The Roles of Different Types of Trichomes in Tomato Resistance to Cold, Drought, Whiteflies, and Botrytis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
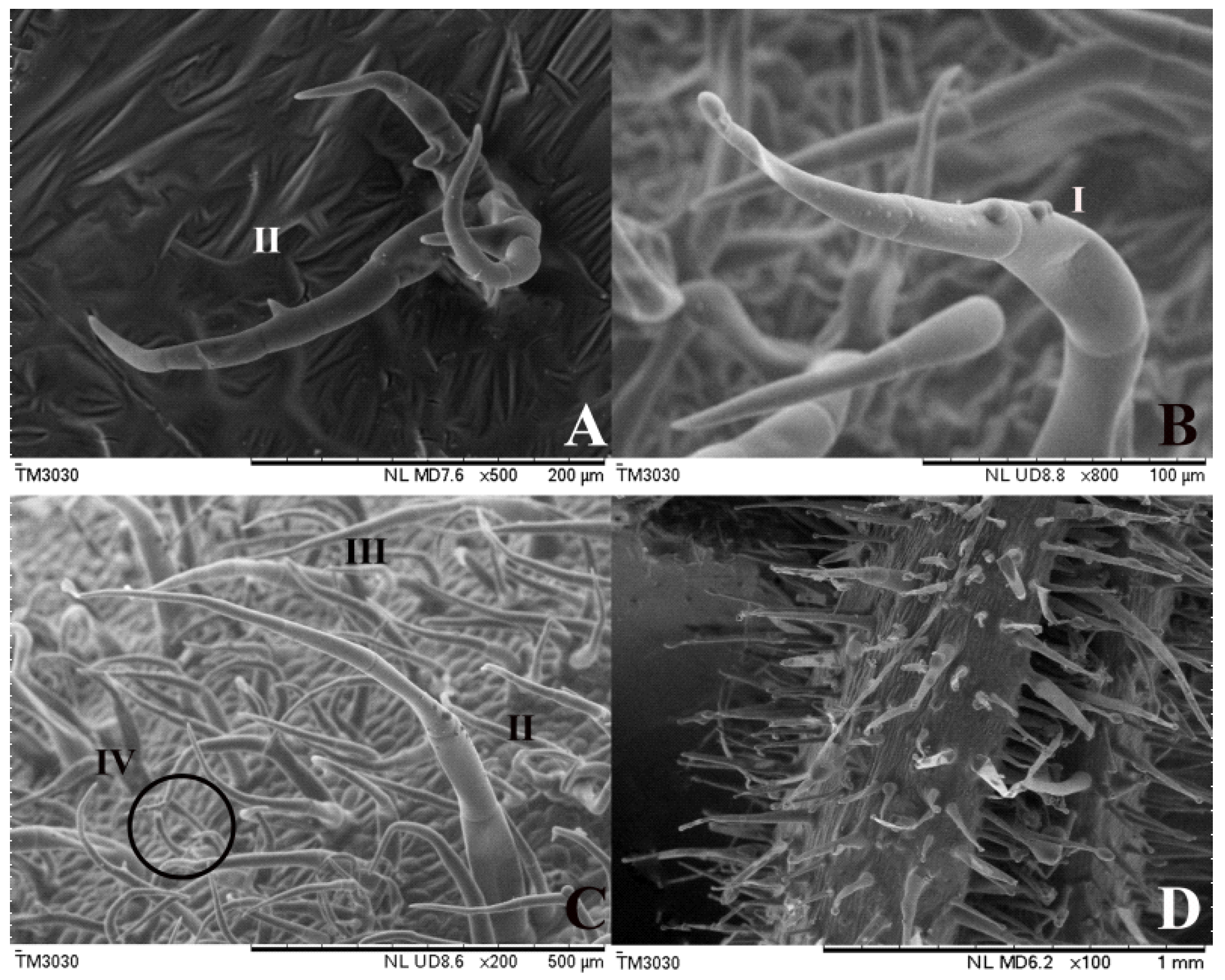
2.2. Observation of Trichomes Difference Using Scanning Electron Microscope
2.3. Abiotic Stress Treatment
2.4. Botrytis Cinerea Inoculation
2.5. Whitefly Treatment
3. Results
3.1. Different Tomato Trichome Types
3.2. The Chloroplast Structure of Four Tomato Trichome Types
3.3. Response to Abiotic Stress
3.4. Resistance to B. Cinerea
3.5. Resistance to Whiteflies
4. Discussion
4.1. Tomatoes with a High Density of Type Ⅰ Glandular and Type Ⅱ Nonglandular Trichomes were Tolerant to Cold Stress
4.2. Abundant Nonglandular Trichomes in Tomatoes can Promote Drought Tolerance
4.3. Abundant Type Ⅲ Nonglandular Trichomes and a Higher Average Height of Tomato Trichomes can Result in Pathogen Resistance
4.4. With Longer Trichomes and a Higher Trichome Density, Particularly Type Ⅰ and Ⅳ Glandular Trichomes, Tomatoes can be Resistant to Whiteflies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, H.B. Plant pubescence: An ecological perspective. Botanical Rev. 1975, 41, 233–258. [Google Scholar] [CrossRef]
- Kang, J.H.; Liu, G.; Shi, F.; Jones, A.D.; Beaudry, R.M.; Howe, G.A. The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 2010, 154, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Kurata, T.; Okada, K.; Wada, T. A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 2008, 59, 365–386. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Physiological and Biochemical Experimental Principles and Techniques; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Yan, A.; Pan, J.; An, L.; Gan, Y.; Feng, H. The responses of trichome mutants to enhanced ultraviolet-B radiation in Arabidopsis thaliana. J. Photochem. Photobiol. B Biol. 2012, 113, 29–35. [Google Scholar] [CrossRef]
- Chandravanshi, S.S.; Singh, B.P.; Thakur, M.P. Persistence of different fungicides used against Alternaria alternata in tomato. Indian Phytopathol. 1994, 47, 241–244. [Google Scholar]
- Chien, J.C.; Sussex, I.M. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996, 111, 1321–1328. [Google Scholar] [CrossRef] [Green Version]
- McDowell, E.T.; Kapteyn, J.; Schmidt, A.; Li, C.; Kang, J.H.; Descour, A.; Shi, F.; Larson, M.; Schilmiller, A.; An, L.; et al. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 2011, 155, 524–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calo, L.; Garcia, I.; Gotor, C.; Romero, L.C. Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma alpha-1, 3-glucanase. J. Expt. Bot. 2006, 57, 3911–3920. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.X.; Huang, Y.F. The morphological structure and disease resistance of plants. Acta Phytopathol. Sinica 1995, 1, 1–3. [Google Scholar]
- Yang, Z.M.; Deng, J.J.; Yu, H.P. Research on the resistance of tomato hair virus and Tm-Znv and their recombinant genotypes to tomato virus disease. Acta Agric. Boreali-Occident. Sinica 1996, 5, 23–26. [Google Scholar]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Kenzo, T.; Yoneda, R.; Azani, M.A.; Majid, N.M. Changes in leaf water use after removal of leaf lower surface hairs on Mallotus macrostachyus (Euphorbiaceae) in a tropical secondary forest in Malaysia. J. For. Res. 2008, 13, 137–142. [Google Scholar] [CrossRef]
- Sandquist, D.R.; Ehleringer, J.R. Population- and family-level variation of brittlebush (Encelia farinosa, Asteraceae) pubescence: Its relation to drought and implications for selection in variable environments. Amer. J. Bot. 2003, 90, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.A.; Fishbein, M. Plant defense syndromes. Ecology 2006, 87, S132–S149. [Google Scholar] [CrossRef]
- Traw, M.B.; Bergelson, J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 2003, 133, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.Z.; Li, J.F.; Wang, A.X. Effect of low temperature stress on physiological and biochemical characteristics of Lycopersicon hirsutum seedlings. Dongbei Nongye Daxue Xuebao. 2011, 42, 57–62. [Google Scholar]
- Xiong, C.; Xie, Q.M.; Yang, Q.H. Woolly, interacting with MYB transcription factor SlMYB31, regulates cuticular wax biosynthesis by modulating SlCER6 expression in tomato. Plant J. 2020. [Google Scholar] [CrossRef]
- Robert, J.P. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosyn. Res. 2002, 73, 149–156. [Google Scholar]
- Corbinenu, F.; Gay-Mathieu, C.; Vinel, D.; Côme, D. Decrease in sunfloiwer (Helianthus annuus)seed viability caused by high temperatures as related to energy metabolism, membrane damage and lipid composition. Physical Plant. 2002, 116, 489–496. [Google Scholar] [CrossRef]
- Fu, S.L.; Zhou, Y.B.; He, X.Y.; Chen, W. Effects of drought stress on photosynthesis physiology of Populus pseudosimonii. Chin. J. Appl. Ecol. 2006, 17, 2016–2019. [Google Scholar]
- Silva, M.D.A.; Jifon, J.L.; Silva, J.A.G.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Begum, M.K.; Alam, M.R.; Islam, M.S.; Arefin, M.S. Effect of water stress on physiological characters and juice quality of sugarcane. Sugar Tech. 2012, 14, 161–167. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Expt. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Hao, Z.B.; Cang, J.; Xu, Z. Physiological Experiment Bottle; Harbin Institute of Technology Press: Harbin, China, 2004. [Google Scholar]
- Foolad, M.R.; Lin, G.Y. Genetic analysis of cold tolerance during vegetative growth in tomato, Lycopersicon esculentum Mill. Plant Breed. 2001, 122, 105–111. [Google Scholar]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2018, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Jones, J.P.; Stall, R.E.; Zitter, T.A. Compendium of Tomato Diseases; The American Phytopathological Society: St. Paul, MN, USA, 1991. [Google Scholar]
- Patil, P.G.; Shashidhar, H.E.; Byregowda, M.; Reena, G.A.M.; Ashok, T.H.; Swamy, H.V.V.; Ramappa, H.K.; Babu, J. Association of leaf micro-morphological features with sterility mosaic disease resistance in pigeonpea. J. Environ. Biol. 2017, 38, 649–656. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, Y.Q.; Shi, L.; Pan, N.S.; Chen, Z.L. Activity of SOD in wheat varieties with head scab bacteria. Plant Physiol. J. 1993, 19, 353–358. [Google Scholar]
- Zhang, J.F.; Xue, Q.Z. Effects of rice planthopper stress on the activities of major protective enzymes in rice plants. Chin. Agric. Sci. 2004, 37, 1487–1491. [Google Scholar]
- Ferreira, P.A.A.; Tiecher, T.; Tiecher, T.L.; De Melo Rangel, W.; Soares, C.R.F.S.; Deuner, S.; Peligrinotti Tarouco, C.; Giachini, A.J.; Nicoloso, F.T.; Brunetto, G.; et al. Effects of Rhizophagus clarus and P availability in the tolerance and physiological response of Mucuna cinereum to copper. Plant Physiol Biochem. 2018, 122, 46–56. [Google Scholar] [CrossRef]
- Sun, L.L.; Cao, C.W.; Xue, X.T.; Du, C.Y. Trichoderma reesei WP preparation and determination of bactericidal activity. J. Beijing For. Univ. 2015, 37, 45–52. [Google Scholar]
- Jia, W.; Mao, L.; Zhang, L.; Jiang, H. Effects of two strobilurins (azoxystrobin and picoxystrobin) on embryonic development and enzyme activities in juveniles and adult fish livers of zebrafish (Danio rerio). Chemosphere 2018, 207, 573–580. [Google Scholar] [CrossRef]
- Juvik, J.A.; Babka, B.A.; Timmermann, E.A. Influence of trichome exudates from species of Lycopersicon on oviposition behavior of Heliothis zea (Boddie). J. Chem. Ecol. 1988, 14, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, S.R.; Oki, Y.; Heleno, V.C.G.; Chaves, J.S.; Nascimento, P.G.B.D.; Lichston, J.E.; Constantino, M.G.; Varanda, E.M.; Da Costa, F.B. Constituents of glandular trichomes of Tithonia diversifolia: Relationships to herbivory and antifeedant activity. Phytochemistry 2008, 69, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Elad, Y.; Shtienberg, D. Botrytis cinerea in greenhouse vegetables: Chemical, cultural, physiological and biological controls and their integration. Integr. Pest Mgt. Rev. 1995, 1, 15–29. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert:glandular trichomes as sensors for insect herbivores. New Phytol. 2009, 184, 644–656. [Google Scholar] [CrossRef]







| Accession | POD Activities /U∙g−1∙min−1 (FW) | SOD Activities /U∙g−1∙min−1 (FW) |
|---|---|---|
| 3186M | 503 ± 3.33d | 270.11 ± 1.62d |
| 3186L | 439 ± 2.52b | 251.82 ± 1.02c |
| 3-071 | 485 ± 0.60c | 230.72 ± 0.85b |
| JR | 403 ± 6.03a | 205.40 ± 1.08a |
| Accession | Chlorophyll a Content /mg·g−1 | Chlorophyll b Content /mg·g−1 | Total Chlorophyll /mg·g−1 |
|---|---|---|---|
| 3186M | 5.47 ± 0.03b | 11.13 ± 0.03b | 16.60 ± 0.06b |
| 3186L | 3.74 ± 0.07a | 7.82 ± 0.05a | 11.56 ± 0.12a |
| 3-071 | 3.79 ± 0.04a | 7.81 ± 0.04a | 11.60 ± 0.08a |
| JR | 3.85 ± 0.07a | 7.79 ± 0.04a | 11.64 ± 0.11a |
| Accession | POD Activity /U∙g−1∙min−1 (FW) | SOD Activity /U∙g−1∙min−1 (FW) |
|---|---|---|
| 3186M | 499 ± 3.33c | 311.73 ± 0.71c |
| 3186L | 690 ± 1.32d | 286.34 ± 0.52bc |
| 3-071 | 478 ± 2.56b | 263.77 ± 1.66b |
| JR | 415 ± 1.05a | 157.98 ± 0.36a |
| Accession | Chlorophyll a Content /mg·g−1 | Chlorophyll b Content /mg·g−1 | Total Chlorophyll /mg·g−1 |
|---|---|---|---|
| 3186M | 3.66 ± 0.03a | 7.95 ± 0.03a | 11.61 ± 0.06a |
| 3186L | 5.98 ± 0.07c | 12.19 ± 0.05c | 18.17 ± 0.12c |
| 3-071 | 4.49 ± 0.04b | 9.21 ± 0.04b | 13.7 ± 0.08b |
| JR | 4.85 ± 0.07b | 9.95 ± 0.04b | 14.80 ± 0.11b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Song, H.; Wang, X.; Zhou, X.; Zhang, K.; Chen, X.; Liu, J.; Han, J.; Wang, A. The Roles of Different Types of Trichomes in Tomato Resistance to Cold, Drought, Whiteflies, and Botrytis. Agronomy 2020, 10, 411. https://doi.org/10.3390/agronomy10030411
Zhang Y, Song H, Wang X, Zhou X, Zhang K, Chen X, Liu J, Han J, Wang A. The Roles of Different Types of Trichomes in Tomato Resistance to Cold, Drought, Whiteflies, and Botrytis. Agronomy. 2020; 10(3):411. https://doi.org/10.3390/agronomy10030411
Chicago/Turabian StyleZhang, Yao, Haihui Song, Xingyuan Wang, Xinan Zhou, Kewei Zhang, Xiuling Chen, Jiayin Liu, Junyou Han, and Aoxue Wang. 2020. "The Roles of Different Types of Trichomes in Tomato Resistance to Cold, Drought, Whiteflies, and Botrytis" Agronomy 10, no. 3: 411. https://doi.org/10.3390/agronomy10030411
APA StyleZhang, Y., Song, H., Wang, X., Zhou, X., Zhang, K., Chen, X., Liu, J., Han, J., & Wang, A. (2020). The Roles of Different Types of Trichomes in Tomato Resistance to Cold, Drought, Whiteflies, and Botrytis. Agronomy, 10(3), 411. https://doi.org/10.3390/agronomy10030411





