Abstract
Pre-harvest climatic conditions and genotype may have important effects on head quality and post-harvest performance of fresh-cut broccoli. The present work evaluates the effect of the growing cycle (summer–autumn (SA), winter (W), winter–spring (WS), and spring (S)) and genotype on qualitative (dry matter, concentration of chlorophylls, carotenoids, and color) and antioxidative (ascorbic acid, dehydroascorbic acid, total phenol concentrations, and antioxidant capacity) traits of broccoli heads and minimally processed florets. The WS raw product showed the best color indices (L* = 38.6, C* = 9.3 and h° = 123.8) as well as the highest chlorophyll (0.23 µg mg−1 fresh weight) but the lowest total phenol concentration (5.5 µg mg−1 dry weight - DW), whereas the ascorbic acid level (2.3 µg mg−1 DW) was comparable to or lower than that the other growing cycles. The WS florets confirmed their best visual quality, even showing an improved total phenol level after 14 days of cold storage. The climatic conditions experienced by broccoli plants grown in SA, W, and S periods were stressful as they resulted in a slight reduction in the visual quality of the heads, though only the SA florets showed a distinctive decay during storage. The lower post-harvest performance of SA grown broccoli was confirmed in all the tested cultivars, despite ‘Naxos’ seeming more tolerant. On the contrary, the greatest content of ascorbic acid (3.2 µg mg−1 DW) in the W heads and of phenols (11.1 µg mg−1 DW) in S heads was maintained during storage, thus preserving floret color.
1. Introduction
Broccoli (Brassica oleracea L. conv. botrytis (L.) Alef. var. cymosa Duch.) is a green vegetable with high nutritional value due to its richness in vitamins, antioxidants, anti-carcinogenic compounds, and health-promoting phytochemicals [1,2].
Broccoli has been traditionally marketed as having whole heads, yet in recent years it has grown to become greatly appreciated by consumers as a ready-to-eat product.
Broccoli heads are harvested before florets anthesis, requiring a continuous supply of water, nutrients, and endogenous hormones to maintain their homeostasis [3]. Thus, after harvesting, these organs experience severe stress that leads to the appearance of senescence symptoms [4,5] with yellowing that occurs quite rapidly during storage. In the case of the ready-to-use product, additional stresses are caused by handling, floret cutting, cold storage, and packaging.
In general, abiotic stressors will induce perturbations in the plant cellular homeostasis, provoking an increase in reactive oxygen species (ROS) generation [6]. The ability of the cell to contrast this oxidative stress depends largely on its endogenous antioxidant capacity [7]. It is known that pre-harvest factors such as climatic conditions and cultural practices act as potential stressors and may affect antioxidative status of raw material, which, as a consequence, impact the shelf-life of the ready-to-use product [8]. In particular, moderate abiotic stresses experienced by the plant during growth potentially work towards enhancing post-harvest resistance through up-regulating genes and anti-oxidative pathways, thus rendering the tissues cross-tolerant to storage processing [9]. However, this response is genotype-specific, since the variability within a species may greatly affect the phytochemical composition of the plant [8].
The prevention or delay of post-harvest de-greening in both intact and minimally processed broccoli has been addressed in various studies mainly focused on post-harvest factors with little research carried out on pre-harvest factors [10,11,12,13,14]. To the best of our knowledge, no information is available on the effects of pre-harvest climatic conditions or cultivar characteristics on post-harvest performance of fresh-cut broccoli.
Italy is one of the major broccoli producers in the world [15] and the Puglia region is the most important area for broccoli cultivation [16]. Typically, under Mediterranean climate, various cropping cycles of broccoli are performed. The transplanting season starts from late August to late March and the harvest from mid-autumn (October–November) to late spring (June) with the crop cycle lasting about 90–100 days. Therefore, broccoli crops are subjected to greatly varying climatic conditions that may have important effects both on the qualitative and antioxidative characteristics of heads and, as a consequence, on its suitability for being processed as fresh-cut florets.
The main aim of the present work is to evaluate the effect of the growing cycle of broccoli on the shelf-life of the ready-to-use product. The bio-morphological (color, chlorophyll, carotenoid concentrations, fresh weight, and dry matter) and anti-oxidative traits (ascorbic acid, total phenols, and antioxidant capacity) were evaluated in the heads of cultivar Parthenon, grown in four growing cycles from summer to spring, along with their changes during the post-harvest storage as fresh-cut florets. Four popular cultivars (‘Moycan’, ‘Naxos’, ‘Parthenon’, and ‘Steel’) were also compared in a summer–autumn cycle in terms of heads quality and floret shelf-life by evaluating their bio-morphological and antioxidative characteristics.
2. Materials and Methods
2.1. Sampling and Sample Preparation
Fresh broccoli heads (stem 10 cm in length, three intact leaves at the head base) of cv. Moycan F1 (Coraseed), ‘Naxos F1′ (Sakata), ‘Parthenon F1′ (Sakata), and ‘Steel F1′ (Monsanto) were sampled in November (summer–-autumn cycle, early-season crop) and the cultivar Parthenon in February (winter cycle, mid-season crop), April (winter–spring cycle, mid-late season crop), and June (spring cycle, late-season crop) at the processing and storage facility of a vegetable growers’ association (Table 1).

Table 1.
Genotypes, growing cycles, harvesting times, and climate parameters.
The heads came from commercial four farms located about 20 km apart in the Foggia area of the Puglia region (Southern Italy) (41°23′49″ N 15°30′33″ E). All farms were managed according to the regional integrated crop management guidelines, which also includes rules on pests and diseases management. The main physical and chemical characteristics of the soil of farms are reported in Table S1. The vegetable growers’ association also adopted standardized procedures for the harvest and post-harvest handling of broccoli, such as the maximum time for heads to reach the storage facility and the temperature setpoint for pre-cooling, during the warm season.
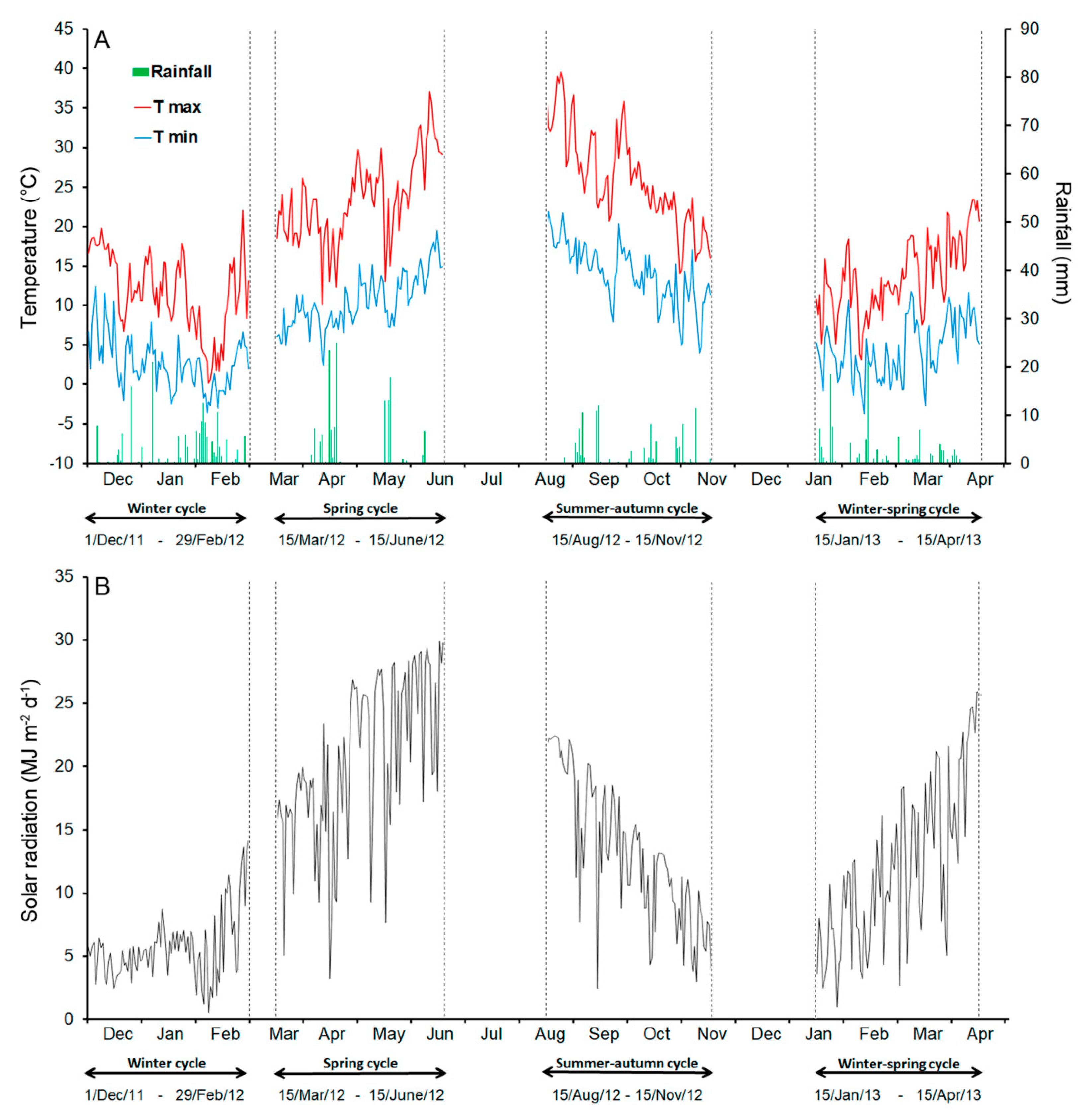
The area is dominated by an accentuated thermo Mediterranean climate (Food and Agriculture Organization-United Nations Educational, Scientific and Cultural Organization classification) with summer temperatures often higher than 40 °C and winter ones lower than 0 °C. Rains are mainly concentrated in the winter months [17]. The minimum and maximum daily temperatures, the distribution of rainfall, and the global radiation of each cropping cycle (three-months before the harvest) were recorded at a nearby weather station located about 7 km from each farm and are reported in Table 1 and Figure 1A,B.
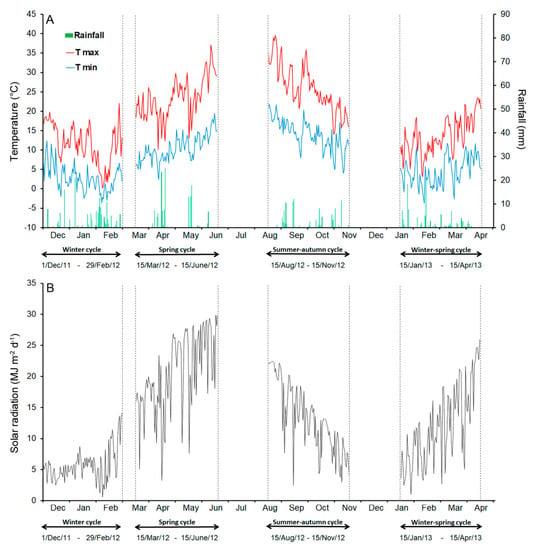
Figure 1.
Climatic data during the four growing periods (winter, spring, summer–autumn, and winter–spring) of the broccoli crops: (A) the temperatures and rainfall; (B) the solar radiation.
In total, 20 to 30 heads collected from three (Farm A, B, and C at each growing cycle) or two (Farm B, D for each genotype) different farms were considered. Samplings were performed at midday of each harvest (on pre-cooled product for April and June harvests) by selecting high-quality standard heads (i.e., free from any physical, disease, or pest injury, uniform in size and color and with tight florets). The heads were transported immediately to the laboratory, were pooled and then three replicates of six heads were randomly selected. Inflorescences were weighed and separated into florets with stems. Florets were then immersed in chlorinated water (sodium hypochlorite, 0.25 g L−1) for 1 min and rinsed by immersion in tap water at room temperature for another minute. The excess surface water was removed by centrifuging in a manual salad spinner (Meliconi s.p.a., Bologna, Italy). After carefully mixing, the florets of each replication, three subsamples (approximately 150 g) were created: (i) the raw product (time 0, T0), (ii) the 7 days (time 7, T7) and (iii) the 14 days (time 14, T14) stored product. The T7 and T14 samples were stored in a refrigerated cooler (5 °C), after packaging them in heat-sealed bags (29.5 × 24.3 cm) made of 20 µm oriented polypropylene (OPP) film (Metalvuoto, Roncello, MI, Italy) characterized by a very high barrier value to water, but very low to gas, mainly to carbon dioxide (water vapor transmission rate 0.61 ± 0.06 mL m−2 d−1; the transmission rate of oxygen and carbon dioxide is 1.014 and 2.700 mL m−2 d−1, respectively).
2.2. Sample Analyses and Measurements
The fresh weight of florets was measured at T0. At T0, T7, and T14, dry matter (DM) concentration, fresh weight losses (at T7 and T14), color, chlorophyll concentration, carotenoid concentration, ascorbic acid (dehydroascorbic acid and vitamin C for SA product), phenolic acids concentrations, and total antioxidant capacity were measured. All samples were analyzed in triplicate. To determine the DM, fresh plant material was dried in a thermo-ventilated oven at 70 °C until reaching a constant mass.
2.2.1. Chemicals and Standards
Ascorbic acid was purchased from (Mallinckrodt Baker B.V., Deventer, The Netherlands). Gallic acid and Folin–Ciocalteau reagent were purchased from Carlo Erba (Rodano, MI, Italy). Methanol was purchased from (VWR International Ltd., Radnor, Pennsylvania, USA). Ethanol, Hexane, ABTS [2,2-Azino-bis(3-ethyl-benzothiazoline-6-sulphonic acid)], potassium persulfate, sodium hypochlorite, magnesium carbonate, celite, pyrogallol, and dithiothreitol were purchased from (Sigma-Aldrich Inc., Milano, Italy). Ultrapure water was used (Millipore Corporate Headquarters, Billerica, MA, USA).
2.2.2. Post-Harvest Weight Losses
At T7 and T14, fresh weight (FW) was measured on packaged (FWp) (closed bag) and on un-packaged (FWup) (after the bag had been opened) samples. Fresh weight losses (WL) were calculated and classified as:
Total, as:
WLTot = [FWup(Tx) − FWup(T0)]/FWup(T0) × 100
By respiration, as:
WLResp = [FWp(Tx) − FWp(T0)]/FWup(T0) × 100
By transpiration, as:
where x is 7 or 14. Since OPP film is characterized by a very high barrier-value to water, but very low to gas, mainly to carbon dioxide, the weight loss of entire packaged bags (WLResp) can be approximately attributed to the loss by respiration (CO2 loss).
WLTransp = WLTot − WLResp
The low water vapor permeability of OPP film results in water condensation on the inside of packaging due to transpiration of the product, and so the difference WLTot− WLResp can be approximately attributable to product transpiration.
Dry matter (DM) change was calculated as: DM = [(DM(Tx) − DM(T0)], where DM = dry weight/fresh weight × 100.
2.2.3. Surface Color
Color indices were measured using a portable tristimulus color-meter (Minolta Chroma Meter CR-200; Minolta Camera Co.Ltd., Osaka, Japan), using the CIE-L*a*b* scale 1976. The chromameter was calibrated using a white color standard and the color was expressed in the tristimulus L* (lightness), a* (green to red), and b* (yellow to blue), from which hue angle (h°) and chroma (C*) were calculated [18]. In total, 30 (10 florets for each subsample × 3 replications) measurements were done per storage time (T0, T7 and T14) and cultivar (‘Parthenon’, ‘Moycan’, ‘Naxos’ and ‘Steel’). Measurements were taken on the top of florets.
2.2.4. Chlorophylls and Carotenoids
The chlorophyll a (CHLa) and b (CHLb) were extracted from previously lyophilized samples by homogenizing in 80% acetone, spectrophotometrically measured, and estimated by the equation provided by Dere et al. [19], and expressed on a fresh weight basis.
As reported by Conversa et al. [20], carotenoids were determined from lyophilized samples. Briefly, 0.05 g of MgCO3 was added to the samples to neutralize cytosolic acids and celite (0.01 g) was used to better disrupt the tissues. The extraction was performed using the following mixture: 10 mL of ethanol:hexane (4:3 by volume) and 1 mL of pyrogallol solution (5%) as an antioxidant. The mixture was shaken and then centrifuged at 6700×g for 10 min. After the collection of the supernatant, the residue was re-extracted, the two extracts were combined and decanted into a 50 mL tube. The supernatant hexane phase was transferred into a new tube and the lower aqueous phase was discarded. To avoid carotenoid overestimation due to the presence of chlorophylls, a saponification phase was introduced during extraction. The total carotenoids in the extract were measured at 450 nm using a UV-visible spectrophotometer (Shimadzu UV-1800, Shimadzu Italia S.r.l., Milano, Italy) and estimated according to the “Method of Mean” reported by Biehler et al. [21]. Results were expressed on a fresh weight basis.
2.2.5. Ascorbic and Dehydroascorbic Acid
The total content of vitamin C-ascorbic acid (AsA) + de-hydro-ascorbic acid (DHAA) was measured in lyophilized samples (0.3 g) using a chromatographic system (Dionex ICS3000 System, Thermo Fisher Scientific, Sunnyvale, CA, USA) equipped with a UV-visible detector (RLSC Diode Array Detector, Dionex), a 10 µL injection loop, and a 5 μm reverse-phase column (C18) (Acclaim 120, Dionex) (temperature set at 30 °C).
Ascorbic acid was extracted according to the modified method of Koh et al. [22] described by Bonasia et al. [23], in order to determine the total content of vitamin C and, indirectly, the DHAA content. DHAA was reduced with dithiothreitol and reduced samples were also injected into the chromatographic system. AsA was identified and quantified by retention time and spectra. The flow rate was fixed at 1 mL min−1. The detection wavelength was 254 nm and the UV spectra were in the 190–350 nm range. The method was calibrated with a curve of standard AsA solution. Results were expressed on a dry weight basis.
2.2.6. Total Polyphenols
Total polyphenols were determined as reported in Bonasia et al. [18]. Briefly, lyophilized florets were double extracted in water/methanol (20:80, v/v) solution and centrifuged. Total phenol (TP) content was determined by mixing the methanolic extracts with Folin–Ciocalteu reagent and the absorbance was read at 750 nm. The results are expressed as gallic acid equivalents (GAE) using a calibration curve.
2.2.7. Antioxidant Capacity
The antioxidant capacity (AC) was determined by ABTS assay based on the formation of the radical ABTS·+ by the reaction of ABTS [2,2-Azino-bis(3-ethyl-benzothiazoline-6-sulphonic acid)] (7mM L−1) with 140 mM L−1 of potassium persulfate as reported in Conversa et al. [24]. In brief, 20 μL of the extracts were reacted with 1 mL of ABTS·+ radical. The hydrophilic fraction was extracted twice from lyophilized samples using 80% methanol in a shaking water bath for 15 min and by centrifugation (13,000 rpm; 10 min). The supernatants were combined. The absorbance of the reaction mixtures was measured at 734 nm and the results of the antioxidant activity were expressed as TEAC (Trolox Equivalent Antioxidant Capacity) according to Pellegrini et al. (2007) [25].
2.3. Statistical Analysis
Data were analyzed as a repeated-measure design using the GLM procedure in SAS software [26] with storage time as the ‘repeated factor’ [27]. The least significant difference (LSD) test (P = 0.05) was used to establish differences between means.
This model was used to separately analyze the effects of (i) growing cycle and Storage time (only with cultivar Parthenon data) and (ii) genotype and storage time (with all the cultivars data in the summer–autumn season). The values expressed as percentages were subjected to arcsine square root transformation before data analysis.
For visual analysis of data of raw and stored material, a principal component analysis (PCA) was performed using PAST3 Software [28] on a mean standardized [((x − mean)/standard deviation]) data before analysis. The data matrix submitted to PCA was made up of 21 data [(four sampling dates (cv. Parthenon) × 3 storage times and three cultivars (‘Naxos’, ‘Steel’ and ‘Moycan’) × 3 storage times]) for each of the considered variables.
3. Results and Discussion
3.1. Effect of Harvest Season and Storage Time on Quality Traits of Cultivar Parthenon
3.1.1. Head Characteristics and Floret Weight Loss
The growing period affected both the fresh weight and the dry mass (DM) content of ‘Parthenon’ heads with the highest weight observed in the winter (W) cycle and the lowest in spring (S) cycle. However, both products showed the highest dry mass content compared with those of the other cycles (Table 2).

Table 2.
Bio-morphological traits of raw and stored broccoli (cultivar Parthenon) as affected by growing cycle and storage time.
The loss of fresh weight (WLTot = WLResp + WLTransp) observed during cold storage was attributable mostly to respiration (WLResp), with a negligible contribution of transpiration (WLTransp) (Table 2). As expected, both WLResp and WLTransp rose after 14 days of storage. However, WLTot was always below 1.5%, except for the florets from the heads harvested in June (spring cycle), which had the highest WLResp and WLTransp (Table 2). Temperatures occurring in this period were above the optimal values for the crop (20 °C) [29,30]. Particularly during the 15-20 days before harvest, when the head formation and enlargement occur [16], daily maximum temperatures were between 27–37 °C (Figure 1A) with a concomitant increase in solar radiation (Figure 1B) (daily values 18-30 MJ m−2).
These stressing conditions probably resulted in the formation of reactive oxygen species (ROS) [31] which notoriously increase the respiration rate. On the contrary, the lowest weight loss was observed for the product harvested in February (winter cycle) when lower temperatures and solar radiation occurred (Figure 1A,B). However, on the whole, the WLTot was below the 3%–4% loss reported for broccoli florets stored in conditions very similar to those of this study [14,32]. The dry mass concentration decreased at T14, while it increased at T7 (Table 2), likely as an initial physiological adaption to cold storage.
3.1.2. Chlorophylls, Carotenoids, and Visual Quality
The ANOVA results for chlorophyll and carotenoid concentrations, color indices L*, h°, and C* affecting the visual quality, are reported in Table 3.

Table 3.
Effect of growing cycle and cold storage time on the color and anti-oxidative traits of broccoli (cv. Parthenon).
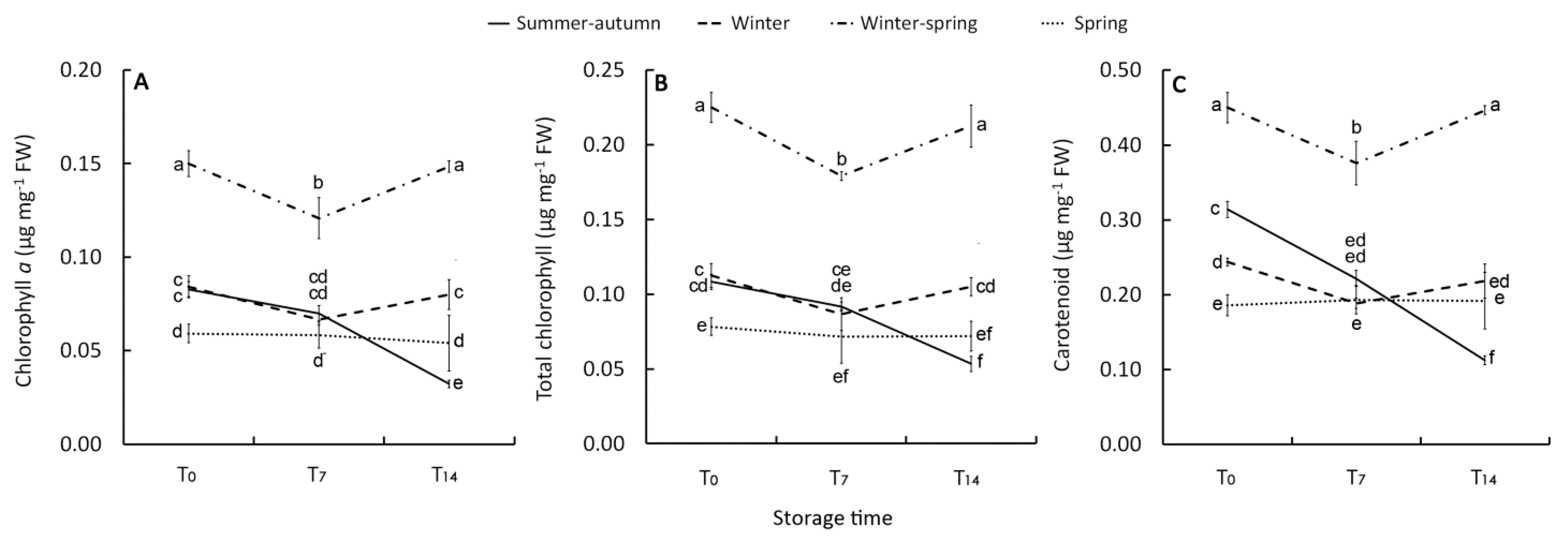
Chlorophyll b (CHLb) was highest in the winter–-spring (WS) heads and lowest in the spring ones. Irrespective of the growing period, CHLb exhibited a reduction in the latter part of the storage phase (T14) (Table 2). The total chlorophyll (CHL) concentration, however, was mostly affected by chlorophyll a (CHLa), both showing a significant growing cycle x storage time (GCxST) interaction (Table 3). In the raw product harvested in April (WS cycle), both CHLa (Figure 2A) and CHL (Figure 2B) concentrations were higher (0.15 and 0.23 µg mg−1 FW, respectively) compared with those of the other harvests (0.07 and 0.11 µg mg−1 FW, on average), particularly in June (S cycle) (0.05 and 0.08 µg mg−1 FW).
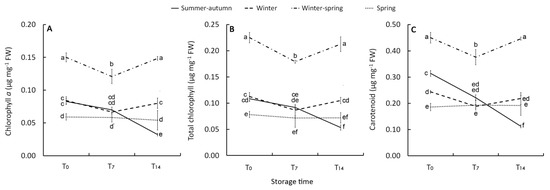
Figure 2.
Effect of growing cycle on chlorophyll a (A), total chlorophyll (B), and carotenoids (C) concentration in the raw heads (T0), 7 days (T7) and 14 days (T14) stored product. Vertical bars (± SE of mean) (n = 9) with different letters are significantly different according to the Least Significant Difference test (P = 0.05).
Except for the clear decrease shown by the florets picked in November (SA cycle), a general retention of CHLa was always detected during storage. In particular, a rise in this pigment in the last phase of the cold storage (T7-T14) was observed in the WS florets, following a reduction in the T0–-T7 period. (Figure 2A,B). A pattern similar to the changes of chlorophyll a was obtained for the level of carotenoids both in raw and stored products with higher content (0.45 µg mg−1 FW) in April-harvested heads (WS cycle) and an almost stable concentration during storage, except for the reduction detected in the summer–-autumn product at T14 (Figure 2C). This decrease could be linked to that in β-carotene since it is the main carotenoid in cold-stored broccoli [33] and it is reported to decline under the more stressing condition of storage, consistently with CHL variation [32]. Chlorophyll pigments have been found to have a high correlation with total carotenoid levels in different Brassica species [20], confirming that the metabolisms of these two pigments are interrelated [34].
The difference observed in the content of pigments between the raw product obtained in the winter–-spring cycle, especially if compared with that from the spring season, may be mostly attributable to the climatic conditions. Likely, the combination of temperatures and radiation that occurred over the January–-April (WS cycle) period were more favourable for the biosynthesis of chlorophylls and carotenoids in broccoli, with a maximum temperature not higher than 20 °C during the head formation (Table 1; Figure 1A,B). Greater light availability combined with higher temperatures, particularly during the head formation (max = 31.1 °C and min = 15.3 °C) in June-harvested broccoli, resulted in a more drastic reduction in these pigments. It is well known that high light intensity impairs chlorophyll biosynthesis [35] with light stress exacerbated when associated with high temperature [31]. The exposure of broccoli plants during the vegetative phase to increasing UV-B radiation levels (from 2.2 to 16.4 kJ m−2) led to a significant reduction of both chlorophylls and carotenoids [12] since UV-B radiation (280–-320 nm) is the most dangerous radiation of the light spectrum inducing severe damage [31]. A similar combination of climatic conditions also occurred in the summer–-autumn cycle (Table 1; Figure 1A,B), explaining the reduced chlorophyll (and carotenoid) content in florets harvested in November. In contrast, the lower level of pigments in the winter product was probably due to the low light intensities, which were close to one-third of those in the winter–-spring period (Table 1; Figure 1A,B). These results are in agreement with Zhu et al. [36] reporting a decrease in chlorophylls and carotenoids in pak-choi (Brassica campestris ssp. Chinensis Makino) when exposed to low light with the effect increasing as the duration of exposure increased.
The post-harvest reduction of chlorophylls is widely reported both for cold-stored [12,14,33,37] and not refrigerated broccoli (15–-20 °C) [33,38] as a consequence of the storage oxidative stress which promotes the enzymatic degradation of chlorophylls [33]. However, no changes in chlorophyll content have been observed in florets stored at 4 °C in perforated polypropylene film [32] and un-packaged heads even showed an improvement in CHL concentration after a few days at 4 °C [39].
In this study, irrespective of the initial amount, a loss of chlorophylls (~45%) was only observed in the florets harvested in November (SA cycle) and stored for 14 days (Figure 2B), underlining that a specific physiological status of this raw material makes it unable to delay the CHL degradation. In contrast, the product harvested in April (WS cycle) exhibited an increase in CHL content, in agreement with the findings of Loi et al. [39]. This result may be explained as an adaption to storage condition, as also reported by Luo et al. [33], with the resumed metabolic activity of the immature florets. A concentration increase of the pigments could be excluded since the water loss during storage was negligible (Table 2).
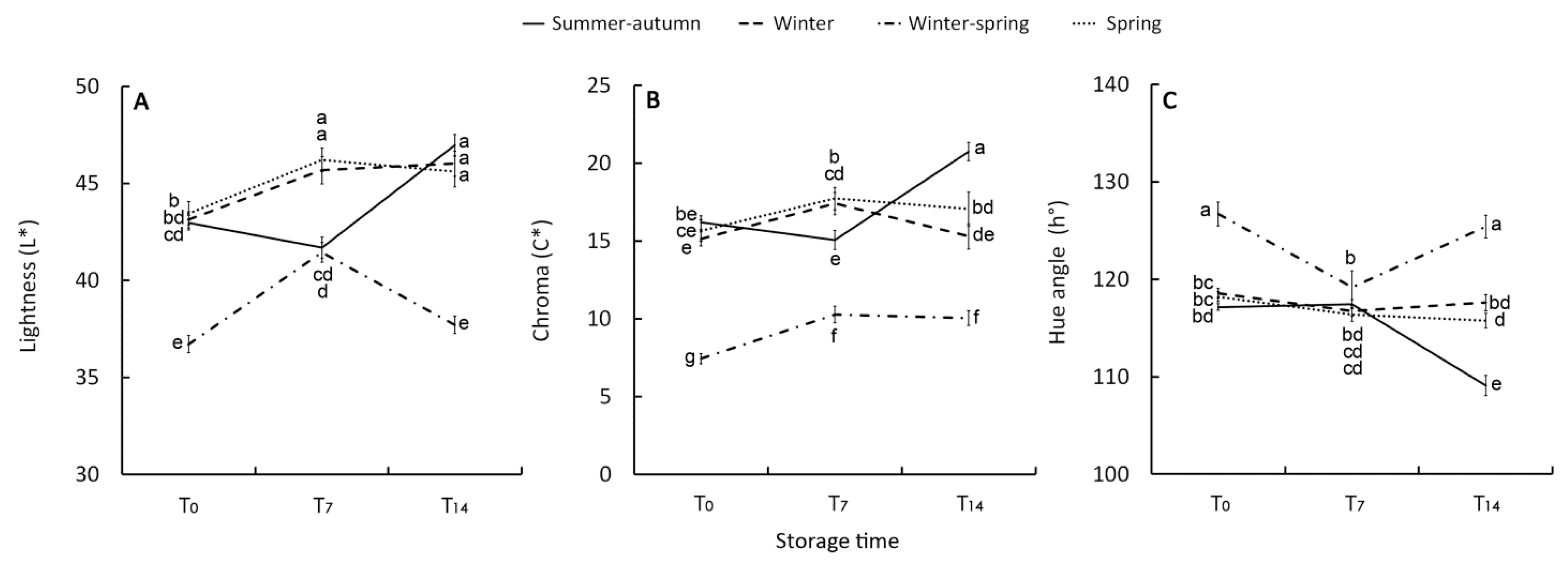
Color of vegetables and its post-harvest change is an important index for assessing their visual quality and consumers acceptance. The retention of the green color in broccoli is the most important factor for marketability and the increase in color lightness (L*) and brightness (C*) along with a reduction of hue angle (h°), representing the tonality, are associated to the post-harvest color de-greening [13,39,40]. In the present research, color indices L*, C*, and h° were consistent with the changes of CHLa and carotenoids being affected in the same way by the interaction between growing cycle and storage time. The best visual quality both of raw and stored florets was shown by the winter–-spring product with, on average, the lowest L* (38.6) (Figure 3A) and C* (9.3) (Figure 3B) values along with the greatest h° (123.8) (Figure 3C). In line with the changes in CHLa and carotenoids, during storage WS florets showed a recovery of visual quality in the T7–-T14 period.
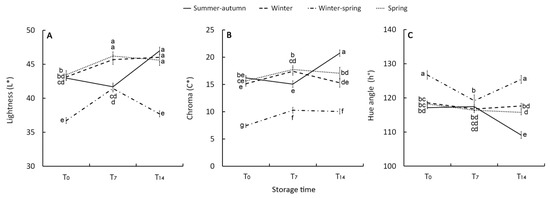
Figure 3.
Effect of growing cycle on lightness (L*) (A), chroma (C*) (B), and hue angle (h°) (C) index of the raw heads (T0), 7 days (T7) and 14 days (T14) stored product. Vertical bars (± SE of mean) (n = 9) with different letters are significantly different according to the Least Significant Difference test (P = 0.05).
The raw materials harvested in the other periods had similar values of L* (42), C*(14), and h° (120) between them. They also showed a slight post-harvest reduction in h° and an increase in brightness (C*) and lightness (L*) underlining a paler green color. The decline in visual quality was more evident in the T14 florets harvested in summer–-autumn season, mirroring the behaviour of CHLa and carotenoids. Despite the above-mentioned differences in visual quality, no evident or negligible (less than 3%) yellowing was observed in any of the stored samples. It occurred to a lesser extent than the 30% reported as the threshold for broccoli shelf-life [38].
3.1.3. Antioxidant Compounds and Antioxidant Capacity
The ANOVA results for ascorbic acid (AsA), total phenol (TP) concentration and antioxidant capacity (AC) are reported in Table 3, all showing significant GCxST interaction.
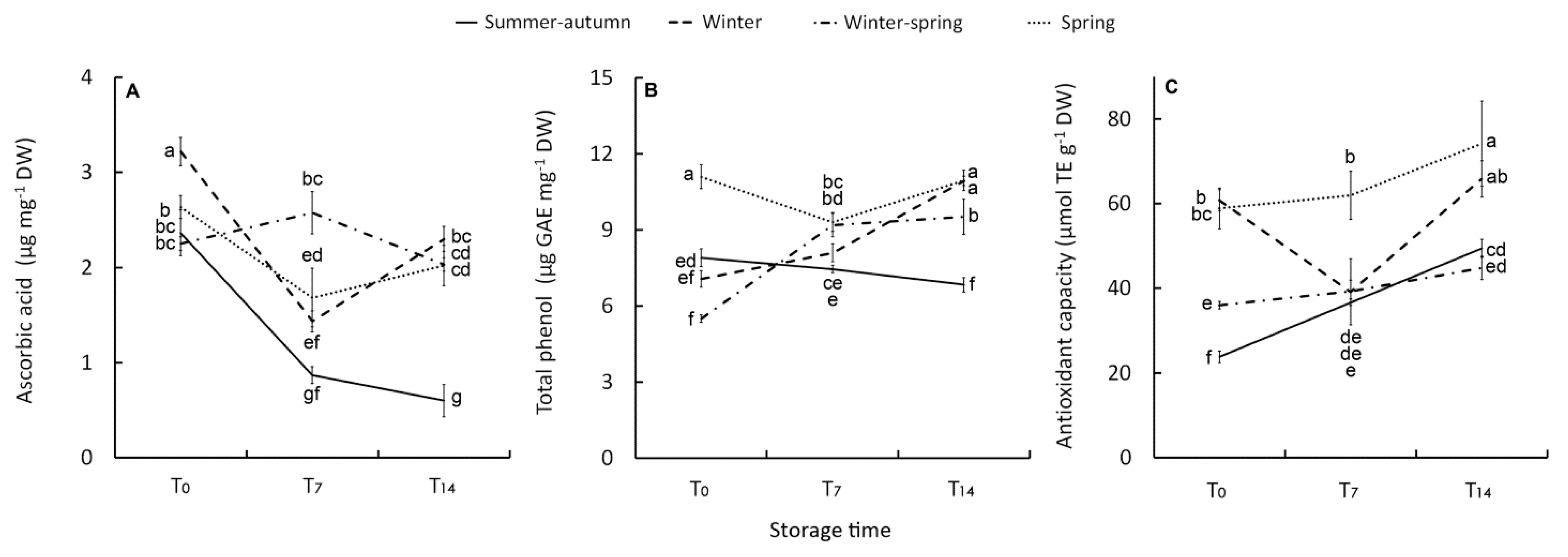
Ascorbic acid is the main biologically active form of vitamin C. It is essential in defence against environmentally induced oxidative stress, interacting with the damaging ROS compounds in cells [41]. Vitamin C amount is modulated by AsA and AsA-regenerating enzymes responsiveness to stress factors such as light and temperature [41]. In particular, ascorbic acid is involved in photoprotection. Thus, in several investigations, an increase in ascorbic acid content has been attributed to high radiation stress [42]. However, under low radiation, growth temperature had a strong influence on the ascorbic acid content in broccoli heads with values of 15–-20 °C negatively affecting the ascorbic acid content compared to 7–-12 °C, indicating that at lower temperatures the plant was under stress [42]. Our results seem to confirm these latter findings with the greatest level of AsA (3.2 µg mg−1 DW) detected in the raw product harvested in February (winter cycle) (Figure 4A) when the lowest values for both temperatures (minimum temperature were frequently 1–-2 °C below zero) (Figure 1A) and radiation availability (Figure 1B) occurred. In the other harvests, the AsA content was 25% lower on average, with no significant difference between growing cycles (Figure 4A), probably due to an overlapping of the effect of light and temperature levels. Seasonal variability in AsA content has been also reported for broccoli grown in California [43] and Spain [44], where AsA content was respectively greater than (13–-110 mg 100 g−1 FW) or in line with (16–-36 mg 100 g−1 FW) our findings (24–-46 mg 100 g−1 FW).
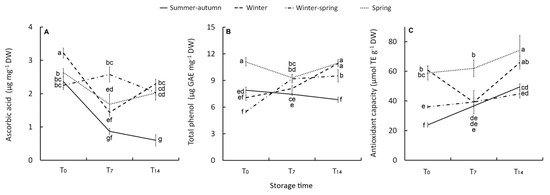
Figure 4.
Effect of growing cycle on ascorbic acid (A) and total phenol concentration (B) and on antioxidant capacity (C) of the raw heads (T0), 7 days (T7) and 14 days (T14) stored product. Vertical bars ( ± SE of mean) (n = 9) with different letters are significantly different according to the Least Significant Difference test (P = 0.05). GAE = gallic acid equivalent. TE = Trolox equivalent.
Except for the winter–-spring product, which maintained an almost constant AsA level throughout the storage period (2.3 g kg−1 DW, on average), changes in the post-harvest phase were detected. The AsA content decreased in the first seven days of storage, much more in the florets from summer–-autumn and winter cycles (–(-63% and –-55%, respectively) compared with the spring florets (–(-36%). At T14 AsA concentration was stable or even rose in winter product (Figure 4A).
On the whole, the regulation of AsA level in plants depends on collective contributions of different metabolic pathways, including both the oxidization of AsA to dehydroascorbic acid and/or recycling using glutathione (GSH) as a reductant (AsA-GSH cycle), and its de novo biosynthesis [45]. The post-harvest reduction of AsA content in broccoli is widely reported in the literature [14,39] due to the partial effectiveness of the AsA-GSH cycle against a large production of ROS caused by post-harvest handling and storage [40,43,46]. On the other hand, a rise in dark cold-stored broccoli has also been observed [47].
This research highlights that the post-harvest changes of AsA in broccoli are affected by the growing conditions that occurred during plant growth. Both the summer–-autumn and winter climatic growing conditions resulted in a rapid and substantial post-harvest depletion in AsA content, but the winter product was able to react to the post-harvest stress by improving AsA content at T14. A similar trend was also observed for the spring product (Figure 4A). This is likely to be due to AsA de novo biosynthesis, as both products have a higher dry biomass concentration (Table 2). Dry biomass, indeed, is reported to be positively correlated with the AsA concentration because of higher availability in sugars which are precursors of ascorbic acid [42].
Similarly, in wild rocket (Diplotaxis spp.) grown in an unheated greenhouse under southern Italy autumn-winter conditions, the initial greater level of AsA [48] further increased during storage [23]. In contrast, the winter–-spring leaves exhibited the greatest post-harvest AsA reduction. Thus, the authors suggested that the winter–-spring radiative and thermal conditions may have compromised the efficiency in de novo biosynthesis of AsA during post-harvest in wild rocket.
The fate of AsA during storage seems to affect post-harvest visual traits of florets with the best performance exhibited by the winter–-spring florets which were the best at retaining AsA, while the visual quality decay observed in summer–-autumn florets (lower CHLa and h°, higher L* and C*) can be associated to a substantial depletion in AsA content. This relationship is also reported for wild rocket [23] and broccoli raab (Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort.) [20], where the reduction in shelf-life (chlorophyll degradation and membrane oxidative damage) of the ready-to-use product was linked to the AsA-GHS cycle degradation.
Phenolic compounds are part of the plant’s natural defence system and they can play an important role in absorbing and neutralizing ROS as they have redox properties [49]. In our research, total phenols (TP) in the raw material were very high in the spring product (11.1 µg mg−1 DW), especially compared with the winter and winter–-spring heads (5.5 µg mg−1 DW) (Figure 4B). This seasonal change in TP content can be explained by the higher temperature and radiative conditions of the spring cycle triggering the secondary metabolism as a plant response [44,50]. The range of TP concentration on a fresh weight basis was 668–-1,317 mg kg−1 FW, which is in line with TP content reported by other authors [3,12,39,40,47,51].
The post-harvest increase in TP content was observed in winter (+55%) and winter–-spring (+73%) florets. A decreasing trend (–(-14%) of TP was only observed in the summer–-autumn product despite having a TP content at harvest quite similar to that of other raw products (Figure 4B). Generally, an increase in TP compounds during post-harvest storage occurs in Brassicas (broccoli sprouts [52], fresh-cut rocket [14], and broccoli raab [20] to contrast post-harvest oxidative stress [53]). After 7–-14 days, storage at 4–-5 °C showed a slight increase for intact broccoli heads [39,47]. Other authors [14,40] have observed a decrease of TP in fresh-cut florets associated with a loss of quality parameters (overall appearance, odour, weight loss, and color). In wild rocket, the post-harvest decrease in TP has also linked to leaves perishability, while there was a retention of visual quality with the rise in TP [23]. Therefore, it is arguable that in our study the quality decline of the summer–-autumn product can be related to the decreasing trend in TP (along with the AsA content) during storage. In contrast, its rise or retention contributed to quality preservation in other products.
Both AsA and phenolic compounds mostly affect antioxidant capacity in broccoli as in other Brassica vegetables [20,49]. The initial antioxidant capacity (AC) (ABTS·+) showed variability according to crop season with the highest values in winter- and spring-harvested heads (Figure 4C), likely to be due to the higher contribution of AsA in the former and TP in the latter. AC was lower in the heads harvested in April (WS crop) and especially in those harvested in November (SA crop) (Figure 4C). Over the whole storage period, only the summer–autumn crop showed a clear increase in AC (+108%). This trend was opposite compared to that of AsA and TP, leading us to suppose that antioxidant compounds other than AsA and TP affected the AC in this product. Glucosinolates represent an important defence system for plants, acting as indirect antioxidants through their break-down products [54] and/or direct antioxidants [55,56]. In general, a rise in glucosinolates due to de novo biosynthesis during storage is reported as a result of physiological stresses in intact broccoli [57]. In fresh-cut florets, however, a reduction in GL content occurs [14,58] because biosynthesis may be counteracted by hydrolysis of GLs due to contact with myrosinase when cellular integrity is lost in stressed tissues [58]. Therefore, these authors reported that retention of GLs was positively correlated to florets shelf-life. The present study did not evaluate glucosinolates, yet it can be speculated that the increase in AC in the stored summer–-autumn product could be associable to the formation of antioxidant break-down products of GLs which suppose a membrane injury. The loss of cellular compartmentation membrane integrity can also be argued due to the degradation of phenolic compounds [59] observed in this product, explaining its greatest decline in quality.
3.2. Effect of Genotype and Storage Time on Floret Quality in Early-Cycle Broccoli
3.2.1. Head Characteristics and Floret Weight Loss
Among the cultivars grown in the summer–autumn cycle, ‘Naxos’ exhibited the lowest head fresh weight and the highest dry mass concentration. In all cultivars, the loss of fresh weight (WLTot) observed during cold storage was attributable mostly to respiration (WLResp) with no significant difference between genotypes, while a lower weight loss due to transpiration was observed for ‘Naxos’. Despite WLResp and WLTransp increased at T14, WLTot was always below 1% (Table 4).

Table 4.
Bio-morphological traits of raw and stored broccoli as affected by growing cycle and genotype.
3.2.2. Chlorophylls, Carotenoids, and Visual Quality of Florets
The ANOVA results of chlorophyll and carotenoid concentrations in broccoli genotypes, color indices L* a*, b*, h°, and C*, affecting the visual quality are reported in Table 5.

Table 5.
Effect of genotype and cold storage time on the visual and anti-oxidative traits of broccoli florets.
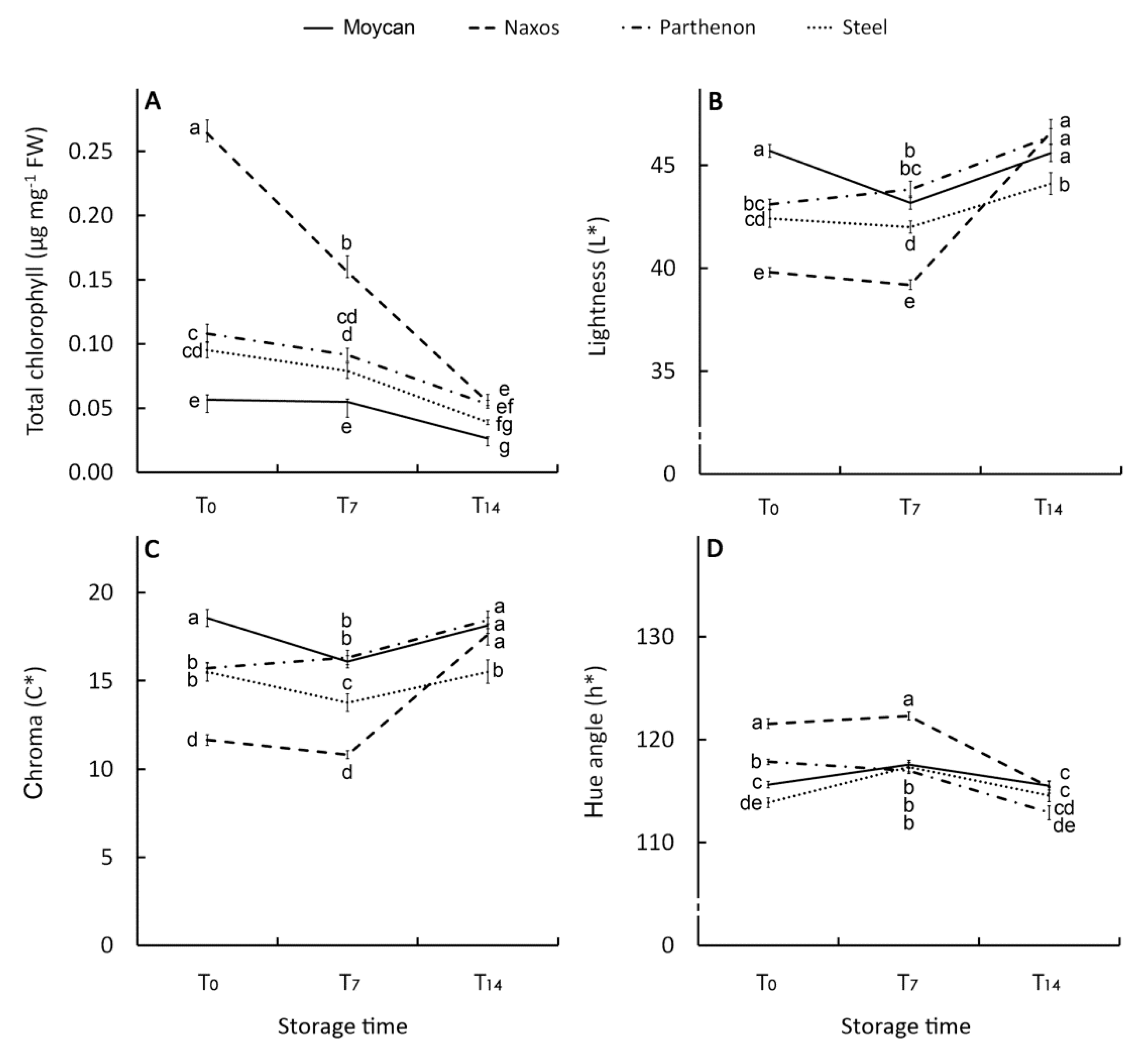
Chlorophyll a, b, and the total showed a significant GexST interaction. The cultivar Naxos exhibited an initial concentration of total CHL (0.26 µg mg−1 FW) 1.6 fold higher than ‘Parthenon’ and ‘Steel’ and 3.7 fold higher than ‘Moycan’ (Figure 5A), in line with levels of CHLa and CHLb (Figure S1A,B). Degradation of CHL during storage was more evident in the T7–-T14 period and was mostly affected by the CHLa changes (Figure S1A), while CHLb (Figure S1B) showed better retention over the storage period. However, despite the hugely decreased CHL in ‘Naxos’, it was still higher (at T7) or comparable (at T14) to other cultivars. The carotenoid levels both in raw and stored products mirrored CHLa (Figure S1C). Our findings are in agreement with studies reporting that CHL and carotenoid levels [60,61] and their post-storage changes [59] in broccoli are affected by genotypes. Consistently with the CHL and carotenoid levels, the best visual quality was shown by ‘Naxos’ at harvest and for a short-storage period (T7) with the lowest L* (39.8) (Figure 5B) and C* (11.7) (Figure 5C) along with the greatest h° (121.5) (Figure 5D) values compared with ‘Parthenon’, ‘Moycan’, and ‘Steel’. At T14, a general visual quality decay was observed in all cultivars.
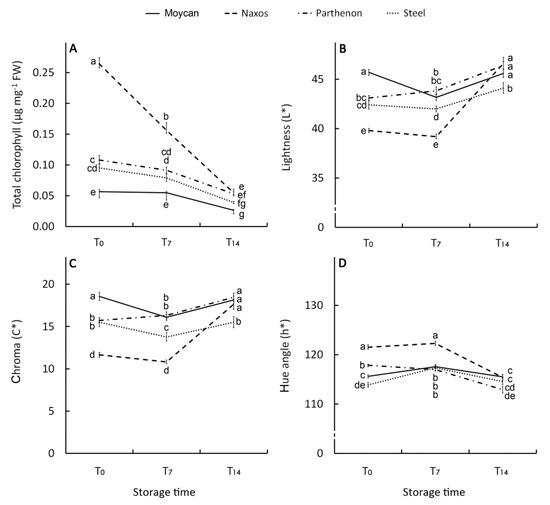
Figure 5.
Effect of genotype on total chlorophyll (A), lightness (L*) (B), chroma (C*) (C), and hue angle (h°) (D) index of the raw heads (T0), 7 days (T7) and 14 days (T14) stored product. Vertical bars (± SE of mean) (n = 9) with different letters are significantly different according to the Least Significant Difference test (P = 0.05).
3.2.3. Antioxidant Compounds and Antioxidant Capacity
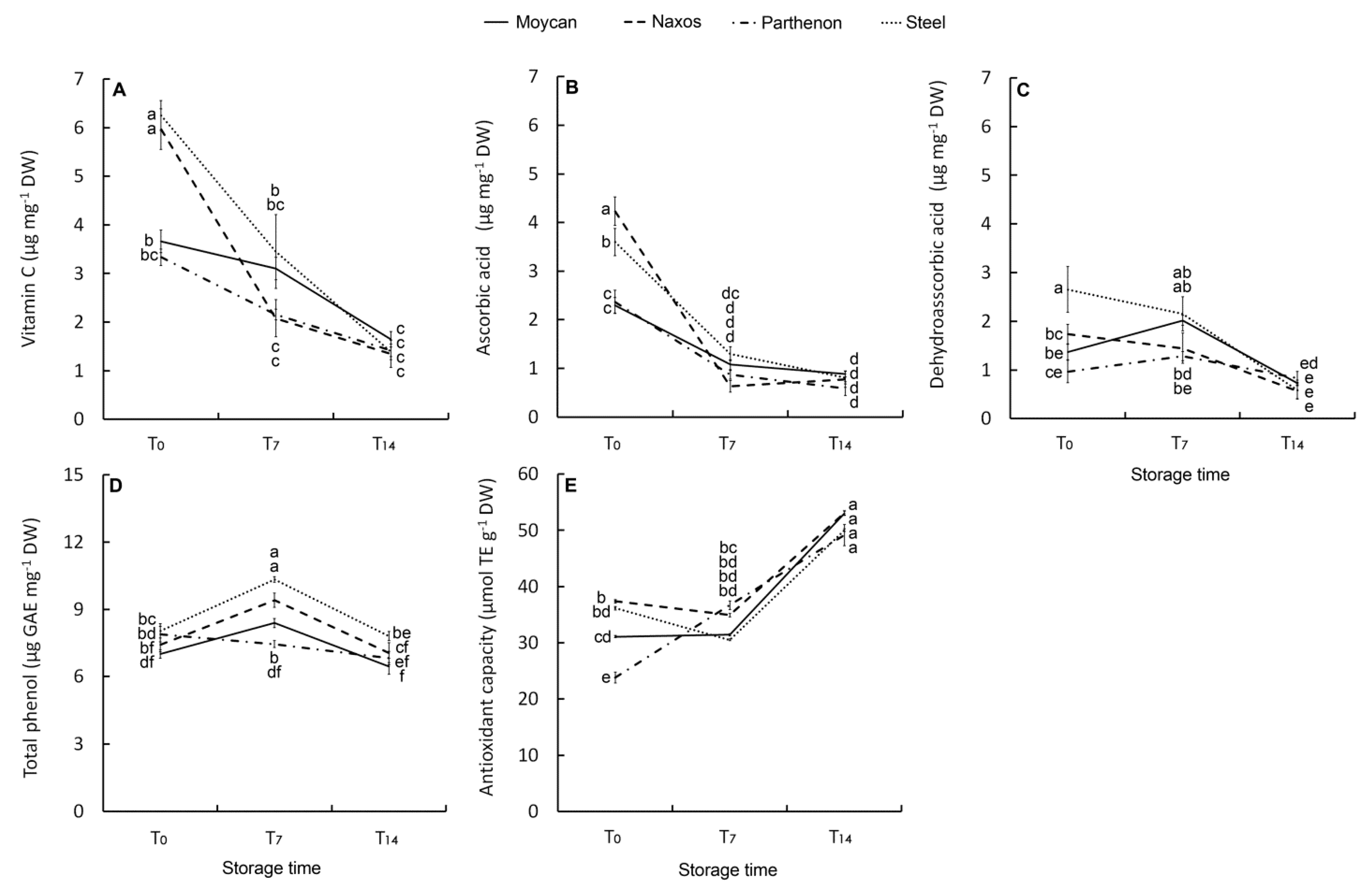
The ANOVA results for florets AsA, dehydroascorbic acid (DHAA), vitamin C, and TP concentrations, and antioxidant capacity (AC) are reported in Table 5, all showing significant interaction GexST. At harvest, both cultivar Naxos and ‘Steel’ had the highest vitamin C concentration (6.2 µg mg−1 DW) (Figure 6A). However, in ‘Naxos’, a greater contribution of AsA was observed (Figure 6B) while in ‘Steel’ that of DHAA was greater (Figure 6C). As DHAA is the oxidized form of vitamin C, its higher level underlines the more stressed physiological condition of ‘Steel’. Intermediate values of AsA, DHAA and vitamin C were observed in ‘Parthenon’ and ‘Moycan’. In all cultivars, AsA dropped at T7 whereas DHAA decreased at T14. This result suggests a prevailing oxidized status of vitamin C in the short-storage period and that the AsA-GSH cycle was definitively impaired at T14 with the loss of DHAA and with no de novo biosynthesis of AsA.
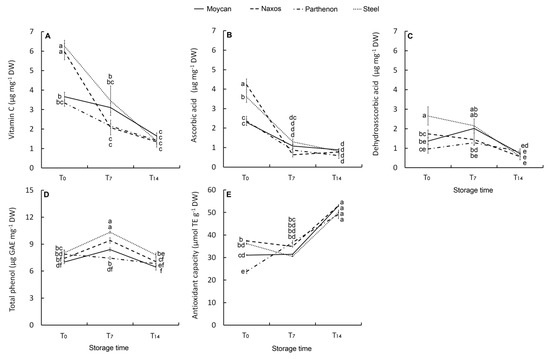
Figure 6.
Effect of genotype on vitamin C (A), ascorbic acid (B), dehydroascorbic acid (C), and total phenol concentration (D), as well as on antioxidant capacity (E) of the raw heads (T0), in 7 days (T7) and 14 days (T14) stored product. Vertical bars (± SE of mean) (n = 9) with different letters are significantly different according to the Least Significant Difference test (P = 0.05). GAE = gallic acid equivalent. TE = Trolox equivalent.
Contrarily to vitamin C, TP content in raw heads was not statistically different between cultivars (7.6 µg mg−1 DW), yet it increased at T7 particularly in ‘Naxos’ and ‘Steel’, but not in ‘Parthenon’ (Figure 6D). The above-described changes in vitamin C components and TP can be linked to the florets shelf-life with ‘Naxos’ being the best performing cultivar for short-term storage due to a greater initial level of AsA and TP post-harvest biosynthesis. It is noteworthy that this cultivar had the largest heads DM (Table 3), supporting the hypothesis of a positive relationship between DM and floret shelf-life. In the last phase of storage, TP content substantially reduced in all cultivars, so prolonged storage resulted in a loss of visual quality with no difference between genotypes (Figure 6D). In this phase, the antioxidant capacity (AC) of florets rose in all the cultivars (Figure 6E), suggesting that the oxidative stress during the T7–-T14 period caused the production of other antioxidant compounds contributing to AC, irrespective of genetic characteristics. On the other hand, a change in qualitative phenolic profile towards compounds at a higher antioxidant activity, such as phenolic acids [62], could have occurred during this period as their post-harvest accumulation has been proved to be high in wounded-tissue [63].
Variability has been reported in broccoli among cultivars in vitamin C [64] and TP [43,58,65]. Balouchi et al. [59] observed differences between genotypes in TP post-harvest changes with cultivars exhibiting an increase in phenols better retaining the green color in broccoli florets.
3.3. Principal Component Analysis
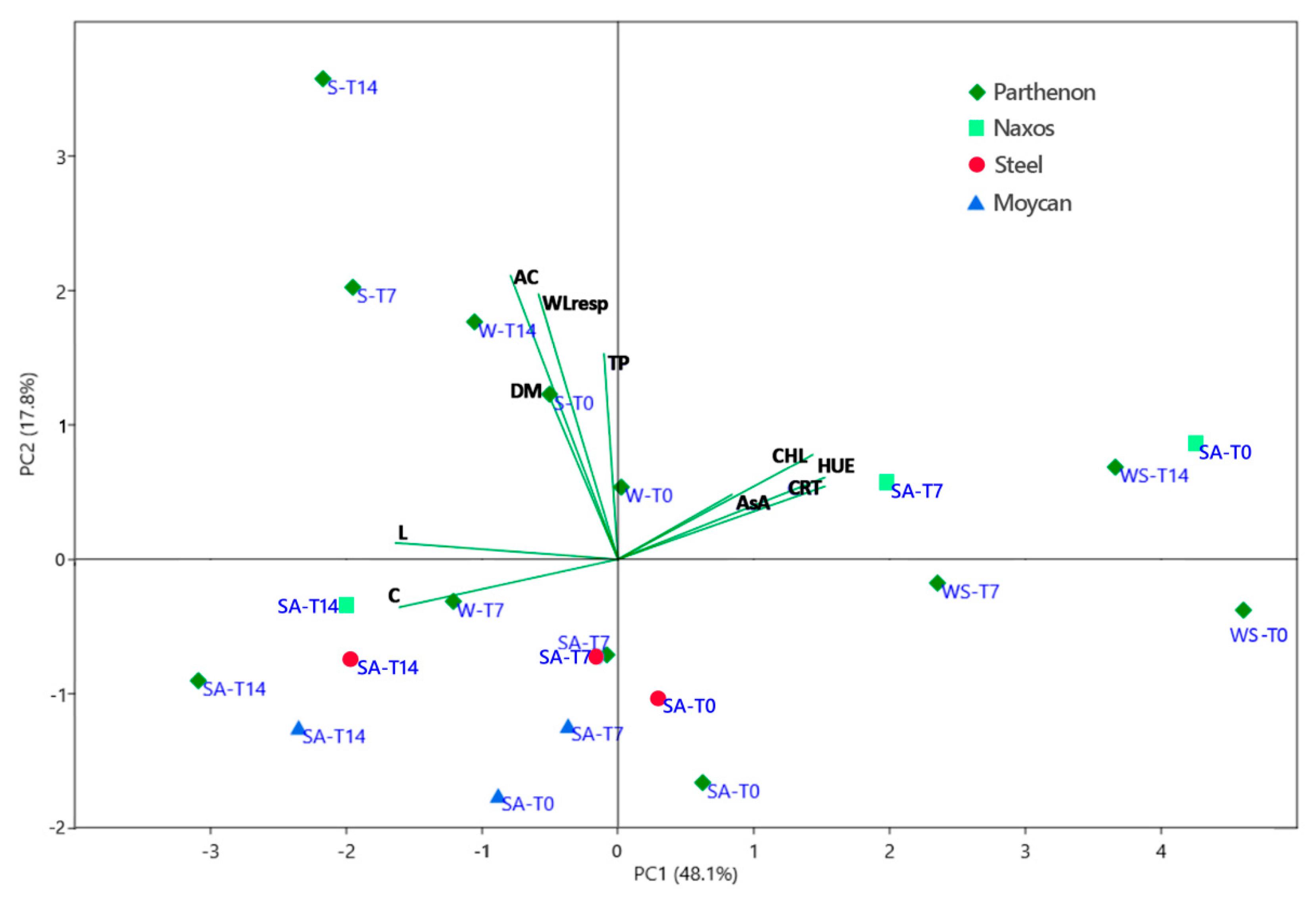
Overall exploration of data was carried out by principal component analysis (PCA) which showed that the first two PCs explained 66% of the total variability, attributing 48% to PC1 and 18% to PC2. The PC1 versus PC2 biplot is presented in Figure 7.
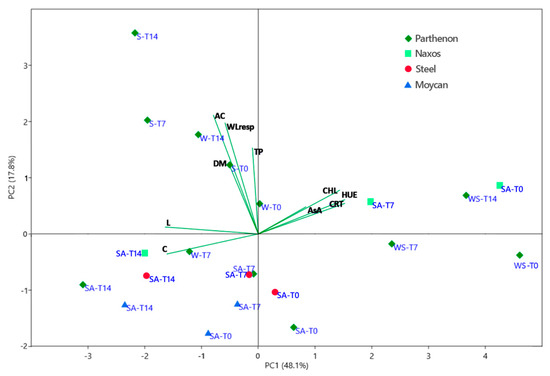
Figure 7.
Principal component analysis bi-plot (PC 1 vs PC 2) showing the spatial distribution of the main examined traits in raw (T0), 7 (T7) and 14 (T14) days stored broccoli [namely: lightness index (L), hue angle (HUE), chroma (C), concentration of dry matter (DM), chlorophylls (CHL), carotenoids (CRTs), total phenols (TP), ascorbic acid (AsA), antioxidant capacity (AC), and losses of fresh weight by respiration (mass losses) (WLresp)], as affected by the growing period [summer–autumn (SA), winter (W), winter–-spring (WS), spring (S)] and the genotype (‘Moycan’, ‘Naxos’, ‘Partenon’, and ‘Steel’).
Parameters indicating a better visual quality (angle of hue, CHLs, and carotenoids) of the product are positively correlated particularly to PC1 (loadings 0.41, 0.38, and 0.40, respectively) and linkable to the concentration of ascorbic acid (loading 0.23). On the contrary, L*(loading –-0.44) and Chroma (loading –-0.43) are negatively correlated to the PC1. PC1 separates on the far right-hand side the raw and stored product of both ‘Parthenon’ grown in winter–spring and ‘Naxos’ grown in the summer–-autumn period. However, prolonged-stored ‘Naxos’ florets are excluded from this group, confirming the ANOVA results (Figure 5). Although with a lower score, the raw heads of ‘Parthenon’ and ‘Steel’ from the summer–-autumn cycle are also located on the right of the PC1 axis. All other samples of ‘Parthenon’ and other genotypes are on the left of the PC1 axis, showing the reduced visual quality of raw heads and stored florets. However, PC2 allows these samples to be distinguished according to the cultivation season, with the summer–-autumn in the lower left-hand side and the spring and some winter products on the upper left-hand side. In the latter, the better visual quality compared to the summer–-autumn broccoli is mostly attributable to the greater antioxidant activity (loading 0.57) correlated to phenols (loading 0.41) and, to a lesser extent (loading 0.13), to AsA concentration. This physiological adaptation to the storage exhibited by spring and winter broccoli seems associable to the greater respiration activity (loading 0.53), which may have been sustained by the higher DM concentration (loading 0.34). Other authors have found that greater sugar content in heads (associable to higher DM) may contrast post-harvest de-greening in broccoli [14] by preventing sugar starvation. Among the summer–-autumn broccoli, the 14-day stored product grouped showing a more evident loss of quality (highest chroma and lowest hue and pigments content), particularly in ‘Parthenon’.
4. Conclusions
In a Mediterranean climate, the temperatures and the photoperiod occurring during the winter–-spring period improve the head color and the shelf-life of the fresh-cut broccoli florets, with a better level and retention of chlorophylls and carotenoids. This response can be linked to a more efficient antioxidative system based on ascorbic acid retention and total phenol biosynthesis during the post-harvest phase. The climatic conditions experienced by broccoli plants grown in the other growing cycles may be considered as abiotic stressors for broccoli resulting in a slight reduction in the visual quality of the heads. However, in the winter and spring product, an improved anti-oxidative status of heads due to a greater level of ascorbic acid (winter product) or phenols (spring product) may be maintained during storage, allowing the qualitative attributes of florets to be preserved for longer. In the summer–-autumn growing period, the decreasing trend in temperature and radiation does not elicit any antioxidant pathways so the physiological capacity of florets to prolong their shelf-life is impaired. The poorer post-harvest behaviour of broccoli grown in this period is, in general, confirmed in all the tested cultivars thus underlining a stronger effect of climate over that of genotype. However, cultivar Naxos seems more tolerant to these conditions, exhibiting a good visual quality until at least seven days of storage.
The observed difference in response to climatic stress condition seems to be mediated by the dry mass content of heads, with greater content playing an important role in supporting florets post-harvest metabolic activities to contrast tissues senescence. However, the relationship between the climate experienced by the broccoli crops, head dry matter, changes in antioxidative compounds and florets shelf-life deserves further investigation.
Supplementary Materials
Table S1: Main physical and chemical characteristics of soil of farms where broccoli crops were performed; Figure S1: Effect of the genotype on chlorophyll a (A), chlorophyll b (B), and carotenoid (C) concentrations of the raw heads (T0), 7 days (T7) and 14 days (T14) stored product is available online at https://www.mdpi.com/2073-4395/10/4/527/s1.
Author Contributions
Conceptualization, G.C. and A.E.; data curation, A.B.; formal analysis, C.L.; funding acquisition, A.E.; supervision, G.C. and A.E.; validation, G.C.; writing-original draft preparation, G.C.; writing-review and editing, A.E. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the MIUR (Italian Ministry of University and Scientific Research) within the PON Program (Research Project “High-Convenience Fruits and Vegetables: New Technologies for Quality and New Products” (OFRALSER), PON 01 01435).
Acknowledgments
The authors are grateful to all the growers who kindly donated the plant material.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Podsȩdek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Chaves, A.R.; Martínez, G.A. End of day harvest delays post-harvest senescence of broccoli florets. Post-Harvest Biol. Technol. 2011, 59, 64–70. [Google Scholar] [CrossRef]
- Forney, C.F.; Song, J.; Fan, L.; Hildebrand, P.D.; Jordan, M.A. Interactive effects of ozone and 1-methylcyclopropene alter the post-harvest quality of broccoli. J. Am. Soc. Hortic. Sci. 2003, 128, 403–408. [Google Scholar] [CrossRef]
- Vasconcelos, I.S.A.; Almeida, D.P.F. Treatment with 1-methylcyclopropene complements temperature management in maintaining post-harvest quality of broccoli. Acta Hortic. 2003, 628, 227–232. [Google Scholar] [CrossRef]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signalling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kader, A.A.; Rolle, R.S. The Role of Post-Harvest Management in Assuring the Quality and Safety Horticultural Crops; Agric. Serv. Bull.; FAO: Rome, Italy, 2004; Volume 152. [Google Scholar]
- Toivonen, P.M.A.; Hodges, D.M. Abiotic stress in harvested fruits and vegetables, abiotic stress in plants—Mechanisms and adaptations. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A.K., Venkateswarlu, B., Eds.; Abiotic Stress in Plants—Mechanisms and Adaptations; InTech: Rijeka, Croatia, 2011; pp. 39–58. [Google Scholar]
- Zaicovski, C.B.; Zimmerman, T.; Nora, L.; Nora, F.R.; Silva, J.A.; Rombaldi, C.V. Water stress increases cytokinin biosynthesis and delays post-harvest yellowing of broccoli florets. Post-Harvest Biol. Technol. 2008, 49, 436–439. [Google Scholar] [CrossRef]
- Cogo, S.L.P.; Chaves, F.C.; Schirmer, M.A.; Zambiazi, R.C.; Nora, L.; Silva, J.A.; Rombaldi, C.V. Low soil water content during growth contributes to preservation of green color and bioactive compounds of cold-stored broccoli (Brassica oleraceae L.) florets. Post-Harvest Biol. Technol. 2011, 60, 158–163. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Kasimoglu, Z.; Sahin-Nadeem, H.; Polat, E.; Erkan, M. The effects of UV radiation during the vegetative period on antioxidant compounds and post-harvest quality of broccoli (Brassica oleracea L.). Plant Physiol. Biochem. 2015, 93, 56–65. [Google Scholar] [CrossRef]
- Casajús, V.; Reyes, A.; Gergoff, G.; Lobato, M.G.; Civello, P.; Martínez, G. The time of the day to harvest affects the degreening, antioxidant compounds, and protein content during post-harvest storage of broccoli. J. Food Biochem. 2019, 43, e12904. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Lemoine, L.; Vicente, A.R.; Chaves, A.R.; Martínez, G.A. Post-harvest senescence of florets from primary and secondary broccoli inflorescence. Post-Harvest Biol. Technol. 2015, 104, 42–47. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Statistic Division. 2018. Available online: http://faostat3.fao.org/ (accessed on 13 April 2018).
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Elia, A. Growth, N uptake and N critical dilution curve in broccoli cultivars grown under Mediterranean conditions. Sci. Hortic. 2019, 244, 109–121. [Google Scholar] [CrossRef]
- Elia, A.; Conversa, G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012, 40, 64–74. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Pre-harvest nitrogen and Azoxystrobin application enhances post-harvest shelf-life in Butterhead lettuce. Post-Harvest Biol. Technol. 2013, 85, 67–76. [Google Scholar] [CrossRef]
- Dere, S.; Gunes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; Elia, A. Bio-physical, physiological, and nutritional aspects of ready-to-use cima di rapa (Brassica rapa L. subsp. sylvestris L. Janch. var. esculenta Hort.) as affected by conventional and organic growing systems and storage time. Sci. Hortic. 2016, 213, 76–86. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, 55–61. [Google Scholar] [CrossRef]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of organic and conventional cropping systems on ascorbic acid, vitamin C, flavonoids, nitrate, and oxalate in 27 varieties of spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Post-harvest performance of ready-to-eat wild rocket salad as affected by growing period, soilless cultivation system and genotype. Post-Harvest Biol. Technol. 2019, 156, 110909. [Google Scholar] [CrossRef]
- Conversa, G.; Lazzizera, C.; Chiaravalle, A.E.; Miedico, O.; Bonasia, A.; La Rotonda, P.; Elia, A. Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Sci. Hortic. 2019, 252, 176–191. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Salvatore, S.; Brenna, O.V.; Galaverna, G.; Del Rio, D.; Bianchi, M.; Bennett, R.N.; Brighenti, F. Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007, 87, 103–111. [Google Scholar] [CrossRef]
- SAS Institute. SAS Version 8.02; SAS Institute Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical Analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Olesen, J.; Grevsen, K. Effects of temperature and irradiance on vegetative growth of cauliflower (Brassica oleracea L. botrytis) and broccoli (Brassica oleracea L. italica). J. Exp. Bot. 1997, 48, 1591–1598. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Knaflewski, M.; Lisiecka, J.; Spiżewski, T.; Frąszczak, B. Effect of temperature on the growth of broccoli (Brassica oleracea L. var. italica Plenck) cv. Fiesta. Veg. Crops Res. Bull. 2012, 77, 129–141. [Google Scholar] [CrossRef]
- Ferrante, A.; Mariani, L. Review. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses: High and Low Values of Temperature, Light Intensity, and Relative Humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Nath, A.; Bagchi, B.; Misra, L.K.; Deka, B.C. Changes in post-harvest phytochemical qualities of broccoli florets during ambient and refrigerated storage. Food Chem. 2011, 1510–1514. [Google Scholar] [CrossRef]
- Luo, F.; Cheng, S.; Cai, J.; Wei, B.; Zhou, X.; Zhou, Q.; Zhao, Y.; Ji, S. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, J.K. Photosynthesis and Abiotic Stress in Plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Biotic and Abiotic Stress Tolerance in Plants; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 27–46. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Chenga, S.; Zhoub, H.; Ji, S. Potential of jasmonic acid (JA) in accelerating post-harvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chem. 2020, 309, 125737. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zuo, J.; Gu, S.; Gao, L.; Hu, W.; Wang, Q.; Jiang, A. Putrescine treatment reduces yellowing during senescence of broccoli (Brassica oleracea L. var. italica). Post-Harvest Biol. Technol. 2019, 152, 29–35. [Google Scholar] [CrossRef]
- Loi, M.; Liuzzi, V.C.; Fanelli, F.; De Leonardis, S.; Maria, T.; Ancona, N.; Paciolla, C.; Mulè, G. Effect of different light-emitting diode (LED) irradiation on the shelf life and phytonutrient content of broccoli (Brassica oleracea L. var. italica). Food Chem. 2019, 283, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; Amodio, M.L.; Colelli, G.; González-Gómez, D. Retention of quality and functional values of broccoli “Parthenon” stored in modified atmosphere packaging. Food Control 2013, 31, 302–313. [Google Scholar] [CrossRef]
- Davey, M.W.; Van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Review: Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Schonhof, I.; Kläring, H.P.; Krumbein, A.; Claußen, W.; Schreiner, M. Effect of temperature increase under low radiation conditions on phytochemicals and ascorbic acid in greenhouse grown broccoli. Agric. Ecosyst. Environ. 2007, 119, 103–111. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Effect of climatic and sulphur fertilisation conditions, on phenolic compounds and vitamin C, in the inflorescences of eight broccoli cultivars. Eur. Food Res. Technol. 2003, 216, 395–401. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433. [Google Scholar] [CrossRef]
- Raseetha, S.; Leong, S.Y.; Burritt, D.J.; Oey, I. Understanding the degradation of ascorbic acid and glutathione in relation to the levels of oxidative stress biomarkers in broccoli (Brassica oleracea L. italica cv. Bellstar) during storage and mechanical processing. Food Chem. 2013, 138, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Mareczek, A.; Starzyńska, A.; Rożek, S. Antioxidant ability of broccoli flower buds during short-term storage. Food Chem. 2001, 72, 219–222. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Florkiewicz, A.F.; Topolsk, K. Sous-vide technique as an alternative to traditional cooking methods in the context of antioxidant properties of Brassica vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Beatriz, M.; Oliveira, P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Post-harvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Vicas, S.I.; Teusdea, A.C.; Carbunar, M.; Socaci, S.A.; Socaciu, C. Glucosinolates profile and antioxidant capacity of Romanian Brassica vegetables obtained by organic and conventional agricultural practices. Plant Foods Hum. Nutr. 2013, 68, 313–321. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Gicquel, M.; Esnault, M.A. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012, 132, 1003–1009. [Google Scholar] [CrossRef]
- Rodriguez, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Jia, C.G.; Xu, C.J.; Wei, J.; Yuan, J.; Yuan, G.F.; Wang, B.L.; Wang, Q.M. Effect of modified atmosphere packaging on visual quality and glucosinolates of broccoli florets. Food Chem. 2009, 114, 28–37. [Google Scholar] [CrossRef]
- Balouchi, Z.; Ghasemnezhad, M.; Saadatian, M.; Peyvast, G. Biochemical characteristic of florets senescence in broccoli cultivars during storage at low and high temperatures EJPAU 2014, 17(2), 12. Available online: http://www.ejpau.media.pl/volume17/issue2/art-12.html (accessed on 13 February 2020).
- Farnham, M.W.; Vegetable, U.S. Importance of Genotype on carotenoid and chlorophyll levels in broccoli heads. HortScience 2009, 44, 1248–1253. [Google Scholar] [CrossRef]
- Balouchi, Z.; Peyvast, G.A.; Ghasemnezhad, M.; Saadatian, M. Changes of antioxidant compounds of broccoli (Brassica oleracea L.var. italica) during storage at low and high temperatures. South West. J. Hortic. Biol. Environ. 2011, 2, 193–212. Available online: https://www.researchgate.net/profile/Mohammad_Saadatian/publication/234055250_changes_of_antioxidant_compounds_of_broccoli_Brassica_oleracea_Lvar_Italica_during_storage_at_low_and_high_temperatures/links/0912f50ea7607baeff000000/changes-of-antioxidant-com (accessed on 13 February 2020).
- Bhandari, S.R.; Kwak, J.H. Chemical composition and antioxidant activity in different tissues of brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, 107–113. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Kaur, C.; Kumar, K.; Anil, D.; Kapoor, H.C. Variations in antioxidant activities in broccoli (Brassica oleracea L.) cultivars. J. Food Biochem. 2007, 31, 621–638. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).