Abstract
Agricultural use of precipitated calcium carbonate (PCC), a byproduct of sugar clarification process, as a possible source of nutrients and pest and disease control in sugar beet (Beta vulgaris subsp. vulgaris.) needs a careful examination of the risk and benefit assessment at various levels of management and production. A series of controlled environment studies were conducted in Scottsbluff, NE, to assess the 1) effect of PCC on root aphids in sugar beet, and 2) risk of the weed kochia spreading by applying PCC to agricultural land and its chemical control strategy, by conducting various dose-response studies. A replicated lab study was conducted twice to determine the effect of PCC on root aphid in sugar beet using three rates of PCC (9, 18, and 27 Mg ha−1) and a control. The results showed that root aphid populations in all PCC-amended treatments were significantly reduced when compared to the control (p < 0.05). Two cycles of dose-response studies using the herbicides Roundup and Clarity at 6 concentrations revealed that kochia biotypes grown on PCC piles at three western sugar production locations were effectively controlled (LD50) with the current rate recommendations administered in this region for both herbicides. More field experiments are needed to confirm the results of these controlled environment studies.
1. Introduction
Sugar beet (Beta vulgaris subsp. vulgaris) is a feedstock root crop for sugar (i.e., sucrose) production that is grown in close proximity to sugar factories. Sugar beet had a production value of about $1.31 billion dollars in the US in the 2015/2016 crop year [1]. The sugar beet crop is primarily grown in five ecological regions of the US; two of the regions are east of the Mississippi River, while the three other areas are in the Great Plains and Far West [2,3,4]. Along with this geographic diversity, sugar beet is often produced in annual rotation with other crops such as soybean, field bean, and corn [2,5]. Therefore, the production practices, abiotic, and biotic conditions under which it is produced vary accordingly.
Precipitated calcium carbonate (PCC) (also known as spent lime) is a byproduct of the sugar clarification process when sugar beet is processed [6]. Calcium oxide and carbon dioxide are injected into extracted juice to form calcium carbonate, precipitating impurities to produce the thin juice from which sugar is extracted. In this process, removal of impurities including organic molecules plus inorganic forms of phosphorus (P), magnesium (Mg), and calcium (Ca) with limited removal of potassium (K), sodium (Na), and nitrates occur [7]. Western sugar factories at Fort Morgan, CO, Scottsbluff, NE, and Torrington, WY alone produce approximately 100,000 tons of PCC per year (Jerry Darnell, Western Sugar Cooperative, personal communication). Historically, PCC has been stockpiled over time near the factory site [2,6,8] and growing PCC piles can be a management problem as they allow the growth of weeds, produce dust during wind storms, and continue to require additional land for storage [2,5]. This is a concern with current EPA regulations on the particulate matter as part of the National Ambient Air Quality Standards (https://www.epa.gov/pm-pollution/table-historical-particulate-matter-pm-national-ambient-air-quality-standards-naaqs).
PCC has been effectively used in production agriculture as a liming source in lieu of agricultural lime. In the Red River Valley (RRV) and southern MN, it has generally had a beneficial effect on growth and yield [2,3,9,10]. In MI as well, PCC significantly increased sugar beet yield [11]. A large field study in MI [5] including sugar beet, soybean (Glycine max), corn (Zea mays), field (dry) bean (Phaseolus vulgaris), and wheat (Triticum aestivum) showed limited effects of PCC on yield up to 5.6 Mg ha−1. These findings were in agreement with a recent greenhouse study conducted in western NE [6], where the authors reported that PCC had no negative effect on the early growth of sugar beet, corn, or dry bean. They suggested that the application of PCC to neutral or slightly alkali soils did not affect the soil chemical characteristics. They also concluded that PCC can be a significant source of several plant nutrients such as N, P, S, and Fe if applied in large quantities. PCC has also been shown to reduce Aphanomyces root rot severity significantly [2,3,12]. Research in CA showed a significant effect of PCC in controlling cabbage clubroot fungus Plasmodiophora brassicae [13]. Similar research on field pea (Pisum sativum) in ND showed fusarium root rot disease control by reducing conidia production, conidia germination, and mycelial growth from the PCC treatment [14].
The sugar beet root aphid, Pemphigus betae Doane (Hemiptera: Aphididae), is a pest of sugar beet throughout the U.S. and Canada [15,16]. Although economically important infestations are sporadic in the northeastern Great Plains [16,17], high populations are common in the Central High Plains [18]. This aphid is thought to be heteroecious with a holocyclic life cycle [19]. However, anholocyclic apterae have been reported to overwinter in MN [16,17]. Its primary hosts are trees in the genus Populus on which it deposits eggs in the fall. These develop into nymphs, which develop colony-filled, petiole cysts in the spring [15,16,19,20].
Pemphigus betae is not known to cause damage on its primary host, Populus spp. [15]. However, following dispersal from the Populus spp. in the spring, this aphid can settle and feed on the roots of a number of Chenopodaceae—including sugar beet and common lambsquarters (Chenopodium album) [19,21]. Lambsquarters is an important weed in sugar beet fields and also acts as an alternate secondary host for P. betae. The root-feeding forms of these aphids secrete a waxy material that is useful in rating the severity of beet-root infestation [17]. This waxy material plus the aphids feeding activity can result in economic losses in beet tonnage, sugar, and quality [15,16,17,19]. Even moderate infestations can result in sugar losses of 30% [18].
Another production aspect that can affect the utilization of PCC is kochia (Kochia scoparia). Kochia is a predominant invasive C4 broadleaf weed that grows on piles of PCC at all western sugar factory sites (Figure 1).

Figure 1.
Kochia weed grown on precipitated calcium carbonate piles at sugar beet processing factory site in Scottsbluff, NE in 2011.
In fact, kochia is the only weed that survives on PCC piles (Figure 1), although the exact reason is unknown. Kochia is native to Eurasia and was introduced into the U.S. as an ornamental plant [22]. Kochia is highly competitive with field crops. For example, kochia produces a large amount of seed (approximately 30,000 seeds/plant) and has been reported to be one of the fast-spreading species in the northwestern U.S. [23]. Kochia is known to disperse seed for long distances through prevailing winds. It has a short life cycle (usually 1 to 2 years), with a brief dormancy pause in winter [24,25]. Kochia, being a C4 crop, possesses high photosynthetic efficiency, tolerance to extreme abiotic stresses, and allelopathic tendency to neighboring crops [22].
Chemical control of kochia has proven to be the most effective practice in the agricultural production systems [26]. However, in the past few years, kochia has been reported to show significant resistance to different classes of herbicides with various modes of action [27,28,29]. Herbicide resistance was largely attributed to mono-cropping and repeated use of the same herbicides for several years [30].
Future utilization of PCC as a potential multi-purpose agronomic material in western sugar producing regions has a limitation of possibly spreading kochia seed (Figure 1) through large amounts of PCC applications. Therefore, a chemical control strategy is needed for kochia before or after applying the PCC. With the prevailing problem of herbicide-resistant kochia in different parts of the western US, it is necessary to test if the kochia growing on PCC piles is herbicide-resistant.
Various components of PCC utilization plan are interconnected and need considerable research in these areas for effectively utilizing this byproduct in agriculture. Thus, the objectives of the study were: (1) To evaluate the effect of PCC on sugar beet root aphids in a laboratory experiment and (2) to evaluate the dose-response of kochia collected from PCC piles at three western sugar factory sites using six rates of Roundup (N-(phosphonomethyl) glycine) and Clarity, (diglycolamine salt of 3,6-dichloro-o-anisic acid) herbicides in order to estimate the current level of herbicide resistance to these herbicides under a controlled environment.
2. Materials and Methods
2.1. Root Aphid Study
A soil mixture of 50% Tripp very fine silty loam (coarse-silty, mixed, superactive, mesic Aridic Haplustolls) and 50% peat moss was used for this study. PCC was collected from the Western Sugar factory at Scottsbluff, NE. Four different rates of PCC (0, 9, 18, 27 Mg/ha) were blended with the soil mixture for tests. These rates were approximately similar to the rates tested in the preliminary study [6]. The chemical composition of PCC was determined by sending a sample to a commercial soil testing laboratory (Ward Laboratories, Kearney, NE). A historical sugar beet root aphid-susceptible variety (HM3035RZ) was used. Ten replications of each lime rate were planted into 8.8 cm plastic pots. Pots were over-planted and then thinned to a single sugar beet plant. The temperatures were maintained at approximately 25 °C. Each pot had 2 earthen root cells established in the root-ball where aphids were allowed to develop. Cells were established by inserting glass tubes (ca. 1.25 cm. diameter) into 2 locations in each pot when sugar beets were 3 weeks old. Glass tubes were removed, leaving 2 holes in the pot and natural corks were placed in the top of each hole to seal the cell. Over a 2-week period, the beets continued to grow, and a significant mass of roots extended into the enclosed cells. At this time, 5 five sugar beet root aphids were placed in each cell (10 aphids per pot), and the corks were immediately replaced to seal the aphids into the cells. Aphids were allowed to multiply in these closed cells for 3 weeks, and then the study was evaluated. This experiment was repeated twice.
Root aphid and root mass were evaluated by a visual rating. Root mass was evaluated by removing corks at aphid evaluation and the roots in the chamber observed and rated according to the following rating system: (0) No roots; (1) 1 to 2 roots filling the cell; (2) About 50% of cell space taken up by roots; (3) No visible empty space in the cell (fully covered roots).
Root aphid evaluation was accomplished by removing the soil/root-ball from the pot and breaking open the ball thus that both of the root cells were visible. The root balls from each sample were placed in Berlese funnels to extract the aphids from the soil, and aphids were counted.
2.2. Herbicide Resistance Study
Kochia seeds were collected in the fall of 2011 from plants growing on PCC piles at sugar factories at Fort Morgan, CO, Scottsbluff, NE, and Torrington, WY. The seed was cleaned by sieving plant debris and air stratification. Kochia seed from each location was kept separate. A peat-moss based potting mix (Sun Gro Horticulture, Agawam, MA, USA) was utilized as a growth medium for kochia. Ten cm. square plastic pots were filled with potting mix. The potting mix was packed firmly into each pot, and 15 kochia seeds were placed on the surface and then moved into the potting mix with fingertips. After planting, pots were moved into a growth chamber (Conviron, Winnepeg, Canada) programmed to provide 14 h of light at 29.4 °C and 10 h of darkness at 23.9 °C. After pots were placed in the growth chamber, they were watered daily. After kochia seeds germinated, seedlings were removed by hand pulling until 3 seedlings remained in each pot. When the average seedling height was 7.5 to 10 cm (approximately 15 days after planting), pots were removed from the growth chamber, and plants were treated with different doses of herbicide.
The experimental design was a randomized complete block with 3 locations; Fort Morgan, Scottsbluff, and Torrington and two herbicides; Roundup and Clarity. Each herbicide was applied at 6 rates and there were 6 replications of each treatment. The experiment was repeated twice. Glyphosate was applied in combination with ammonium sulfate at 7.7 kg per 379 L of spray solution plus nonionic surfactant (NIS) at 0.25% v/v at doses of 0, 0.385, 0.770, 1.541, 3.082, and 6.164 kg a.e. ha−1 (Roundup PowerMAX, Monsanto Company, St. Louis, MO, USA) per hectare. The range of concentrations was selected based on local data to test the level of resistance. Dicamba was applied in combination with NIS at 0.25% v/v at doses of 0, 0.070, 0.140, 0.280, 0.560, and 1.120 kg a.e. (Clarity, BASF Chemical Company, Research Triangle Park, NC, USA) per hectare. Herbicides were applied in a greenhouse spray chamber set to deliver 250 L of water per ha at 193 KPa, using an 8002 E spray nozzle.
After treatment, plants were returned to the growth chamber and allowed to grow for 15 days. During the 30-day growth period, plants were temporarily removed from the growth chamber on 3 occasions and rearranged to address small differences in temperature and light intensity that existed in the growth chamber. Plants were fertilized 10 and 20 days after planting with a 20 (5.4% NH4-N, 5.5% NO3-N, 9.1% Urea-N)-20 (P2O5)-20 (K2O) dry fertilizer (Miller Chemical & Fertizer, LLC., Hanover, PA, USA) that was dissolved in water to supplement 10 kg of N, 10 kg of P2O5, and 10 kg of K2O per ha.
Kochia injury from herbicides was evaluated 7 days after spraying on a scale from 0 to 100 with 0 equal to no injury and 100 was equal to death of the plant. Kochia plants were clipped at the base (soil surface) 15 days after treatment, put in a paper sack, placed in a dryer set at 37.8 °C for 24 h, and the dry weight recorded. LD50 for each herbicide was estimated as the dose required to have 50% visual injury or percent reduction in kochia dry weight.
2.3. Statistical Methods
Data were analyzed using SAS 9.4 software (SAS Institute, Cary, NC, USA) using the Proc GLM procedure. The locations were treated as blocks. Data were combined based on Bartlett’s homogeneity of variance test [P[χ2] 0.4653, alpha = 0.05] for root aphid number and root area from the 2 experiments and means were reported. When the F-test was found to be significant (alpha = 0.05), a mean separation test was conducted using Fisher’s protected LSD. The means for the herbicide rate response study was reported by averaging all 3 seed sampling sites. The experimental runs were not combined due to heterogeneity of variance [P[χ2] 0.0077, alpha = 0.05]. A mean separation test was conducted using Fisher’s protected LSD, when the F-statistic was significant (alpha = 0.05). Additionally, the residual plots were checked for autocorrelation, random pattern, and normality (Shapiro-Wilk) [31].
A regression analysis was conducted in SAS 9.4 using the PROC REG procedure. The average of % reduction in dry wt. for the 2 experimental runs was used to develop the dose-response regression models. To determine the best model of fit between dose and % reduction in kochia dry weight, maximum R2 and minimum RMSE values were used. Based on the best-fit regression model, the LD50 values for glyphosate and dicamba were extrapolated.
3. Results and Discussion
3.1. Root Aphid Study
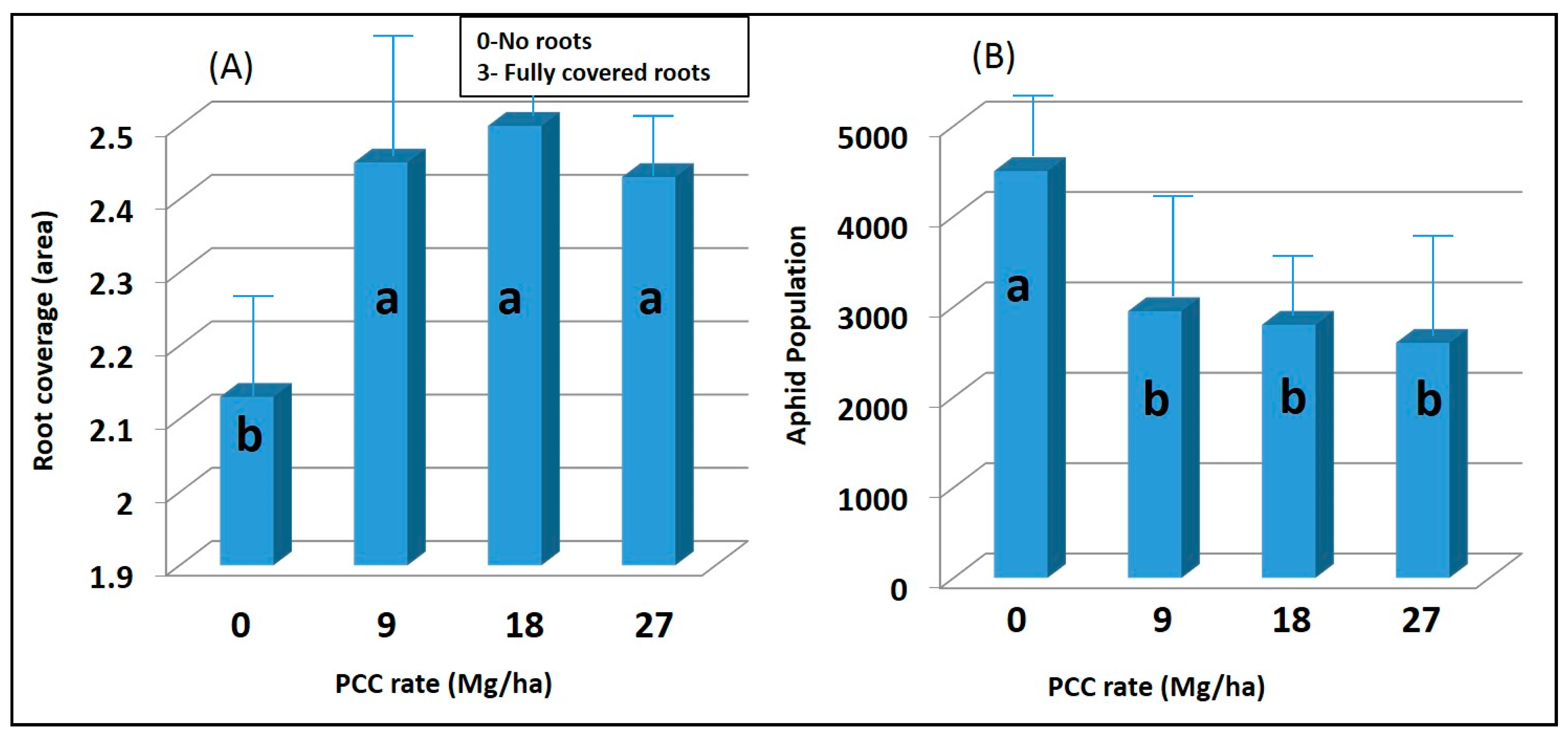
Root area generally was greater in lime-amended treatments (p < 0.05), which was most evident at the final evaluation (Figure 2A). Roots were evaluated because it might be expected that soil amendments would affect the root growth and development [32,33,34]. Because of the greater root area, one might expect aphid production to be higher [16]. However, this was not the case. All rates of PCC significantly (p < 0.05) reduced root aphid numbers as compared to an untreated control (Figure 2B). No significant differences in aphid populations were found between the tested rates of lime (p > 0.05). Although an exact reason for reduced aphid populations in the PCC-amended treatments is unknown, it is believed that high calcium and sulfur concentrations (Table 1) present in the PCC reduced aphid populations.
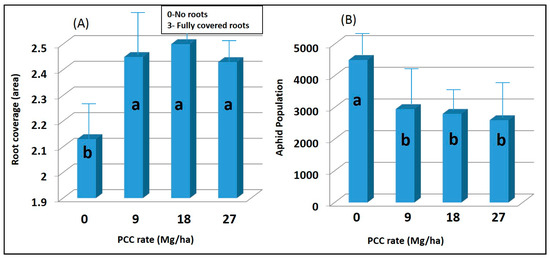
Figure 2.
(A) Average root coverage rating (0–3) of sugar beet plants exposed to 0,9,18, and 27 Mg ha−1 rates of precipitated calcium carbonate 6 weeks after seeding. Mean separation test (LSD) indicates that the mean values with the same letters are not significantly different at a 0.05 probability level. Note: Ratings: (0) No roots; (1) 1 to 2 roots filling the cell; (2) About 50% of cell space taken up by roots; (3) No visible empty space in cell (fully covered roots). (B) The average number of root aphids on sugar beet roots following exposure to 0,9,18, and 27 Mg ha−1 rates of precipitated calcium carbonate. Mean separation test (LSD) indicates that the mean values with the same letters are not significantly different at a 0.05 probability level. Error bars indicate the standard error of the means.

Table 1.
Chemical characteristics of precipitated calcium carbonate (PCC) used in the root aphid study.
3.2. Herbicide Resistance Study
For glyphosate dose-response, the visual injury of kochia in both experiments was almost similar in trend (Table 2).

Table 2.
Herbicide dose response of kochia grown on PCC piles averaged across three locations.
Kochia populations were nonresponsive to the lowest dose of glyphosate. About 50% visual injury was observed at 1.541 kg a.e. ha−1. As the dose increased from 1.541 to 6.164 kg a.e. ha−1, the visual injury was almost doubled. These results were consistent with an earlier study [28], where the authors reported that the amount of glyphosate required to cause 50% visible injury 21 days after treatment (DAT) ranged from 0.470 kg a.e. ha−1 to 2.149 kg a.e. ha−1. In the current study, the 50% herbicide visual injury was observed at 1.541 kg a.e. ha−1 of glyphosate, which suggests that the kochia grown on the PCC piles can be controlled with current dosage regimes [28,35]. Percent reduction of the dry weight of kochia 15 days after herbicide treatment is shown in Table 2. When averaged over two experiments, glyphosate caused about 50% reduction in kochia dry weight at a concentration of 1.541 kg a.e. ha−1; when the dose increased to 6.164 kg a.e. ha−1, glyphosate only caused approximately 75% reduction in dry weight.
In the first experiment, dicamba had about 75% of visual injury at 0.280 kg a.e. ha−1 dosage rate but a dose increase beyond 0.280 kg a.e. ha−1 did not increase visual injury significantly (Table 2). However, the results in the second experiment suggested that dose increment from 0.280 to 1.120 kg a.e. ha−1 caused a slight increase in the visual injury of kochia (statistically not significant). Additionally, the dicamba treatment had about a 60% reduction in kochia dry weight at 1.121 kg a.e. ha−1. Therefore, the tested kochia grown on PCC plies was not resistant to dicamba when compared to the reports of other dose-response studies [36,37], where the authors reported 50% damage at the concentration range of 0.045-1.331 kg a.e. ha−1 for various susceptible and resistant accessions in kochia.
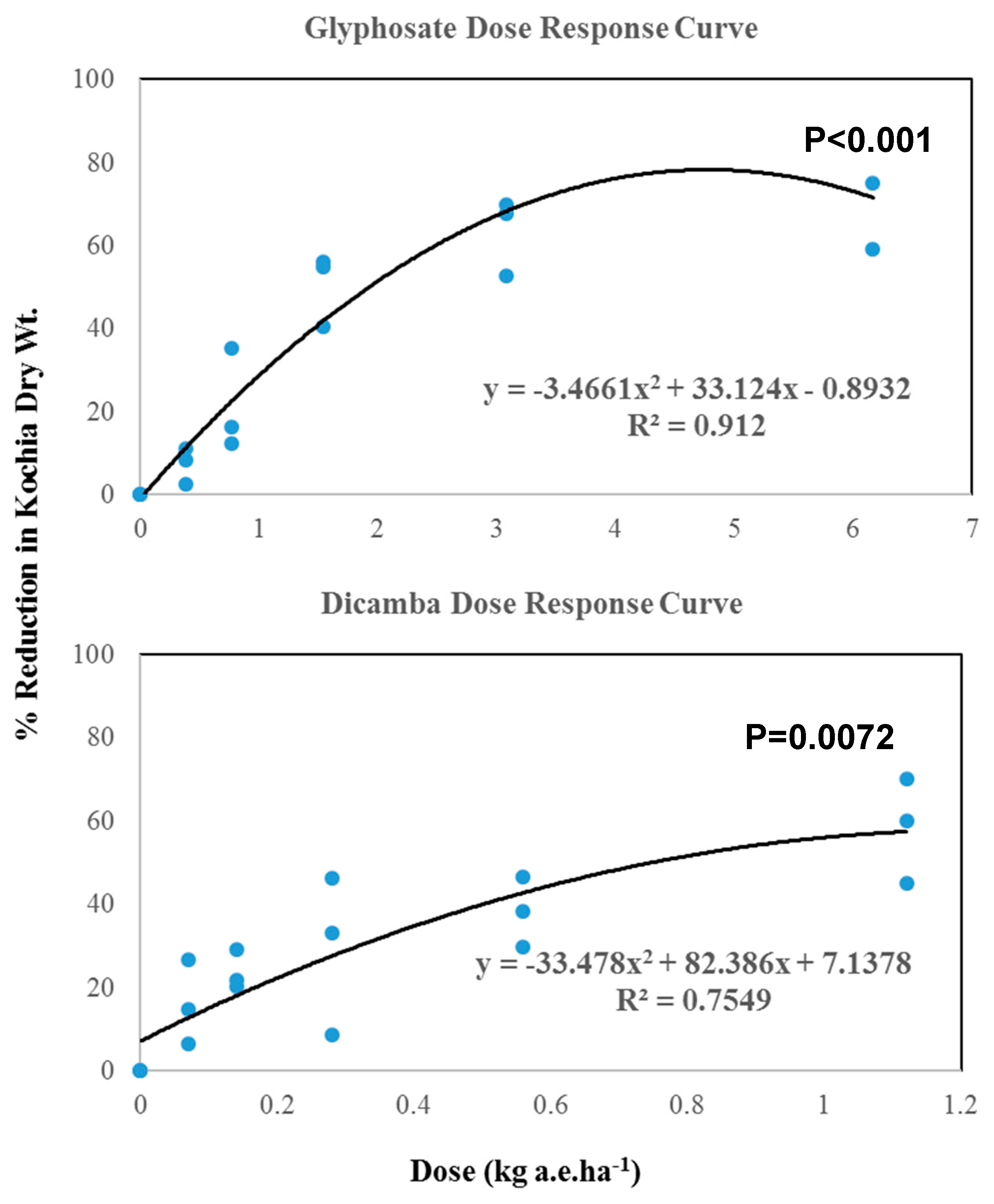
The results of regression analysis showed a polynomial relation between dosage and % reduction of dry weight for both glyphosate and dicamba (Figure 3).
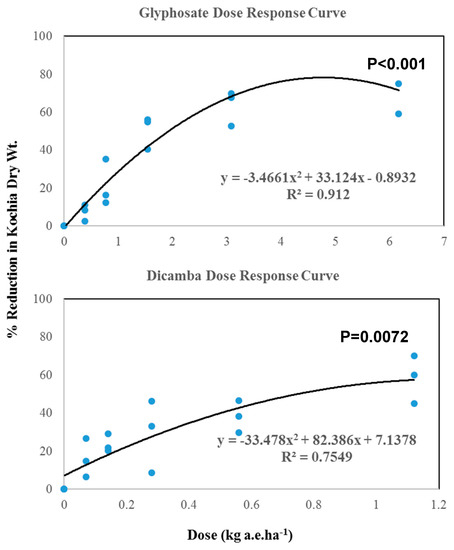
Figure 3.
Regression analysis for dose-response of glyphosate and dicamba in kochia grown on PCC piles.
The magnitude of regression coefficient (R2) values revealed that a better prediction existed in glyphosate response (R2 = 0.91). Based on regression prediction models (Figure 3), the estimated LD50 values for glyphosate and dicamba were 2.014 and 1.042 kg a.e. ha−1, respectively. The study in Montana reported that LD50 values of glyphosate for different resistant kochia accessions ranged from 2.350 to 3.640 kg a.e. ha−1; whereas, susceptible populations showed LD50 at only 0.330 kg a.e. ha−1 [29]. The current study suggests that kochia grown on PCC piles is moderately resistant, based on the levels from reference [29], but still within the range of the current dosage regime [35] to control the kochia effectively. However, the relatively high dosage of glyphosate to control kochia in the current study might be attributed to the formation of the less active Ca-glyphosate chemical complex [38,39,40], which could compromise the effectiveness of glyphosate on kochia control. LD50 values for dicamba was still within the threshold range to control kochia grown on PCC piles [36,37].
In summary, the kochia populations growing on PCC piles at Fort Morgan, Scottsbluff, and Torrington did not exhibit significant herbicide resistance and were controlled with current recommended dosages of glyphosate and dicamba.
4. Conclusions
Agronomic utilization of PCC in Western Sugar regions requires a careful examination of various aspects of logistical and production considerations. In this study, the two potential production concerns related to the PCC utilization were evaluated. One aspect of the research studied a potential benefit of PCC to control root aphids in the sugar beet crop; while the other aspect focused on examining herbicide control strategy and level of herbicide resistance (risk assessment) in kochia biotypes grown on PCC piles at three Western Sugar. The PCC-root aphid study concluded that the PCC is an effective material in reducing the root aphid populations. In the herbicide study, kochia biotypes grown on PCC piles can be controlled with current recommended dosages of glyphosate and dicamba. Although it is hypothesized that high concentrations of Ca and S in the PCC may have contributed to the aphid control, this assumption should be further investigated. Before considering the PCC as a soil amendment material, it is important to assess the cost effectiveness of PCC benefits in terms of root aphid control (from the current study), nutrient supply [6], and disease control [8] against the costs associated with PCC spreading and kochia weed control. Additionally, more field studies are needed to confirm the results of current controlled environment studies.
Author Contributions
Conceptualization, M.D., G.W.H., J.B., and R.H.; data curation, M.D., and A.A.; Formal analysis, M.D., L.L.; funding acquisition, M.D., G.W.H., J.B., and A.A.; investigation, M.D., A.A., and R.N.; methodology, G.W.H., J.B., R.W. and A.A.; project administration, M.D., G.W.H., J.B.; resources, J.B., R.H., and R.N.; software, M.D.; supervision, G.W.H., J.B., and R.W.; validation, M.D.; writing—original draft, M.D.; writing—review and editing, M.D., G.W.H., L.L., and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Western Sugar’s Joint Research Committee
Acknowledgments
We sincerely thank the Western Sugar’s Joint Research Committee for funding this project. We also thank all the farmer cooperators plus UNL Panhandle Research and Extension Center personnel who directly and indirectly contributed to this project
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
PCC = precipitated calcium carbonate.
References
- USDA-ERS. Table 12 and 17: Sugarbeet: Price Per Ton, by State and United States & U.S. Sugarbeet Area, Yield, and Production. 2018. Available online: https://www.ers.usda.gov/data-products/sugar-and-sweeteners-yearbook-tables/ (accessed on 07 December 2018).
- Windels, C.E.; Brantner, J.R.; Sims, A.L.; Bradley, C.A. Long-term effects of a single applicationof spent lime on sugarbeet, Aphanomyces root rot, rotation crops, and antagonistic microorganisms. Sugarbeet Res. Ext. Rept. 2007, 38, 251–262. [Google Scholar]
- Brantner, J.R.; Windels, C.E.; Sims, A.L.; Bradley, C.A. Ten years after a single field application of spent lime: Effects on soil pH, Aphanomyces root rot, and sugarbeet yield and quality. Sugarbeet Res. Ext. Rept. 2015, 45, 168–173. [Google Scholar]
- Brantner, J.R.; Crane, E.A.; Chanda, A.K. Lime amendment reduces infection of sugar beet by Aphanomyces cochlioides in soils over a wide range of pH. J. Sugar Beet Res. 2015, 50, 77. [Google Scholar]
- Christenson, D.R.; Brimhall, R.B.; Hubbel, L.; Bricker, C.E. Yield of sugar beet, soybean, corn, field bean, and wheat as affected by lime application on alkaline soils. Commun. Soil Sci. Plant Anal. 2000, 31, 1145–1154. [Google Scholar] [CrossRef]
- Hergert, G.W.; Darapuneni, M.K.; Aqeel, A.M.; Wilson, R.G.; Harveson, R.M.; Bradshaw, J.D.; Nielsen, R.A. Agronomic potential of using precipitated calcium carbonate on early plant growth and soil quality in the inter mountain west-greenhouse studies. J. Sugar Beet Res. 2017, 54, 35–49. [Google Scholar]
- Dutton, J.; Huijbregts, T. Root quality and processing. In Sugar Beet; Draycott, A.P., Ed.; Blackwell Publ.: Oxford, UK, 2006; pp. 409–442. [Google Scholar]
- Windels, C.E.; Brantner, J.R.; Sims, A.L.; Bradley, C.A. Five-year effect of a single fieldapplication of various rates of spent lime on Aphanomyces, sugarbeet and rotation crops. Sugarbeet Res. Ext. Rept. 2009, 39, 237–249. [Google Scholar]
- Bredehoeft, M.W.; Dunsmore, C.; Lamb, J.A. PCC use in Southern Minnesota—A success story of collaboration between research and production. ASSBT Proc. J. Sugar Beet Res. 2013, 50, 30. [Google Scholar]
- Giles, J.F.; Smith, L.J. Effect of spent lime on sugar production and crops following sugarbeet in the Red River Valley of the North. ASSBT Proc. J. Sugar Beet Res. 2005, 42, 36. [Google Scholar]
- Clark, G.M.; Hubbell, L.A.; Stewart, J.F.; Groullx, B.J. Influence of various precipitated calcium carbonate (PCC) “spent” lime rates on sugarbeet production, rotational crops and soil characteristics. ASSBT Proc. 2015 J. Sugar Beet Res. 2015, 52, 78. [Google Scholar]
- Lien, A.K.; Brantner, J.R.; Chanda, A.K. Understanding the effects of spent lime on Aphanomyces cochliodes. Sugarbeet Res. Ext. Rept. 2015, 45, 1–5. [Google Scholar]
- Campbell, R.N.; Greathead, A.S. Control of clubroot of crucifers by liming. In Soilborne Plant Pathogens: Management of Diseases with Macro- and Micronutrients; Engelhard, A.W., Ed.; APS Press: St. Paul, MN, USA, 1989; pp. 90–101. [Google Scholar]
- Chittem, K.; Khan, M.F.R.; Goswami, R.S. Efficacy of precipitated calcium carbonate in managing Fusarium root rot of field pea. Phytoparasitica 2016, 44, 295–303. [Google Scholar] [CrossRef]
- Summers, C.G.; Newton, A.S. Economic significance of sugarbeet root aphid, Pemphigus populivenae Fitch. (Homoptera: Aphididae) in California. Appl. Agric. Res. 1989, 4, 162–167. [Google Scholar]
- Hutchison, W.D.; Campbell, C.D. Economic impact of sugarbeet root aphid (Homoptera: Aphididae) on sugarbeet yield and quality in southern Minnesota. J. Econ. Entomol. 1984, 87, 465–475. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Campbell, C.D. Overwintering biology of the sugarbeet root aphid: Development and validation of a spring phenology forecasting model. In 1992 Sugarbeet Research and Extension Reports; North Dakota State University Extension Service, North Dakota State University: Fargo, ND, USA, 1983; Volume 23, pp. 129–144. [Google Scholar]
- Hein, G.L.; Boetel, M.A.; Godfrey, L.D. Part IV. Major insect and arthropod pests. In Compendium of Beet Diseases and Pests; Harveson, R.M., Hanson, L.E., Hein, G.L., Eds.; APS Press: St. Paul, MN, USA, 2009; pp. 95–117. [Google Scholar]
- Harper, A.M. Sugar-beet root aphid, Pemphigus betae Doane (Homoptera: Aphididae) in southern Alberta. Can. Entomol. 1963, 95, 863–873. [Google Scholar] [CrossRef]
- Foottit, R.G.; Floate, K.; Maw, E. Molecular evidence for sympatric taxa within Pemphigus betae (Hemiptera: Aphididae: Eriosomatinae). Can. Entomol. 2010, 142, 344–353. [Google Scholar] [CrossRef]
- Pretorius, R.J.; Hein, G.L.; Bradshaw, J.D. Ecology and Management of Pemphigus betae (Hemiptera: Aphididae) in Sugar Beet. J. Integr. Pest Manag. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Friesen, L.F.; Beckie, H.J.; Warwick, S.I.; Van Acker, R.C. The biology of Canadian weeds. 138. Kochia scoparia (L.) Schrad. Can. J. Plant Sci. 2009, 89, 141–167. [Google Scholar] [CrossRef]
- Stallings, G.P.; Thill, D.C.; Mallory-Smith, C.A.; Shafii, B. Pollen-mediated gene flow of sulfonylurea-resistant kochia (Kochia scoparia). Weed Sci. 1995, 43, 95–102. [Google Scholar] [CrossRef]
- Anderson, R.L.; Nielsen, D.C. Emergence pattern of five weeds in the central Great Plains. Weed Technol. 1996, 10, 744–749. [Google Scholar] [CrossRef]
- Schwinghamer, T.D.; Van Acker, R.C. Emergence timing and persistence of kochia (Kochia scoparia). Weed Sci. 2008, 56, 37–41. [Google Scholar] [CrossRef]
- Blackshaw, R.E. Russian thistle (Salsola iberica) and kochia (Kochia scoparia) control in dry land corn (Zea mays). Weed Technol. 1990, 4, 631–634. [Google Scholar] [CrossRef]
- Beckie, H.J.; Blackshaw, R.E.; Low, R.; Hall, L.M.; Sauder, C.A.; Martin, S.; Brandt, R.N.; Shirriff, S.W. Glyphosate-and acetolactate synthase inhibitor-resistant kochia (Kochia scoparia) in western Canada. Weed Sci. 2013, 61, 310–318. [Google Scholar] [CrossRef]
- Waite, J.; Thompson, C.R.; Peterson, D.E.; Currie, R.S.; Olson, B.L.; Stahlman, P.W.; Al-Khatib, K. Differential kochia (Kochia scoparia) populations response to glyphosate. Weed Sci. 2013, 61, 193–200. [Google Scholar] [CrossRef]
- Kumar, V.; Jha, P.; Reichard, N. Occurrence and characterization of kochia (Kochia scoparia) accessions with resistance to glyphosate in Montana. Weed Technol. 2014, 28, 122–130. [Google Scholar] [CrossRef]
- Regehr, D.L.; Morishita, D.W. Questions and Answers on Managing Herbicide-Resistant Weeds; MF-926; Kansas State University Coop. Ext. Service: Manhattan, KS, USA, 1989. [Google Scholar]
- SAS Institute. The SAS system for Windows. Release 9.4; SAS Inst.: Cary, NC, USA, 2013. [Google Scholar]
- Foy, C.D. Soil Chemical Factors Limiting Plant Root Growth. In Limitations to Plant Root Growth; Hatfield, J.L., Stewart, B.A., Eds.; Springer: New York, NY, USA, 1992; Volume 19. [Google Scholar]
- Carvalho, M.C.S.; Van Raij, B. Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 1997, 192, 37–48. [Google Scholar] [CrossRef]
- Mosaddeghi, M.R.; Mahboubi, A.A.; Safadoust, A. Short-term effects of tillage and manure on some soil physical properties and maize root growth in a sandy loam soil in western Iran. Soil Tillage Res. 2009, 104, 173–179. [Google Scholar] [CrossRef]
- Samuelson, S.L. Response of Problematic Weed Populations in Nebraska to Glyphosate. Master’s Thesis, Digital Commons University of Nebraska-Lincoln, Lincoln, NE, USA, 2017. [Google Scholar]
- Cranston, H.J.; Kern, A.J.; Hackett, J.L.; Miller, E.K.; Maxwell, B.D.; Dyer, W.E. Dicamba resistance in kochia. Weed Sci. 2001, 49, 164–170. [Google Scholar] [CrossRef]
- Preston, C.; Belles, D.S.; Westra, P.H.; Nissen, S.J.; Ward, S.M. Inheritance of resistance to the auxinic herbicide dicamba in kochia (Kochia scoparia). Weed Sci. 2009, 57, 43–47. [Google Scholar] [CrossRef]
- Sandberg, C.L.; Meggitt, W.F.; Penner, D. Effect of diluent volume and calcium on glyphosate phytotoxicity. Weed Sci. 1978, 26, 476–479. [Google Scholar] [CrossRef]
- Stahlman, P.W.; Phillips, W.M. Effects of water quality and spray volume on glyphosate phytotoxicity. Weed Sci. 1979, 27, 38–41. [Google Scholar] [CrossRef]
- Shea, P.J.; Tupy, D.R. Reversal of cation-induced reduction in glyphosate activity with EDTA. Weed Sci. 1984, 32, 802–806. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).