Abstract
In this work, the effect of dyes extracted through the Naviglio method (an eco-innovative solid-liquid extraction technique) was tested, which proved interesting for such an extraction. The dyes extracted from Opuntia ficus-indica (L.) Miller waste were treated to maximize the extraction of the coloring molecules. The extraction method was compared with conventional methods under the same conditions. The results showed that the extracts obtained with the different techniques, in the same extraction conditions (solvent, pH, temperature, time), were richer in the pigments for the extract obtained with the Naviglio method. The stability of the dyes was tested before the staining. A plant-based fiber, cotton, as well as an animal-based fiber, wool, were chosen for the staining tests. For the two types of fiber, two etching methods were used: one with rock alum, a method widely used to fix a color and one with lemon juice, a method used for a long time by rural populations who practiced the coloring of hides and tissues. In addition, the qualitative content of the pigments was also verified with an HPLC profile of the Opuntia ficus-indica (L.) Miller extracts. Ultimately, the results suggest that the Opuntia ficus-indica (L.) Miller fruit can be of great interest as a natural source of dyes and be used for tinctures.
1. Introduction
The agricultural and food industries are particularly sensitive to the disposal and/or reuse of waste, especially due to the increasing amounts of waste in their production processes. In Italy only, in 2012, with increasing data up until 2018, it was estimated that 3,037,092 tons of vegetable waste were produced and that 96% of it was derived from the food, beverage and tobacco industries [1,2].
Recent studies have shown that there is an increasing interest in the nutri-energetics area, an industrial compartment which plans to transform agro-food waste into nutraceutical products, protein and organic fertilizers as well as textile fiber products. According to Heather Youngs, a bioenergetics analyst at the Energy Bioscience Institute of Berkley University, the possibility to create new food products, from the residues of agro-processing industries, could be an interesting way to solve the problem of the management of non-hazardous waste and could be more cost effective and profitable than the production of biodiesel [3].
The Opuntia ficus-indica (L.) Miller belongs to the Cactaceae family and is found in nature as a weed edible plant (WEP), but there are also large commercial plantations in South America (Mexico, Brazil and Chile) and Southern Europe (Italy and Spain).
The parts of Opuntia ficus-indica (L.) Miller are divided into fruits, cladodes and flowers; these parts have been frequently used as food for humans and farm animals, but also in folk medicine, thanks to their nutritional properties and beneficial activities [4]. The fruits of the prickly pear can be of various colors, ranging from white to purple, passing through yellow, orange and red: this is due to the combination of two betalain-based pigments [5]. Physical chemical differences exist between the cultivars of the different colors confirmed, in particular between the orange and the green fruit [6]; the difference in color also induces variability in the nutritional and antioxidant properties of the fruit [7,8].
In this context, the red-purple prickly pear peels are suggested as a precious source of betalain [9]. In general, betalains are water-soluble, nitrogen-containing and include red-purple betacyanines and yellow betaxantines [10,11]. They are the conjugated compounds of the ammonia of betalamic acid with cyclo-DOPA (cyclo-dihydroxyphenylalanine) and amines or aminoacids [12], where the chromophore is a system of 1,7-diazaeptametinium. These compounds represent one of the most important classes of natural pigments [13].
Furthermore, even if numerous studies have demonstrated that the prickly pear peels have a good antioxidant and anti-inflammatory effect (because of the presence of polyphenolic compounds such as flavonoids quercetin glycoside and luteolin glycoside), today they are used only in composting [14]. Therefore, it could be interesting to enhance the health and dye properties of prickly pear peels by extracting the bioactive molecules to produce other products [15].
The main aim of this work was to recover the by-products and waste of prickly pear peels (Opuntia ficus-indica (L.) Miller) to produce natural colorants, following the new trends and market demands, but at the same time to produce energy, biofuels and edible lignin for textiles from biomasses. In fact, after extraction the residues can be used for animal feedstuff or for the production of biogas and energy using an anaerobic digestion process, or to obtain edible lignin for the production of textile fibers [16].
2. Materials and Methods
2.1. Chemicals and Solvents
All solvents and reagents were purchased from commercial sources at a high grade of purity and used as received unless stated otherwise. Ethanol 96% RPE for analysis, hydrochloric acid 37% RPE for analysis, potassium bicarbonate, anhydrous sodium carbonate for analysis and gallic acid ACS for analysis were obtained from Carlo Erba (Milano, Italy). Methanol for HPLC, n-hexane anhydrous at 95%, chloroform for HPLC, 1-butanol ACS reagent ≥ 99.5%, aluminum potassium sulfate dodecahydrate, pure sodium chloride and betanin (red beet extract diluted with dextrin) were purchased from Sigma-Aldrich Chemical Company (Milano, Italy). Acetonitrile hypergrade for LC–MS was purchased from Merck Millipore GmbH (Milano, Italy). Sodium hydroxide pellets were obtained from J.T. Baker and 2,2-diphenyl-1-picrylhydrazyl (free radical) was obtained from Alfa Aesar. J.T. Baker and Alfa Aesar are Thermo Fisher Scientific companies (Rodano, Milano, Italy). All analytical standards, such as quercetin, luteolin, kaempferol and isorhamnetin, were obtained from Supelco, a Sigma-Aldrich Chemical Company (Milano, Italy).
Bidistilled water was employed throughout the preparation of the solutions. Extracts obtained from the different parts of the Opuntia ficus-indica (L.) Miller were applied without any purification. Stock solutions of extracts were prepared by diluting them in water or in a hydroalcoholic solution.
2.2. Plant Materials
Prickly pears without apparent physical damage were collected from farms in Sicily, from Roccapalumba (Palermo, Italy), from September to October 2018. They were immediately sealed under a vacuum in clean polyethylene bags and stored in refrigerated boxes at −4 °C; samples were transported to the laboratory within a maximum of 3 h after harvest. Subsequently, the pears were washed thoroughly with distilled water to remove impurities and thorns. All the samples were left frozen at −24 °C until they were analyzed. The samples were prepared manually; the peel was separated from the pulp and the seeds together with the mucilage were separated.
2.3. Preparation of Prickly Pear Peel Extracts
The sample to solvent ratio was kept constant (plant material:solvent = 1:10, w/v) for the two methods of extraction and for all experiments.
The plant materials were extracted by two different extraction methods; moreover, two different pure solvents and a mix of them were used: ethanol (EtOH), bidistilled water (H2O) and a solution of EtOH and H2O in a ratio of 8:2 (v/v). The latter ratio was selected on the basis of previous extraction studies found in the literature, which testified that this EtOH and H2O ratio was the most promising for extracting the various components present within the matrix [9,10,17,18,19,20,21,22,23].
Extractions were conducted at room temperature for 2 h.
2.4. Apparatus
The Extractor Naviglio® represents an eco-innovative technological solution in solid-liquid extraction. This extraction was achieved through the discovery of a new principle of solid-liquid extraction, called “Principio di Naviglio” (Naviglio’s Principle) [24]. The Extractor Naviglio® is based on a suction effect, with a pressure of approximately 9–10 bar for a specific time (the defined cycle) and is followed by an immediate decompression at atmospheric pressure. The rapid release of the liquid extract from the inside of a solid matrix, because of the pressure gradient, mechanically transports the extractable compounds contained in the solid matrix outwards (Naviglio’s Principle). An extractive cycle includes a static phase followed by a dynamic phase. During the static phase, the system is subjected to pressure for a time which is determined by the composition of the solid matrix from which molecules have to be extracted; in this phase the liquid penetrates into the solid and it permeates all the empty spaces passing through the cellular membranes of vegetables in a manner more efficacious than maceration. When the static phase is finished, the dynamic phase starts immediately. It accomplishes two objectives; the first is the generation of a negative pressure gradient between the inside and the outside of the solid matrix, which represents the dragging effect of the extraction (Naviglio’s Principle), while the second objective is to stir the liquid in the entire system again to prevent the formation of a super saturation zone of extracted substances near the solid surface.
All absorbance measurements were carried out using a Merck Pharo 300 spectrophotometer (Merck Millipore GmbH, Milano, Italy). A 1.0 cm long optical path glass cell was used in all measurements.
The HPLC analyses were performed using a LC-20ADxr SHIMADZU HPLC (Shimadzu Italia S.r.l., Milano, Italy) system equipped with two pumps, a SIL-20AHT autosampler, an SPD-M20A diode array detector and a Dr. Maisch GmbH (Ammerbuch-Entringen, Germany) 250 mm C-18 column.
A Metrohm 827 pH meter (Metrohm Italiana S.r.l., Origgio (VA), Italy), a two-channel laboratory pH measuring instrument for measuring pH/mV and temperature, was used for the pH measurement.
Specific gravities were measured with a Brix & Gravity Aichose Refractometer (Qingdao Xindacheng Plastic Machinery Co. ltd, Jiaozhou City, Qingdao, China) with automatic temperature compensation (ATC) that features a 0%–32% Brix Scale and a Specific Gravity Scale from 1.000–1.130.
2.5. Maceration Extraction (ME)
Ten grams of prickly pear peel were extracted by macerations with 100 mL of EtOH, H2O and their mixture in a ratio of 8:2 (v/v) under continuous stirring and in the dark, for 24 h. After maceration, the extracts were filtered through paper to remove any floating matter.
2.6. Extractor Naviglio® Extraction (NE)
The extraction was then repeated on another sample (with the pulp and peel of prickly pears) using the Extractor Naviglio® (ATLAS FILTRI ITALIA, Limena, Padova, Italy). A quantity of 60 g of the sample was placed in a 50 µm filtering membrane bag and placed into the 500 mL chamber of the Extractor Naviglio®. Then, the chamber was filled with the solvent. The extractions were carried out with solutions of EtOH, H2O and their mixture in a ratio of 8:2 (v/v). Each extraction cycle consisted of a 2 min static phase, followed by a 2 min dynamic phase; the total number of cycles was 360 for a total time of 24 h. After filtration, the clear solution extract obtained was stored in a dark container.
2.7. Soluble Solid Contents and pH
The soluble solid content (°Bx) and pH were measured in all extracts by means of a refractometer and Metrohm pH meter.
2.8. Liquid-Liquid Extraction
The extracts obtained with the different techniques were analyzed by HPLC. After the evaluation of the chromatograms, the separation of the pigments and the bioactive components was carried out, including a liquid-liquid separation of the separating funnel. Solvents with increasing polarity were used, such as n-hexane, chloroform and butanol. The liquid-liquid separation led to obtaining four fractions in different solvents which were desolvated with a rotary evaporator. The remaining fraction in double distilled water was concentrated and stored at 4 °C in sealed dark glass bottles. These samples were used as dyes for the coloration of animal and vegetable type textile fibers, wool and cotton, respectively.
The fractions that were obtained through the liquid-liquid separation, carried out with solvents with increasing polarity, allowed for the extraction of several compounds of well known biological interest and that could have nutraceutical uses: compounds such as fatty acids, small molecules like polyphenols and amino acids were found.
2.9. Chemical Characterization of the Extracted Dyes
The extracts of Opuntia ficus-indica (L.) Miller were chemically characterized with spectroscopic and chromatographic techniques. The dye extracts were subjected to UV-VIS spectrophotometry (Merck Pharo 300) after appropriately diluting the solution containing the extracted dye with water (0.5 mg/mL) [25]. UV-VIS spectra were acquired in a visible region of 350–650 nm. For the quantitative internal standard, a calibration curve was prepared using final concentrations of 0.015, 0.032, 0.065, 0.130, 0.25, 0.5 and 1.0 mg/mL in triplicate. Quantitative determination was made taking into account the maximum absorbance of the absorption signal, at the wavelength of the analyzed colored compound reported and expressed in milligrams per gram of the prickly pear peels: the estimated amount of coloring compounds was 5 × 10−3 g/gpeel. The R2 interval was between 0.9849 and 0.9990 and the relative standard deviation (RSD) slope values between the measured samples were less than 5%.
In addition, all samples were examined by high performance liquid chromatography (HPLC) and by thin layer chromatography (TLC) on silica gel plates, Analtech TLC Uniplates (Merck). Chromatography on TLC was performed in the following solvent systems: methanol–n-hexane (6:4, v/v), ethyl acetate–methanol–water (8:2:1, v/v) and chloroform–isopropanol (95:5, v/v). The spots were visualized by exposure to UV radiation (253 nm).
The HPLC separation of the active compounds was carried out by using a C-18 column (Dr. Maisch GmbH, 250 mm × 4.6 mm, particle size 5 µm) set for a gradient elution. Eluent A was water (0.1% trifluoroacetic acid) and eluent B was acetonitrile (0.1% trifluoroacetic acid). The gradient program was 15% B for 5 min, 15%–20% B over 5 min, 20%–25% B over 10 min, 25%–35% B over 10 min and 35%–50% for 10 min. The flow rate was 1.0 mL∙min−1 and the injection volume was 20 µL at 25 °C. The identification of these compounds was carried out by comparing the retention time in the extracts of the injected samples to those of the standard HPLC compounds. The qualitative determinations were made taking into account the absorbance signal of each peak relative to the analytical standards (betalain, quercetin, luteolin, kaempferol and isorhamnetin) of the compounds found at the same wavelength. Chromatograms were observed at a 340–540 nm wavelength.
2.10. Dyeing Fiber with Opuntia ficus-indica (L.) Miller Dye Extract
Cotton and wool were the fibers selected for the study. They were previously leached in an aqueous solution containing 0.5 g/L of sodium carbonate and 2 g/L of non-ionic detergent at 50 °C for 60 min. Subsequently, threads of fiber material, between 20 cm (wool fibers) and 35 cm (cotton fibers) were prepared for dyeing. The dyes obtained from Opuntia ficus-indica (L.) Miller were fixed to the fibers with the use of mordants. The mordants used were rock alum (potassium alum sulphate) and lemon juice; both methods were reported by popular tradition. The fibers were dyed with the extract dye of Opuntia ficus-indica (L.) Miller without the use of mordants in obscured balloons, sealed and placed under agitation with a magnetic stir bar.
The etching solutions were prepared by placing 245 mg of rock alum in 500 mL of deionized and double distilled water (solution 1) and placing 100 mL of lemon juice in 500 mL of deionized and double distilled water (solution 2). The solutions were placed under magnetic stirring and heated for 60 min at 80 °C.
Two processes were used: pre-mordant, a process in which the sample is first mordanted and subsequently dyed and a process in which the fibers were dipped directly into the coloring solution.
The fiber samples were weighed (1 g) and immersed in the solutions.
For the pre-mordant method, the tissues were first immersed in the aqueous solutions of each of the aforementioned mordants at 80 °C for 60 min. The samples were then removed and without drainage were immersed in an aquatic extract of Opuntia ficus-indica (L.) Miller (10 mL) at 80 °C for 24 h with constant stirring (Figure 1, Figure 2 and Figure 3). Subsequently, the drained and dried fabrics were washed twice (2 min) at 40 °C with distilled water.

Figure 1.
Images of the cotton (right) and the wool (left) fibers before the etching and the dyeing treatments.

Figure 2.
Image of the post-etching cotton and wool fibers: the treatment with aluminum potassium sulfate (left) and lemon juice (right).

Figure 3.
Image of the samples immersed in an aquatic extract of Opuntia ficus-indica (L.) Miller.
For direct coloring without mordants, the fibers were immersed in a bath containing the coloring extract of Opuntia ficus-indica (L.) Miller at room temperature (25 °C) and under stirring for 24 h. After dyeing, the surplus of dye was removed from the colored materials by rinsing twice with water at 40 °C.
Samples of the non-colored fibers were used as controls. The experiments were performed by triplicates.
2.11. Colorimetric and Fastness Properties of Fiber Dyed with Opuntia ficus-indica (L.) Miller Dye Extract
The tissues dyed with the Opuntia ficus-indica (L.) Miller extracts (Figure 4) discussed in this study (see Section 2.10) were subjected to reflectance color measurements using a FRU WR-10 QC colorimeter from ShenZhen Wave Optoeletronics Technology with a viewing area of 4 mm in diameter. The following measurement conditions were selected: light source D65, which represented a daylight phase, with a correlated color temperature (CCT) between 5000–6500 K and a standard CIE (International Commission on Illumination, from its French name, "Commission internationale de l’Éclairage") 10° observer. The measurements were made using the method reported in the work of Chan-Bacab et al., 2015 [26], that reported the right method to apply to the analysis of fiber coloring using CIE LAB (also known as CIE L*a*b*, expresses the color through three values L, a and b as explained below) standards [27].
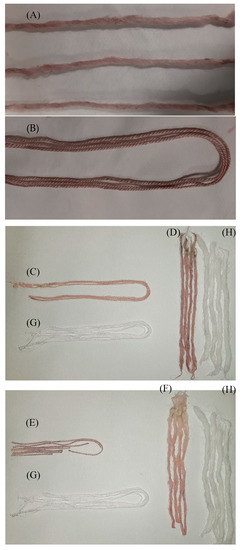
Figure 4.
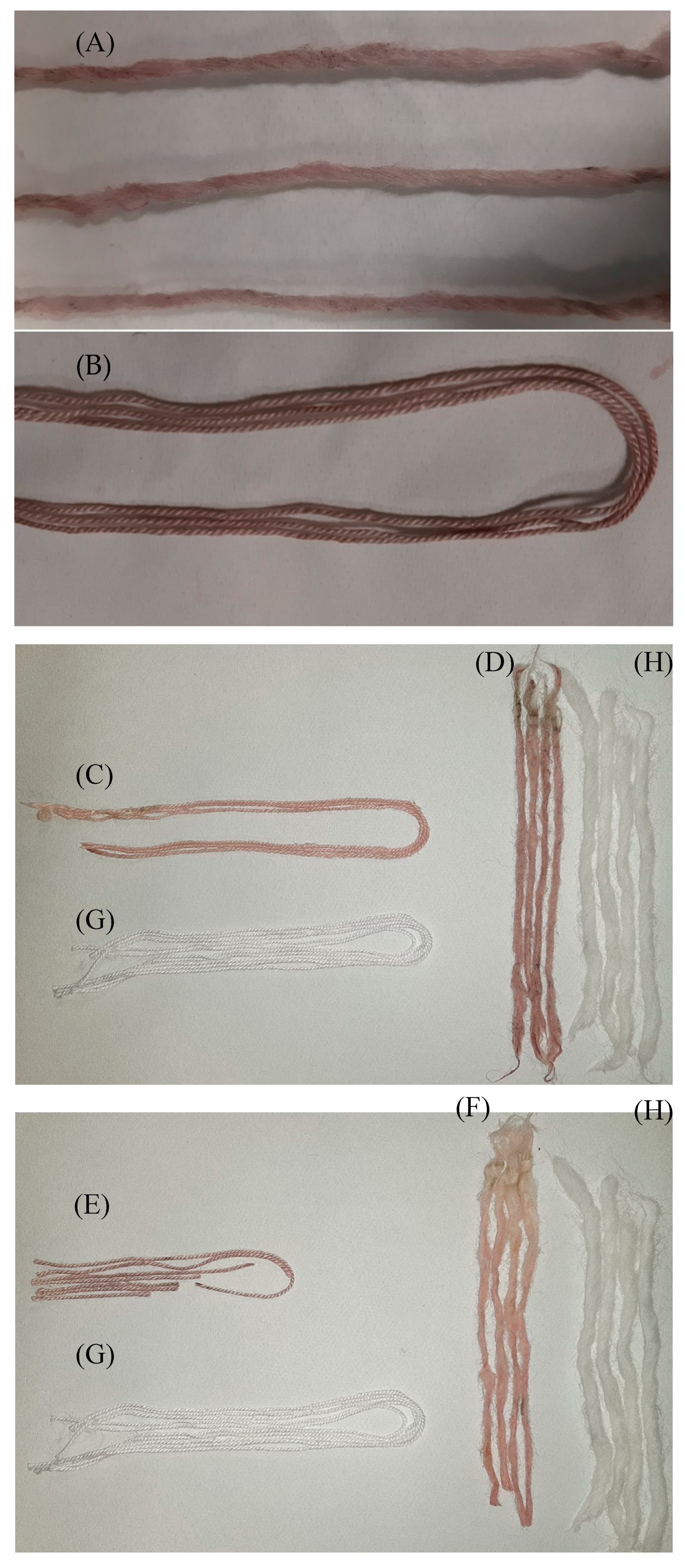
Series of pictures of the dyed fibers with and without mordanting. (A) The cotton and (B) wool without mordanting; (C) the cotton and (D) wool mordanting with lemon juice; (E) the cotton and (F) wool mordanting with aluminum potassium sulphate. The controls appear and are represented with letter (G) and (H) for the cotton and wool fibers, respectively.
Therefore, the color measurements were analyzed considering the CIE L* a* b* or CIELAB color system (CIE 1986) [27], widely endorsed by both the scientific community and industry [28,29,30,31]. The CIELAB color system organized the perception of the three-dimensional color space (which appears to be approximately uniform in color) represented in rectangular coordinates: L*, a*, b*. The L* axis is associated with the lightness of the color going from value 100, corresponding to white, to value 0, corresponding to black, while the axes a* and b* are associated with the changes of red–green (where when a* is positive it corresponds to red and when a* is negative it corresponds to green) and yellow–blue (where when b* is positive it corresponds to yellow and when b* is negative it corresponds to blue). These reading parameters allow to easily calculate, through the use of tabulated equations, the attributes of the perceived coloring: intensity and tonality. These attributes are calculated as follows:
defined in CIELAB 1976 (CIELAB) like ‘chroma’ and:
defined in CIELAB 1976 (CIELAB) like ‘hue angle’
C*ab = [a*2 + b*2]1/2
hab = arctan (b* / a*)
Where the ‘Chroma’ (C*ab) corresponds to the color saturation and the ‘hue angle’ (hab) corresponds to the angle or tone of the color tone, in accordance with Boonsong et al., 2012, Wyszecki and Stiles, 1968 works.
Color differences were calculated in the same way, partial (ΔL*, Δa* and Δb*), ‘chroma’ (ΔC*ab), ‘hue angle’ (Δhab) and the total (ΔE*ab), between the tissue samples. As far as the hue angle is concerned, if the line joining the two colors crosses the positive axis a*, the value of Δhab must be corrected by adding or subtracting 360° to bring it into the range ±180°.
The difference in color between the two-color stimuli is calculated as the Euclidean distance between the points representing them in space, as shown in CIELAB 1976, for the evaluation of the parameter ΔE*ab, according to the equation:
ΔE*ab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2
The compared samples were the colored samples with the non-colored ones and the colored samples with and without the use of mordants. The samples compared were the colored samples with the non-colored samples and the colored samples with and without the use of stain. Between ten and fifteen measurements were made, carried out in random positions of the textile fibers. On the basis of these repeated measurements, the minimum number of measurements necessary to quantify the color of the colored textile material under examination was established, taking into account the cumulative averages of the coordinates of the CIELAB color system as also defined according to work by Prieto et al., 2010 [32].
The washing and light fastness properties of the colored samples were evaluated according to the ISO Test (ISO105C06) and the methods (ISO105BO2) reported, in the works of Erdem Işmal et al., 2014 and in Haddar et al., 2014, respectively [33,34].
3. Results and Discussions
With a view to the circular economy, the dyes obtained from natural sources and specifically from waste matrices deriving from the agro-food industry are increasingly at the center of research for their ecological value and non-toxicity. For example, in 2018, the global market for dyes from natural sources was estimated to be around 2.3 billion for natural food dyes alone, for their application in beverages. This shows the economic importance of these biomolecules that lies in modern bioeconomies.
In this context, the Opuntia ficus-indica (L.) Miller and in particular its waste identified in its peel, was selected for the extraction of the dyes [12].
It is possible to identify within the literature of the studies carried out on the use of dyes extracted from other vegetable waste matrices, such as the study on pomegranate peel (Punica granatum L.) [35], the use of different dye extraction and fixing methods.
Different parameters of the extracts obtained with the different extraction techniques were measured [36]. The values of pH, specific gravities and dry residue were collected for all extracts at 24 h. The values are shown in Table 1.

Table 1.
pH, specific gravity and dry residue of the different Opuntia ficus-indica (L.) Miller fruit extracts at 24 h.
An important result (in degrees Brix) was obtained in the evaluation of the soluble solid, that within the extracts, the specific gravity was very low in relation to the other components present [37].
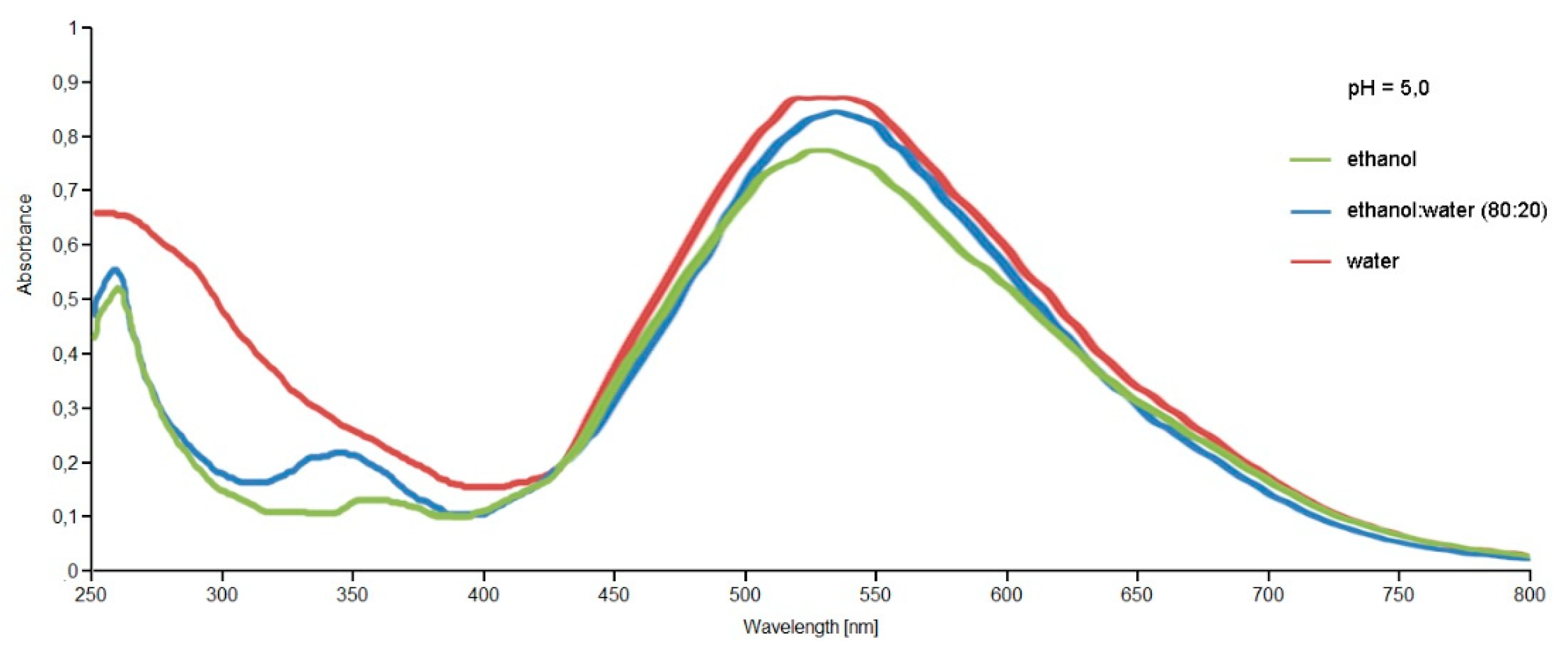
The extracts were primarily analyzed by UV-VIS spectrophotometry to evaluate the extraction of the pigments present within the extracts obtained with different extraction techniques and different solvents. The spectra obtained showed the presence of coloring molecules belonging to the betalain family. By comparing the spectra obtained for the extracts obtained through conventional maceration and the Naviglio method (data not shown for brevity), they showed that the extracts obtained under the same conditions (solvent, time, pH, ratio sample:solvent) with the Naviglio method had a higher content of coloring molecules compared to those obtained by maceration. Thus, it was concluded that the use of the Naviglio method allowed to obtain richer extracts in less time, as reported in the literature for other applications [38,39,40]. Figure 5 shows the spectra obtained from the analysis of the extracts of Opuntia ficus-indica (L.) Miller with the Naviglio method, which shows a better content of coloring molecules, even if only slightly, for the extract obtained with water.
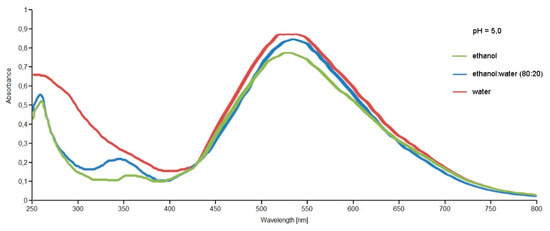
Figure 5.
UV-VIS spectra, between 250–800 nm, of the Opuntia ficus-indica (L.) Miller extracts obtained with the Extractor Naviglio® in different solvent conditions and at the same pH value.
The extracts were dried with a rotavapor and dissolved in bidistilled and deionized water to study their stability according to some parameters.
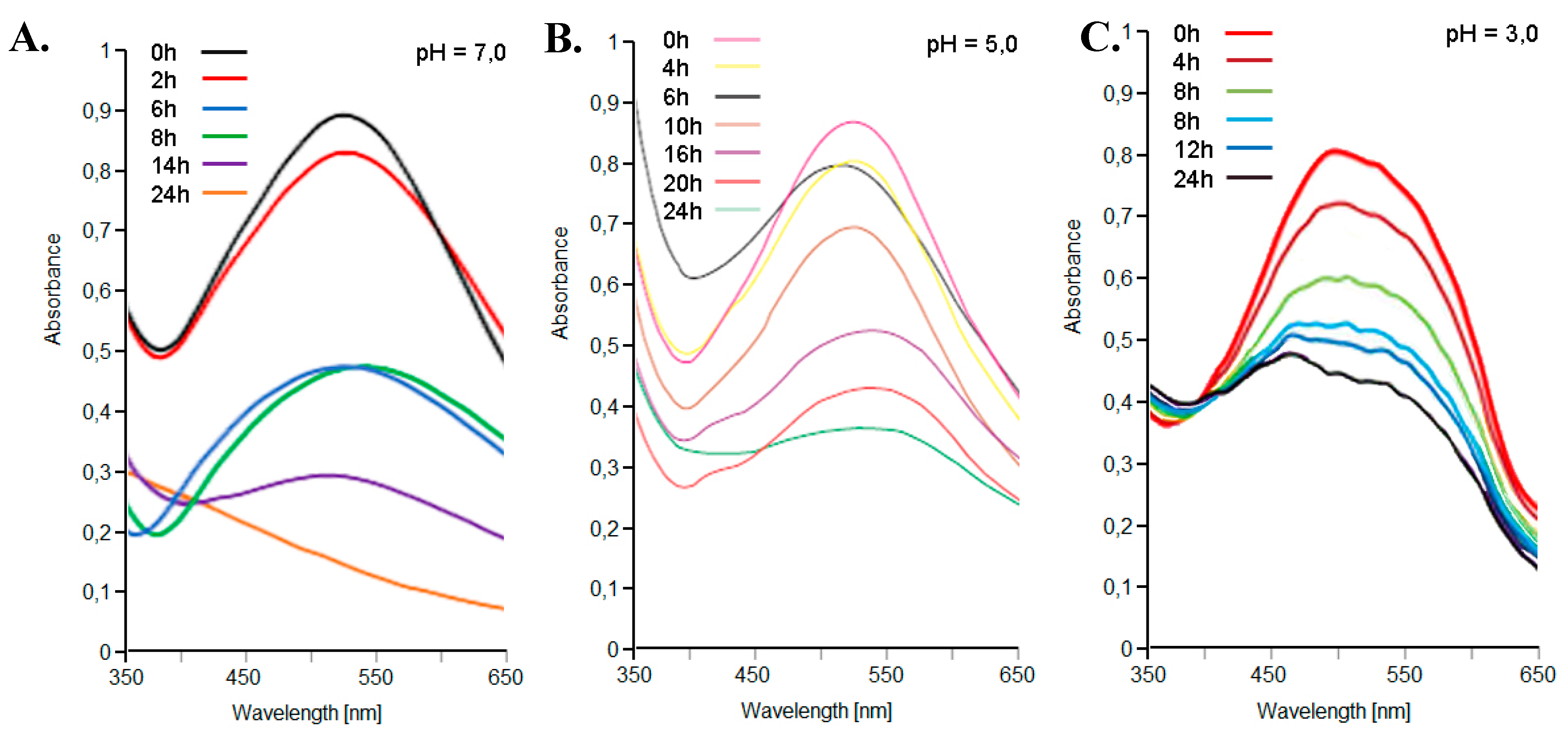
The storage stability of the coloring extracts was studied. Storage stability was studied at different pH values (3 < pH < 7) and at 4°C and 25 °C. The pigment extracts showed a very high storage stability, which was at its maximum at pH 5 and 4 °C (Figure 6). A t1/2 of 20 days was found for red-violet peel extract at 25 °C and pH 5.
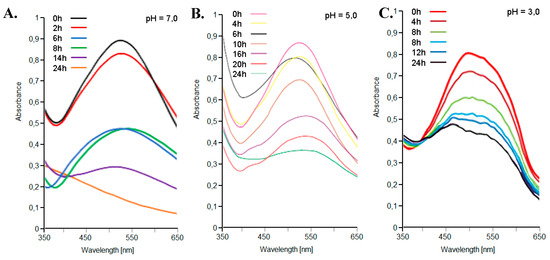
Figure 6.
UV-VIS spectra, between 350–650 nm, of the red-violet peels from the Opuntia ficus-indica (L.) Miller extracts with the Extractor Naviglio® in water solvent, stored at 4 °C and different pH values: 7.0 (A), 5.0 (B) and 3.0 (C).
The visible absorption spectra of the extracts of the red-violet peels from the Opuntia ficus-indica (L.) Miller with the Extractor Naviglio® in water solvent, stored at 4 °C, are shown in Figure 6. Initially, only one peak at 535 nm could be detected, due to the presence of pigment molecules. These compounds were progressively degraded, while absorbance at 535 nm decreased at the same time. At pH 7, a small shoulder was observed with a maximum absorbance close to 410 nm, as a result of the degradation process. At pH 5, a new shoulder in the region between 455–465 nm could be detected, which was more intense in the samples incubated for longer times (more than 22 h). At pH 3, no shoulders were observed, while a peak in the 455–465 nm region was clearly depicted, especially in samples incubated from more than 6 h. The action of air and light, as well known, over time play an important role in the degradation of the pigment molecules of natural extracts [41].
Commercial betanin diluted with dextrin (which contains a mixture of betanin and isobetanin) purchased from Sigma-Aldrich represents one of the main betalainic sources commercially used for coloring purposes in North America and Europe.
The comparison between the UV-VIS spectra obtained from the Opuntia ficus-indica (L.) Miller extracts and the commercial betanin solution allowed to identify its presence, as also reported in the literature [42].
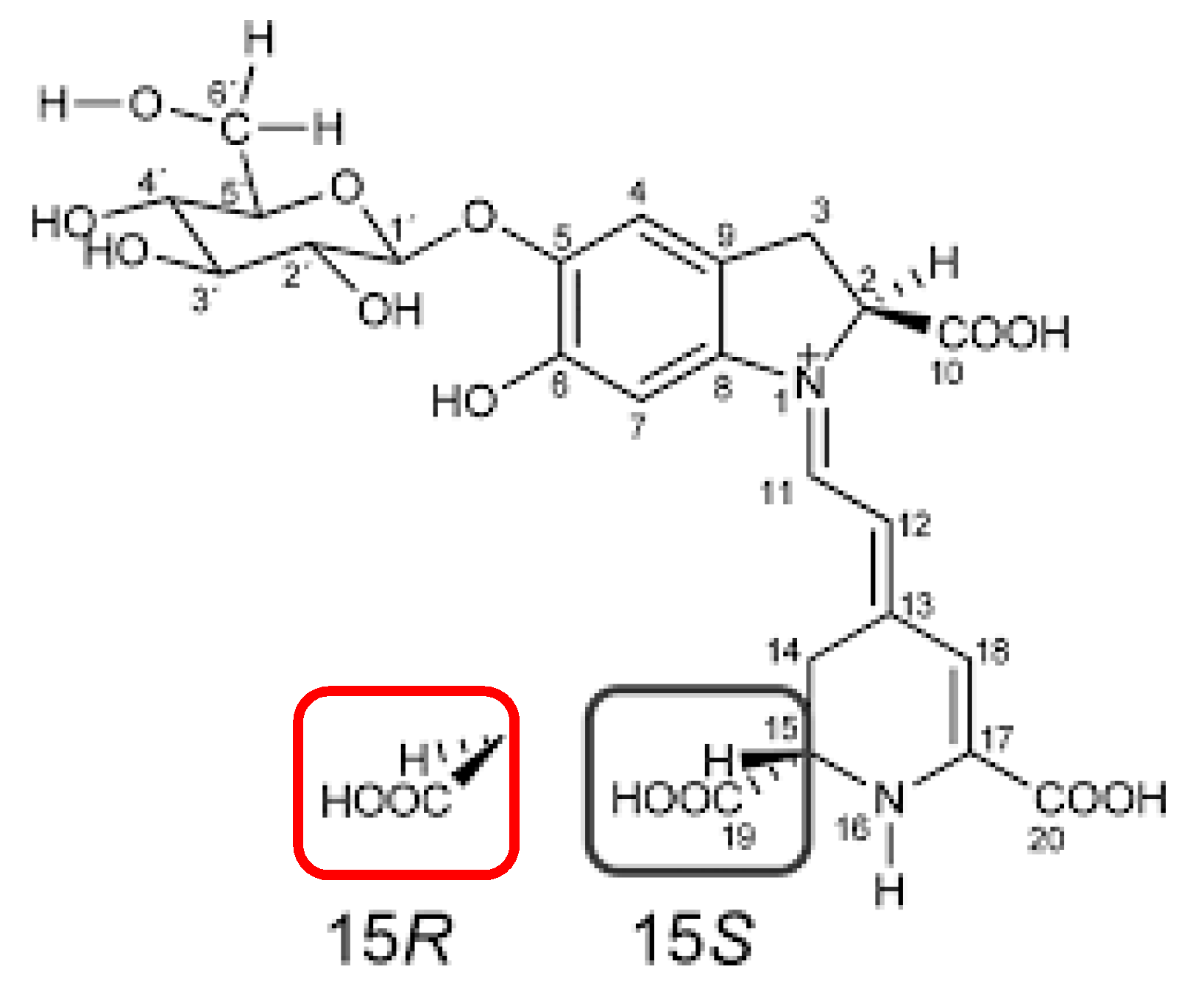
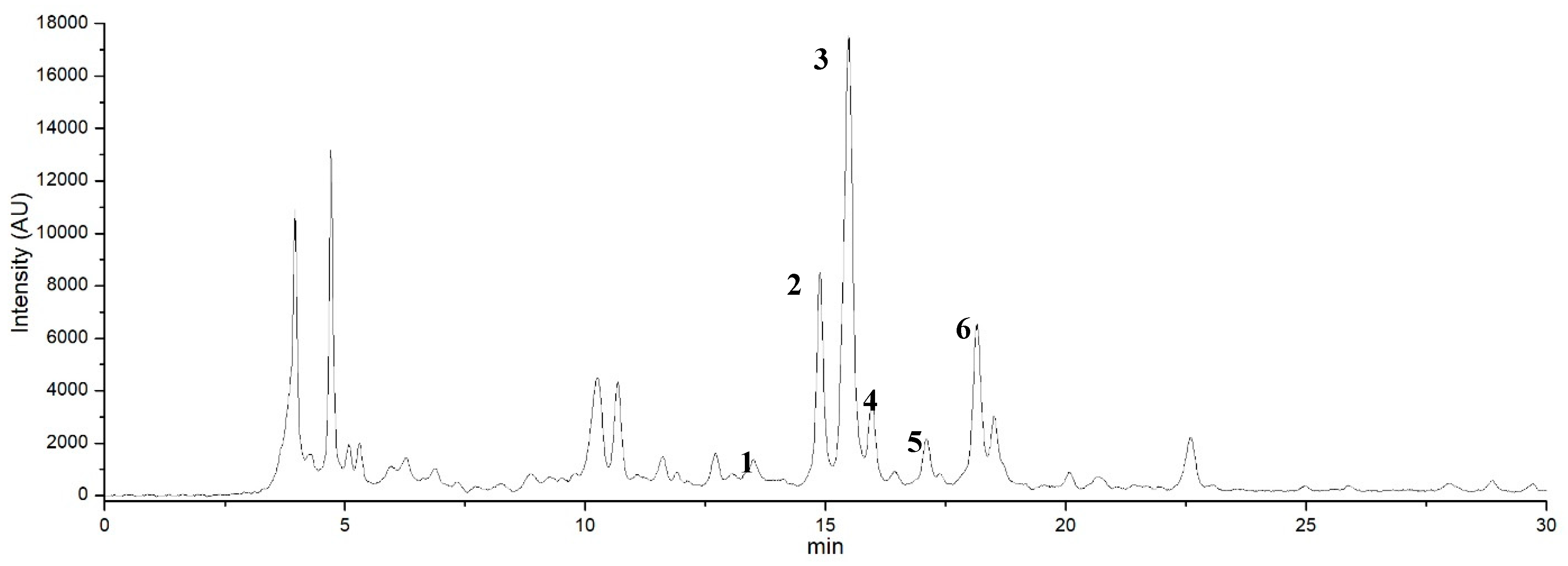
A series of chromatographic analyses was performed to define the presence of the specific molecular components of coloring pigments. The thin layer chromatography (TLC) profiles of the Opuntia ficus-indica (L.) Miller extracts (data not shown) revealed and confirmed the presence of natural dyes in the extract and the data were consistent with those obtained in the absorption spectra (Figure 5), in accordance with the literature [43]. In all the extracts obtained, the presence of betacyanins was detected, which may be responsible for the signals observed in the absorption spectra, which are responsible for the metabolites responsible for staining. The presence of betacyanins such as betanin and isobetanin (Figure 7) was expected as shown in the chromatograms obtained by HPLC (Figure 8). The chromatogram, obtained at 340 nm, showed the presence of other compounds of the flavonoids family. The selected wavelength of 340 nm was a compromise to show the presence of the extracted compounds. Other analyses were performed by HPLC (data not shown) at wavelengths more suitable to only show the presence of the coloring compounds. Under the same conditions it was established that for the sample containing commercial betanin there are only two more important peaks, associated precisely with betanin and isobetanin; it was calculated that betanin has a shorter retention time than isobetanin (ΔtR = 0.4 min) in the HPLC analysis, as reported in this work. This difference is explained by the fact that isobetanin has a stronger interaction with the non-polar-stationary phase of the chromatographic column, as also shown in the literature [44,45].
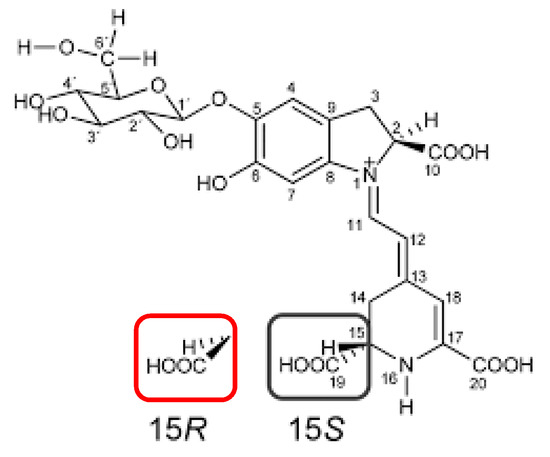
Figure 7.
Chemical structures of betanin and isobetanin. The two structures differ in the spatial arrangement of the groups in position 15: betanin 15S (black), isobetanin 15R (red).
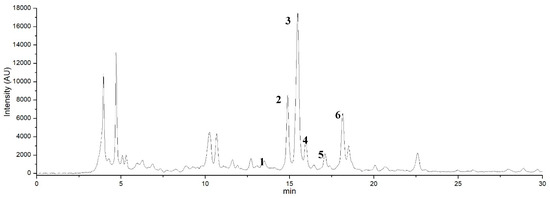
Figure 8.
HPLC chromatogram detected at 340 nm the molecules contained in the aqueous extract of the peel of the fruits of Opuntia ficus-indica (L.) Miller obtained by the Extractor Naviglio®. Peaks 2 and 3 were identified as pigment molecules such as betanin and isobetanin. Peaks 1, 4, 5 and 6 were identified as luteolin, kaempferol, quercetin and isorhamnetin according to the standards available in the laboratory; the other peaks were unknown compounds.
Cotton and wool fibers colored with the extracts of Opuntia ficus-indica (L.) Miller were subjected to colorimetric measurement and the color measurement parameters were recorded, L*, a*, b*, C*ab and hab, according to the CIELAB color system.
It was verified that the minimum number of measurements required to characterize these parameters in each sample of colored textile material was in line according to what has already been shown in the literature [46], in which this minimum parameter was defined by the fixed section of the inverted exponential decay graph, obtained by CIELAB color coordinates (L*, a* and b*) with respect to the number of measurements. Eight measurements were made to characterize an area between 4.00 and 7.50 cm2 approximately, with an opening diameter of 4 mm.
The color of each tissue sample stained with the Opuntia ficus-indica (L.) Miller extract was characterized by making the eight measurements in random positions on three replicates of each type of sample, following the conditions described in Section 2.11. The values of the CIELAB color coordinates (L*, a*, b*, C*ab and hab) for the colored fibers treated in this study are shown in Table 2.

Table 2.
Colorimetric data and the CIELAB color coordinate values (CIELAB units) of the cotton plant fibers and the wool animal fibers colored using the extracts of the peel of the fruits of Opuntia ficus-indica (L.) Miller.
The L* parameter (color lightness) varied between 49.39 and 40.10 CIELAB units for colored cotton fiber samples and between 38.73 and 50.14 for colored wool fiber samples, having 99.11 and 92.46 CIELAB units, as the control L* value for the cotton and wool fibers, respectively (identified as non-colored fabric, U), which indicates a clearly perceptible change in the color of the fibers due to dyeing, or ΔL* > 40 CIELAB units, indicating a significant color difference. The value of a* (associated with the change from greenness (−) to redness (+)) varies in a data range from −0.54 (U) to 2.59 (DWM) and 25.82 (DPA), for cotton fiber and in a data range from −1.79 (U) to 7.49 (DWM) and 27.47 (DPA), for wool fiber: this numerical identification means that the fabric was given a reddish color. The variation in b* (associated with the variation in the range from blueness (−) to yellowness (+)) occurs in a data range from 1.75 (U) to 5.18 (DWM) and 17.40 (DPA), for cotton fiber and in a data range from 8.37 (U) to 12.04 (DPA) and 13.74 (DPL), for wool fiber: an increase ranging, for cotton fibers, between 3–15 units and 4–5 units for wool fibers. The C*ab (color saturation) parameter varies in a data range from 1.86 (U) to 5.88 (DWM) and 31.14 (DPA), for cotton fiber and in a data range from 8.56 (U) to 15.48 (DWM) and 30.74 (DPL), for wool fiber; in both cases there was an increase in the value between 4–30 CIELAB units, which indicates a more marked color saturation.
The hue angle, hab, for fibers colored with and without mordants, fell within the range 25.53° (DPL) and 65.45° (DWM), therefore, they were in the area of the red, red-pink hue.
The extraction of the coloring pigment molecules was compared with the maceration and the Naviglio extraction methods. The Naviglio method proved, as expected, to be a more effective extraction method that acts in a shorter time.
The use of two mordants, rock alum and lemon juice, was compared, together with the natural extract from the skins of Opuntia ficus-indica (L.) Miller. Rock alum is widely used by industries that apply dyes to fabrics and fibers, while the use of lemon juice has been traced within testimonies provided by rural populations who in the past practiced the coloring of skins and fabrics with natural methods.
It was therefore observed that the color components isolated from Opuntia ficus-indica (L.) Miller led to a specific color of the type of fruit used. The two mordants used influenced the depth of the shades, as the literature suggests [47,48,49]. The mordants allowed for the fixing of the dye obtained from the extracts of Opuntia ficus-indica (L.) Miller to the vegetable cotton fibers and the animal wool fibers. This was certainly due to the formation of a chemical bridge between the dye and the fibers [50,51].
Stain-free dyed fabrics (DWM) had lighter shades, i.e., higher L* values, with the exception of the lemon juice stained wool fiber (DPL).
The hab value represents the main attribute of color perception, therefore these results led us to conclude that the use of mordants had no significant alteration effects on the color values. As can be seen in Table 3, the differences in the absorption of the dye, between the cotton and the wool, is higher for cotton, which seems to be a good support for the dye obtained from Opuntia ficus-indica (L.) Miller.

Table 3.
Partial color differences (ΔL*, Δa* and Δb*), the ‘chroma’ differences (ΔC*ab), the ‘hue angle’ differences (Δhab) and the total color difference (ΔE*ab) between the dyed fabrics.
An evaluation of the resistance of the coloring to washing and exposure to light was performed [52,53]. The washing and light fastness values with a brief description of the fastness evaluation are shown in Table 4. The dye of the extract of Opuntia ficus-indica (L.) Miller itself, without the use of stains (DWM), produced properties of washing fastness equivalent but not superior (6–7) to stained fabrics. Its light fastness (7) was lower than that of most samples.

Table 4.
Washing and light fastness values for the colored fibers.
Considering these results, we can indicate that the fastness properties were not significantly influenced by the use of mordants and therefore, the use of the natural pigment without mordants can be a good solution and alternative to the coloring treatment.
4. Conclusions
This work highlights the possibility of using waste matrices such as those of prickly pear peels which within the agro-food chain, represent an important cost for companies in terms of disposal. Therefore, using such scraps or more ‘secondary raw materials’ to obtain natural dyes, but not only, can represent a step towards reducing the important costs for companies and increasingly directing them towards a circular economy. The use of the Naviglio extractor, an eco-innovative equipment, would allow various companies working in the agro-food sector to obtain molecules with a high intrinsic value from the ‘secondary raw materials’ (waste of by-products) to be used in various sectors, such as that of natural dyes.
The compounds contained within the fractions obtained from the liquid-liquid extractions could have an important role. Their recovery, also linked to the extractive method used, is of fundamental interest in the qualitative and quantitative evaluation that can be done. These bioactive compounds are a valid choice for use in the nutraceutical field and they could be an important step for innovation within the agro-food chain and an added value for companies willing to innovate their systems and productions; ultimately, this process can also be considered environmentally friendly.
In recent years, natural dyes have become an interesting alternative to synthetic dyes, all thanks to their best properties. They are incredibly more healthy for humans and the environment. For this reason, they fall into the category of important molecules with a view to a circular and zero-impact economy, since they are obtained from processing waste. With this study, we want to open a line of analysis on other endemic and non-endemic plants, present within the Campania landscape. The fibers dyed with the Opuntia ficus-indica (L.) Miller extract show a good receptivity to absorb the color both with the aid of the mordants and without.
In fibers dyed with the Opuntia ficus-indica (L.) Miller extract, the mordant influenced the depth of the shades, although it did not have significant effects on the color values. The fastness properties were not significantly affected by the use of mordants. All dyed fibers had a similar color, with the hue angle values indicating a pinkish color.
The use of a natural mordant such as lemon juice, widely used in popular tradition, shows excellent etching and solidity characteristics for the color extracted from Opuntia ficus-indica (L.) Miller.
Author Contributions
Conceptualization, C.G. and D.N.; methodology, R.S.; investigation, P.S.; data curation, A.P., M.T. and A.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Adella, L.; Mariotta, C. Rapporto Rifiuti Speciali; ISPRA-Settore Editoria: Roma, Italy, 2014; ISBN 978-88-448-0643-9. [Google Scholar]
- Vulcano, G.; Ciccarese, L. Spreco Alimentare: Un Approccio Sistematico per la Prevenzione e la Riduzione strutturali; ISPRA: Roma, Italy, 2018; ISBN 978-88-448-0882-2. [Google Scholar]
- Ali, M.; Watson, I.A. Comparison of oil extraction methods, energy analysis and biodiesel production from flax seeds. Int. J. Energy Res. 2014, 38, 614–625. [Google Scholar] [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Almela, L. Application of high-performance liquid chromatography to the characterization of the betalain pigments in prickly pear fruits. J. Chromatogr. A 2001, 913, 415–420. [Google Scholar] [CrossRef]
- Méndez, L.P.; Flores, F.T.; Martín, J.D.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Physicochemical characterization of cactus pads from Opuntia dillenii and Opuntia ficus indica. Food Chem. 2015, 188, 393–398. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, C.; Estévez, A.M.; Sepúlveda, E.; Mecklenburg, P. Cactus pear fruit: A new source for a natural sweetener. Plant Foods Hum. Nutr. 1998, 52, 141–149. [Google Scholar] [CrossRef]
- Lamia, I.; Zouhir, C.; Youcef, A. Characterization and transformation of the Opuntia ficus indica fruits. J. Food Meas. Charact. 2018, 12, 2349–2357. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Stintzing, F.; Carle, R. Betalains in food: Occurrence, stability, and postharvest modification. In Food Colorants-Chemical and Functional Properties; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Dumbravǎ, A.; Enache, I.; Oprea, C.I.; Georgescu, A.; Gîrţu, M.A. Toward a more efficient utilisation of betalains as pigments for Dye-Sensitized solar cells. Dig. J. Nanomater. Biostruct. 2012, 7, 339–351. [Google Scholar]
- Mannai, F.; Ammar, M.; Yanez, J.G.; Elaloui, E.; Moussaoui, Y. Cellulose fiber from Tunisian Barbary Fig “Opuntia ficus-indica” for papermaking. Cellulose 2016, 23, 2061–2072. [Google Scholar] [CrossRef]
- Souza, C.M.P.; Almeida, F.S.; Junior, V.F.V.; De Lima, B.P.G. Characterization of atomized extract of Opuntia ficus- indica (L.) Mill. and assessment of its pharmaceutical potential. J. Basic Appl. Pharm. Sci. 2014, 35, 195–203. [Google Scholar]
- Zhao, M.; Yang, N.; Yang, B.; Jiang, Y.; Zhang, G. Structural characterization of water-soluble polysaccharides from Opuntia monacantha cladodes in relation to their anti-glycated activities. Food Chem. 2007, 105, 1480–1486. [Google Scholar] [CrossRef]
- Abou-Elella, F.M.; Ali, R.F. Antioxidant and anticancer activities of different constituents extracted from egyptian prickly pear cactus (Opuntia ficus-indica) peel. Biochem. Anal. Biochem. 2014, 3, 1. [Google Scholar] [CrossRef]
- Guesmi, A.; Ben Hamadi, N.; Ladhari, N.; Sakli, F. Dyeing properties and colour fastness of wool dyed with indicaxanthin natural dye. Ind. Crops Prod. 2012, 37, 493–499. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia spp. as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef]
- Paliwal, H.; Goyal, S.; Singla, S.; Daksh, S. Pigments from natural sources: An overview. Int. J. Res. Pharm. Pharm. Sci. Issue 2016, 1, 1–12. [Google Scholar]
- Naviglio, D. Naviglio’s principle and presentation of an innovative solid-liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y.; Kasheva, T.N.; Villa, A.E.P. Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices. J. Chem. Inf. Comput. Sci. 2001, 41, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Chan-Bacab, M.J.; Sanmartín, P.; Camacho-Chab, J.C.; Palomo-Ascanio, K.B.; Huitz-Quimé, H.E.; Ortega-Morales, B.O. Characterization and dyeing potential of colorant-bearing plants of the Mayan area in Yucatan Peninsula, Mexico. J. Clean. Prod. 2015, 91, 191–200. [Google Scholar] [CrossRef]
- Alessi, P.J.; Carter, E.C.; Fairchild, M.D.; Hunt, R.W.; McCamy, C.S.; Kranicz, B.; Moore, J.R.; Morren, L.; Nobbs, J.H.; Ohno, Y.; et al. CIE 15:200-Technical Report-Colorimetry; Internation Commission on Illumination: Vienna, Austria, 2004. [Google Scholar]
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Völz, H.G. Industrial Color Testing, 2nd ed.; Wileye-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Wyszecki, G.; Stiles, W.S. Colour Science: Concepts and Methods, Quantitative Data and Formulas, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1968. [Google Scholar]
- Boonsong, P.; Laohakunjit, N.; Kerdchoechuen, O. Natural pigments from six species of Thai plants extracted by water for hair dyeing product application. J. Clean. Prod. 2012, 37, 93–106. [Google Scholar] [CrossRef]
- Prieto, B.; Sanmartín, P.; Silva, B.; Martínez-Verdú, F. Measuring the color of granite rocks: A proposed procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Erdem Işmal, Ö.; Yildirim, L.; Özdoǧan, E. Use of almond shell extracts plus biomordants as effective textile dye. J. Clean. Prod. 2014, 70, 61–67. [Google Scholar] [CrossRef]
- Haddar, W.; Ben Ticha, M.; Guesmi, A.; Khoffi, F.; Durand, B. A novel approach for a natural dyeing process of cotton fabric with Hibiscus mutabilis (Gulzuba): Process development and optimization using statistical analysis. J. Clean. Prod. 2014, 68, 114–120. [Google Scholar] [CrossRef]
- Sinha, K.; Das Saha, P.; Datta, S. Response surface optimization and artificial neural network modeling of microwave assisted natural dye extraction from pomegranate rind. Ind. Crops Prod. 2012, 37, 408–414. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Naviglio, D.; Pizzolongo, F.; Romano, R.; Ferrara, L.; Naviglio, B.; Santini, A. An innovative solid-liquid extraction technology: Use of the naviglio extractor for the production of lemon liquor. Afr. J. Food Sci. 2007, 1, 42–50. [Google Scholar]
- Naviglio, D.; Franchi, G.G.; Rossi, I.; Fiore, G.; Massarelli, P.; Nencini, C.; Santini, A. Preparation of an elixir from common juniper (Juniperus communis L.) berries: The new Naviglio Extractor versus the traditional maceration technique. Food Manuf. Effic. 2009, 2, 41–47. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Ciaravolo, M.; Cirino, P.; Toscano, A.; Salvatore, F.; Gallo, M.; Naviglio, D.; Andolfi, A. Fatty acids from paracentrotus lividus sea urchin shells obtained via rapid solid liquid dynamic extraction (Rslde). Separations 2019, 6, 50. [Google Scholar] [CrossRef]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Gonalves, L.C.P.; Trassi, M.A.D.S.; Lopes, N.B.; Dörr, F.A.; Dos Santos, M.T.; Baader, W.J.; Oliveira, V.X.; Bastos, E.L. A comparative study of the purification of betanin. Food Chem. 2012, 131, 231–238. [Google Scholar] [CrossRef]
- Bilyk, A. Thin-layer chromatographic separation of beet pigments. J. Food Sci. 1981, 46, 298–299. [Google Scholar] [CrossRef]
- Schwartz, S.J.; von Elbe, J.H. Quantitative determination of individual betacyanin pigments by high-performance liquid chromatography. J. Agric. Food Chem. 1980, 28, 540–543. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 3483–3487. [Google Scholar] [CrossRef] [PubMed]
- Prieto, B.; Ferrer, P.; Sanmartín, P.; Cárdenes, V.; Silva, B. Color characterization of roofing slates from the Iberian Peninsula for restoration purposes. J. Cult. Herit. 2011, 12, 420–430. [Google Scholar] [CrossRef]
- Singh, S.V.; Purohit, M.C.; Pauri, C.; Garhwal, P. Applications of eco-friendly natural dye on wool fibers using combination of natural and chemical mordants abstract: 1.2 recent trends of natural dyeing: 2.0 materials and methods: 1.1 status of natural dyes and dye-yielding plants in India. Univers. J. Environ. Res. Technol. 2012, 2, 48–55. [Google Scholar]
- Sanjeeda, I.; Taiyaba, A.N. Natural dyes: Their sources and ecofriendly use as textile materials. J. Environ. Res. Dev. 2014, 8, 683–688. [Google Scholar]
- Zubairu, A.; Mshelia, Y.M. Effects of selected mordants on the application of natural dye from onion skin (Allium cepa). Sci. Technol. 2015, 5, 26–32. [Google Scholar]
- Meksi, N.; Haddar, W.; Hammami, S.; Mhenni, M.F. Olive mill wastewater: A potential source of natural dyes for textile dyeing. Ind. Crops Prod. 2012, 40, 103–109. [Google Scholar] [CrossRef]
- Khan, A.A.; Iqbal, N.; Adeel, S.; Azeem, M.; Batool, F.; Bhatti, I.A. Extraction of natural dye from red calico leaves: Gamma ray assisted improvements in colour strength and fastness properties. Dye. Pigment. 2014, 103, 50–54. [Google Scholar] [CrossRef]
- Fabrics, S.; Mordants, E.; Janani, L.; Winifred, D. Suitability of dyes from mulberry and coffee leaves on silk fabrics using eco-friendly mordants. Int. J. Sci. Res. Publ. 2013, 3, 1–4. [Google Scholar]
- Singh, S.V.; Purohit, M.C. Evaluation of colour fastness properties of natural dye extracted from Symplocos racemosa (Lodh) on wool fibres using combination of natural and synthetic mordants. Indian J. Fibre Text. Res. 2014, 39, 97–101. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).