Root Contact between Maize and Alfalfa Facilitates Nitrogen Transfer and Uptake Using Techniques of Foliar 15N-Labeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
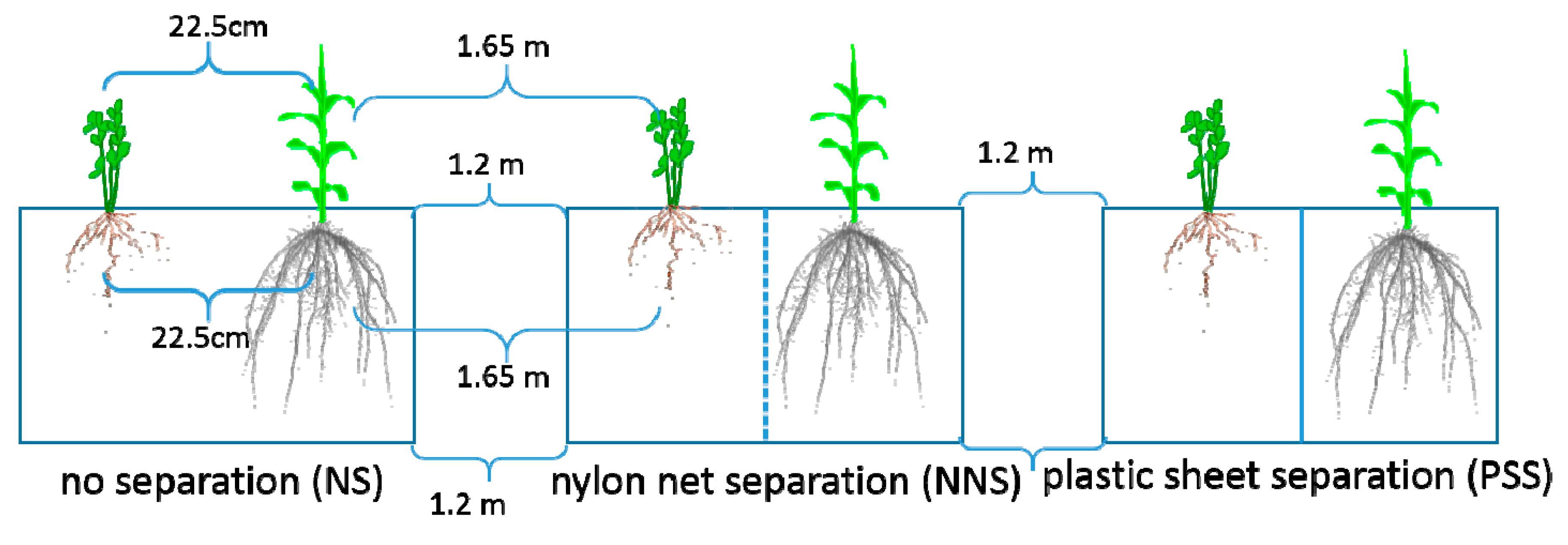
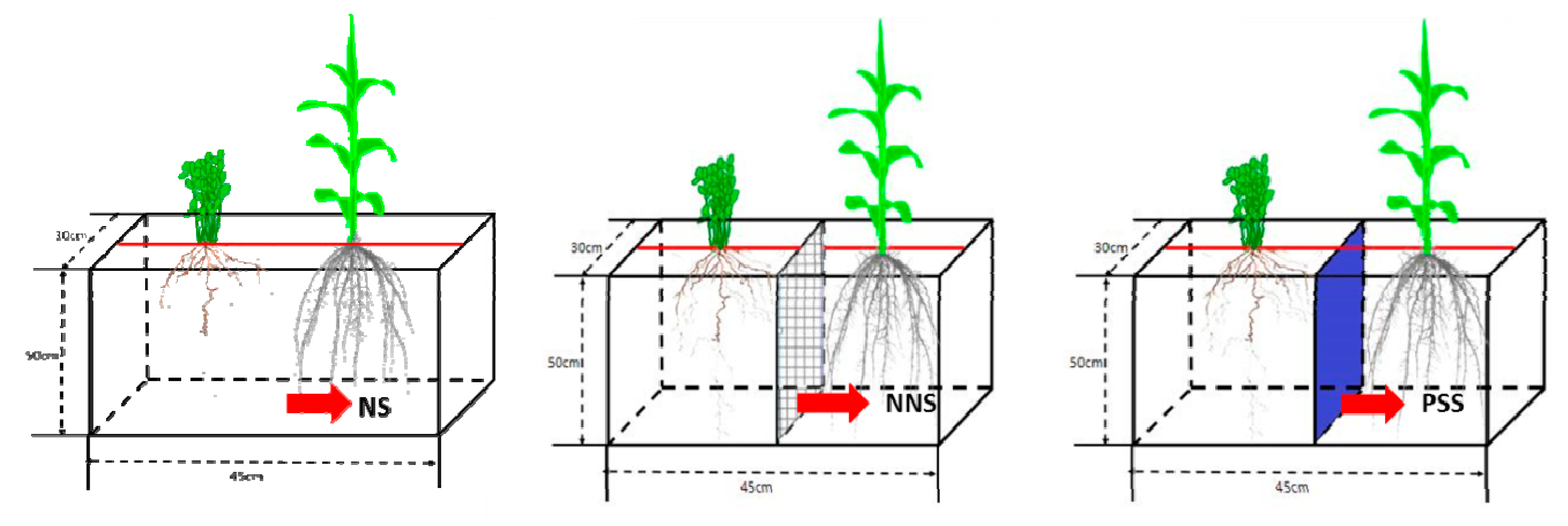
2.2. Experimental Design
2.2.1. Experiment I. Isotope (15N) Labeling of Alfalfa Leaves (Alfalfa as the 15N Donor)
2.2.2. Experiment II. Isotope (15N) Labeling of Maize Leaves (Maize as the 15N Donor)
2.3. Sampling and Measurements
2.4. Data Calculations
2.5. Statistical Analysis
3. Results
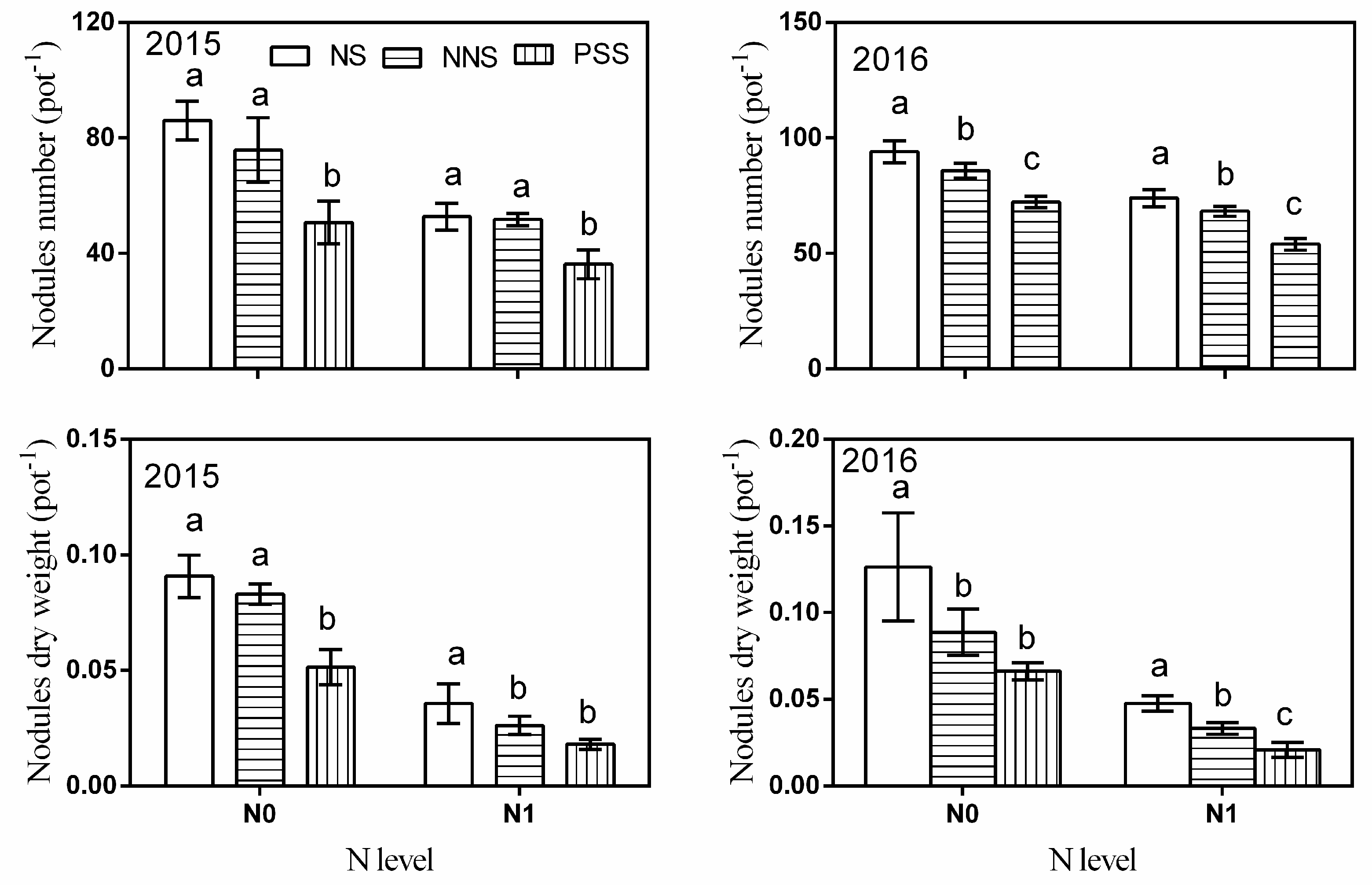
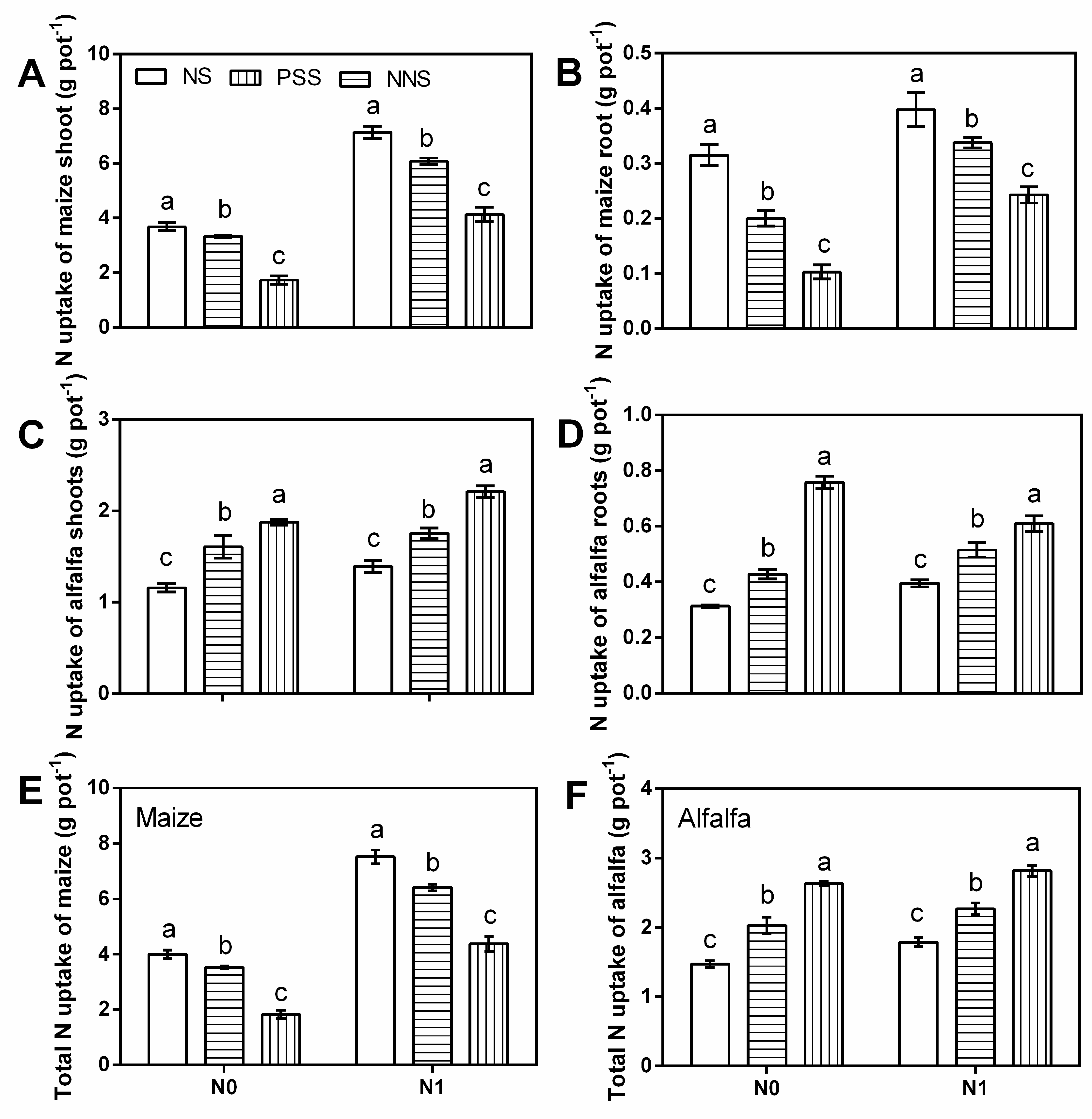
3.1. Dry Biomass
3.2. N Uptake
3.3. 15N Abundance
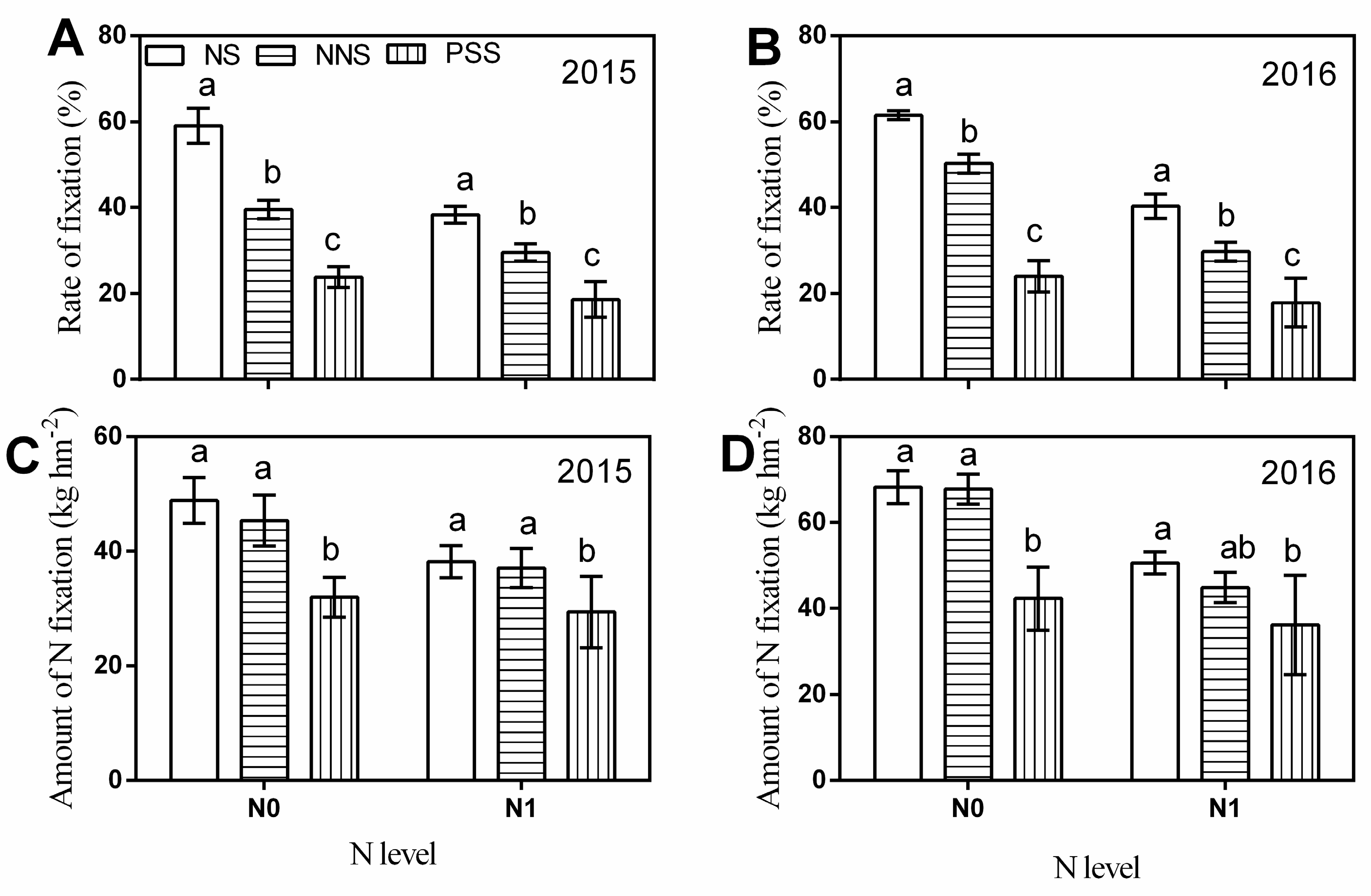
3.4. N Transfer
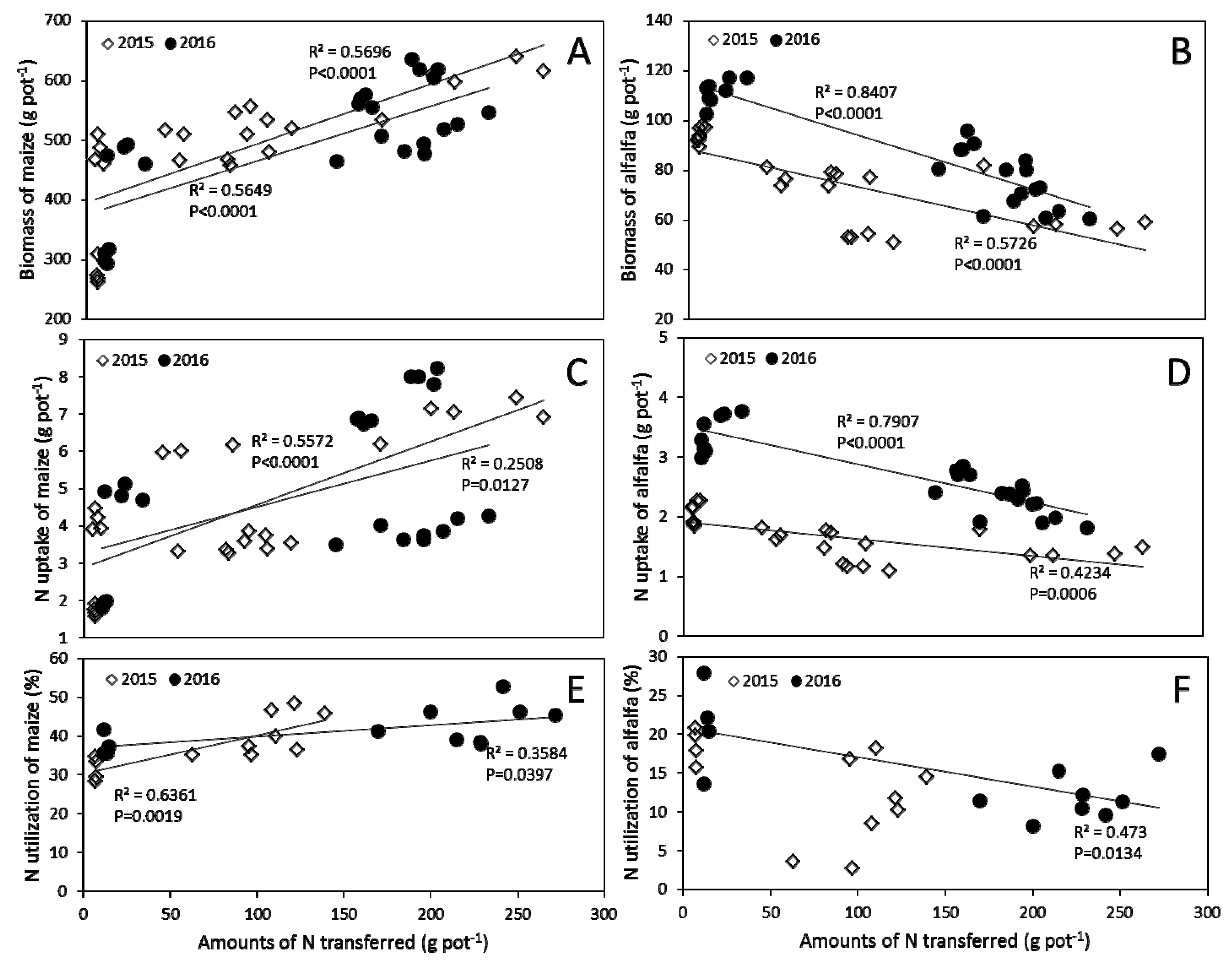
3.5. Correlation Analysis
4. Discussion
4.1. Crop Biomass and N Uptake in the Intercropping System of Maize and Alfalfa
4.2. N Transfer between Maize and Alfalfa
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Years | Treatment | Ama | NCRma | |
|---|---|---|---|---|
| 2015 | N0 | NS | 2.30 | 4.46 |
| NNS | 1.53 | 2.90 | ||
| PSS | ||||
| N1 | NS | 1.48 | 2.39 | |
| NNS | 0.55 | 1.65 | ||
| PSS | ||||
| 2016 | N0 | NS | 2.07 | 3.88 |
| NNS | 1.46 | 2.49 | ||
| PSS | ||||
| N1 | NS | 2.26 | 3.05 | |
| NNS | 1.59 | 2.03 | ||
| PSS | ||||
| Factors | Nodule Number | Dry Weight | ||
|---|---|---|---|---|
| Y | 106.39 | <0.001 ** | 16.44 | <0.001 ** |
| N | 191.63 | <0.001 ** | 285.27 | <0.001 ** |
| RS | 82.74 | <0.001 ** | 41.94 | <0.001 ** |
| Y × N | 3.02 | 0.091 | 3.14 | 0.085ns |
| Y × RS | 1.59 | 0.218 | 2.90 | 0.068ns |
| N × RS | 3.74 | 0.033 * | 6.25 | 0.005 ** |
| Y × N × RS | 2.59 | 0.089 | 1.27 | 0.293ns |
| Factors | Alfalfa %Ndfa | Alfalfa Ndfa | ||
|---|---|---|---|---|
| Y | 7.42 | <0.001 ** | 74.15 | <0.001 ** |
| N | 239.16 | <0.001 ** | 54.60 | <0.001 ** |
| RS | 339.12 | <0.001 ** | 44.47 | <0.001 ** |
| Y × N | 4.77 | 0.036 * | 7.39 | <0.001 ** |
| Y × RS | 3.33 | 0.047 * | 2.30 | 0.114ns |
| N × RS | 24.55 | <0.001 ** | 5.28 | 0.009 ** |
| Y × N × RS | 3.28 | 0.049 * | 1.15 | 0.328ns |
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Celette, F.; Findeling, A.; Gary, C. Competition for nitrogen in an unfertilized intercropping system: The case of an association of grapevine and grass cover in a Mediterranean climate. Eur. J. Agron. 2009, 30, 41–51. [Google Scholar] [CrossRef]
- Miriti, J.M.; Kironchi, G.; Esilaba, A.O.; Heng, L.K.; Gachene, C.K.K.; Mwangi, D.M. Yield and water use efficiencies of maize and cowpea as affected by tillage and cropping systems in semi-arid Eastern Kenya. Agric. Water Manag. 2012, 115, 148–155. [Google Scholar] [CrossRef]
- Xia, H.Y.; Wang, Z.G.; Zhao, J.H.; Sun, J.H.; Bao, X.G.; Christie, P.; Zhang, F.S.; Li, L. Contribution of interspecific interactions and phosphorus application to sustainable and productive intercropping systems. Field Crop. Res. 2013, 154, 53–64. [Google Scholar] [CrossRef]
- Sun, B.R.; Peng, Y.; Yang, H.Y.; Li, Z.J.; Gao, Y.Z.; Wang, C.; Yan, Y.L.; Liu, Y.M. Alfalfa (Medicago sativa L.)/maize (Zea mays L.) intercropping provides a feasible way to improve yield and economic incomes in farming and pastoral areas of northeast China. PLoS One 2014, 9, e110556. [Google Scholar] [CrossRef]
- Kermah, M.; Franke, A.C.; Adjei-Nsiah, S.; Ahiabor, B.D.K.; Abaidoo, R.C.; Giller, K.E. N2-fixation and N contribution by grain legumes under different soil fertility status and cropping systems in the guinea savanna of northern ghana. Agric. Ecosyst. Environ. 2018, 261, 201–210. [Google Scholar] [CrossRef]
- Chu, G.X.; Shen, Q.R.; Cao, J.L. Nitrogen fixation and N transfer from peanut to rice cultivated in aerobic soil in an intercropping system and its effect on soil N fertility. Plant Soil 2004, 263, 17–27. [Google Scholar] [CrossRef]
- Sierra, J.; Daudin, D. Limited 15N transfer from stem-labelled leguminous trees to associated grass in an agroforestry system. Eur. J. Agron. 2010, 32, 240–242. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.S.; Li, X.L.; Christie, P.; Sun, J.H.; Yang, S.C.; Tang, C.X. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosyst. 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Jensen, E.S. Barley uptake of N deposited in the rhizosphere of associated field pea. Soil Biol. Biochem. 1996, 28, 159–168. [Google Scholar] [CrossRef]
- Xiao, Y.B.; Li, L.; Zhang, F.S. Effect of root contact on interspecific competition and N transfer between wheat and fababean using direct and indirect 15N techniques. Plant Soil 2004, 262, 45–54. [Google Scholar] [CrossRef]
- Sakai, R.H.; Ambrosano, E.J.; Negrini, A.C.A.; Trivelin, P.C.O.; Schammass, E.A.; Melo, P.C.T.D. N transfer from green manures to lettuce in an intercropping cultivation system. Acta Sci. Agron. 2011, 33, 679–686. [Google Scholar] [CrossRef]
- Olujobi, O.J.; Oyun, M.B. Nitrogen Transfer from Pigeon Pea [Cajanus Cajan (L.) Misllp.] to Maize (Zea mays L.) In a Pigeon Pea/Maize Intercrop. Am. Int. J. Contemp. Res. 2012, 2, 115–120. [Google Scholar]
- Zhang, H.; Zeng, F.P.; Zou, Z.G.; Zhang, Z.Q.; Li, Y.Z. Nitrogen uptake and transfer in a soybean/maize intercropping system in the karst region of southwest China. Ecol. Evol. 2017, 7, 8419–8426. [Google Scholar] [CrossRef]
- Tang, Q.X.; Haile, T.; Liu, H.B.; Ren, T.Z.; Jiang, P.A.; Zhai, L.M.; Lei, B.K.; Liu, T.; Liu, E.K. Nitrogen uptake and transfer in broad bean and garlic strip intercropping systems. J. Integr. Agric. 2018, 1, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Moyer-Henry, K.A.; Burton, J.W.; Israel, D.W.; Rufty, T.W. Nitrogen transfer between plants: A 15N natural abundance study with crop and weed species. Plant Soil 2006, 282, 7–20. [Google Scholar] [CrossRef]
- He, X.H.; Xu, M.G.; Qiu, G.Y.; Zhou, J.B. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant. Ecol. 2009, 2, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Chalk, P.M.; Peoples, M.B.; Mcneill, A.M.; Boddey, R.M.; Unkovich, M.J.; Gardener, M.J.; Silva, C.F.; Chen, D.L. Methodologies for estimating nitrogen transfer between legumes and companion species in agro-ecosystems: A review of 15N-enriched techniques. Soil Biol. Biochem. 2014, 73, 10–21. [Google Scholar] [CrossRef]
- Erice, G.; Louahlia, S.; Irigoyen, J.J.; Sanchez-Diaz, M.; Avice, J.C. Biomass partitioning, morphology and water status of four alfalfa genotypes submitted to progressive drought and subsequent recovery. J. Plant. Physiol. 2010, 167, 114–120. [Google Scholar] [CrossRef]
- Zhang, G.G.; Yang, Z.B.; Dong, S.T. Interspecific competitiveness affects the total biomass yield in an alfalfa and corn intercropping system. Field Crop. Res. 2011, 124, 66–73. [Google Scholar] [CrossRef]
- Meng, L.B.; Zhang, A.Y.; Wang, F.; Han, X.G.; Wang, D.J.; Li, S.M. Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant. Sci. 2015, 6, 339. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.R.; Chu, G.X. Bi-directional nitrogen transfer in an intercropping system of peanut with rice cultivated in aerobic soil. Biol. Fertil. Soil. 2004, 40, 81–87. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Rasmussen, J.; HøGh-Jensen, H.; Eriksen, J.; Søegaard, K.; Rasmussen, J. Nitrogen transfer from forage legumes to nine neighbouring plants in a multi-species grassland. Plant Soil 2012, 350, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Danso, S.K.A.; Hardarson, G.; Zapata, F. Misconceptions and practical problems in the use of 15N soil enrichment techniques for estimating N2 fixation. Plant Soil 1993, 152, 25–52. [Google Scholar] [CrossRef]
- Yu, H.L.; Gao, Q.; Shao, Z.Q.; Ying, A.N.; Sun, Y.Y.; Liu, J.W.; Mao, W.; Zhang, B. Decreasing nitrogen fertilizer input had little effect on microbial communities in three types of soils. PLoS One 2016, 11, e0151622. [Google Scholar] [CrossRef]
- Cui, T.T.; Li, Z.H.; Wang, S.J. Effects of in-situ straw decomposition on composition of humus and structure of humic acid at different soil depths. J. Soil Sediment. 2017, 17, 2391–2399. [Google Scholar] [CrossRef]
- ISO. Soil Quality-Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations Using a Hexamminecobalt Trichloride Solution; ISO: Geneva, Switzerland, 2007; p. 23470. [Google Scholar]
- Schlindwein, J.A.; Miotti, A.A.; Fioreliperira, E.C.; Pequeno, P.L.D.L.; Bortolon, L.; Marcolan, A.L. Adjustment of the expedite method for clay content determination in rondônia soils. Ciência Rural. 2011, 41, 2096–2100. [Google Scholar] [CrossRef] [Green Version]
- FAO-ISRIC Guidelines for soil description, thirded, revised, food and agricultural organization. Rome. 1990.
- Fageria, N.K.; Baligar, V.C. Methodology for evaluation of lowland rice genotypes for nitrogen use efficiency. J. Plant Nutr. 2003, 26, 1315–1333. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Papadopoulos, Y.A.; Rodd, A.V.; Grimmett, M.; Fillmore, S.A.E.; Crouse, M.; Prithiviraj, B. Nitrogen fixation and transfer of red clover genotypes under legume–grass forage based production systems. Nutr. Cycl. Agroecosyst. 2016, 106, 233–247. [Google Scholar] [CrossRef]
- Fan, J.L.; McConkey, B.G.; Wang, H.; Janzen, H.H. Root distribution by depth for temperate agricultural crops. Field Crop. Res. 2016, 189, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Yu, C.B.; Eng, X.C.; Li, C.J.; Sun, J.H.; Zhang, F.S.; Lambers, H.; Li, L. Intercropping alleviates the inhibitory effect of N fertilization on nodulation and symbiotic N2 fixation of faba bean. Plant Soil 2009, 323, 295–308. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zhao, X.N.; Wu, P.T.; He, J.Q.; Chen, X.L.; Gao, Y.; Cao, X.C. Radiation interception and utilization by wheat/maize strip intercropping systems. Agric. For. Meteor. 2015, 204, 58–66. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.Z.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exper. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef] [Green Version]
- Gylfadóttir, T.; Helgadóttir, Á.; Høgh-Jensen, H. Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil 2007, 297, 93–104. [Google Scholar] [CrossRef]
- Ledgard, S.F. Nitrogen cycling in low input legume-based agriculture, with emphasis on legume/grass pastures. Plant Soil 2001, 228, 43–59. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Unkovich, M. Nitrogen fixation in Australian dairy systems: review and prospect. Crop Pasture Sci. 2012, 63, 787–804. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Gao, Y.Z.; Zhang, H.L.; Shao, Z.Q.; Sun, B.R.; Gao, Q. Enhancement of rhizosphere citric acid and decrease of NO3−/NH4+ ratio by root interactions facilitate N fixation and transfer. Plant Soil 2019. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Gao, Y.; Sun, B. Short-term N transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than root exudates in N deficient soil. Plant Soil 2020, 446, 23–41. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Wu, Q.; Sheng, T.; Du, M. Effects of alfalfa intercropping on crop yield, water use efficiency, and overall economic benefit in the Corn Belt of Northeast China. Field Crop. Res. 2018, 216, 109–119. [Google Scholar] [CrossRef]
- Li, L. Intercropping enhances agroecosystem services and functioning: Current knowledge and perspectives. Chin. J. Eco-Agric. 2016, 24, 403–415. [Google Scholar]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Li, L. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]

| Factor | Df | Maize dry Biomass (g pot−1) | Alfalfa Dry Biomass (g pot−1) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Y | 1 | 6.43 * | 0.77ns | 116.38 ** | 234.64 ** |
| N | 1 | 556.16 ** | 421.09 ** | 36.40 ** | 42.69 ** |
| RS | 2 | 470.64 ** | 545.17 ** | 905.97 ** | 310.23 ** |
| Y × N | 1 | 0.00ns | 4.71 * | 2.36ns | 15.54 ** |
| Y × RS | 2 | 2.51ns | 6.21 ** | 5.71 ** | 16.82 ** |
| N × RS | 2 | 62.28 ** | 11.66 ** | 1.14ns | 0.19ns |
| Y × N × RS | 4 | 2.15ns | 10.50 ** | 1.24ns | 0.86ns |
| Factors | Df | Maize nitrogen Uptake (g pot−1) | Alfalfa Nitrogen Uptake (g pot−1) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Y | 1 | 33.63 ** | 2.09ns | 450.14 ** | 101.10 ** |
| N | 1 | 4444.12 ** | 544.04 ** | 153.68 ** | 67.15 ** |
| RS | 2 | 1044.18 ** | 286.31 ** | 613.31 ** | 408.66 ** |
| Y × N | 1 | 10.86 ** | 43.58 ** | 0.48ns | 54.72 ** |
| Y × RS | 2 | 0.27ns | 3.81 * | 17.24 ** | 2.63ns |
| N × RS | 2 | 36.38 ** | 7.42 ** | 6.46 ** | 11.81 ** |
| Y × N × RS | 4 | 0.89ns | 14.56 ** | 0.50ns | 25.63 ** |
| Factors | Df | 15N Abundance (Alfalfa as 15N-donor Plant) | 15N Abundance (Maize as 15N-donor Plant) | ||||
|---|---|---|---|---|---|---|---|
| Maize Shoot | Maize Root | Alfalfa Shoot | Alfalfa Root | Alfalfa Shoot | Alfalfa Root | ||
| Y | 1 | 0.26 ns | 3.69 ns | 68.27 ** | 374.08 ** | ||
| N | 1 | 0.68 ns | 26.42 ** | 1.30 ns | 13.58 ** | 6.94 * | 24.14 ** |
| RS | 2 | 47.13 ** | 221.05 ** | 2.44 ns | 5.16 * | 1.63 ns | 2.71 ns |
| Y × N | 1 | 2.50 ns | 13.59 ** | 8.09 ** | 5.94 * | ||
| Y × RS | 2 | 3.00 ns | 12.73 ** | 4.94 * | 5.20 * | ||
| N × RS | 2 | 1.18 ns | 9.47 ** | 0.63 ns | 6.68 ** | 3.34 ns | 1.86 ns |
| Y × N × RS | 4 | 2.11 ns | 1.73 ns | 1.68 ns | 3.35 * | ||
| Years | Treatment | 15N Abundance (Alfalfa as 15N-donor Plant) | 15N Abundance (maize as 15N-donor Plant) | |||||
|---|---|---|---|---|---|---|---|---|
| Maize Shoot | Maize Root | Alfalfa Shoot | Alfalfa Root | Alfalfa Shoot | Alfalfa Root | |||
| 2015 | N0 | NS | 0.386 ± 0.003ab | 0.392 ± 0.003a | 0.918 ± 0.071a | 0.601 ± 0.062ab | ||
| NNS | 0.384 ± 0.006ab | 0.387 ± 0.005b | 0.879 ± 0.099ab | 0.576 ± 0.004b | ||||
| PSS | 0.372 ± 0.005c | 0.371 ± 0.001d | 0.783 ± 0.096b | 0.583 ± 0.006b | ||||
| N1 | NS | 0.391 ± 0.006a | 0.394 ± 0.005a | 0.844 ± 0.037ab | 0.571 ± 0.024b | |||
| NNS | 0.382 ± 0.011b | 0.381 ± 0.004c | 0.856 ± 0.022ab | 0.637 ± 0.035a | ||||
| PSS | 0.372 ± 0.001c | 0.371 ± 0.001d | 0.794 ± 0.077b | 0.577 ± 0.009b | ||||
| 2016 | N0 | NS | 0.388 ± 0.003a | 0.393 ± 0.003a | 0.627 ± 0.052c | 0.432 ± 0.001c | 0.369 ± 0.008a | 0.368 ± 0.006a |
| NNS | 0.390 ± 0.002a | 0.396 ± 0.003a | 0.717 ± 0.027ab | 0.448 ± 0.001b | 0.370 ± 0.010a | 0.369 ± 0.004a | ||
| PSS | 0.372 ± 0.000c | 0.373 ± 0.000c | 0.679 ± 0.055bc | 0.457 ± 0.001a | 0.369 ± 0.007a | 0.369 ± 0.008a | ||
| N1 | NS | 0.382 ± 0.001b | 0.387 ± 0.001b | 0.734 ± 0.048ab | 0.452 ± 0.000c | 0.368 ± 0.015a | 0.368 ± 0.013a | |
| NNS | 0.384 ± 0.004b | 0.384 ± 0.003b | 0.735 ± 0.012ab | 0.493 ± 0.001b | 0.368 ± 0.011a | 0.367 ± 0.010a | ||
| PSS | 0.374 ± 0.002c | 0.371 ± 0.000c | 0.754 ± 0.033a | 0.511 ± 0.017a | 0.369 ± 0.007a | 0.368 ± 0.011a | ||
| Control (2015) | 0.372 ± 0.004 | 0.371 ± 0.010 | 0.373 ± 0.006 | 0.370 ± 0.007 | ||||
| Control (2016) | 0.370 ± 0.003 | 0.371 ± 0.006 | 0.370 ± 0.009 | 0.368 ± 0.006 | ||||
| Years | Treatment | Rate of N Transferred (%) | Amount of N Transferred (mg pot−1) | N Transferred as of Maize N (%) | N Transferred as of Alfalfa N (%) | |
|---|---|---|---|---|---|---|
| 2015 | N0 | NS | 14.52 ± 1.01a | 167.92 ± 13.82a | 3.53 ± 0.40a | 8.93 ± 0.12a |
| NNS | 7.48 ± 2.06b | 119.89 ± 34.31b | 3.18 ± 0.43a | 5.09 ± 0.32b | ||
| N1 | NS | 11.86 ± 1.48a | 165.83 ± 25.60a | 4.41 ± 0.26a | 16.55 ± 0.45a | |
| NNS | 5.14 ± 0.14b | 90.40 ± 1.99b | 1.96 ± 1.04b | 4.89 ± 0.36b | ||
| 2016 | N0 | NS | 15.58 ± 2.29a | 241.19 ± 30.45a | 5.90 ± 0.64a | 12.69 ± 1.85a |
| NNS | 11.12 ± 1.30b | 210.36 ± 27.94a | 5.78 ± 0.65a | 8.62 ± 1.07b | ||
| N1 | NS | 13.07 ± 1.00a | 229.56 ± 8.32a | 2.87 ± 0.12a | 10.09 ± 0.68a | |
| NNS | 8.89 ± 0.39b | 187.67 ±4.17b | 2.75 ± 0.08a | 6.82 ± 0.24b | ||
| 2 year average | N0 | NS | 15.05 ± 1.51a | 204.56 ± 18.79a | 4.72 ± 0.42a | 10.81 ± 1.42a |
| NNS | 9.30 ± 0.71b | 165.12 ± 15.97b | 4.48 ± 0.22a | 6.86 ± 0.33b | ||
| N1 | NS | 12.47 ± 0.93a | 197.70 ± 12.76a | 3.64 ± 0.26a | 13.32 ± 0.77a | |
| NNS | 7.02 ± 1.70b | 139.04 ± 11.97b | 2.36 ± 0.60b | 5.86 ± 1.50b | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Wang, X.; Gao, Q.; Zhang, H.; Yu, H.; Wang, Y.; Zhang, J.; Nasar, J.; Gao, Y. Root Contact between Maize and Alfalfa Facilitates Nitrogen Transfer and Uptake Using Techniques of Foliar 15N-Labeling. Agronomy 2020, 10, 360. https://doi.org/10.3390/agronomy10030360
Shao Z, Wang X, Gao Q, Zhang H, Yu H, Wang Y, Zhang J, Nasar J, Gao Y. Root Contact between Maize and Alfalfa Facilitates Nitrogen Transfer and Uptake Using Techniques of Foliar 15N-Labeling. Agronomy. 2020; 10(3):360. https://doi.org/10.3390/agronomy10030360
Chicago/Turabian StyleShao, Zeqiang, Xinyu Wang, Qiang Gao, Hualiang Zhang, Hailing Yu, Yin Wang, Jinjing Zhang, Jamal Nasar, and Yingzhi Gao. 2020. "Root Contact between Maize and Alfalfa Facilitates Nitrogen Transfer and Uptake Using Techniques of Foliar 15N-Labeling" Agronomy 10, no. 3: 360. https://doi.org/10.3390/agronomy10030360







