Starch Accumulation and Granule Size Distribution of Cassava cv. Rayong 9 Grown under Irrigated and Rainfed Conditions Using Different Growing Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Design and Crop Management
2.2. Data Collection
2.2.1. Meteorological Condition and Soil Properties
2.2.2. Storage Root Dry Weight and Sample Preparation for Laboratory Analyses
2.2.3. Determination of Starch Content and Starch Yield
2.2.4. Determination of Starch Granule Size Distribution
2.2.5. Determination of Amylose and Amylopectin
2.3. Statistical Analysis
3. Results
3.1. Weather Conditions and Soil Water Status
3.2. Starch Content, Starch Yield and Storage Root Dry Weight
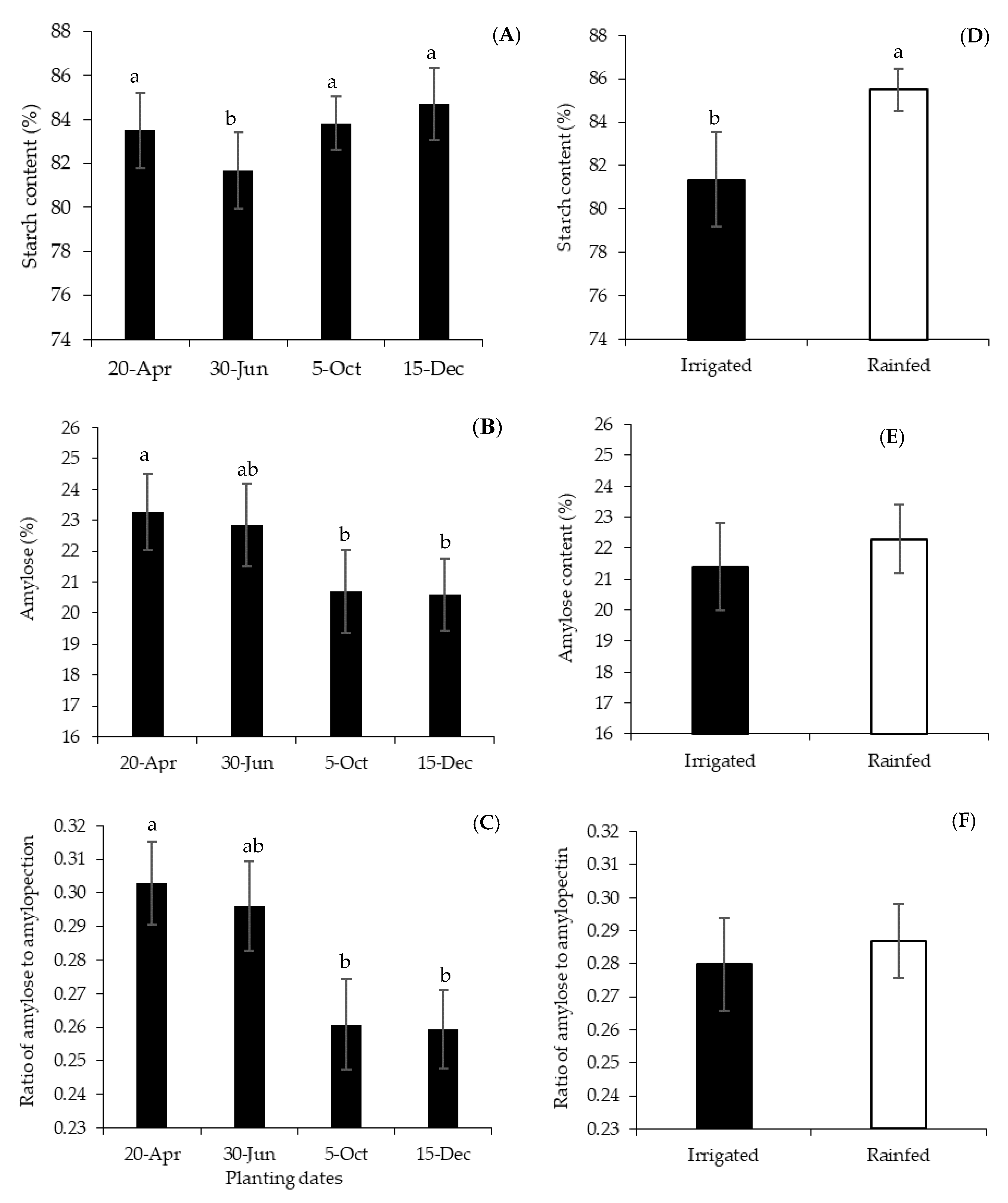
3.3. Starch Granule Size Distribution, Amylose and Ratio of Amylose to Amylopectin
3.4. Combined Analysis of Variance
3.5. Stepwise Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef]
- Howeler, R.; Lutaladio, N.; Thomas, G. Save and Grow: Cassava—A Guide to Sustainable Production Intensification; Food and Agriculture Organization: Rome, Italy, 2013. [Google Scholar]
- Howeler, R.H. Sustainable Soil and Crop Management of Cassava in Asia: A Reference Manual; CIAT Publication: Hanoi, Vietnam, 2014. [Google Scholar]
- Office of Agricultural Economics. Available online: http://www.oae.go.th (accessed on 13 April 2018).
- Connor, D.J.; Cock, J.H. Response of cassava to water shortage. II. Canopy dynamics. Field Crops Res. 1981, 4, 285–296. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Drought-tolerant cassava for Africa, Asia, and Latin America. BioScience 1993, 43, 441–451. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava Botany and Physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 115–147. [Google Scholar] [CrossRef] [Green Version]
- Janket, A.; Vorasoot, N.; Kesmala, T.; Jogloy, S. Influence of zinc, copper and manganese on dry matter yield and physiological traits of three cassava genotypes grown on soil micronutrient deficiencies. Pak. J. Bot. 2018, 50, 1719–1725. [Google Scholar]
- Sawatraksa, N.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Hoogenboom, G. Chlorophyll fluorescence and biomass of four cassava genotypes grown under rain-fed upper paddy field condition in tropics. J. Agro. Crop Sci. 2018, 204, 554–565. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Growth rates and yields of cassava at different planting dates in a tropical savanna climate. Sci. Agric. 2018, 76, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.V.S.; Ramesh, S.; Reddy, P.S. Sorghum breeding research at ICRISAT-goals, strategies, methods and accomplishments. Int. Sorghum Millets Newsl. 2004, 45, 5–12. [Google Scholar]
- Santisopasri, V.; Kurotjanawong, K.; Chotineeranat, S.; Piyachomkwan, K.; Sriroth, K. Impact of water stress on yield and quality of cassava starch. Ind. Crops Prod. 2001, 13, 115–129. [Google Scholar] [CrossRef]
- Teerawanichpan, P.; Lertpanyasampatha, M.; Netrphan, S.; Varavinit, S.; Boonseng, O.; Narangajavana, J. Influence of cassava storage root development and environmental conditions on starch granule size distribution. Starch Stärke 2008, 60, 696–705. [Google Scholar] [CrossRef]
- Defloor, I.; Dehing, I.; Delcour, J.A. Physico-chemical properties of cassava starch. Starch Stärke 1998, 50, 58–64. [Google Scholar] [CrossRef]
- Prammanee, S.; Kamprerasart, K.; Salakan, S.; Sriroth, K. Growth and starch content evaluation on newly released cassava cultivars, Rayong 9, Rayong 7 and Rayong 80 at different harvest times. Kasetsart J. 2010, 44, 558–563. [Google Scholar]
- Good Agricultural Practices for Cassava. Available online: http://www.acfs.go.th/standard /download/eng/GAP_cassava.pdf (accessed on 12 August 2018).
- Howeler, R.H. Cassava Mineral Nutrition and Fertilization. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 281–300. [Google Scholar]
- Hoover, R.; Ratnayake, W.S. Current Protocols in Food Analytical Chemistry; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Freed, R.D.; Nissen, O. MSTAT-C Version 1.42; Michigan State University: East Lansing, MI, USA, 1992. [Google Scholar]
- Polthanee, A.; Srisutham, M. Supplementary irrigation for cassava planted in the late rainy season of Northeastern Thailand. Asian J. Crop Sci. 2017, 9, 100–108. [Google Scholar] [CrossRef]
- Mahakosee, S.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P.; Banterng, P.; Kesmala, T.; Holbrook, C.; Kvien, C. Seasonal variations in canopy size and yield of rayong 9 cassava genotype under rainfed and irrigated conditions. Agronomy 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Sriroth, K.; Piyachomkwan, K.; Santisopasri, V.; Oates, C.G. Environmental conditions during root development: Drought constraint on cassava starch quality. Euphytica 2001, 120, 95–101. [Google Scholar] [CrossRef]
- Lahai, M.T.; Ekanayake, I.J.; Koroma, J.P.C. Influence of canopy structure on yield of cassava cultivars at various toposequences of an inland valley agro ecosystem. J. Agric. Biotech. Sustain. Dev. 2013, 5, 36–47. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H. Response of cassava to water stress. Plant Soil 1987, 100, 345–360. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; De Tafur, S.M. Genotypic and within canopy variation in leaf carbon isotope discrimination and its relation to short-term leaf gas exchange characteristics in cassava grown under rain-fed conditions in the tropics. Photosynthetica 2007, 45, 515–526. [Google Scholar] [CrossRef]
- Geigenberger, P.; Reimholz, R.; Geiger, M.; Merlo, L.; Canale, V.; Stitt, M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 1997, 201, 502–518. [Google Scholar] [CrossRef]
- Cuellar-Ortiz, S.M.; De La Paz Arrieta Montiel, M.; Acosta-Gallegos, J.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409. [Google Scholar] [CrossRef]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environment effects on growth and development of cassava (Manihot esculenta Crantz) III. Assimilate distribution and storage organ yield. Field Crops Res. 1982, 5, 293–303. [Google Scholar] [CrossRef]
- Fukai, S.; Alcoy, A.B.; Llamelo, A.B.; Patterson, R.D. Effects of solar radiation on growth of cassava (Manihot esculenta Crantz.). I. Canopy development and dry matter growth. Field Crops Res. 1984, 9, 347–360. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H.; Held, A.A. Photosynthetic responses of cassava cultivars (Manihot esculenta Crantz) from different habitats to temperature. Photosynth. Res. 1984, 5, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Saithong, T.; Rongsirikul, O.; Kalapanulak, S.; Chiewchankaset, P.; Siriwat, W.; Netrphan, S.; Suksangpanomrung, M.; Meechai, A.; Cheevadhanarak, S. Starch biosynthesis in cassava: A genome-based pathway reconstruction and its exploitation in data integration. BMC Syst. Boil. 2013, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irikura, V.; Cock, J.H.; Kawano, K. The physiological basis of genotype-temperature interactions in cassava. Field Crops Res. 1979, 2, 227–239. [Google Scholar] [CrossRef]
- Okoli, P.S.; Wilson, G.F. Response of cassava (Manihot esculenta Crantz) to shade under field conditions. Field Crops Res. 1986, 14, 349–359. [Google Scholar] [CrossRef]
- Aresta, R.B.; Fukai, S. Effects of solar radiation on growth of cassava Manihot esculenta Crantz II. Fibrous root length. Field Crop. Res. 1984, 9, 361–371. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Effect of humidity and wind on leaf conductance of field grown cassava. Rev. Bras. Fisiol. Vegetal. 1990, 2, 17–22. [Google Scholar]
- El-Sharkawy, M.A. Stress tolerant cassava: The role of integrative ecophysiology breeding research in crop improvement. OJSS 2012, 162–186. [Google Scholar] [CrossRef] [Green Version]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Seasonal variation in photosynthesis performance of cassava at two different growth stages under irrigated and rain-fed conditions in a tropical savanna climate. Photosynthetica 2018, 56, 1398–1413. [Google Scholar] [CrossRef]
- Boerboom, B.W. A model of dry matter distribution in cassava (Manihot esculenta Crantz). Neth. J. Agri. Sci. 1978, 26, 267–277. [Google Scholar]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environmental effects on growth and development of cassava (Manihot esculenta Crantz) II. Crop growth rate and biomass yield. Field Crops Res. 1982, 5, 283–292. [Google Scholar] [CrossRef]
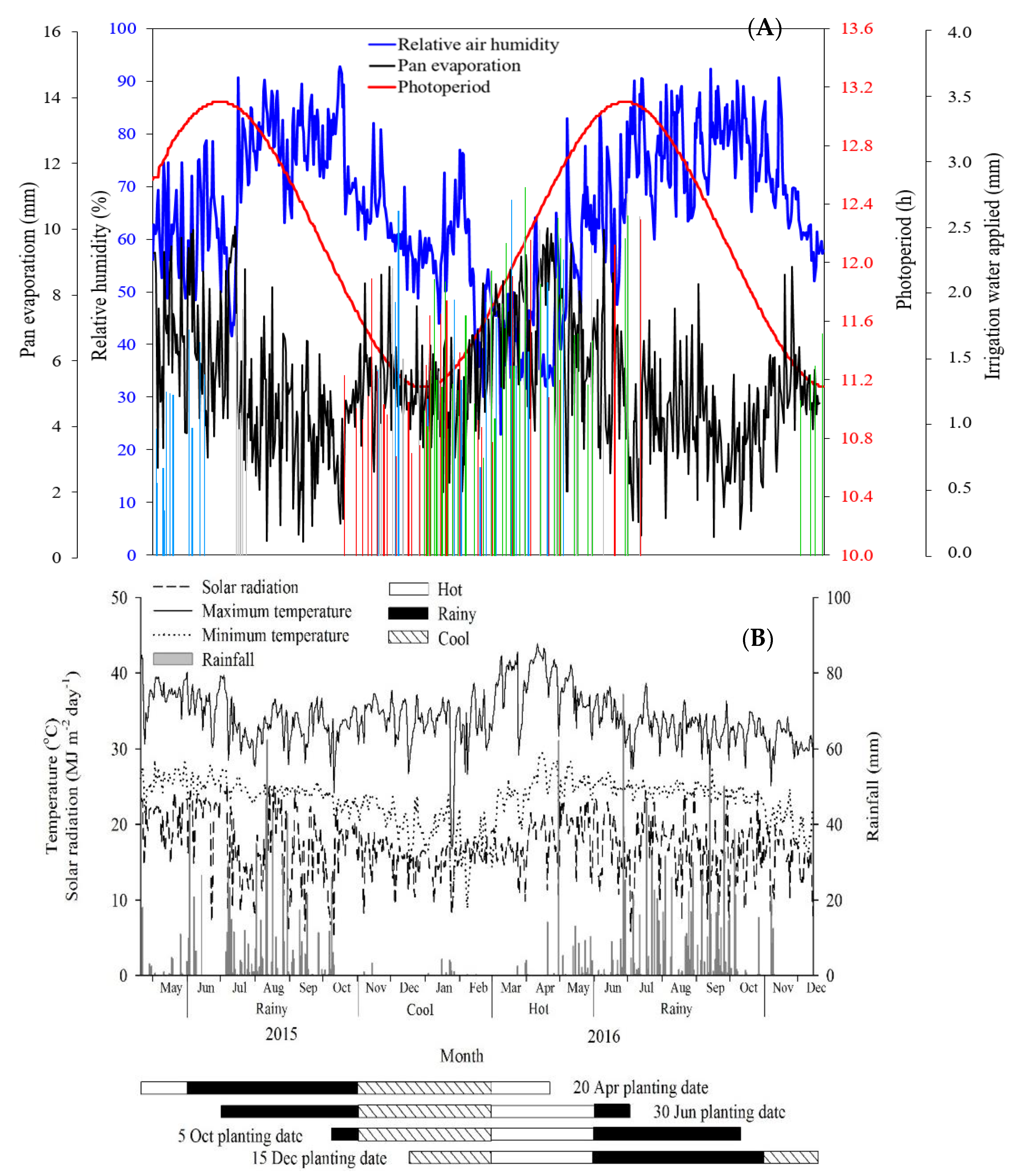
| Soil Physicochemical Property | 20-Apr | 30-Jun | 5-Oct | 15-Dec | ||||
|---|---|---|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | |
| Physical property | ||||||||
| Sand (%) | 83.9 | 84.4 | 85.4 | 78.5 | 85.5 | 73.0 | 85.8 | 78.3 |
| Silt (%) | 10.0 | 9.5 | 7.6 | 7.5 | 8.5 | 10.0 | 10.1 | 12.3 |
| Clay (%) | 6.1 | 6.1 | 7.0 | 14.0 | 6.0 | 17.0 | 4.1 | 9.5 |
| Chemical properties at preplanting | ||||||||
| Total N (%) | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 |
| Available P (mg kg−1) | 61.2 | 56.5 | 24.5 | 17.2 | 62.9 | 22.7 | 25.7 | 17.4 |
| Exchangeable K (mg kg−1) | 54.6 | 35.6 | 34.2 | 20.2 | 49.0 | 12.8 | 41.4 | 16.6 |
| Exchangeable Ca (mg kg−1) | 339 | 387 | 335 | 379 | 200 | 287 | 245 | 225 |
| Mg (mg kg−1) | 36.3 | 34.9 | 50.4 | 46.3 | 39.7 | 40.1 | 30.8 | 38.4 |
| S (mg kg−1) | 53.5 | 43.2 | 47.5 | 41.1 | 5.4 | 19.3 | 3.0 | 8.3 |
| Exchangeable Na (mg kg−1) | 47.4 | 42.4 | 24.8 | 25.3 | 26.2 | 24.9 | 25.7 | 24.0 |
| pH (1:1 H2O) | 6.6 | 6.7 | 5.8 | 6.1 | 5.6 | 5.3 | 5.6 | 5.5 |
| EC (dS m−1) | 0.05 | 0.04 | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 |
| OM (%) | 0.44 | 0.43 | 0.53 | 0.38 | 0.46 | 0.33 | 0.44 | 0.34 |
| CEC (cmol kg−1) | 3.3 | 5.3 | 1.8 | 2.8 | 2.0 | 5.3 | 3.0 | 4.8 |
| Months after Planting | 20-Apr | 30-Jun | 5-Oct | 15-Dec | ||||
|---|---|---|---|---|---|---|---|---|
| Irrigation | Rainfed | Irrigation | Rainfed | Irrigation | Rainfed | Irrigation | Rainfed | |
| 1–3 | 0 | 10 | 0 | 0 | 0 | 50 | 0 | 56 |
| 4–6 | 0 | 0 | 0 | 42 | 0 | 90 | 0 | 65 |
| 7–9 | 0 | 90 | 0 | 90 | 0 | 35 | 0 | 6 |
| 10–12 | 0 | 81 | 0 | 45 | 0 | 1 | 0 | 18 |
| Sum | 0 | 181 | 0 | 177 | 0 | 176 | 0 | 145 |
| Planting Date | Starch Yield (kg ha−1) | LSD | Storage Root Dry Weight (kg ha−1) | LSD | ||
|---|---|---|---|---|---|---|
| Irrigated | Rainfed | Irrigated † | Rainfed | |||
| 20-Apr | 14,489a | 9022c | ** | 20,240a | 10,538c | ** |
| 30-Jun | 9227b | 9339c | ns | 11,601b | 11,161c | ns |
| 5-Oct | 14,974a | 15,004b | ns | 18,437a | 17,350b | ns |
| 15-Dec | 15,929a | 17,779a | ns | 19,114a | 20,628a | ns |
| Mean | 14,155 | 12,786 | 17,348 | 14,919 | ||
| Planting Date | Granule Size Distribution (µm) | |||||
|---|---|---|---|---|---|---|
| Irrigated | Rainfed | |||||
| d (0.1) | d (0.5) | d (0.9) | d (0.1) | d (0.5) | d (0.9) | |
| 20-Apr | 7.21 | 15.94 | 29.39b | 6.71 | 15.82 | 30.04b |
| 30-Jun | 7.81 | 16.47 | 31.81a | 7.32 | 15.89 | 30.80b |
| 5-Oct | 7.82 | 16.24 | 31.16a | 7.96 | 17.33 | 33.78a |
| 15-Dec | 7.31 | 16.16 | 30.96a | 8.54 | 17.19 | 31.34ab |
| Mean | 7.54 | 16.2 | 30.83 | 7.63 | 16.56 | 31.49 |
| Source of Variance | df | Starch Content (%) | Starch Yield (kg ha−1) | Granule Size (d (0.05)) | Amylose (%) | Ratio of Amylose to Amylopectin |
|---|---|---|---|---|---|---|
| Planting date (D) | 3 | 12.91 (14.8) * | 84,800,000 (60.8) ** | 0.721 (38.2) * | 15.66 (50.5) ** | 0.00461 (53.7) ** |
| Reps within D | 12 | 2.81 (12.9) | 2,190,282 (6.3) | 0.081 (5.2) | 1.79 (15.8) | 0.00050 (16.3) |
| Water regime (W) | 1 | 136.13 (52.1) ** | 14,990,000 (3.6) * | 0.501 (7.9) ns | 6.46 (6.3) ns | 0.00151 (5.3) ns |
| D × W | 2 | 1.88 (2.2) ns | 34,460,000 (24.7) ** | 0.695 (33.0) * | 2.16 (6.3) ns | 0.00055 (5.8) ns |
| Pooled error | 12 | 3.94 (18.1) | 1,639,082 (4.7) | 0.310 (15.6) | 1.82 (21.1) | 0.00045 (18.8) |
| CV a (%) | 2.01 | 10.99 | 1.74 | 6.12 | 8.00 | |
| CV b (%) | 2.38 | 9.50 | 3.40 | 6.17 | 7.52 |
| Months after Planting | Variable | Coefficient | t | Determination Coefficient (R2) |
|---|---|---|---|---|
| Starch content (%) for irrigated crops | ||||
| 1–3 | Constant | 81.31 | 8.82 * | 0.38 |
| Solar radiation | 2.85 | 2.88 * | ||
| 3–6 | Constant | 80.62 | 19.8 * | 0.45 |
| Solar radiation | 1.82 | 2.8 * | ||
| 6–9 | Constant | 53.41 | 4.98 ** | 0.35 |
| Photoperiod (h) | 3.10 | 4.15 * | ||
| 9–12 | Constant | 68.55 | 18.1 ** | 0.44 |
| Relative humidity (%) | 1.09 | 3.44 * | ||
| Starch content (%) for rainfed crops | ||||
| 1–3 | Constant | 85.4 | 84.5 * | 0.42 |
| Rainfall | −1.38 | −1.89 * | ||
| 3–6 | Constant | 98.1 | 79.88 * | 0.38 |
| Solar radiation | 1.42 | 2.10 * | ||
| 6–9 | Constant | 80.70 | 18.30 ** | 0.40 |
| Solar radiation | 0.01 | 4.17 ** | ||
| Maximum air temperature (%) | −0.31 | −2.85 * | ||
| 9–12 | Constant | 101.1 | 23.27 ** | 0.38 |
| Solar radiation | −9.71 | −3.61 ** | ||
| Months after Planting | Variable | Coefficient | t | Determination Coefficient (R2) |
|---|---|---|---|---|
| Starch yield (kg ha−1) for irrigated crops | ||||
| 1–3 | Constant | −2.53 | −6.26 ** | 0.82 |
| Solar radiation | −74,033 | −5.25 ** | ||
| Mean air temperature | 3.18 | 5.25 ** | ||
| Rainfall | −57,471 | −4.28 ** | ||
| 3–6 | Constant | −400,029 | −4.24 ** | 0.83 |
| Solar radiation | −36.61 | −7.67 ** | ||
| Photoperiod (h) | 10,073.7 | 4.13 ** | ||
| Relative humidity (%) | −182.73 | −3.97 ** | ||
| 6–9 | Constant | −125,540 | −5.10 ** | 0.82 |
| Photoperiod (h) | 8724 | 5.97 ** | ||
| Relative humidity (%) | 731.7 | 6.70 ** | ||
| Rainfall | −39.7 | −5.21 ** | ||
| 9–12 | Constant | 44,122 | 3.64 ** | 0.84 |
| Solar radiation | −70.1 | −3.29 ** | ||
| Photoperiod (h) | 8223 | 4.55 ** | ||
| Relative humidity (%) | −254.5 | −4.16 ** | ||
| Starch yield (kg ha−1) for rainfed crops | ||||
| 1–3 | Constant | 78,631 | 5.88 | 0.83 |
| Mean air temperature | −5364 | −4.93 | ||
| 3–6 | Constant | −12,547 | −1.78 ns | 0.92 |
| Photoperiod (h) | 3624 | 6.18 ** | ||
| Relative humidity (%) | −285 | −11.82 ** | ||
| 6–9 | Constant | −46,399 | −8.18 ** | 0.91 |
| Photoperiod (h) | 4224 | 7.73 ** | ||
| Relative humidity (%) | 140 | 3.46 ** | ||
| 9–12 | Constant | 41,178 | 5.09 ** | 0.91 |
| Photoperiod (h) | −3835 | −5.70 ** | ||
| Relative humidity (%) | 280 | 11.19 ** | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Jogloy, S.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Banterng, P. Starch Accumulation and Granule Size Distribution of Cassava cv. Rayong 9 Grown under Irrigated and Rainfed Conditions Using Different Growing Seasons. Agronomy 2020, 10, 412. https://doi.org/10.3390/agronomy10030412
Janket A, Vorasoot N, Toomsan B, Kaewpradit W, Jogloy S, Theerakulpisut P, Holbrook CC, Kvien CK, Banterng P. Starch Accumulation and Granule Size Distribution of Cassava cv. Rayong 9 Grown under Irrigated and Rainfed Conditions Using Different Growing Seasons. Agronomy. 2020; 10(3):412. https://doi.org/10.3390/agronomy10030412
Chicago/Turabian StyleJanket, Anon, Nimitr Vorasoot, Banyong Toomsan, Wanwipa Kaewpradit, Sanun Jogloy, Piyada Theerakulpisut, C. Corley Holbrook, Craig K. Kvien, and Poramate Banterng. 2020. "Starch Accumulation and Granule Size Distribution of Cassava cv. Rayong 9 Grown under Irrigated and Rainfed Conditions Using Different Growing Seasons" Agronomy 10, no. 3: 412. https://doi.org/10.3390/agronomy10030412







