Abstract
Two Parthenium hysterophorus populations resistant (R) and susceptible (S) harvested in banana crop from the Dominican Republic were studied. All S plants died when the herbicides were applied at field dose, except with paraquat. For the R population, the order of plant survival was as follows: glyphosate and paraquat > flazasulfuron > glufosinate > fomesafen > 2,4-D. The resistance factors obtained in the dose–response assays showed a high resistance to glyphosate, flazasulfuron, and fomesafen, medium resistance to glufosinate and 2,4-D, and a natural tolerance to paraquat (resistance factor (RF) = 1.0). The I50 values obtained in the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), acetolactate synthase (ALS), and glutamine synthetase (GS) activity studies with glyphosate, flazasulfuron, and glufosinate, respectively, were greater in R than in S. The effect of fomesafen was measured by the Proto IX levels, obtaining five times more Proto IX in the S than in the R population. The resistance to 2,4-D in the R was determined by the lower accumulation of ethylene compared to the S population. The studies with 14C-paraquat conclude that the lower absorption and translocation in both the R and S populations would explain the natural tolerance of P. hysterophorus. This is the first case of multiple resistance to herbicides with different mechanisms of action confirmed in P. hysterophorus.
1. Introduction
Parthenium hysterophorus is a weed that is widely distributed in Africa, Asia, and Oceania and is native to tropical America. For example, in Cuba, it is considered as one of the most noxious species [1,2]. The International Union for Conservation of Nature (IUCN) considers it to be one of the 100 most invasive species in the world [3].
This weed has a high seed production capacity (130,000–200,000 seeds m−2), as well as persisting in the soil and germinating in a wide range of temperatures at any time of the year. These characteristics have contributed to the propagation via flowing water, the movement of vehicles, animals, and machinery, or it can be blown by wind (presence of achenes), facilitating its dissemination and distribution [1,2,4,5]. It is an allelopathic species that releases phytotoxic substances, such as parthenin, hymenin, hysterin, and ambrosin, which inhibit the germination and growth of some cultivable plants and trees [6,7,8,9]. These characteristics make it a successful weed in agricultural and non-agricultural areas.
The banana is the second most important crop in the Dominican Republic. Currently, this crop has more than 21,000 ha under production, with the majority exported to the United Kingdom, Sweden, Belgium, the Netherlands, and Germany, among other countries. [10,11].
The appearance of weeds with possible herbicide resistance in this crop is causing a low production yield; increasing the amount of labor and supplies dedicated to other weed controls. This is a very important situation because it affects other agricultural practices that require labor, such as harvesting and fertilization [12,13,14]. This situation has been reflected in an increase in production costs in order to control the weeds and avoid these effects [15]. The main species considered as weeds present in the banana crops of countries such as Brazil, Colombia, Costa Rica, Cuba, Guatemala, Honduras, India, Indonesia, Malaysia, and Puerto Rico are Conyza canadensis, Echinochloa colona, Eleusine indica, Sorghum halepense, Setaria viridis, Euphorbia helioscopia, Bidens pilosa, Amaranthus sp., Paspalum paniculatum, and Pathenium sp., among others [16,17,18]. The management of weeds in banana crops has been conducted through chemical control using herbicides, such as glyphosate (5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) inhibitor), as well as paraquat and/or diquat (PSI photosystem inhibitor) [14,19]. However, it is common to repeatedly use these herbicides because of their high control efficacy, imposing a selection pressure that leads to the evolution of resistance to herbicides with different modes of action (MOA) [20]. Due to the emergence of resistance to previous herbicides, Dominican banana growers have implemented the use of other herbicides with different MOAs. In this region, farmers make two to three applications per year of other herbicides such as glufosinate (glutamine synthetase (GS) inhibitor), 2,4-D (synthetic auxin), flazasulfuron (acetolactate synthase (ALS) inhibitor), and fomesafen (protoporphyrinogen oxidase (PPO) inhibitor) mainly.
The use of herbicides with different MOAs, alone or in mixture, is the main tool to combat resistance to a specific group of herbicides, which allows us extensive control of mono and dicotyledonous weeds [21,22,23]. Despite the large number of herbicides and their different MOAs, a poorly designed weed control program that is mainly dependent on chemical control and frequently uses the same herbicides, even if they have different MOAs, has given rise to multiple resistance [24]. Thus, multiple resistance is one of the biggest problems that resistance presents since it makes it difficult to choose alternative herbicides, especially when several resistance mechanisms are found in the same population (target and non-target sites) [25,26]. Some studies have claimed that herbicides are involved in the evolution of resistance [24,27,28,29,30,31]. Many plants, particularly weeds, reach this condition because they contain a tremendous amount of genetic variation that allows them to survive under different biotic and abiotic conditions [32].
There are three reports on the website weedscience.org regarding the P. hysterophorus herbicide resistance cases, two of them for EPSPS inhibitors in Colombia and the United States, and one for ALS inhibitors in Brazil, including the active ingredients chlorimuron-ethyl, chloratram-methyl, foramsulfuron, imazethapyr, and iodosulfuron-methyl-sodium [33]. Additionally, glyphosate resistance has been reported in the Everglades Agricultural Area of South Florida, in the Caribbean Islands of Cuba and the Dominican Republic, and in México [19,34,35]. Additionally, multiple resistance to synthetic auxin and glyphosate has been reported in Dominican Republic citrus orchards [36].
The objective of this research is (1) to evaluate resistance in a population of P. hysterophorus from the Monte Cristi region suspected of having multiple resistance to glyphosate, glufosinate, flazasulfuron, fomesafen, and 2,4-D and its natural tolerance to paraquat and (2) to confirm resistance and investigate possible resistance mechanisms using biochemical analysis.
2. Materials and Methods
2.1. Plant Material
Seeds of P. hysterophorus from the provinces of Monte Cristi and Azua (Dominican Republic) were used in this study. The seeds of populations considered to be resistant (R) were collected during 2016 from banana orchards located in Monte Cristi, where the technicians and farmers of the area reported difficulty in controlling outbreaks with different herbicides and MOAs (EPSPS, GS, ALS, PSI, PPO, and synthetic auxin). The seeds of the population considered to be susceptible (S) were collected from organic banana orchards in a region of Azua that has never been exposed to glyphosate or other herbicide treatment. The seeds were germinated in plastic containers (10 × 10 cm x 6.3 cm) that were filled with moistened peat and covered with parafilm. The plastic containers were kept in a growth chamber (28/18 °C (day/night), 16 h photoperiod, 850 μmol m−2 s−1 light density and 60% relative humidity) until germination. Seedlings were transplanted into 250 cm3 pots (1 plant/pot) filled with sand/peat (1:1 v/v). Throughout the experiments, pots were placed in a room with controlled conditions and were watered daily.
2.2. Chemicals
Table 1 shows the commercial herbicides used in this study to determine the physiological effects on the populations of P. hysterophorus. To perform the absorption and translocation test in P. hysterophorus, we used 14C-paraquat (specific activity 6.2789 MBq/mg, American Radiolabeled Chemicals, Inc., Saint Louis, MO, USA). The commercial formulation of paraquat is described in Table 1.

Table 1.
Main characteristics of the herbicides used in this study.
2.3. Fast Screening Assays
To ascertain whether these populations survive the application of herbicides by a mechanism of resistance and not because of incorrect application, greenhouse fast screening assays were carried out. The characteristics of the applied herbicides are shown in Table 1. All herbicides were applied in post-emergence plants with four to six true leaves. The experiments were arranged in a completely randomized design using ten plants per herbicide, applying the field doses (Table 1) in a treatment chamber (De Vries Manufacturing, Hollandale, MN, USA) equipped with a Tee Jet 8002 EVS flat fan nozzle calibrated to deliver 200 L ha−1 at 200 kPa at a height of 50 cm. Ten untreated plants were used as a control for all herbicides. This was done for both the resistant (R) and susceptible (S) P. hysterophorus populations. Three weeks after application (WAA), the number of surviving plants was counted. The experiments were conducted twice at different times (spring and fall) and the average of the two was obtained.
2.4. Dose–Response Assays
After the fast screening assays, we created a dose–response curve for each populationof P. hysterophorus (R and S) for the previously described herbicides (Table 1). Seven doses were used (0, X/16, X/8, X/4, X/2, X, 2X), where X is the field dose for each herbicide and zero (0) indicates untreated plants as control. The treatments were applied in the laboratory chamber mentioned above using the same application calibration. The experiment was arranged in a completely randomized design using five plants that were selected for each dose, for a total of 35 plants for each herbicide, for each population, for both the resistant population (R) and the susceptible population (S). After the treatments were completed, the plants were taken to a greenhouse and held under a temperature regime of 26/18 °C day/night, with water irrigation controlled at appropriate levels. Three WAA, the plants were weighed, and they were dried at 60 °C for four days in a climatic chamber.
2.5. EPSPS Enzyme Activity
The extraction of the EPSPS enzyme was carried out according to the methodology described by Bracamonte et al. [34]. Five grams of young leaf tissue from the R and S populations of P. hysterophorus were ground to a fine powder using liquid nitrogen in a cold mortar for EPSPS extraction and total protein content measurement [37]. The specific activity of EPSPS was tested in plants of both populations in the presence of different concentrations of glyphosate (0, 0.1, 1, 10, 50, 100, 200, 500, and 1000 μM) and in the absence (basal activity) of glyphosate. The EPSPS enzyme activity was expressed as percentage of enzymatic activity in the presence of glyphosate with respect to the control (without glyphosate). The EPSPS activity was determined by the amount of phosphate (μmol) released per μg of total soluble protein (TSP) per min. Three replicates for each concentration were tested in a completely randomized design and the experiment was conducted twice.
2.6. GS Enzyme Activity
The response of the glutamine synthetase enzyme (S) to glufosinate was determined using extracts of crude protein isolated from the R and S populations of P. hysterophorus following the methodology described by Tahmasebi et al. [38]. The following glufosinate concentrations were applied: 0, 15.6, 31.25, 62.5, 125, 250, 500, 1000, 2000, and 4000 μM. The concentration of glufosinate that reduced the S activity by 50% (I50) was used to calculate the resistance factor (RF) values. The total protein was also measured following the Bradford method [37]. This experiment was conducted twice using three replicates per herbicide concentration in a completely randomized design.
2.7. ALS Enzyme Activity
The acetolactate synthase (ALS) activity was determined following the methodology described by Hatami et al. [39] and Tahmasebi et al. [38], using 3 g of young leaf tissue per each population. The crude extract of the ALS enzyme was used to measure the enzyme activity at increasing concentrations (0, 0.1, 1, 10, 100, 1000, and 10,000 µM) of flazasulfuron. The acetoin absorbance, obtained from the decarboxylation of acetolactate, was measured with a spectrophotometer (Beckman DU-640, Fullerton, CA, USA) at 520 nm. The concentration of flazasulfuron that reduced the ALS activity by 50% (I50) was used to calculate the RF values. The total content of protein in the crude extract was also measured by the Bradford colorimetric method [37]. The experiment was carried out twice with three replicates per herbicide concentration and per population, following a completely randomized design.
2.8. 14C-Paraquat Absorption and Translocation
14C-paraquat absorption and translocation were evaluated at 6, 12, 24, 48, and 96 h after treatment (HAT). Once the R and S plants had four leaves, they were treated with a solution of 14C-paraquat plus a commercial paraquat formulation. The solution applied contained 14C-paraquat, providing 0.834 kBq µL−1 at a final concentration of 100 g ia ha−1 of paraquat in 300 L. One microliter per plant of solution was applied on the adaxial surface of the second youngest fully expanded leaf. After treatment, the plants were maintained in the growth chamber for 12 h in the dark before being exposed to light [38,40].
To determine the absorption, the 14C-paraquat-treated plants were harvested, and the treated leaves were washed three times with 1 mL of distilled water to recover the non-absorbed 14C-paraquat. The washing solution was mixed with 2 mL of scintillation liquid (Ultima Gold, Perkin-Elmer, BV BioScience Packard Groningen, Netherlands, MA). The samples were reserved for radioactivity analysis. To determine translocation, the whole plants were carefully removed from the pot and washed, mainly the roots. The plants were individually divided into treated leaf, the root system, and the remainder of the plant. The samples were stored in flexible combustion cones (Perkin-Elmer, BV BioScience Packard) and dried in an oven at 60 °C for 48 h. Subsequently, the samples were combusted in a Packard Tri Carb 307 biological oxidizer (Packard Instrument Co., Downers Grove, IL, USA). The CO2 released from the combustion was captured in 18 mL of a mix of Carbo-Sorb E and Permafluor (1:1 v/v) (Perkin-Elmer, BV BioScience Packard, Groningen, Netherlands). The radioactivity in the washing solution and combustion samples was quantified by liquid scintillation spectrometry in an LS 6500 scintillation counter (Beckman Coulter Inc., Fullerton, CA, USA) for 5 min per sample, and the measurement was repeated again 24 h later. The radioactive values were used to calculate the recovery percentage as % 14C-paraquat recovered = (kBq in treated leaf + kBq remaining in plant + kBq in root system + kBq from washes/total kBq applied) x 100. The experiments were arranged in a completely random design with four replicates per population at each evaluated time point, and the experiment was conducted twice.
2.9. Proto IX Levels
Determination of the Proto IX levels was carried out following the methodology described by Dayan et al. [41]. Leaf discs (approximately 0.1 g each) from the two populations were incubated individuality in a 6 cm diameter Petri dish containing 6 mL of 2% (wt/v) sucrose, 1 mM 2-(N-morpholin) ethanesulfonic acid, and 1000 µM fomesafen (technical grade) for 20 h at 25 °C in the dark. The same experiment was conducted but without herbicide for the control. All Proto IX extractions from the leaf discs were carried out under a dim, green light source. The extracts were stored in light tight glass vials at −20 °C until analysis. The analysis was carried out by LC using a 126 Gold HPLC system from Beckman Coulter (Fullerton, California, USA) equipped with a Jasco FP−1520 fluorescence detector (Easton, Pennsylvania, USA, excitation and emission wavelengths at 400 and 630 nm, respectively) and a SphereClone® ODS column (25 cm x 4.6 mm, 5 micron particle size) from Phenomenex (Torrance, California, CA, USA).
Proto IX levels in the extracts were quantified using a calibration curve obtained with a commercially available Proto IX standard from Sigma Aldrich (St. Louis, Missouri, USA). The data are expressed as nmol per g of fresh weight. All treatments were carried out three times using three replicates with and without herbicide in a completely randomized design.
2.10. 2,4-D and Ethylene Production
R and S plants with 6 leaves were treated with 2,4-D at 0, 125, 250, 500, 1000, and 2000 g ai ha−1 following the methodology described above for the dose–response assays. After 24 h of treatment, 400 g of leaf tissue was placed in a 10 mL syringe with 1 mL of distilled water and then sealed [42]. The syringes were placed in a dark incubator at 27 °C for 4 h. The ethylene (C2H4) in 1 mL of the gas at the top of the syringe was analyzed by gas chromatography [38,43]. The C2H4 content was expressed as nanoliters per gram of fresh weight per hour. There were five replicates per treatment, and the experiment was conducted twice.
2.11. Statistical Analysis
The herbicide concentration causing 50% growth reduction (GR50) of the plants and the herbicide concentration causing 50% inhibition of enzyme activity (I50) were calculated by non-linear regression analysis using the R package drc (R Core Team) [44]. A three-parameter log-logistic model was selected for modeling growth reduction or enzyme inhibition (Equation (1)):
where Y is the dry weight of the harvested plants or enzyme activity, d is the coefficient corresponding to the upper asymptote, c the lower limit (fixed at 0), the coefficient b is the slope at the inflection point, e the herbicide concentration required to inhibit shoot growth or enzyme activity by 50% (i.e., GR50 or I50, respectively), and X the herbicide dose. The resistance factors (RF = R/S) were computed as R-to-S GR50 or I50 ratios.
The effects of the population, time (HAT), and 14C-paraquat absorption and translocation were tested using ANOVA. The population was treated as a fixed factor, and time was considered as a random factor. The means and standard errors of 14C-paraquat absorption and translocation were computed for all plant parts, and the means were tested for group differences and then compared. For each analysis, the assumptions of equality of variance and normal error distribution were evaluated and they met after angular transformation of the response variable (arcsine of the square root of fractions). The Tukey HSD test at 5% probability was used to separate the means. The ANOVAs were performed using the Statistix software (version 10.0) from Analytical Software.
3. Results
3.1. Fast Screening Assays
The fast screening test at field doses showed that the S populations were 100% controlled by glyphosate, glufosinate, fomesafen, flazasulfuron, and 2,4-D herbicides (Table 2). The herbicides 2,4-D and fomesafen showed the lowest values of plant survival with respect to the control when they were applied at post-emergence, while the other herbicides (glufosinate and flazasulfuron) had higher percentages of plants that survived at field doses. Glyphosate had zero effectiveness on the R population. Paraquat had no effect on the R and S P. hysterophorus populations, which leads us to suspect that this species has a natural tolerance to paraquat.

Table 2.
Plant survival at field doses expressed as a percentage with respect to control (untreated) plants for different herbicides in two populations of Parthenium hysterophorus (resistant (R) and susceptible (S)) collected from banana plantations of the Dominican Republic.
3.2. Dose–Response Assays
Dose–response assays showed the existence of the first case of multiple resistance to herbicides with different mechanisms of action in P. hysterophorus collected from banana orchards in the Dominican Republic (Table 3). The GR50 values obtained for the different herbicides (except paraquat) showed a high efficiency in reducing the 50% dry weight of the S population of P. hysterophorus, which is in good agreement with the results obtained in the fast screening test (Table 2 and Table 3). Based on the GR50 values of the R population, we calculated resistance factors (RF: GR50 of R/GR50 of S) from 4.9 to 27.1, which indicated the following order of resistance to the tested herbicides: glyphosate > flazasulfuron > fomesafen > glufosinate > 2,4-D (Table 3).

Table 3.
Parameters for estimating the log-logistic model a of the growth reduction in response to herbicide concentration for the R and S P. hysterophorus populations treated with the main herbicides used in banana crops in the Dominican Republic.
Paraquat is a contact herbicide that shows very rapid efficacy in the appearance of chlorotic leaves after 24 HAA and necrosis one week later. However, in tolerant species, the plant begins to regrow in meristematic areas. In our study, the GR50 results for the R and S populations showed very high and similar values (Table 3). These results, together with those obtained in the fast screening test, allow us to conclude that there is a natural tolerance to paraquat in this species (Table 2 and Table 3).
3.3. EPSPS Enzyme Activity
The EPSPS was inhibited in situ by increasing glyphosate concentrations. The required glyphosate concentration to inhibit EPSPS by 50% (I50) was approximately 24 times greater in the R population than in the S population (Table 4). However, no differences were found between R and S in the enzyme basal activity (128.46 and 119.11 nmol Pi/ µg TSP/min, respectively).

Table 4.
Parameters of the log-logistic model a of the inhibition of enzymatic activity in response to herbicide concentration for R and S P. hysterophorus populations.
3.4. GS Enzyme Activity
The glufosinate doses required to reduce GS activity by 50% (I50) were 946.6 and 32.2 μM for the R and S populations, respectively. The RF value for R was 29.3 times greater that of S (Table 4). No differences were found in the basal enzyme activity (226.29 and 240.01 nmol of glutamine/mg TSP/h, respectively).
3.5. ALS Enzyme Activity
The quantity of flazasulfuron needed to inhibit the ALS activity by 50% (I50) in the R population was 624.4 μM, while that in the S population was 46.3 μM. In other words, the R population was 13.5 fold more resistant to flazasulfuron than the S population (Table 4). No differences were found in the basal enzyme activity (332.34 and 317.20 nmol of acetoine/mg TSP/h, respectively).
3.6. 14C-Paraquat Absorption and Translocation
The average total recovery of the applied 14C-paraquat was >90% in both the R and S P. hysterophorus populations. The 14C-paraquat absorption was not significantly different between the R and S plants throughout the time periods and increased over time up to 70% at 96 HAT (Table 5). In addition, the translocation of 14C-paraquat from the treated leaf to the rest of the shoot and roots was minimal and similar in both populations. There was a minimal translocation of 1.2% to 4.6% to the rest of the shoot plant, and a negligible amount of 14C-paraquat translocation to the root system during experimental time (Table 5).

Table 5.
14C-paraquat absorption and translocation from 6 to 96 h after treatment (HAT) in R and S P. hysterophorus plants.
3.7. Proto IX Levels
After the application of 1000 μM fomesafen, the P. hysterophorus R population accumulated significantly less Proto IX than the S population. The accumulation values were 0.50 ± 0.03 and 2.75 ± 0.14 nmol g−1 fresh weight in the R and S populations, respectively.
3.8. 2,4-D and Ethylene Production
The ethylene accumulation in the R and S P. hysterophorus plants increased along with the dose of herbicide applied (Figure 1). The R and S control plants produced similar amounts of ethylene (data not shown). The ethylene accumulation at 1000 g ai ha−1 of 2,4-D was 5.0 times smaller in the R population than in the S population.
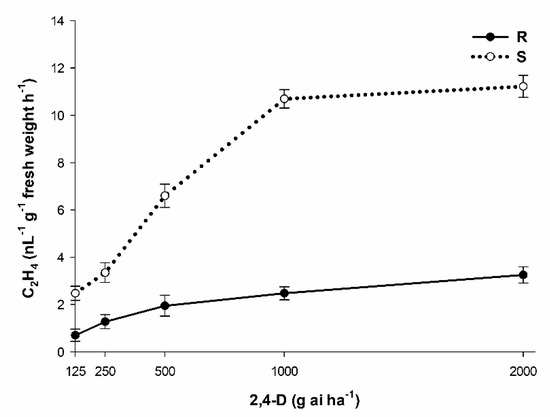
Figure 1.
Ethylene accumulatation induced by 2,4-D in R and S P. hysterophorus populations. Vertical bars indicate ± standard errors of the mean (n = 5).
4. Discussion
Our results demonstrated different levels of resistance in P. hysterophorus to the tested herbicides. The highest resistance factor was for glyphosate, followed by flazasulfuron, fomesafen, and glufosinate. The lower values of GR50 for the susceptible population showed that all of the herbicides except paraquat were useful to control the susceptible population of this weed in banana crops [34]. The natural tolerance to paraquat in this species was confirmed in this study; it was reported for the first time in 1991 in coffee crops [45].
Compared with previous glyphosate research, the level of P. hysterophorus resistance was four times higher than reported in Colombia and 1.5 times lower than in the United States [35,46]. According to a study conducted in Cuba and the Dominican Republic, the values are similar if the greatest resistance factor reported in that study is taken into account [34]. The difference in resistance values can be attributed to the doses used in each country and crop, environmental factors, soil, tillage practices, and management practices [47,48,49].
Regarding 2,4-D, the resistance factor was similar to that reported in citrus orchards (also in the Dominican Republic) [36]. Finally, for ALS inhibitors, Gazziero et al. [50] reported RF values ranging between 17 and 21 for the different active ingredients evaluated (chloransulam-methyl, chlorimuron-ethyl, imazethapyr, iodosulfuron + foramsulfuron), and although flazasulfuron is not included, the values are similar.
The current study is the first report regarding glufosinate and fomesafen resistance in P. hysterophorus. Glufosinate resistance has been confirmed in Eleusine indica, Lolium perenne and Lolium perenne ssp. multiflorum, and Lolium rigidum [51,52,53,54]. Fomesafen resistance was previously confirmed in Acalypha australis, Amaranthus retroflexus, Amaranthus palmeri, and Ambrosia artemisiifolia, among others [33,55,56,57,58].
When there is no significant difference in the EPSPS activity and basal activity, it demonstrates the absence of Target-Site Resistance (TSR) as a resistance mechanism in P. hysterophorus, according to the conclusions made by Palma-Bautista et al. [19]. However, in the study conducted in the Caribbean Islands, accessions showed different resistance mechanisms. Some of them showed significant differences in the EPSPS activity and basal activity between susceptible and resistant populations, concluding that resistance levels depend on the TSR mechanism. Furthermore, in other accessions, the results indicated that Non-Target-Site Resistance (NTSR) mechanisms that accumulated in this adhesion were also involved [34].
A similar GS (glutamine synthetase) basal activity was found in this study in both the R and S populations. However, the enzyme activity inhibition (I50) was 29-fold greater in the susceptible population than in the resistance population. This result indicates that glufosinate resistance in P. hysterophorus is likely to be target-site based, although this hypothesis needs to be investigated. Similar results were found for Epilobium ciliatum in Chilean olive orchards [38]. Avila-Garcia and Mallory-Smith [59] and Jalaludin et al. [60] noted a similar sensitivity of the GS enzyme between susceptible and resistant populations, indicating that glufosinate resistance is not conferred by the target site.
Similar results were determined for the ALS enzyme activity, where there were no differences in the basal activity but differences between the populations were found in the quantity and needed to inhibit the ALS activity by 50%. These results demonstrate the presence of a TSR mechanism for flazasulfuron. Similar studies were conducted in Rapistrum rugosum for different ALS herbicides, in Euphorbia heterophylla and soft wheat for imazamox, and in Conyza canadensis and Epilobium ciliatum for flazasulfuron [38,39,61,62,63].
The poor control, resistance factors, and lack of differences in the absorption and translocation of paraquat between susceptible and resistant populations confirm the natural tolerance of P. hysterophorus to paraquat, explained by the low absorption and translocation of the herbicide [38,40,64]. This natural tolerance was reported in Kenya for the first time [45]. By contrast, in perennial ryegrass (Lolium perenne L.), the tolerance was explained due to the higher activities of catalase and superoxide dismutase, along with elevated peroxidase activity, in paraquat-tolerant lines [65,66]. In young leaves of squash cultivars, the tolerance to paraquat is due to oxidative and abiotic stresses, such as the change in catalase, peroxidase, ascorbate peroxidase, and glutathione reductase activities [67]. In Chenopodium rubrum, the tolerance is explained by the activity of superoxide dismutase and peroxidase [68].
A useful methodology to determine the effectiveness of PPO herbicides is to evaluate the accumulation of protoporphyrin IX, where susceptible plants will accumulate more of this enzyme [41]. The lower accumulation of Proto IX in the R population confirmed the resistant to fomesafen. Similar results were found by Tahmasebi et al. [38], which supported the evolution of resistance to pyraflufen-ethyl by recurrent selection in E. ciliatum.
In the case of 2,4-D, the accumulation of ethylene was evidenced in the R and S plants treated with 2,4-D and has been reported by several authors [38,42,69,70,71]. The lower accumulation of ethylene in R plants may be due to the herbicide not reaching its nuclear protein receptor complex to suppress the genes responsible for ethylene production, which respond to auxins [71,72,73]. Susceptible plants exposed to 2,4-D die due to unregulated auxin activity and the accumulation of reactive oxygen species (ROS), ABA, and ethylene [72,74]. In previous P. hysterophorus studies, resistance to 2,4-D was found to be due to two simultaneous mechanisms of NTSR—less translocation of the herbicide and enhanced metabolism—in which cytochorme-P450 may be involved [36]. The mechanism of resistance to synthetic auxins in Papaver rhoeas is due to the reduced translocation of 2,4-D, which leads to a lower production and accumulation of ethylene and the survival of resistant plants [73]. However, in a more recent study in the same species, an additional mechanism was found to be due to enhanced metabolism mediated by cytochrome P450 [42].
5. Conclusions
The first cases of multiple resistance to EPSPS, GS, ALS, PPO, synthetic auxin, and natural tolerance to PSI in Parthenium hysterophorus harvested from banana orchards in the Dominican Republic has been confirmed. Biochemical analyses suggest that resistance to glyphosate, glufosinate, flazasulfuron, and fomesafen is based on TSR and 2,4-D on NTSR, while tolerance to paraquat is also based on NTSR. Due to the complex evolution of multiple resistance to five MOAs and naturally tolerant to paraquat in this species, farmers should implement integrated weed management with non-chemical alternatives for the management of these weed populations.
Author Contributions
Conceptualization, J.R. and R.D.P.; methodology, C.P.-B., V.H., G.P., J.G.V.-G., and A.M.R.-D.; validation, C.P.-B., V.H., J.G.V.-G., and A.M.R.-D.; formal analysis, C.P.-B. and J.G.V.-G.; investigation, C.P.-B., V.H., G.P., J.G.V.-G., J.R., and A.M.R.-D.; resources, R.D.P.; writing—Original draft preparation, C.P.-B., V.H., G.P., J.G.V.-G., J.R., A.M.R.-D., and R.D.P.; writing—Review and editing, C.P.-B., V.H., G.P., J.G.V.-G., J.R., A.M.R.-D., and R.D.P.; supervision, A.M.R.-D. and R.D.P.; project administration, R.D.P.; funding acquisition, R.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy and Competitiveness (AGL2016-78944-R) and the Asociación de Agroquímicos y Medioambiente.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Labrada, R.; Caseley, J.C.; Parker, C. Malezas de hoja ancha. In Manejo de Malezas Para Países en Desarrollo; FAO, Ed.; FAO: Rome, Italy, 1996. [Google Scholar]
- Kaur, M.; Aggarwal, N.K.; Kumar, V.; Dhiman, R. Effects and Management of Parthenium hysterophorus: A Weed of Global Significance. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar]
- GISD Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/species.php?sc=153 (accessed on 29 November 2019).
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Navie, S.C.; Panetta, F.D.; McFadyen, R.E.; Adkins, S.W. Germinable soil seedbanks of central Queensland rangelands invaded by the exotic weed Parthenium hysterophorus L. Weed Biol. Manag. 2004, 4, 154–167. [Google Scholar] [CrossRef]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K. Residue allelopathy in Parthenium hysterophorus L.-Does parthenin play a leading role? Crop Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Belz, R.G. Investigating a Potential Auxin-Related Mode of Hormetic/Inhibitory Action of the Phytotoxin Parthenin. J. Chem. Ecol. 2016, 42, 71–83. [Google Scholar] [CrossRef]
- Hassan, G.; Rashid, H.U.; Amin, A.; Khan, I.A.; Shehzad, N. Allelopathic effect of Parthenium hysterophorus on germination and growth of some important crops and weeds of economic importance. Planta Daninha 2018, 36, e018176372. [Google Scholar] [CrossRef]
- Belz, R.G. Stimulation versus inhibition-bioactivity of parthenin, a phytochemical from parthenium hysterophorus L. Dose-Response 2008, 6, 80–96. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Trade and Markets: Bananas. Available online: http://www.fao.org/home/en/ (accessed on 15 February 2019).
- FAOSTAT FAO (Food and Agriculture Organization of the United Nations). Statistical Databases. Available online: http://www.fao.org/home/es (accessed on 15 February 2019).
- Plaza, G. Manejo de Malezas en Frutales. In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2012; pp. 238–251. [Google Scholar]
- Quintero-Pértuz, I.; Carbonó-DelaHoz, E. Panorama del manejo de malezas en cultivos de banano en el departamento del Magdalena, Colombia. Rev. Colomb. Ciencias Hortícolas 2016, 9, 329. [Google Scholar] [CrossRef]
- Pinilla, C.; Garcia, J. Manejo integrado de arvenses en plantaciones de banano (Musa AAA). In Proceedings of the XV Reunión Asociación de Bananero de Colombia (Acorbat); Augura: Cartagena, Colombia, 2002; pp. 222–235. [Google Scholar]
- Martinez, A.M.; Hoyos, L.M. Banano (Musa AAA. Simmonds). In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2012; pp. 349–369. [Google Scholar]
- Rodríguez, A.M.; Agüero, R. Identificación de malezas trepadoras del banano (Musa sp.) en la zona caribe de Costa Rica. Agron. Mesoam. 2000, 11, 123–125. [Google Scholar] [CrossRef]
- De la Cruz, R.; Rojas, C.E.; Lobón, H.; Burgos, C. El papel de las malezas en la reducción de la lixiviación de nutrimentos en cultivos de banano en el trópico húmedo. Manejo Integr. Plagas 2001, 62, 29–37. [Google Scholar]
- Amaya, A.; Santos, M.; Morán, I.; Vargas, P.; Comboza, W.; Lara, E. Malezas Presentes en Cultivos del Cantón Naranjal, Provincia Guayas, Ecuador. Investig. Res. Rev. 2018, 11, 1–16. [Google Scholar]
- Palma-Bautista, C.; Gherekhloo, J.; Domínguez-Martínez, P.A.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; De Prado, R. Characterization of three glyphosate resistant Parthenium hysterophorus populations collected in citrus groves from Mexico. Pestic. Biochem. Physiol. 2019, 155, 1–7. [Google Scholar] [CrossRef]
- Radosevich, S.R.; Holt, J.S.; Ghersa, C. Weed Ecology: Implications for Management, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997; ISBN 0471116068. [Google Scholar]
- Tornisielo, V.L.; Botelho, R.G.; Alves, P.A.D.T.; Bonfleur, E.J.; Monteiro, S.H. Pesticide Tank Mixes: An Environmental Point of View, Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Ganie, Z.A.; Jhala, A.J. Interaction of 2,4-D or dicamba with glufosinate for control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in glufosinate-resistant maize (zea mays L.). Front. Plant Sci. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Bracamonte, E.R.; Fernández-Moreno, P.T.; Bastida, F.; Osuna, M.D.; Alcántara-de la Cruz, R.; Cruz-Hipolito, H.E.; De Prado, R. Identifying Chloris Species from Cuban Citrus Orchards and Determining Their Glyphosate-Resistance Status. Front. Plant Sci. 2017, 8, 1977. [Google Scholar] [CrossRef] [PubMed]
- De Prado, R.; Franco, A.R. Cross-resistance and herbicide metabolism in grass weeds in Europe: Biochemical and physiological aspects. Weed Sci. 2004, 52, 441–447. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Shaner, D.L.; Lindenmeyer, B.; Ostlie, M.H. What have the mechanisms of resistance to glyphosate taught us? Pest Manag. Sci. 2012, 68, 3–9. [Google Scholar] [CrossRef]
- Alcántara de la Cruz, R.; Barro, F.; Domínguez-Valenzuela, J.A.; De Prado, R. Physiological, morphological and biochemical studies of glyphosate tolerance in Mexican Cologania (Cologania broussonetii (Balb.) DC.). Plant Physiol. Biochem. 2016, 98, 72–80. [Google Scholar] [CrossRef]
- Busi, R.; Vila-Aiub, M.M.; Powles, S.B. Genetic control of a cytochrome P450 metabolism-based herbicide resistance mechanism in Lolium rigidum. Heredity 2011, 106, 817–824. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Osuna, M.D.; Heredia, A.; Ruiz-Santaella, J.P.; De Prado, R. Nontarget mechanims involved in glyphosate tolerance found in Canavalia ensiformis plants. J. Agric. Food Chem. 2009, 57, 4844–4848. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Rojano-Delgado, A.; Domínguez-Valenzuela, J.A.; Heredia, A.; de Castro, M.D.L.; de Prado, R. Glyphosate tolerance by Clitoria ternatea and Neonotonia wightii plants involves differential absorption and translocation of the herbicide. Plant Soil 2011, 347, 221–230. [Google Scholar] [CrossRef]
- González-Torralva, F.; Gil-Humanes, J.; Barro, F.; Brants, I.; De Prado, R. Target site mutation and reduced translocation are present in a glyphosate-resistant Lolium multiflorum Lam. biotype from Spain. Plant Physiol. Biochem. 2012, 58, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Neve, P.; Vila-aiub, M.; Roux, F. Evoltionary-Thinking in Agricultural Weed Managment. New Phytol. 2009, 184, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 29 October 2019).
- Bracamonte, E.; Fernández-Moreno, P.T.; Barro, F.; De Prado, R. Glyphosate-resistant Parthenium hysterophorus in the Caribbean Islands: Non target site resistance and target site resistance in relation to resistance levels. Front. Plant Sci. 2016, 7, 1845. [Google Scholar] [CrossRef]
- Fernandez, J.V.; Odero, D.C.; Macdonald, G.E.; Ferrell, J.; Gettys, L.A. Confirmation, Characterization, and Management of Glyphosate-Resistant Ragweed Parthenium (Parthenium hysterophorus L.) in the Everglades Agricultural Area of South Florida. Weed Technol. 2015, 29, 233–242. [Google Scholar] [CrossRef]
- Mora, A.D.; Rosario, J.; Rojano-Delgado, A.M.; Palma-Bautista, C.; Torra, J.; Alcántara-De La Cruz, R.; De Prado, R. Multiple Resistance to Synthetic Auxin Herbicides and Glyphosate in Parthenium hysterophorus Occurring in Citrus Orchards. J. Agric. Food Chem. 2019, 67, 10010–10017. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tahmasebi, B.K.; Alcántara-de la Cruz, R.; Alcántara, E.; Torra, J.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; De Prado, R. Multiple Resistance Evolution in Bipyridylium-Resistant Epilobium ciliatum After Recurrent Selection. Front. Plant Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple Mechanisms Increase Levels of Resistance in Rapistrum rugosum to ALS Herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Moretti, M.L.; Hanson, B.D. Reduced translocation is involved in resistance to glyphosate and paraquat in Conyza bonariensis and Conyza canadensis from California. Weed Res. 2017, 57, 25–34. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. Biochemical Markers and Enzyme Assays for Herbicide Mode of Action and Resistance Studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D metabolism in two resistant Papaver rhoeas populations from Spain. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, R.H.; Hoffer, B.L. Induction of ethylene as an indicator of senescence in the mode of action of diclofop-methyl. Pestic. Biochem. Physiol. 1996, 54, 146–158. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Njoroge, J.M. Tolerance of Bidens pilosa L. and Parthenium hysterophorus L. to paraquat (Gramoxone) in Kenya coffee. Kenya Coffee 1991, 56, 999–1001. [Google Scholar]
- Rosario, J.; Fuentes, C.; De Prado, R.; Cruz-Hipolito, H. Resistance of Parthenium hysterophorus L. to the glyphosate: A new case of resistance in Colombia. In Proceedings of the XIIIème Colloque International Sur la Biologie des Mauvaises Herbes, Dijon, France, 8–10 September 2009; pp. 1–8. [Google Scholar]
- Jussaume, R.A., Jr.; Ervin, D. Understanding Weed Resistance as a Wicked Problem to Improve Weed Management Decisions. Weed Sci. 2016, 64, 559–569. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-smith, C.; Burgos, N.R.; Johnson, W.G.; Mcclelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the Risks of Herbicide Resistance: Best Management Practices and Recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Gazziero, D.L.P.; Brighenti, A.M.; Voll, E. Resistência cruzada da losna-branca (Parthenium hysterophorus) aos herbicidas inibidores da enzima acetolactato sintase. Planta Daninha Vicosa-MG 2006, 24, 157–162. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C.; James, T.K. Glyphosate-resistant Lolium multiflorum and Lolium perenne populations from New Zealand are also resistant to glufosinate and amitrole. Crop Prot. 2015, 78, 1–4. [Google Scholar] [CrossRef]
- Jalaludin, A.; Yu, Q.; Powles, S.B. Multiple resistance across glufosinate, glyphosate, paraquat and ACCase-inhibiting herbicides in an Eleusine indica population. Weed Res. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Fernández, P.; Alcántara, R.; Osuna, M.D.; Vila-Aiub, M.M.; Prado, R. De Forward selection for multiple resistance across the non-selective glyphosate, glufosinate and oxyfluorfen herbicides in Lolium weed species. Pest Manag. Sci. 2017, 73, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Travlos, I.S.; Cheimona, N.; De Prado, R.; Jhala, A.J.; Chachalis, D.; Tani, E. First case of glufosinate-resistant rigid ryegrass (Lolium rigidum gaud.) in Greece. Agronomy 2018, 8, 35. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zhang, L.; Zhao, K.; Ge, L.; Lv, X.; Liu, W.; Wang, J. Multiple resistance to thifensulfuronmethyl and fomesafen in redroot pigweed (Amaranthus retroflexus L.) from China. Chil. J. Agric. Res. 2017, 77, 311–317. [Google Scholar] [CrossRef]
- Rousonelos, S.L.; Lee, R.M.; Moreira, M.S.; VanGessel, M.J.; Tranel, P.J. Characterization of a Common Ragweed (Ambrosia artemisiifolia) Population Resistant to ALS- and PPO-Inhibiting Herbicides. Weed Sci. 2012, 60, 335–344. [Google Scholar] [CrossRef]
- Salas, R.A.; Burgos, N.R.; Tranel, P.J.; Singh, S.; Glasgow, L.; Scott, R.C.; Nichols, R.L. Resistance to PPO-inhibiting herbicide in Palmer amaranth from Arkansas. Pest Manag. Sci. 2016, 72, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, V.K.; Brabham, C.; Norsworthy, J.K. Confirmation and Characterization of Non-target site Resistance to Fomesafen in Palmer amaranth (Amaranthus palmeri). Weed Sci. 2018, 66, 702–709. [Google Scholar] [CrossRef]
- Avila-Garcia, W.V.; Mallory-Smith, C. Glyphosate-Resistant Italian Ryegrass (Lolium perenne) Populations also Exhibit Resistance to Glufosinate. Weed Sci. 2011, 59, 305–309. [Google Scholar] [CrossRef]
- Jalaludin, A.; Yu, Q.; Zoellner, P.; Beffa, R.; Powles, S.B. Characterisation of glufosinate resistance mechanisms in Eleusine indica. Pest Manag. Sci. 2017, 73, 1091–1100. [Google Scholar] [CrossRef]
- Domínguez-Mendez, R.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; da Silveira, H.M.; Portugal, J.; Cruz-Hipolito, H.E.; De Prado, R. Stacked traits conferring multiple resistance to imazamox and glufosinate in soft wheat. Pest Manag. Sci. 2019, 75, 648–657. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Tahmasebi, B.K.; Fernández-Moreno, P.T.; Rojano-Delgado, A.M.; de la Cruz, R.A.; De Prado, R. First case of Conyza canadensis from Hungary with multiple resistance to glyphosate and flazasulfuron. Agronomy 2018, 8, 157. [Google Scholar] [CrossRef]
- Rojano-Delgado, A.M.; Portugal, J.M.; Palma-Bautista, C.; Alcántara-de la Cruz, R.; Torra, J.; Alcántara, E.; De Prado, R. Target site as the main mechanism of resistance to imazamox in a Euphorbia heterophylla biotype. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tehranchian, P.; Nandula, V.; Jugulam, M.; Putta, K.; Jasieniuk, M. Multiple resistance to glyphosate, paraquat and ACCase-inhibiting herbicides in Italian ryegrass populations from California: Confirmation and mechanisms of resistance. Pest Manag. Sci. 2018, 74, 868–877. [Google Scholar] [CrossRef]
- Harvey, B.M.R.; Muldoon, J.; Harper, D.B. Mechanism of paraquat tolerance in perennial ryegrass. I. Uptake, metabolism and translocation of paraquat. Plant Cell Environ. 1978, 1, 203–209. [Google Scholar] [CrossRef]
- Harper, D.B.; Harvey, B.M.R. Mechanism of paraquat tolerance in perennial ryegrass. II. Role of superoxide dismutase, catalase and peroxidase. Plant Cell Environ. 1978, 1, 211–215. [Google Scholar] [CrossRef]
- Kuk, Y.-I.; Burgos, N.R.; Talbert, R.E. Evaluation of rice by-products for weed control. Weed Sci. 2001, 49, 141–147. [Google Scholar] [CrossRef]
- Bhargava, S. Paraquat tolerance in a photomixotrophic culture of Chenopodium rubrum. Plant Cell Rep. 1993, 12, 230–232. [Google Scholar] [CrossRef]
- Christoffoleti, P.J.; De Figueiredo, M.R.A.; Peres, L.E.P.; Nissen, S.; Gaines, T. Auxinic herbicides, mechanisms of action, and weed resistance: A look into recent. Sci. Agric. 2015, 72, 356–362. [Google Scholar] [CrossRef]
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of Resistance to Auxinic Herbicides: Historical Perspectives, Mechanisms of Resistance, and Implications for Broadleaf Weed Management in Agronomic Crops. Weed Sci. 2011, 59, 445–457. [Google Scholar] [CrossRef]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rey-Caballero, J.; Menéndez, J.; Giné-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 2016, 133, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Romero-Puertas, M.C.; McCarthy, I.; Gomez, M.; Sandalio, L.M.; Corpas, F.J.; Del Rio, L.A.; Palma, J.M. Reactive oxygen species-mediated enzymatic systems involved in the oxidative action of 2,4-dichlorophenoxyacetic acid. Plant Cell Environ. 2004, 27, 1135–1148. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).