crops Res. 2017, 203, 86–93. [Google Scholar] [CrossRef]
- Lv, B.S.; Li, X.W.; Ma, H.Y.; Sun, Y.; Wei, L.X.; Jiang, C.J.; Liang, Z.W. Differences in growth and physiology of rice in response to different saline-alkaline stress factors. Agron. J. 2013, 105, 1889. [Google Scholar] [CrossRef]
- Carden, D.E.; Walker, D.J.; Flowers, T.J.; Miller, A.J. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol. 2003, 131, 676. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. UK 2017, 7, 42958. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, Q.X.; Gong, Z.Y.; Li, Y.; Xu, H.Y.; Wan, X.F.; Bi, S.Y.; Cao, J.; He, F.; Wan, L.Q.; et al. Analysis of morphological and physiological responses to low Pi stress in different alfalfas. Sci. Agric. Sin. 2017, 50, 3898–3907. [Google Scholar]
- Shi, D.C.; Zhao, K.F. Effects of NaCl and Na2CO3 on growth of Puccinellia tenuiflora and on present state of mineral elements in nutrient solution. Acta Pratacu. Sin. 1997, 6, 51–61. (in Chinese). [Google Scholar]
- Scasta, J.D.; Trostle, C.L.; Foster, M.A. Evaluating alfalfa (Medicago sativa L.) cultivars for salt tolerance using laboratory, greenhouse and field methods. J. Agri. Sci. 2012, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Zahaf, O.; Blanchet, S.; de Zélicourt, A.; Alunni, B.; Plet, J.; Laffont, C.; de Lorenzo, L.; Imbeaud, S.; Ichanté, J.L.; Diet, A.; et al. Comparative transcriptomic analysis of salt adaptation in roots of contrasting Medicago truncatula genotypes. Mol. Plant. 2012, 5, 1068–1081. [Google Scholar] [CrossRef] [Green Version]
- Tani, E.; Sarri, E.; Goufa, M.; Asimakopoulou, G.; Psychogiou, M.; Bingham, E.; Skaracis, G.N.; Abraham, E.M. Seedling growth and transcriptional responses to salt shock and stress in Medicago sativa L., Medicago arborea L., and Their Hybrid (Alborea). Agronomy 2018, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, L.B.; Li, Z.Y.; Wang, M.Z.; Guo, S.J. Effect of temperature in late autumn on free proline, soluble sugar and POD in alfalfa. Pratac. Sci. 2009, 26, 89–93. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Cui, L. Comprehensive Evalution of saline-alkali resistance and study on physiological adaption mechanism of alfalfa. Master’s Thesis, Inner Mongolia University for Nationalities, Agronomy College, Tongliao, China, 2014. [Google Scholar]
- Li, B.; Yu, H.L. Comprehensive evaluation on salt tolerance of different alfalfa varieties under soda salt and alkaline stress. Acta Agrestia. Sin. 2017, 25, 204–208. [Google Scholar]
- Xiong, X.; Gui, W.Y.; Liu, M.H.; Chen, J.H.; Zhang, Y.J. Evaluation of salt tolerance in different alfalfa under uniform and non-uniform salt stress. Acta Pratacu. Sin. 2018, 27, 67–76. [Google Scholar]
- Miao, L.H.; Yuan, Q.H.; Wang, Y. Evaluation on salt tolerance of 41 alfalfa varieties at seedling stage. Seed 2016, 35, 81–84. [Google Scholar]
- Wang, R.F.; Wang, T.M.; Jin, X.M.; Lu, X.S. Evaluation of salt tolerance of 11 approved alfalfa cultivars at seed germination stage. Pratac. Sci. 2012, 29, 213–218. (in Chinese). [Google Scholar]
- Ventroni, L.M.; Volenec, J.J.; Cangiano, C.A. Fall dormancy and cutting frequency impact on alfalfa yield and yield components. Field Crops Res. 2010, 119, 252–259. [Google Scholar] [CrossRef]
- Veatch, M.E.; Smith, S.E.; Vandemark, G. Shoot biomass production among accessions of exposed to NaCl. Crop Sci. 2004, 44, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Rumbaugh, M. Effects of population density on some components of yield of alfalfa1. Crop Sci. 1963, 3. [Google Scholar]
- Brown, P.; Tanner, C.B. Alfalfa stem and leaf growth during water stress1. Agron. J. 1983, 75, 799–805. [Google Scholar] [CrossRef]
- Rengel, Z. Role of calcium in aluminum toxicity. New Phytol. 1992, 121, 499–513. [Google Scholar] [CrossRef]
- Suarez, D.L.; Grieve, C.M. Predicting cation ratios in corn from saline solution composition. J. Exp. Bot. 1988, 39, 605–612. [Google Scholar] [CrossRef]
- Lynch, J.; Läuchli, A. Salinity affects intracellular calcium in corn root protoplasts. Plant Physiol. 1988, 87, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K.; Shi, J.; Singh, U.; Wyatt, S.E.; Bressan, R.A.; Hasegawa, P.M.; Carpita, N.C. Enrichment of vitronectin- and fibronectin-like proteins in NaCl-adapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant J. 1993, 3, 637–646. [Google Scholar] [CrossRef]
- Bernstein, N.; Silk, W.K.; Läuchli, A. Growth and development of sorghum leaves under conditions of NaCl stress: possible role of some mineral elements in growth inhibition. Planta 1995, 196, 699–705. [Google Scholar] [CrossRef]
- Walter, J.; Edwards, J.; McDonald, G.; Kuchel, H. Photogrammetry for the estimation of wheat biomass and harvest index. Field Crops Res. 2018, 216, 165–174. [Google Scholar] [CrossRef]
- Vadez, V.; Krishnamurthy, L.; Serraj, R.; Gaur, P.M.; Upadhyaya, H.D.; Hoisington, D.A.; Varshney, R.K.; Turner, N.C.; Siddique, K.H.M. Large variation in salinity tolerance in chickpea is explained by differences in sensitivity at the reproductive stage. Field Crops Res. 2007, 104, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, L.; Francois, L.E. Leaching requirement studies: sensitivity of alfalfa to salinity of irrigation and drainage waters. Soil Sci. Soc. Am. J. 1973, 37, 931–943. [Google Scholar] [CrossRef]
- Guo, Z.G.; Liu, H.X.; Wang, S.M.; Tian, F.P.; Cheng, G.D. Biomass, persistence and drought resistance of nine lucerne varieties in the dry environment of west China. Aust. J. Exp. Agr. 2005, 45, 59–64. [Google Scholar] [CrossRef]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef]
- Passioura, J.B. Scaling up: the essence of effective agricultural research. Funct. Plant Biol. 2010, 37, 585–591. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Dubey, R.S.; Singh, A.K. Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol. Plantarum. 1999, 42, 233–239. [Google Scholar] [CrossRef]
- Yu, P.J.; Liu, S.W.; Xu, Q.; Fan, G.H.; Huang, Y.X.; Zhou, D.W. Response of soil nutrients and stoichiometric ratios to short-term land use conversions in a salt-affected region, northeastern China. Ecol. Eng. 2019, 129, 22–28. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhou, M.X.; Newman, I.A.; Mendham, N.J.; Zhang, G.P.; Shabala, S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007, 34, 150–162. [Google Scholar] [CrossRef]
- Fakhrfeshani, M.; Shahriari-Ahmadi, F.; Niazi, A.; Moshtaghi, N.; Zare-Mehrjerdi, M. The effect of salinity stress on Na+, K+ concentration, Na+/K+ ratio, electrolyte leakage and HKT expression profile in roots of Aeluropus littoralis. J. Plant Mol. Breeding. 2015, 3, 1–10. [Google Scholar]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef]
- Maas, E.V.; Grieve, C.M. Sodium-induced calcium deficiency in salt-stressed corn. Plant Cell Environ. 1987, 10, 559–564. [Google Scholar]
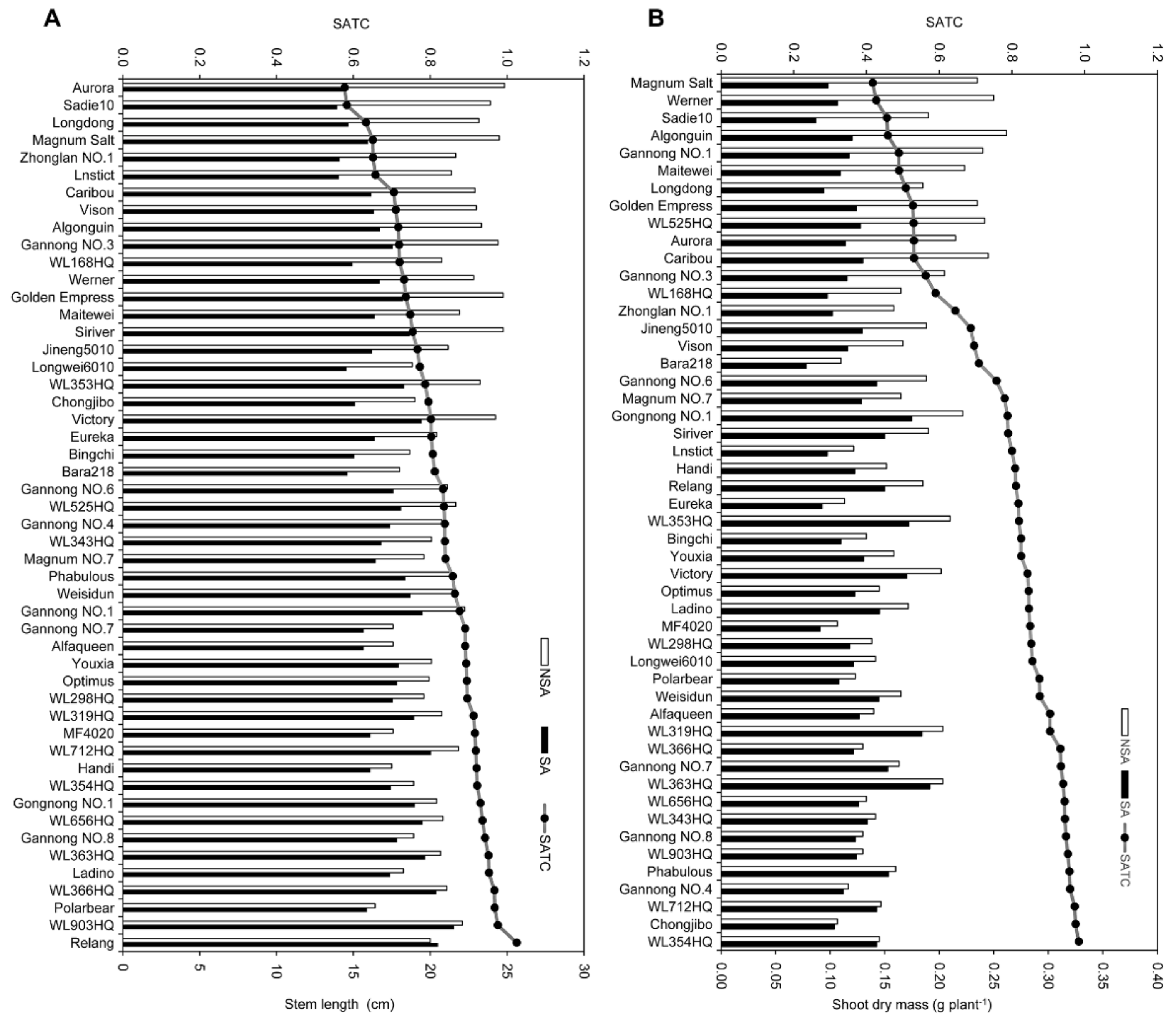


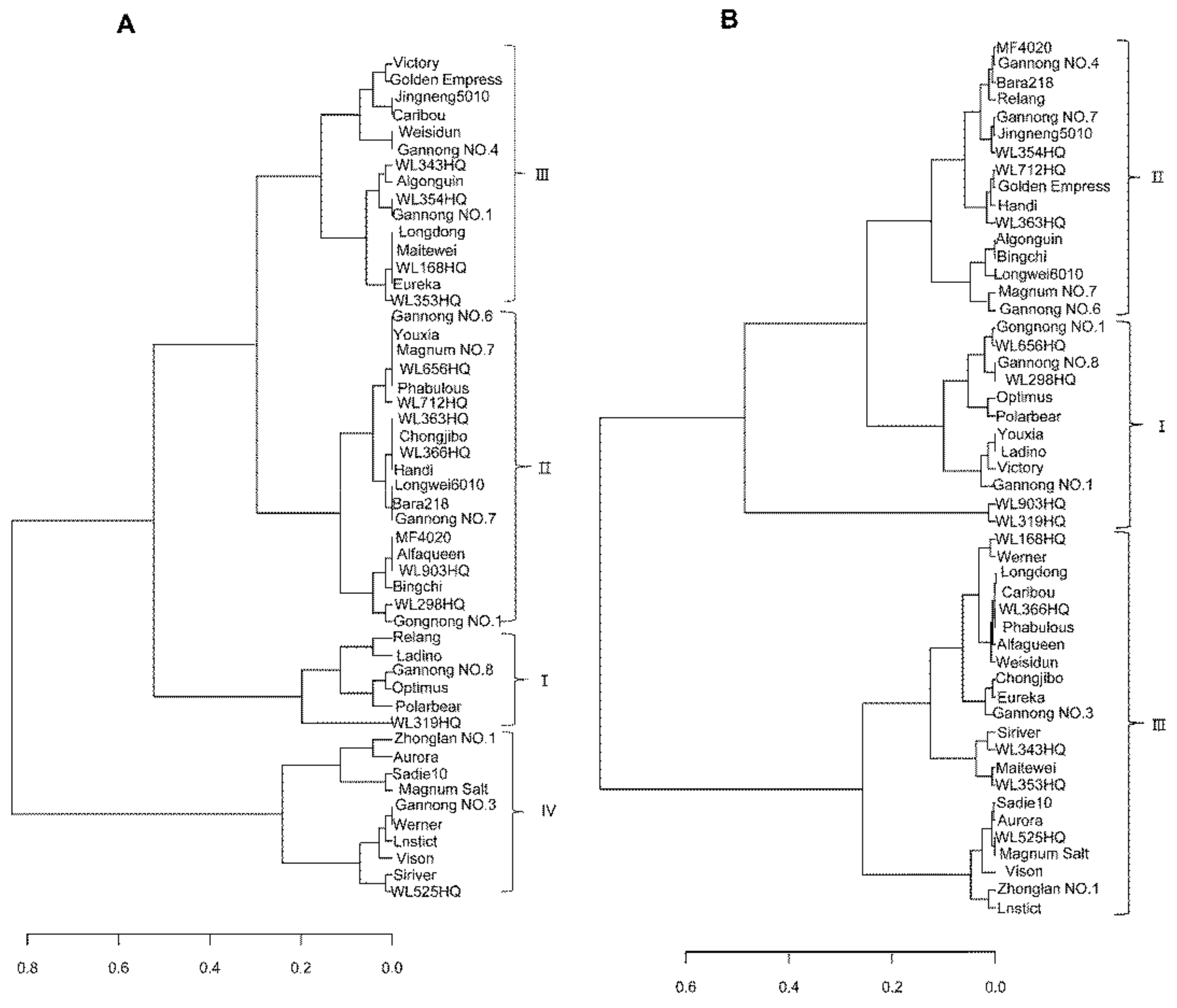
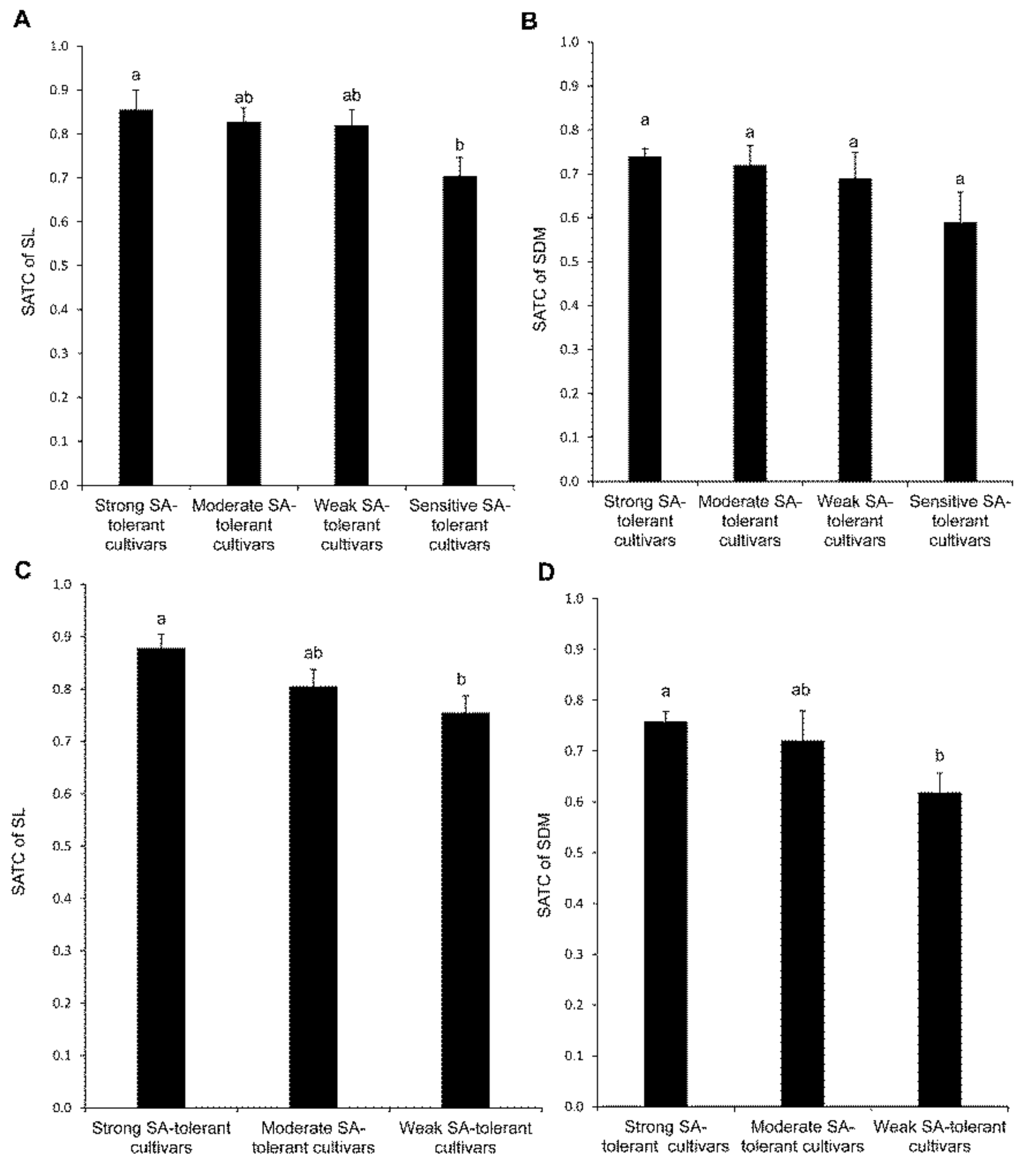
| Treatment | pH | EC | CO32− | HCO3- | Cl | SO42− | Na+ | Mg2+ | K+ | Ca2+ | Organic | Available | Available | Available |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μS cm−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | Matter (%) | N (mg kg−1) | P (mg kg−1) | K (mg kg−1) | ||
| NSA | 7.73 | 166.50 | 0.00 | 368.40 | 126.60 | 97.80 | 105.80 | 16.10 | 16.20 | 118.00 | 3.20 | 73.90 | 15.00 | 139.10 |
| SA | 9.09 | 347.22 | 0.00 | 633.20 | 192.30 | 316.80 | 502.60 | 12.70 | 4.70 | 51.60 | 1.10 | 25.50 | 27.20 | 60.50 |
| Treatment | SL | SDM | SS | PRO | Na+ | K+ | Ca2+ | Mg2+ | K+/Na+ | Ca2+/Na+ | Mg2+/Na+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (g plant−1) | (%) | (mg g−1) | (mg g−1) | (mg g−1) | (mg g−1) | (mg g−1) | ratio | ratio | ratio | |
| NSA | 21.01 | 0.17 | 3.25 | 0.12 | 4.23 | 14.68 | 19.71 | 5.79 | 4.39 | 5.59 | 1.61 |
| SA | 17.13 * | 0.13 * | 7.52 * | 0.29 * | 8.18 * | 21.56 * | 10.62 * | 4.31 * | 5.05 NS | 1.36 * | 0.55 * |
| Traditional Method | New Method | ||
|---|---|---|---|
| Indices | R2 | Indices | R2 |
| [SL] | 67.90% | [Ca2+/Na+] | 62.50% |
| [PRO] | 13.60% | [SL] | 43.40% |
| [K+/Na+] | 25.60% | [Mg2+] | 3.20% |
| [SDM] | 43.50% | [Ca2+] | 55.00% |
| [SS] | 18.20% | [SDM] | 16.70% |
| [K+] | 19.20% | [PRO] | 8.60% |
| [Na+] | 15.20% | [Na+] | 28.20% |
| [SL] + [PRO] | 82.80% | [SS] | 13.90% |
| [SL] + [PRO] + [K+/Na+] | 89.70% | [K+] | 9.40% |
| [SL] + [PRO] + [K+/Na+] + [SDM] | 96.20% | [Ca2+/Na+] + [SL] | 84.30% |
| [SL] + [PRO] + [K+/Na+] + [SDM] + [SS] | 99.00% | [Ca2+/Na+] + [SL] + [Mg2+] | 91.40% |
| [SL] + [PRO] + [K+/Na+] + [SDM] + [SS] + [K+] | 99.40% | [Ca2+/Na+] + [SL] + [Mg2+] + [Ca2+] | 96.60% |
| [SL] + [PRO] + [K+/Na+] + [SDM] + [SS] + [K+] + [Na+] | 99.90% | [Ca2+/Na+] + [SL] + [Ca2+] + [Mg2+] + [SDM] | 97.70% |
| __ | __ | [Ca2+/Na+] + [SL] + [Ca2+] + [Mg2+] + [SDM] + [PRO] | 99.00% |
| __ | __ | [Ca2+/Na+] + [SL] + [Ca2+] + [Mg2+] + [SDM] + [PRO] + [Na+] | 99.30% |
| __ | __ | [Ca2+/Na+] + [SL] + [Ca2+] + [Mg2+] + [SDM] + [PRO] + [Na+] + [SS] | 99.80% |
| __ | __ | [Ca2+/Na+] + [SL] + [Ca2+] + [Mg2+] + [SDM] + [PRO] + [Na+] + [SS] + [K+] | 99.90% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.-J.; Jiang, C.-J.; Jin, Y.-Y.; Zhang, G.-H.; Wang, M.-M.; Liang, Z.-W. Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.). Agronomy 2020, 10, 191. https://doi.org/10.3390/agronomy10020191
Wei T-J, Jiang C-J, Jin Y-Y, Zhang G-H, Wang M-M, Liang Z-W. Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.). Agronomy. 2020; 10(2):191. https://doi.org/10.3390/agronomy10020191
Chicago/Turabian StyleWei, Tian-Jiao, Chang-Jie Jiang, Yang-Yang Jin, Guo-Hui Zhang, Ming-Ming Wang, and Zheng-Wei Liang. 2020. "Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.)" Agronomy 10, no. 2: 191. https://doi.org/10.3390/agronomy10020191
APA StyleWei, T.-J., Jiang, C.-J., Jin, Y.-Y., Zhang, G.-H., Wang, M.-M., & Liang, Z.-W. (2020). Ca2+/Na+ Ratio as a Critical Marker for Field Evaluation of Saline-Alkaline Tolerance in Alfalfa (Medicago sativa L.). Agronomy, 10(2), 191. https://doi.org/10.3390/agronomy10020191




